ABSTRACT
Much research focuses on producing maximal intervention effects. This has generally not resulted in interventions being rapidly or widely adopted or seen as feasible given resources, time, and expertise constraints in the majority of real-world settings. We present a definition and key characteristics of a minimum intervention needed to produce change (MINC). To illustrate use of a MINC condition, we describe a computer-assisted, interactive minimal intervention, titled Healthy Habits, used in three different controlled studies and its effects. This minimal intervention produced modest to sizable health behavior and psychosocial improvements, depending on the intensity of personal contacts, producing larger effects at longer-term assessments. MINC comparison conditions could help to advance both health care and health research, especially comparative effectiveness research. Policy and funding implications of requiring an intervention to be demonstrated more effective than a simpler, less costly MINC alternative are discussed.
KEYWORDS: Intervention, Costs, Research methods, Comparative effectiveness
INTRODUCTION
Health care costs in the US were $2.6 trillion in 2010 [1], were 17.4 % [2] of the gross domestic product in 2009, and are expected to rise to 25 % [3]. These costs have increased substantially over the past few decades (http://www.healthcare.gov) [3]; with the aging of the population, as well as developments in personalized medicine and enhanced diagnostic techniques, likely to increase in the future.
Unfortunately, numerous effective interventions are available but not widely or equitably distributed [4]. Low-income populations and low-resource settings face challenges in finding the time, resources, and infrastructure supports necessary to produce successful outcomes from intensive interventions. Thus, many existing, evidence-based interventions have low or limited reach among individual patients and reduced adoption by clinical and public health settings [5].
A number of interventions have been shown to be effective, but it is often difficult for consumers, practitioners, and researchers to know which is best—and to distinguish any meaningful differences in outcomes and cost-effectiveness [6]. These concerns have, in part, given rise to comparative effectiveness research (CER) [6]. The use of aspirin for cardiovascular disease prevention offers a useful example. Aspirin is a widely available, minimal, low-cost, effective intervention. A pragmatic CER perspective suggests that more expensive, intensive, or aggressive interventions should be proven to be more clinically and cost-effective than aspirin to be recommended for use.
A newer CER strategy for identifying effective interventions is based on the concept of “minimal intervention needed to produce change,” or MINC (Robert Croyle, personal communication, 2010) [7]. In this article, we define MINC as “the minimal or lowest level of intervention intensity, expertise, and resources needed to achieve a clinically significant improvement in a specified outcome for a particular target population under a particular set of conditions, when delivered by a specified type of staff or interactive modality.” The goal is to provide an anchor or standard to help compare different interventions to determine the relative improvements based on their relative costs. By defining and documenting the MINC in a given content area, it is possible to identify the least costly, easiest-to-administer interventions that are most likely to be disseminated [7].
Several designs have been used to establish the MINC in different phases of implementation, including maintenance. For example, adaptively designed interventions [7–10] have been used in studies of mammography adherence, in which intervention components and intensity change are linked specifically with patient and care system response [11, 12]. Other designs have included stepped-care approaches [13] that directly compare more and less intense intervention components, manipulation of reminders and encouragement to understand how to motivate people to engage in the desired behavior, and variations of follow-up support to understand how to sustain the behavior change over time [7]. Thus, finding the MINC presents a promising strategy for applying the principles of CER.
The purposes of this paper are to define MINC for practical use (see above), including its key characteristics; provide applied example applications; and discuss the potential use and implications of MINC for health care research and policy.
Key characteristics
Table 1 summarizes key features of MINCs. As can be seen, a MINC includes a minimal number of theoretical and empirically based components [14]. A MINC should contain only enough components to produce meaningful change, as defined above. Ideally, each intervention component has low intensity and low cost. For example, effective MINCs incorporate the minimum number of sessions, and personal contacts or doses needed for success, and utilize the least trained and least expensive staff (e.g., health educators instead of physicians; community health workers instead of professional staff) that can effectively implement a program. Finally, as recommended by Rogers [15], to increase widespread adoption, implementation, and sustainability, MINCs should have low design and application complexity. From a Reach, Effectiveness, Adoption, Implementation, Maintenance [16, 17] or access and scalability perspective, all of the above features tend to make MINCs more broadly adoptable and to increase reach and quality of implementation, and are theoretically more likely to be sustained than more costly, complex, and intensive interventions.
