Because the adult mammalian brain has a very limited capacity to replace neurons lost after lesion (1), understanding the mechanisms regulating their survival or elimination is of special significance. A study in this issue of PNAS (2) reveals that cutting the axons of a neuronal population involved in movement control leads to a progressive and dramatic increase of pro-nerve growth factor (NGF) in brain fluids. Pro-NGF kills injured neurons by virtue of its high affinity binding to a receptor that is induced after axotomy, the neurotrophin receptor p75. Another recent study also indicates that neurons may not be the only targets of pro-NGF-mediated killing: after partial transaction of the spinal cord, oligodendrocytes may also be eliminated by the mechanism described by Harrington and colleagues (3).
In mammals, the neurotrophin family consists of four genes that encode structurally related proteins that are proteolytically processed and secreted in the extracellular space (for review, see ref. 4). In the brain, the secretion of neurotrophins is regulated by the activity of the neurons that synthesize them, and after secretion, these proteins affect many important aspects of neuronal function, including synaptic transmission and excitability (for review, see ref. 5). As their names suggest, neurotrophins are best known for their “trophic” roles on neurons, typically including the promotion of nerve growth and the prevention of the death of embryonic neurons. These two properties were used to purify NGF and brain-derived neurotrophic factor (BDNF), respectively (for review, see ref. 4). Protein sequencing work revealed that the activity of these two neurotrophins is contained in what was shown by cDNA cloning to correspond to the C-terminal half of a larger precursor protein (Fig. 1). A first big surprise came when an intriguing report indicated that the neurotrophin receptor p75 causes the death of the cell line in which it was overexpressed (6). It was later found that the application of antibodies to NGF (including those used in the present study) or to the p75 receptor both blocked the elimination of cells in the developing chick retina (7). These results suggested that even in vivo, neurotrophins may not always prevent the death of neurons, but also precipitate it. More recently, another surprising report indicated that unprocessed, pro-NGF binds with high affinity to the p75 receptor but not to TrkA, the tyrosine kinase receptor mediating the trophic actions of NGF (8). The current report by Harrington and colleagues (2) is the first to demonstrate that pro-NGF can actually be detected in extracellular fluids, suggesting a significant biological role for this protein in the adult brain after lesion.
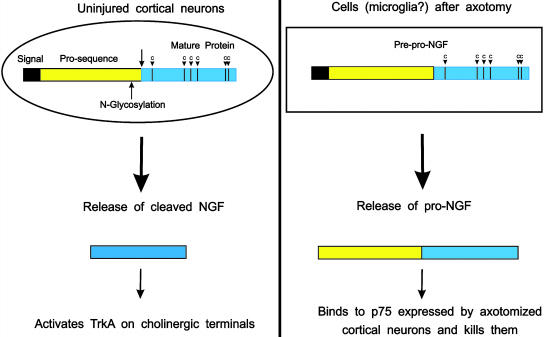
Fig. 1.
(Left) In the cortex, neurons release the “classical” (i.e., processed) form of NGF in an activity-dependent fashion. This is thought to positively feedback on TrkA-positive cholinergic terminals (Right). After section of their axons, cortical neurons express the neurotrophin receptor at much higher levels than normal. This receptor binds with high affinity pro-NGF released by cells of unknown origin (perhaps microglial cells activated by the lesion). Pro-NGF is detected in the cerebrospinal fluid, but only after lesion.
