Abstract
Objective
To investigate a diagnostic value of ultrasonography in carpal tunnel syndrome (CTS) patients and to evaluate a correlation of sonographic measurements with the degree of electrodiagnostic abnormalities and clinical severity.
Methods
Two-hundred-forty-six symptomatic hands in 135 patients and 30 asymptomatic hands in 19 healthy individuals as control group were included. In ultrasonographic study, we measured the cross-sectional area (CSA) and flattening ratio (FR) of the median nerve at the pisiform as well as palmar bowing (PB) of the flexor retinaculum. Sensitivity and specificity of ultrasonographic measurements were evaluated and ultrasonographic data from the symptomatic and control hands were compared to the grade of electrodiagnostic and clinical severity.
Results
The mean CSA was 13.7±4.2 mm2 in symptomatic hands and 7.9±1.3 mm2 in asymptomatic hands. The mean FR was 4.2±1.0 in symptomatic hands and 3.4±0.4 in asymptomatic hands. The mean PB was 3.5±0.5 mm in symptomatic hands and 2.6±0.3 mm in asymptomatic hands. Statistical analysis showed differences of the mean CSA, FR and PB between groups were significant. A cut-off value of 10 mm2 for the mean CSA was found to be the upper limit for normal value. Both the mean CSA and PB are correlated with the grade of electrophysiological abnormalities and clinical severity, respectively.
Conclusion
Ultrasographic measurement of the CSA and PB is helpful to diagnose CTS as a non-invasive and an alternative modality for the evaluation of CTS. In addition, ultrasonography also provides a reliable correlation with the grade of electrodiagnostic abnormalities and clinical severity.
Keywords: Carpal tunnel syndrome, Ultrasonography, Diagnosis, Severity
INTRODUCTION
Carpal tunnel syndrome (CTS) is the most frequent entrapment neuropathy, which occurs as a consequence of compression of the median nerve at the wrist. The diagnosis is usually based on characteristic symptoms and signs, and electrophysiological studies4,21). The most reliable method to confirm clinical diagnosis of CTS is electrophysiological study18,19) but false negatives can be seen in the variable range of 10-20%7). The electrophysiological studies usually show the level of the lesion, but do not provide anatomical information about the nerve or its surroundings. In the last few years, ultrasonography (US) has been shown to be useful diagnostic tools in CTS, providing information on the median nerve and surrounding structures4,6,13,23). A few papers have been published in neurosurgery literature regarding diagnostic utility of ultrasonography in carpal tunnel syndrome1,6). To our knowledge, publication has not been available, describing measurements of ultrasonographic parameters for the diagnosis of CTS, compared with electrodiagnostic findings as well as clinical severity, assessed by historical-objective scale9). The purpose of the present study is to identify the efficacy of ultrasonographic measurements in a consecutive sample of patients with clinically diagnosed CTS and to analyze the correlation of ultrasonographic findings with electrophysiological abnormalities and clinical severity.
MATERIALS AND METHODS
Participants and clinical grade
The clinical diagnostic criterion of CTS is based on the American Academy of Neurology21). One-hundred thirty-five patients who had the symptoms of CTS between January 2007 and December 2009 were included in this study. Nineteen persons with no clinical symptoms and signs of CTS were classified as the control group. Patients with diabetes mellitus, collagen disease, thyroid disease, peripheral neuropathy, rheumatic arthritis, traumatic nerve injury were excluded from the study. The patient group contained total of 246 hands, whereas 30 hands were used in the control group. The clinical grade in the patient group was classified into 6 stages according to the historical-objective (Hi-Ob) clinical scale as follows9); stage 0 (no symptoms), stage 1 (paresthesia only at night), stage 2 (paresthesia even for short time in the daytime), stage 3 (hypesthesia in the finger of the median nerve distribution), stage 4 (accompanying weakness or thenar muscle atrophy), and stage 5 (thenar muscle complete atrophy or paralysis).
Mild cases in Hi-Ob scale were defined as those patients who reported only symptoms without objective motor deficit of thenar eminence muscles and normal objective sensory function in the median nerve territory of the hand. Grades 1 or 2 of a Hi-Ob scale belong to mild case9).