Table 1.
Characteristics and examples of MINCs
| Characteristic | MINC Status | Healthy Habits/Leap Ahead example MINC |
|---|---|---|
| Intensity | Low–moderate: Just sufficient for change | Largely automated; no in-person contact required; only phone follow-up |
| Cost and Resources Needed to Implement | Low: Developed to require few new resources of setting and participants | Only require laptop computer in clinic; no time from primary care staff; minimal training for research assistant |
| Theoretical Components | Few effective strategies: Use of common intervention techniques | Assessment; feedback; nonspecific support; follow-up |
| Complexity | Low: Only what is needed for change | Easy to implement |
A MINC example
To illustrate development and use of MINC interventions, we describe a MINC chronic disease self-management intervention used in three controlled diabetes studies. The computerized intervention, called variously Healthy Habits or Leap Ahead, consisted of a health risk appraisal based on recommendations from the US Preventive Services Task Force [18]. Healthy Habits/Leap Ahead provided a realistic minimal intervention to enhance usual care for diabetes self-management. This condition was initially developed primarily to provide a credible attention control condition for the automated interactive diabetes self-management interventions. It was only at the time of our third funded study (Reducing Distress and Enhancing Effective Management (REDEEM)) that we began conceptualizing this as a MINC condition, given some of our findings below. Targeted to the user's age and gender, it assessed diabetes-specific and generic preventive activities. Upon completion of Healthy Habits/Leap Ahead, a printable report was generated with standardized recommendations [19], reinforcing diabetes self-management practices (e.g., checking one's feet, not smoking, monitoring blood glucose levels, getting flu shots) and general preventive activities (e.g., wearing a seat belt, getting a mammogram) [18]. Because Healthy Habits/Leap Ahead was developed to provide a realistic but minimal intervention comparison to more intensive intervention, it did not provide assistance in the hypothesized key intervention processes of goal setting or developing an action plan for the specific health behaviors under study (diet, medication adherence, and exercise).
Healthy Habits was created in Macromedia Director and was deployed as a stand-alone desktop application for Windows computers. It was developed in English and translated into Spanish. To maximize ease of use, keyboard input was required only during the registration when participants entered name and study ID. Thereafter, all user inputs were via mouse clicks.
Participants completed a 19- to 21-item health risk assessment that included 14 questions related to their diabetes care, and 5–7 questions related to general preventive care targeted to their gender and age. Questions were presented with onscreen text as well as voiced by a female narrator (Fig. 1). The narration could be turned on or off at any time. The program then generated a two-page printout, as shown in Fig. 2. The first page listed the participant's positive health habits with a brief description of each, followed by a listing of areas where the participant was at risk and ways to address those risks. The second page listed recommended preventive care procedures grouped by age range, with the participant's age range highlighted.
Fig 1.
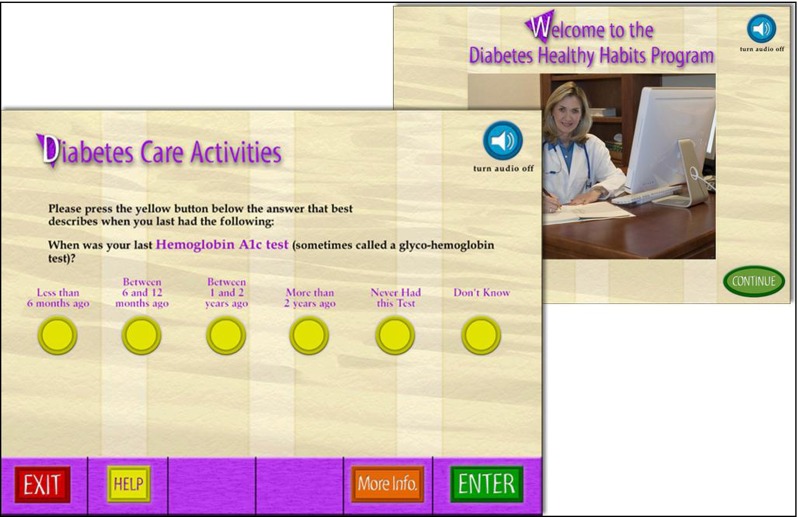
User interface screens from Healthy Habits
Fig 2.