Like many other secreted growth factors, neurotrophins are synthesized as prepro-proteins. Until recently, essentially all of the work on neurotrophins was performed with the processed proteins. It was generally assumed that the N-glycosylated precursors merely represent transient intermediates allowing proper folding and appropriate disulfide bridging of the mature proteins. In addition, unlike processed neurotrophins that are highly conserved between different species, the sequences of the neurotrophin precursors differ substantially. With regard to biosynthesis, these sequences seem to be interchangeable, at least when tested in heterologous systems. For example, substituting the prosequence of pro-NT3 by that of pro-BDNF (or vice versa) does not change the yield of mature, biologically active neurotrophin dimers (9). An additional reason why little attention has been paid to pro-neurotrophins in a physiological context is the presence of a typical cluster of basic amino acids immediately preceding the mature sequence, a potential target for several, widely distributed proteases including plasmin, furins, and matrix metalloproteinases (8). This cleavage site was thought to make it unlikely that pro-neurotrophins would be secreted from intact cells in significant amounts. However, experiments with hippocampal neurons infected with vaccinia virus encoding prepro-BDNF revealed that these neurons release substantial amounts pro-BDNF (10). In addition, a frequent polymorphism was recently detected in the pro-sequence of the human bdnf gene that is predictive of memory performance. This Val–Met substitution is thought to affect the intracellular trafficking of pro-BDNF, an important finding that also contributes to increasing the interest in the pro-sequences of neurotrophins (11).
One of the questions raised by the present study is the cellular origin of pro-NGF. In the cortex before lesion, NGF cannot be detected at appreciable levels by Western blot analysis. However, results obtained by using sensitive immunoassays indicate that cortical cells, presumably neurons innervated by the forebrain cholinergic neurons, do synthesize NGF (12) and that these levels can be increased by various stimuli augmenting the activity of cortical neurons (13). After proteolytic processing, mature NGF is thought to be released and to act in a positive, “trophic” fashion on TrkA-positive, cholinergic terminals, as reflected, for example, by an increase in the levels of choline acetyl transferase. Interestingly, these cholinergic neurons also express the receptor p75, thus putting them at risk should they be exposed to pro-NGF. Also, Western blot analyses performed with extracts prepared from Alzheimer's brain revealed increased levels of pro-NGF (14), and it has long been known that forebrain cholinergic neurons are lost in Alzheimer's disease (15). The loss of these neurons has been proposed as part of the explanation for the cognitive deficits observed in this disease.
The appearance reported by Harrington and colleagues (2) of pro-NGF in the cerebrospinal fluid may be a consequence of the recruitment of cells such as microglial cells, long known to be attracted by lesioned neurons (16). These cells have been shown to express the NGF gene, and although it remains to be seen whether they can release pro-NGF, they have already been shown to cause the death of retinal cells during normal development by NGF- and p75-dependent mechanisms (17). In recent reports, the possible role of microglial cells as active “killers” has been examined, as opposed to cells merely removing the remnants of dead cells (for review, see ref. 18). The results clearly indicate that pro-NGF and p75 are not the only mechanisms used by microglial cells to kill neurons: the generation of superoxide ions or of tumor necrosis factor have been shown to cause the death of Purkinje cells (19) and motoneurons, respectively (20).
Death of neurons may not be a necessary consequence of their axons' being cut.
An intriguing aspect of the work of Harrington and colleagues (2) is that neither pro-BDNF nor pro-NT3 can be detected in the cerebrospinal fluid after section of the corticospinal tract, indicating an interesting specificity in the regulation of the NGF gene and the processing of the protein. In this context, it is of note that BDNF and NGF are differently regulated following lesion of the sciatic nerve (21).
Perhaps the most important practical implication of these new results is that death of neurons may not be a necessary consequence of their axons' being cut and that interfering with the action of a molecule released in the extracellular space may prevent cell death in the lesioned central nervous system. Whether this may also be relevant to neurodegenerative diseases is unclear, but in a broader physiopathological context, it is worth noting the similarities between the pro-NGF/p75 signaling system to the tumor necrosis factor receptors and the FAS signaling system (22). These receptors are all structurally related and often expressed by cells activated during the course of tissue injury of inflammation. In the context of Alzheimer's disease, retrospective studies have indicated that drugs decreasing inflammatory reactions decrease incidence of the disease (23). Whether they interfere directly or indirectly with the secretion of pro-NGF in the cerebrospinal fluid remains to be investigated.
See companion article on page 6226.