Ultrasonographic examination
Ultrasonographic examination was performed using Sonix RP (Ultrasonix Medical Corporation, Richmond, Canada) by the same experienced examiner (HSN). All wrists were evaluated in the neutral position with the palm up and the fingers semi-extended. The full course of the median nerve in the carpal tunnel was evaluated in both the transverse and sagittal planes. The weight of the probe was applied without additional pressure. The cross-sectional area (CSA) of the median nerve (measured in mm2) was measured to proximal carpal tunnel at the level of the pisiform bone. The CSA of the median nerve was measured by direct methods as suggested by Duncan et al.7), which was calculated automatically by tracing the inner margin of the epineurium of median nerve, assuming that it has an elliptical shape (Fig. 1A). The flattening ratio (FR) was defined as the ratio of the nerve's transverse axis to the antero-posterior axis (Fig. 1B) and was assessed at the level of the pisiform bone. The palmar bowing (PB) of the flexor retinaculum is displacement (measured in mm) of the retinaculum from the attachments of a ligament connecting the pisiform bone with the scaphoid bone (Fig. 1C)5).
Fig. 1.
Transverse sonographic section of an enlarged median nerve (indicated by arrow) at the level of the pisiform bone in a subject with CTS. The cross-sectional area (CSA) of the median nerve (direct method within the oval dotted line) (A). The flattening ratio (FR) is defined as the ratio of the nerve's transverse axis to the antero-posterior axis (B). The palmar bowing (PB) of the flexor retinaculum is displacement of the retinaculum from the attachments of a ligament connecting the pisiform bone with the scaphoid bone (C). S: scaphoid bone, P: pisiform bone.
Electrophysiological study
The electrophysiological study was performed using a Cadwell Sierra Wave® (Cadwell Laboratories, Kennewick, WA, USA). The study consisted of motor and sensory median nerve conduction tests using standard techniques according to the practice parameters for the electrodiagnosis of CTS of the American Association of Electrodiagnostic Medicine, the American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation3). The electrophysiological abnormalities were classified into three grades according to Stevens' classification as follows23): Mild; prolonged median sensory distal latency, Moderate; prolonged median sensory and motor distal latency, and Severe; abnormal needle electromyography other than the above two abnormalities, or no response in sensory and motor distal latency.
Statistical analysis
Measurements by using US in both the CTS patients and control group were compared and the accuracy of diagnostic techniques was calculated. The arithmetic mean, range and the standard deviation of the results were determined and both groups were compared using independent t-tests. Sensitivity and specificity of US measurements in CTS patients were obtained by determining the cut-off point using the receiver operating characteristics (ROC) curve. The correlation between US measurements and Hi-Ob scale and between the electrophysiological study and US measurements were assessed by using Spearman's test. According to Hi-Ob scale and electrophysiological severity, accuracy of US was evaluated. SPSS statistical software (v. 11.0, SPSS Inc., Chicago, IL, USA) was used in all statistical analyses. A p value <0.05 was considered significant.
RESULTS
Demographic data
Of a total of 246 hands in the patient group, 30 hands were male and 216 hands were female. The mean age was 53.0 years. Of a total 30 hands in the control group, 27 hands were female and 3 hands were male, and the mean age was 41.7 years.
Ultrasonographic measurements
Results of this study showed the significant increase in the measures of the CSA, FR and PB in the CTS, compared with the control group (p<0.05) (Table 1). The accuracy of ultrasonographic measurements was evaluated by using cut off points of ROC curve. The area under the curve (AUC) of CSA was 0.95, indicating a sensitivity and specificity of 88.5% and 90.0% respectively, at cut off value of 10.0 mm2. The AUC of FR was 0.74 at cut off value of 3.4 indicating a sensitivity and specificity of 77.8% and 50.0% respectively. The AUC of PB was 0.94 at cut off value of 3.0 mm indicating a sensitivity and specificity of 87.2% and 93.3% respectively (Table 2, Fig. 2).
Table 1.