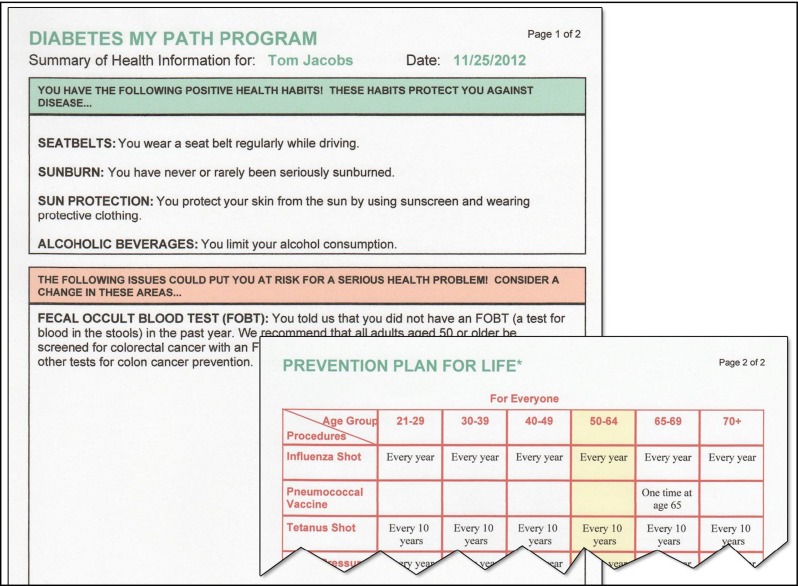
Two-section printout from Healthy Habits
Participant data were stored locally in an SQLite database. If a participant entered a study ID that was already in the database, the program presented several options—resuming the program, retaking the program, or reprinting the printout—to maximize its ability to be worked into a typical practice's patient flow. Healthy Habits/Leap Ahead provided an opportunity for systematically asking patients about diabetes-related care procedures (e.g., foot exams, eye exams) and both tailored and targeted advice on recommended preventive care (e.g., alcohol use, vaccines) using minimal patient and staff time.
Illustrations using Healthy Habits/Leap Ahead
Following is a brief description of three studies in which the Healthy Habits/Leap Ahead MINC has been used (Table 2).
Table 2.
Characteristics of participants assigned to the MINC in three diabetes studies
| DHC (n = 82) Mean (SD) or % | MyPath (n = 132) Mean (SD) | REDEEM (n = 96) Mean (SD) or % | |
|---|---|---|---|
| Age (years) | 63.36 (9.49) | 58.58 (9.05) | 55.23 (10.88) |
| Sex (% female) | 39.0 % | 51.5 % | 59.4 % |
| Years with diagnosis | 7.72 (7.63) | 7.95 (estimated) | 7.60 (6.44) |
| Education | |||
| % ≤ high school | 24.7 % | 10.6 % | 10.4 % |
| % Tech school | 38.3 % | 45.5 % | 28.1 % |
| % College | 33.3 % | 41.7 % | 61.5 % |
| No. of comorbidities | 3.00 (1.86) [of 9] | NR | 3.55 (2.75) [of 12] |
| % on insulin | 15.2 % | 30.0 % | 19.8 % |
| Ethnicity (% White) | 77.2 % | 70.6 % | 35.4 % |
| % smoke | 11.1 % | 10.6 % | 10.4 % |
| BMI (kg/m2) | 31.61 (7.00) | 34.45 (6.29) | 34.83 (6.54) |
NR Not Reported
Diabetes Health Connection
The Diabetes Health Connection (DHC) study [20] was a randomized trial that evaluated the effectiveness of a CD-ROM-based diabetes self-management intervention relative to Healthy Habits. The more complex self-management intervention used a computer-assisted behavior-change program to facilitate healthful, patient-selected dietary and physical activity behaviors. All patients, regardless of treatment condition, completed four study visits facilitated by a research staff member. Participants in the self-management condition received a tailored intervention that combined interactive technology with one-on-one health counseling and telephone follow-up support between visits, and featured tailored goal setting, creation of a personalized diabetes self-management plan, and barriers-based problem solving. Self-management participants also received 10- to 15-min follow-up calls 1 week and 1 month later to review their goals, barriers, and strategies and to revise their plan (Healthy Habits MINC participants did not receive these added contacts).