References
- 1.Nakatomi, H., Kuriu, T., Okabe, S., Yamamoto, S., Hatano, O., Kawahara, N., Tamura, A., Kirino, T. & Nakafuku, M. (2002) Cell 110, 429–441. [DOI] [PubMed] [Google Scholar]
- 2.Harrington, A. W., Leiner, B., Blechschmitt, C., Arevalo, J. C., Lee, R., Mörl, K., Meyer, M., Hempstead, B. L., Yoon, S. O. & Giehl, K. M. (2004) Proc. Natl. Acad. Sci. USA 101, 6226–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beattie, M. S., Harrington, A. W., Lee, R., Kim, J. Y., Boyce, S. L., Longo, F. M., Bresnahan, J. C., Hempstead, B. L. & Yoon, S. O. (2002) Neuron 36, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibel, M. & Barde, Y. A. (2000) Genes Dev. 14, 2919–2937. [DOI] [PubMed] [Google Scholar]
- 5.Poo, M.-M. (2001) Nature 2, 24–32. [DOI] [PubMed] [Google Scholar]
- 6.Rabizadeh, S., Oh, J., Zhong, L. T., Yang, J., Bitler, C. M., Butcher, L. L. & Bredesen, D. E. (1993) Science 261, 345–348. [DOI] [PubMed] [Google Scholar]
- 7.Frade, J. M., Rodríguez-Tébar, A. & Barde, Y. A. (1996) Nature 383, 166–168. [DOI] [PubMed] [Google Scholar]
- 8.Lee, R., Kermani, P., Teng, K. K. & Hempstead, B. L. (2001) Science 294, 1945–1948. [DOI] [PubMed] [Google Scholar]
- 9.Jungbluth, S., Bailey, K. & Barde, Y.-A. (1994) Eur. J. Biochem. 221, 677–685. [DOI] [PubMed] [Google Scholar]
- 10.Mowla, S. J., Pareek, S., Farhadi, H. F., Petrecca, K., Fawcett, J. P., Seidah, N. G., Morris, S. J., Sossin, W. S. & Murphy, R. A. (1999) J. Neurosci. 19, 2069–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan, M. F., Kojima, M., Callicott, J. H., Goldberg, T. E., Kolachana, B. S., Bertolino, A., Zaitsev, E., Gold, B., Goldman, D., Dean, M., et al. (2003) Cell 112, 257–269. [DOI] [PubMed] [Google Scholar]
- 12.Korsching, S., Auburger, G., Heumann, R., Scott, J. & Thoenen, H. (1985) EMBO J. 4, 1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thoenen, H., Zafra, F., Hengerer, B. & Lindholm, D. (1991) Ann. N.Y. Acad. Sci. 640, 86–90. [DOI] [PubMed] [Google Scholar]
- 14.Fahnestock, M., Michalski, B., Xu, B. & Coughlin, M. D. (2001) Mol. Cell. Neurosci. 18, 210–220. [DOI] [PubMed] [Google Scholar]
- 15.Terry, A. V., Jr., & Buccafusco, J. J. (2003) J. Pharmacol. Exp. Ther. 306, 821–827. [DOI] [PubMed] [Google Scholar]
- 16.Kreutzberg, G. W. (1996) Trends Neurosci. 19, 312–318. [DOI] [PubMed] [Google Scholar]
- 17.Frade, J. M. & Barde, Y. A. (1998) Neuron 20, 35–41. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, A. R. & Oppenheim, R. W. (2004) Neuron 41, 491–493. [DOI] [PubMed] [Google Scholar]
- 19.Marin-Teva, J. L., Dusart, I., Colin, C., Gervais, A., Van Rooijen, N. & Mallat, M. (2004) Neuron 41, 535–547. [DOI] [PubMed] [Google Scholar]
- 20.Sedel, F., Bechade, C., Vyas, S. & Triller, A. (2004) J. Neurosci 24, 2236–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer, M., Matsuoka, I., Wetmore, C., Olson, L. & Thoenen, H. (1992) J. Cell Biol. 119, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dechant, G. & Barde, Y. A. (2002) Nat. Neurosci. 5, 1131–1136. [DOI] [PubMed] [Google Scholar]
- 23.Aisen, P. S. (2002) Lancet Neurol. 1, 279–284. [DOI] [PubMed] [Google Scholar]