Comparisons of the ultrasonographic measurements between CTS patients and control
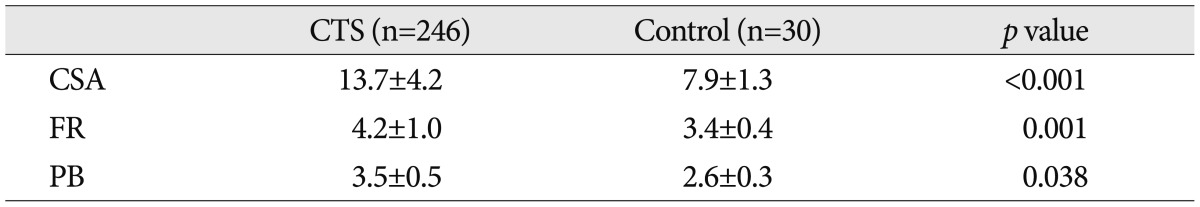
Cross sectional area unit: (mm2), Palmar bowing unit: (mm). CTS: carpal tunnel syndrome, CSA: cross sectional area, FR: flattening ratio, PB: palmar bowing
Table 2.
Accuracy of the statistics for the cut-off points of the ultrasonographic measurements contained using the receiver operating characteristic curve

Cross sectional area unit: (mm2), Palmar bowing unit: (mm). CSA: cross sectional area, FR: flattening ratio, PB: palmar bowing, AUC: area under curve
Fig. 2.
ROC curve of ultrasonographic measurements. ROC: receiver operating characteristic, CSA: cross sectional area, FR: flattening ratio, PB: palmar bowing.
Correlation of the ultrasonography with Hi-Ob scale and electrophysiological study
The CSA and PB of US measurements, and Hi-Ob scale appeared to show a significant positive correlation and as the Hi-Ob score increased, the CSA and PB was increased (r=0.45: p<0.001, r=0.37: p<0.001) (Table 3). The increase in electrophysiological severity, and the CSA and PB of US measurements also showed a significant correlation (r=0.59: p<0.001, r=0.51: p<0.001) (Table 4).
Table 3.
Correlation of ultrasonographic measurements according to Hi-Ob scale
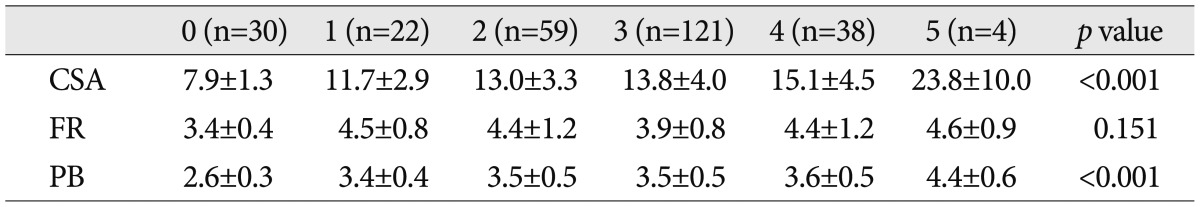
Cross sectional area unit: (mm2), Palmar bowing unit: (mm). Hi-Ob scale: historical-objective scale, CSA: cross sectional area, FR: flattening ratio, PB: palmar bowing
Table 4.
Correlation of ultrasonographic measurements according to electrophysiologic grade

Cross sectional area unit: (mm2), Palmar bowing unit: (mm). CSA: cross sectional area, FR: flattening ratio, PB: palmar bowing
Accuracy of ultrasonography according to Hi-Ob and electrophysiological grade
Sensitivity of CSA and PB for mild patients with less than 3 Hi-Ob scales were 83.9% and 85.1%, respectively. However, the cases with 3 or more Hi-Ob scale showed higher sensitivity of CSA (92.0%) and PB (89.4%). In relation to electrophysiological study, the mean CSA and PB of ultrasonographic measurement usually appeared to be decreased in the mild cases. In mild 65 hands confirmed to the electrophysiological study, 15 hands (22.4%) were found to be negative by CSA and 18 hands (26.9%) by PB. However, all of very severe five hands with no response to the electrophysiological study showed positive results in ultrasonographic measurements (Table 5).
Table 5.