MyPath to Healthy Life
MyPath to Healthy Life (MyPath) was a pragmatic trial [21] conducted at Kaiser Permanente Colorado with 463 adults with type 2 diabetes (Table 3.) Participants were randomized to one of three conditions: Healthy Habits; a web-based diabetes self-management support program (MyPath) that included online assistance for setting tailored goals for diet, physical activity, and medication taking; or the same web-based diabetes self-management support program plus additional in-person support (MyPath-Plus). The online MyPath program was similar to the DHC program, with added features for tracking progress on self-management behaviors, a library of educational materials, and an “ask an expert” feature.
Table 3.
Behavior change results across three diabetes studies on common measures (Healthy Habits/Leap Ahead MINC only)1
| DHC (n = 82) | MyPath (n = 132) | REDEEM (n = 96) | |
|---|---|---|---|
| Mean (SD) [F; p] | Mean (SD) [F; p] | Mean (SD) [F; p] | |
| Food Behav/Habits | Kristal score | STC score | STC score |
| T1–T2 | 2.28 (0.39) to 2.21 (0.41) [4.84; 0.031] | 2.13 (0.31) to 2.18 (0.27) [6.74; 0.011] | 2.07 (0.29) to 2.14 (0.27) [3.38; 0.07] |
| T1–T3 | 2.27 (0.39) to 2.19 (0.39) [5.64; 0.020] | 2.14 (0.30) to 2.23 (0.30) [13.10; <0.001] | 2.07 (0.29) to 2.21 (0.25) [11.66; 0.001] |
| T2–T3 | 2.21 (0.40) to 2.18 (0.39) [0.59; 0.45] | 2.18 (0.26) to 2.23 (0.30) [4.42; 0.038] | 2.14 (0.27) to 2.21 (0.25) [3.73; 0.057] |
| Fat intake | NCI; grams | NCI; % | NCI; % |
| T1–T2 | 30.94 (14.56) to 26.67 (14.54) [7.28; 0.009] | 35.10 (4.91) to 35.42 (6.77) [0.30; 0.59] | 32.08 (4.21) to 31.25 (2.79) [4.23; 0.042] |
| T1–T3 | 30.60 (15.00) to 31.91 (18.27) [0.33; 0.57] | 35.28 (4.92) to 33.98 (4.30) [12.67; 0.001] | 32.08 (4.21) to 30.78 (3.50) [5.77; 0.018] |
| T2–T3 | 26.87 (14.91) to 31.69 (17.71) [5.59; 0.021] | 35.61 (6.99) to 33.95 (4.12) [5.86; 0.017] | 31.25 (2.79) to 30.78 (3.50) [1.22; 0.27] |
| Physical activity | Freq/week all PA | Freq/week all PA | Cals/week all PA |
| T1–T2 | 30.80 (26.85) to 30.43 (21.88) [0.02; 0.89] | 45.48 (51.86) to 41.69 (42.2) [1.53; 0.22] | 3,240 (2,384) to 4,030 (3,054) [8.73; 0.004] |
| T1–T3 | 30.90 (27.49) to 32.54 (29.65) [0.22; 0.64] | 45.18 (47.92) to 43.41 (48.02) [0.22; 0.64] | 3,240 (2,384) to 3,464 (2,928) [0.46; 0.50] |
| T2–T3 | 31.73 (22.11) to 32.04 (29.66) [0.01; 0.92] | 41.04 (39.09) to 40.87 (45.40) [0.00; 0.95] | 4,030 (3,054) to 3,464 (2,928) [3.15; 0.08] |
| Diabetes distress | Total distress | Total distress | |
| T1–T2 | 2.88 (1.21) to 2.80 (1.19) [0.86; 0.36] | 2.48 (0.85) to 2.27 (0.92) [8.65; 0.004] | |