Results of the ultrasonographic measurements in relation to mild and very severe cases with no response to electrophysiologic study
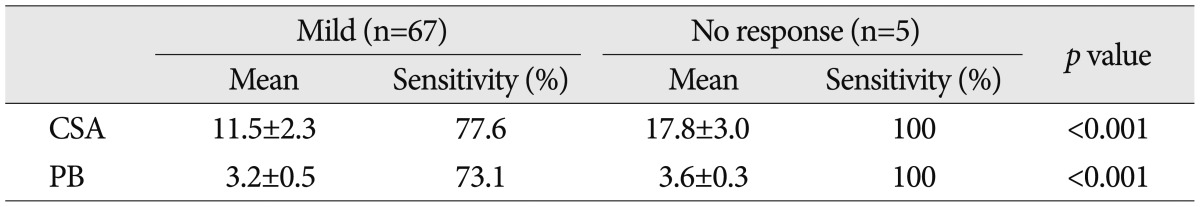
Cross sectional area unit: (mm2), Palmar bowing unit: (mm). CSA: cross sectional area, PB: palmar bowing
DISCUSSION
The diagnosis of CTS is made mainly on the basis of the patient's history and the clinical features10,12). The Hi-Ob scale is a reliable measurement in CTS evaluation, based on clinical history and physical examination9). This scale has no diagnostic purpose but it has a good correlation with the patient-oriented findings and the median nerve electrophysiological impairment9). Confirmation of CTS is usually evaluated by electrophysiological study3). However, sometimes, it is difficult to diagnose CTS using only the electrophysiological study in cases of early and mild CTS, even severe CTS that show no response to the study, elderly patients and associated peripheral polyneuropathy patients.
Recently, US techniques came into advancement as a tool to complement the diagnosis of CTS. US provide anatomical images of the median nerve, neighboring structures, and space-occupying lesion in the carpal canal as a painless and non-invasive study and can offer dynamic images. However, US is operator dependent that can show high reproducibility after adequate training of the examiners4). In our study, sonographic examination was done by one experienced examiner to ensure unbiased imaging. The US measurements used in CTS diagnosis are the CSA of the median nerve at various levels of the carpal canal, the FR and PB of the flexor retinaculum. In most studies, the CSA was performed at a single level at the proximal carpal tunnel7,15,26,27). The increase in the CSA at the tunnel inlet demonstrated the highest sensitivity and specificity8,26,27) and moreover, the measurement at this level was easier to perform. Therefore, we measured the CSA at the proximal tunnel inlet. There was also disagreement about the exact localization of tunnel inlet. Some authors considered the proximal edge of the flexor retinaculum, approximately at the level of the distal radioulnar joint, as the tunnel inlet but others considered the level of the pisiform bone as the landmarks11). We evaluated the CSA at the level of the pisiform bone.
The sensitivity of ultrasonographic measurements varies widely among studies. The sensitivity of the CSAs ranged from 48% to 89%4,7,8,26,27) and the CSA cutoff at which the value was considered abnormal, varied from 9 mm2 to 15 mm1,2,7,14,18,26). Our study showed a sensitivity of 88.5% at cut off value of 10.0 mm2 for the mean CSA. The sensitivities of increased PB of the flexor retinaculum varied from 40% to 81%2,5,14,24), and sensitivities of FR ranged from 37% to 100%5,28). Our results showed a sensitivity of 87.2% at cut off value of 3.0 mm2 for the PB and a sensitivity of 77.8% at cut off value of 3.4 mm2 for the FR. These data of sensitivity corresponds with the findings reported in earlier studies.
In our study, CSA and FR, PB of US were significantly increased in the CTS group than the control group. Among them, CSA and PB were evaluated to have a relatively higher accuracy than FR according to the ROC curve. Therefore, measurement of CSA and/or PB can be considered as an alternative modality to distinguish CTS patients from asymptomatic controls2,5,14,24,28).
Many other studies showed good correlation between the Hi-Ob scale and US, and between electrophysiological grade and US indicating that the nerve swelling detected by calculation of US reflects in itself the degree of nerve damage: the greater the severity of electrophysiological findings or clinical severity, the greater the CSA of median nerve13,16,20,29). Results of this study showed there is a significant correlation between Hi-Ob scale and CSA as well as PB, respectively. In addition, degree of electrodiagnostic abnormalities is also well correlated with CSA and PB, respectively in our study.
Mild CTS cannot show abnormal findings on US study. In our data, US could not detect abnormalities in 22.4% of patients with mild CTS to electrophysiological study. For patients without abnormal findings on US and electrophysiological study, the diagnosis of CTS should be made, based on the patient's history and the clinical findings. However, our data also demonstrated ultrasonic measurement is helpful to diagnose extremely severe CTS patients with no response to electrophysiological studies and these data agree with earlier study14).
CONCLUSION
Ultrasonographic measurements of the CSA and PB of the median nerve offer comparatively high diagnostic accuracy for CTS and ultrasonographic study could be considered as a non-invasive, alternative and complimentary diagnostic modality for the evaluation of CTS. In addition to diagnosis of CTS, ultrasonographic measurements of the median nerve could also give information about severity of CTS.
Acknowledgements
The authors wish to acknowledge the contribution of Sooa Nam, R.N., for editorial support.
References
- 1.Ahn SY, Hong YH, Koh YH, Chung YS, Lee SH, Yang HJ. Pressure measurement in carpal tunnel syndrome: correlation with electrodiagnostic and ultrasonographic findings. J Korean Neurosurg Soc. 2009;46:199–204. doi: 10.3340/jkns.2009.46.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altinok T, Baysal O, Karakas HM, Sigirci A, Alkan A, Kayhan A. Ultrasonographic assessment of mild and moderate idiopathic carpal tunnel syndrome. Clin Radiol. 2004;59:916–925. doi: 10.1016/j.crad.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 3.American Association of Electrodiagnostic Medicine; American Academy of Neurology; American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. 2002;25:918–922. doi: 10.1002/mus.10185. [DOI] [PubMed] [Google Scholar]
- 4.Beekman R, Visser LH. Sonography in the diagnosis of carpal tunnel syndrome: a critical review of the literature. Muscle Nerve. 2003;27:26–33. doi: 10.1002/mus.10227. [DOI] [PubMed] [Google Scholar]
- 5.Buchberger W, Schön G, Strasser K, Jungwirth W. High-resolution ultrasonography of the carpal tunnel. J Ultrasound Med. 1991;10:531–537. doi: 10.7863/jum.1991.10.10.531. [DOI] [PubMed] [Google Scholar]
- 6.Deniz FE, Oksüz E, Sarikaya B, Kurt S, Erkorkmaz U, Ulusoy H, et al. Comparison of the diagnostic utility of electromyography, ultrasonography, computed tomography, and magnetic resonance imaging in idiopathic carpal tunnel syndrome determined by clinical findings. Neurosurgery. 2012;70:610–616. doi: 10.1227/NEU.0b013e318233868f. [DOI] [PubMed] [Google Scholar]
- 7.Duncan I, Sullivan P, Lomas F. Sonography in the diagnosis of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;173:681–684. doi: 10.2214/ajr.173.3.10470903. [DOI] [PubMed] [Google Scholar]
- 8.El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology (Oxford) 2004;43:887–895. doi: 10.1093/rheumatology/keh190. [DOI] [PubMed] [Google Scholar]
- 9.Giannini F, Cioni R, Mondelli M, Padua R, Gregori B, D'Amico P, et al. A new clinical scale of carpal tunnel syndrome: validation of the measurement and clinical-neurophysiological assessment. Clin Neurophysiol. 2002;113:71–77. doi: 10.1016/s1388-2457(01)00704-0. [DOI] [PubMed] [Google Scholar]
- 10.Grundberg AB. Carpal tunnel decompression in spite of normal electromyography. J Hand Surg Am. 1983;8:348–349. doi: 10.1016/s0363-5023(83)80179-8. [DOI] [PubMed] [Google Scholar]
- 11.Hammer HB, Hovden IA, Haavardsholm EA, Kvien TK. Ultrasonography shows increased cross-sectional area of the median nerve in patients with arthritis and carpal tunnel syndrome. Rheumatology (Oxford) 2006;45:584–588. doi: 10.1093/rheumatology/kei218. [DOI] [PubMed] [Google Scholar]
- 12.Iyer VG. Understanding nerve conduction and electromyographic studies. Hand Clin. 1993;9:273–287. [PubMed] [Google Scholar]
- 13.Karadağ YS, Karadağ O, Ciçekli E, Oztürk S, Kiraz S, Ozbakir S, et al. Severity of Carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int. 2010;30:761–765. doi: 10.1007/s00296-009-1061-x. [DOI] [PubMed] [Google Scholar]
- 14.Keleş I, Karagülle Kendi AT, Aydin G, Zöğ SG, Orkun S. Diagnostic precision of ultrasonography in patients with carpal tunnel syndrome. Am J Phys Med Rehabil. 2005;84:443–450. doi: 10.1097/01.phm.0000163715.11645.96. [DOI] [PubMed] [Google Scholar]