| T1–T3 | 2.87 (1.18) to 2.64 (1.15) [5.50; 0.021] | 2.48 (0.95) to 1.98 (0.88) [34.25; <0.001] | |
| T2–T3 | 2.80 (1.17) to 2.63 (1.16) [2.73; 0.10] | 2.27 (0.92) to 1.98 (0.88) [8.77; 0.004] | |
| PHQ | 9 items | 8 items | 8 items |
| T1–T2 | 5.16 (4.85) to 5.11 (4.67) [0.01; 0.91] | 5.77 (4.02) to 5.89 (4.58) [0.16; 0.69] | 5.35 (3.84) to 3.54 (4.44) [17.15; <0.001] |
| T1–T3 | 5.03 (4.73) to 5.35 (5.57) [0.40; 0.53] | 5.85 (4.26) to 5.26 (4.17) [4.53; 0.036] | 5.35 (3.84) to 3.82 (3.91) [12.61; 0.001] |
| T2–T3 | 5.06 (4.72) to 5.41 (5.59) [0.54; 0.47] | 5.93 (4.68) to 5.22 (4.13) [5.37; 0.022] | 3.54 (4.44) to 3.82 (3.91) [0.52; 0.47] |
| Medication adherence | Adherence | Nonadherence | |
| T1–T2 | 3.78 (0.29) to 3.80 (0.37) [0.45; 0.50] | 1.19 (0.27) to 1.18 (0.35) [0.25; 0.62] | |
| T1–T3 | 3.79 (0.29) to 3.84 (0.22) [8.20; 0.005] | 1.19 (0.27) to 1.17 (0.23) [0.49; 0.48] | |
| T2–T3 | 3.80 (0.39) to 3.85 (0.21) [1.31; 0.26] | 1.18 (0.35) to 1.17 (0.23) [0.02; 0.89] | |
| CIRS total score | No T1 | ||
| T1–T2 | 1.88 (0.61) to 1.89 (0.64) [0.00; 0.95] | 2.12 (0.64) to 2.05 (0.72) [1.38; 0.24] | |
| T1–T3 | 1.91 (0.63) to 1.92 (0.67) [0.09; 0.77] | 2.12 (0.64) to 2.22 (0.68) [2.75; 0.10] | |
| T2–T3 | 2.40 (0.67) to 2.47 (0.55) [1.07; 0.31] | 1.89 (0.65) to 1.91 (0.67) [0.12; 0.73] | 2.05 (0.72) to 2.22 (0.68) [5.41; 0.022] |
| Patient engagement | Physician autonomy support | PACIC | PACIC |
| T1–T2 | 5.72 (1.24) to 5.72 (1.36) [0.00; 0.99] | 3.20 (0.44) to 3.34 (0.42) [11.28; 0.001] | 2.84 (1.03) to 2.81 (1.10) [0.06; 0.81] |
| T1–T3 | 5.71 (1.22) to 5.41 (1.45) [3.86; 0.053] | 3.20 (0.44) to 3.40 (0.46) [17.09; <0.001] | 2.84 (1.03) to 3.11 (1.11) [7.36; 0.008] |
| T2–T3 | 5.70 (1.39) to 5.43 (1.46) [2.53; 0.12] | 3.33 (0.42) to 3.41 (0.44) [2.99; 0.09] | 2.81 (1.10) to 3.11 (1.11) [6.12; 0.015] |
Repeated Measures ANOVA; F and p reported for the time effect. T1 = baseline; T2 = 2 months for DHC and 4 months for MyPath and REDEEM; T3 = 6 months for DHC and 12 months for My Path and REDEEM
For those receiving the MyPath intervention, goals and tailored behavioral action plans were shared with their providers via electronic health record. All participants completed three study appointments, and MyPath/MyPath-Plus participants also received two follow-up calls and interactive voice response contact and email prompts to view new features of the MyPath website. MyPath-Plus participants were also invited to attend three group seminars. Healthy Habits participants did not have access to the MyPath website, calls, or seminars.
Reducing Distress and Enhancing Effective Management
The REDEEM project was a three-arm randomized comparative trial to reduce diabetes-related distress [22]. The study compared the effectiveness of the MyPath program with or without Problem-Solving Therapy, relative to Healthy Habits/Leap Ahead, the previously described MINC condition. Patients with self-reported problems with diabetes management and moderate to high levels of diabetes distress (but who were not clinically depressed) were recruited from five San Francisco Bay Area community medical group and diabetes education centers. Leap Ahead patients received the computerized MyPath program and feedback report and, like intervention participants, a booster session 5 months later, as well as mailed general diabetes information about healthy living, diet, and physical activity that coincided with eight live phone calls at weeks 2, 4, 7, 12, 24, 28, 36, and 48.
Measures
The three studies included many of the same measures, which helped to facilitate interpretation of outcomes. Assessment points for the three studies reported here are time 1 = baseline, time 2 = 2 months for DHC and 4 months for MyPath and REDEEM, and time 3 = 6 months for DHC and 12 months for MyPath and REDEEM. Instruments for measuring eating behaviors were: a semiquantitative food frequency questionnaire [23] to estimate percent of calories from saturated fat; the eating habits score from the seven-item Starting The Conversation instrument, which measures the frequency of consuming sugary beverages and fast foods (MyPath, REDEEM) [24]; and estimated fat intake assessed by the National Cancer Institute's Percentage Energy from Fat Short Screener [25]. In all cases, higher scores indicated more healthful eating habits [24–35]. For physical activity, the Community Healthy Activities Model Program for Seniors [32] instrument was used. The Diabetes Distress Scale was used to measure regimen distress (MyPath, REDEEM) [29]. Measures of medication adherence to diabetes, blood pressure, and cholesterol medications were collected in the MyPath and REDEEM studies using the Hill-Bone Compliance Scale [31]. The Chronic Illness Resources Survey (CIRS) [33] was collected in the MyPath and REDEEM studies to assess use of supportive resources across proximal and distal social ecological domains. The Patient Assessment of Care for Improving Chronic Conditions [34] was used to measure patient engagement in their care in MyPath and REDEEM, while Physician Autonomy Support [35] (a measure of the patient's perception of support for diabetes self-management from his/her health care team, and patient self-confidence in ability to self-manage his/her diabetes) was collected in DHC.
Analyses
Descriptive statistics were calculated to ensure that distributions of the data were appropriate for the tests used. Separately for each of the three studies, one-way analyses of variance (ANOVA) were performed to compare baseline characteristics of participants in the Healthy Habits/Leap Ahead group to participants assigned to more intensive intervention conditions. Using data only from Healthy Habits/Leap Ahead participants, repeated measures ANOVA models were conducted separately for each study to determine the degree to which participants improved on key outcomes over time.
RESULTS
Participants in these three studies were generally similar to most community-based adult type 2 diabetes patients. Table 2 presents characteristics of MINC participants across all studies. In general, these participants were older, had had diabetes for a number of years, were overweight or obese, and also had multiple chronic conditions. There were no differences among conditions on any of these baseline measures in any of the three studies.
Behavior change
As can be seen in Table 3 (in italics), Healthy Habits/Leap Ahead in two studies produced significant behavior changes in eating patterns and fat intake reduction, and in one study yielded significant physical activity increases and medication-taking increases.
In general, Healthy Habits/Leap Ahead produced less short-term behavior change than the more interactive and intensive tailored conditions in the DHC and MyPath studies (see Table 3), but produced equivalent behavior change to the active interventions in the REDEEM study, when accompanied by more frequent phone contacts (data not shown due to complexity and number of comparisons involved; these comparative outcome data have been published elsewhere [20–22]). Healthy Habits/Leap Ahead produced excellent maintenance results, almost always sustaining initial gains and, in some cases, continued increases between the midpoint assessment and the final follow-up.