- 15.Koyuncuoglu HR, Kutluhan S, Yesildag A, Oyar O, Guler K, Ozden A. The value of ultrasonographic measurement in carpal tunnel syndrome in patients with negative electrodiagnostic tests. Eur J Radiol. 2005;56:365–369. doi: 10.1016/j.ejrad.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH, Kim TK, Yoon ES, Dhong ES. Correlation of high-resolution ultrasonographic findings with the clinical symptoms and electrodiagnostic data in carpal tunnel syndrome. Ann Plast Surg. 2005;54:20–23. doi: 10.1097/01.sap.0000141942.27182.55. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, van Holsbeeck MT, Janevski PK, Ganos DL, Ditmars DM, Darian VB. Diagnosis of carpal tunnel syndrome. Ultrasound versus electromyography. Radiol Clin North Am. 1999;37:859–872. doi: 10.1016/s0033-8389(05)70132-9. [DOI] [PubMed] [Google Scholar]
- 18.Nathan PA, Keniston RC, Meadows KD, Lockwood RS. Predictive value of nerve conduction measurements at the carpal tunnel. Muscle Nerve. 1993;16:1377–1382. doi: 10.1002/mus.880161217. [DOI] [PubMed] [Google Scholar]
- 19.Padua L, LoMonaco M, Gregori B, Valente EM, Padua R, Tonali P. Neurophysiological classification and sensitivity in 500 carpal tunnel syndrome hands. Acta Neurol Scand. 1997;96:211–217. doi: 10.1111/j.1600-0404.1997.tb00271.x. [DOI] [PubMed] [Google Scholar]
- 20.Padua L, Pazzaglia C, Caliandro P, Granata G, Foschini M, Briani C, et al. Carpal tunnel syndrome: ultrasound, neurophysiology, clinical and patient-oriented assessment. Clin Neurophysiol. 2008;119:2064–2069. doi: 10.1016/j.clinph.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Practice parameter for carpal tunnel syndrome (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1993;43:2406–2409. [PubMed] [Google Scholar]
- 22.Sarría L, Cabada T, Cozcolluela R, Martínez-Berganza T, García S. Carpal tunnel syndrome: usefulness of sonography. Eur Radiol. 2000;10:1920–1925. doi: 10.1007/s003300000502. [DOI] [PubMed] [Google Scholar]
- 23.Stevens JC. AAEM minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. American Association of Electrodiagnostic Medicine. Muscle Nerve. 1997;20:1477–1486. doi: 10.1002/(sici)1097-4598(199712)20:12<1477::aid-mus1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Stewart JD, Eisen A. Tinel's sign and the carpal tunnel syndrome. Br Med J. 1978;2:1125–1126. doi: 10.1136/bmj.2.6145.1125-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai TW, Wu CY, Su FC, Chern TC, Jou IM. Ultrasonography for diagnosing carpal tunnel syndrome: a meta-analysis of diagnostic test accuracy. Ultrasound Med Biol. 2012;38:1121–1128. doi: 10.1016/j.ultrasmedbio.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 26.Wiesler ER, Chloros GD, Cartwright MS, Smith BP, Rushing J, Walker FO. The use of diagnostic ultrasound in carpal tunnel syndrome. J Hand Surg Am. 2006;31:726–732. doi: 10.1016/j.jhsa.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Wong SM, Griffith JF, Hui AC, Tang A, Wong KS. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002;46:1914–1921. doi: 10.1002/art.10385. [DOI] [PubMed] [Google Scholar]
- 28.Yesildag A, Kutluhan S, Sengul N, Koyuncuoglu HR, Oyar O, Guler K, et al. The role of ultrasonographic measurements of the median nerve in the diagnosis of carpal tunnel syndrome. Clin Radiol. 2004;59:910–915. doi: 10.1016/j.crad.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 29.Ziswiler HR, Reichenbach S, Vögelin E, Bachmann LM, Villiger PM, Jüni P. Diagnostic value of sonography in patients with suspected carpal tunnel syndrome: a prospective study. Arthritis Rheum. 2005;52:304–311. doi: 10.1002/art.20723. [DOI] [PubMed] [Google Scholar]