Psychosocial Changes
Healthy Habits/Leap Ahead produced relatively consistent improvements in psychosocial measures, including diabetes distress (two of two studies), depression (two of three studies), and patient engagement (two of three studies). Also, Healthy Habits/Leap Ahead generally produced psychosocial improvements equivalent to those of the more complex, tailored conditions (data on this comparison presented elsewhere) [22]. The magnitude of these improvements was clinically significant and generally larger than the initial behavior change improvements.
DISCUSSION
We have provided a MINC definition, discussed key MINC characteristics, and presented examples from three studies using the Healthy Habits/Leap Ahead minimal intervention. Analyses from these three randomized diabetes self-management studies consistently indicated that Healthy Habits/Leap Ahead produced modest to moderate long-term behavior changes and fairly sizable improvements in psychosocial measures. The more substantial improvements in psychosocial measures seen especially in the REDEEM trial are clinically significant, and suggest that a low-intensity intervention with increased follow-up contact can produce sizable and meaningful improvement compared to initially more intensive and costly interventions.
Given both conceptual and methodological perspectives, and the considerably reduced costs and complexity of Healthy Habits/Leap Ahead, a strong argument can be made that, to be recommended or reimbursed, a new diabetes self-management intervention should be shown to be significantly better than Healthy Habits/Leap Ahead on these pre-specified outcomes. In a time of spiraling health care costs, using a MINC strategy routinely in CER would allow stakeholders and consumers to know that a new intervention being tested is worth the added cost, time, and resources. More frequent and standard use of the same MINC across different studies, as demonstrated in this article, along with harmonized outcome measures [36], would also aid systematic reviews, comparison across studies, and judgment of significance of change.
One may ask whether the behavioral medicine field is sufficiently advanced for this type of comparison condition. Our answer is that it is. A strong case can be made, on the basis of both conservation of limited health care resources and translation into practice, for such an approach. New innovations, which are almost always more intensive, expensive, and time consuming than existing aspirin-like MINC interventions [37], should be able to demonstrate that their incremental benefits—if any—are worth the added costs, training, supervision, and organizational changes required for adoption. Some may feel that requiring a MINC comparison condition is overly stringent, but we note that it is congruent with both requirements and definitions for CER and PCORI [6]. Use of a MINC vs. usual care comparison condition may or may not require larger sample sizes to detect intervention effects, depending on how effective usual care is and how variable it is across participating sites. This is because usual care in some settings is currently evidence-based and in many others changes over time. If usual care varies across settings, this will substantially increase the variance compared to what might be expected from a standard MINC intervention, and thus could actually require larger sample sizes to detect a given effect size.
The greatest potential advantage of MINCs was not investigated in this article. Because of known relationships between intervention cost, complexity, and demand, with reduced adoption by settings, reach (participation among individuals), implementation, and sustainability [15], these implementation outcomes [38] may be the areas in which MINCs show their strongest effects. Research is needed to specifically assess the reach, adoption, implementation, and relative costs of a MINC to other more intensive conditions.
The MINC intervention described here evolved only slightly from the initial study to include more frequent phone follow-up support in the third study, the REDEEM trial. Although there was not a direct comparison, the magnitude of improvements observed in REDEEM in a very comparable population, especially at follow-up compared to the earlier studies, suggested that the added time and costs of the phone follow-up were warranted. As discussed in the “Introduction” [7–10], there are numerous approaches to development of MINCs and identification of the “essential components”: It seems premature to recommend one approach or development method over others at this time. The approaches themselves vary in speed, cost, level of experimental control, and likely acceptability to stakeholders. Reviewers raised the issue of whether a MINC should be required to produce a significant biological outcome. While acknowledging that some reviewers, study sections, and funders may require this, we think it overly restrictive to require this as part of the definition of a MINC. Some areas do not have agreed-upon biomarkers (e.g., pain, depression, anxiety); for many conditions, quality of life or functional outcomes may be more important outcomes than a biomarker; and it seems inappropriate to assert that any given class of outcome is always required across all conditions, questions, and types of research. It may be that certain areas of research, e.g., diabetes management, might decide upon added criteria for a MINC, but even then, there are many complications concerning which biomarker should be required.
This report has limitations. Specifically, we report data on only one MINC condition and studied only patients with diabetes. Our sample sizes precluded subgroup analyses to identify which patient groups benefited the most and least from Healthy Habits/Leap Ahead; and some of the findings, while statistically significant improvements, were of modest magnitude. Strengths include the provision of an explicit MINC definition, use of the same analytic strategy across three controlled trials, common primary outcome measures across studies, the consistency of results, and example applications. We encourage additional research on characteristics of MINCs, application to other areas, and use of other outcomes to evaluate MINC results. Such use has high potential to advance CER, enhance understanding of outcomes, facilitate systematic reviews and knowledge syntheses, produce more efficient interventions with broader reach and adoption, and reduce costs.
Acknowledgments
The opinions expressed are those of the authors and do not necessarily represent those of the National Cancer Institute. We thank Drs. Barbara Rimer and Robert Croyle for first bringing the MINC concept to our attention. Partial support was provided by NIDDK R01 grants: DK35524, DK035524, and DK061937.
Footnotes
Implications
Practice: MINC conditions have the potential to produce outcomes equivalent to more intensive and expensive conditions; to enhance reach, adoption, implementation, and sustainability; and reduce costs.
Policy: To help address unsustainable health care spending increases, funders should consider if a potential intervention is demonstrably more cost-effective than a MINC.
Research: Consistent use of MINC conditions would aid comparisons across studies, provide a common metric, and aid systematic reviews.
References
- 1.Centers for Medicare and Medicaid Services, Office of the Actuary, National Health Statistics Group. National health care expenditures data. January 2012.
- 2.Naylor CD, Naylor KT. Seven provocative principles for health care reform. JAMA. 2012;307(9):919–920. doi: 10.1001/jama.2012.252. [DOI] [PubMed] [Google Scholar]
- 3.Billheimer LT (2013) Congressional Budget Office. Implications of rising health care costs: presentation to the Heritage Foundation. http://www.cbogov/sites/default/files/cbofiles/attachments/HeritagePresentation_Bilheimer.pdf. Accessed 13 April 2013.
- 4.Woolf SH, Johnson RE, Fryer GE, Jr, Rust G, Satcher D. The health impact of resolving racial disparities: an analysis of US mortality data. Am J Public Health. 2008;98(9 Suppl):S26–S28. doi: 10.2105/AJPH.98.Supplement_1.S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn EA. An evidence-based national quality measurement and reporting system. Med Care. 2003;41(1 Supp):I 8–I 15. doi: 10.1097/00005650-200301001-00002. [DOI] [PubMed] [Google Scholar]
- 6.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307(15):1583–1584. doi: 10.1001/jama.2012.500. [DOI] [PubMed] [Google Scholar]
- 7.Gierisch JM, DeFrank JT, Bowling JM, Rimer BK, Matuszewski JM, Farrell D, et al. Finding the minimal intervention needed for sustained mammography adherence. Am J Prev Med. 2010;39(4):334–344. doi: 10.1016/j.amepre.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu HS, Gupta G (2010). An overview of the FDA draft guidance on adaptive design clinical trials. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/UCM209179.pdf. (MPCC Seminar Series, April 6, 2010). Accessed 17 April 2013.
- 9.Brown CH, Ten Have TR, Jo B, Dagne G, Wyman PA, Muthen B, et al. Adaptive designs for randomized trials in public health. Annu Rev Public Health. 2009;30:1–25. doi: 10.1146/annurev.publhealth.031308.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown JH. Breaking symmetry in protein dimers: designs and functions. Protein Sci. 2006;15(1):1–13. doi: 10.1110/ps.051658406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. doi: 10.1186/1471-2458-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiore MC, Bailey WC, Cohen SJ, et al. (2000) Treating tobacco use and dependence. Clinical Practical Guidelines. Rockville, MD, U.S. Department of Health and Human Services, Public Health Service
- 13.Carels RA, Darby L, Cacciapaglia HM, Douglass OM, Harper J, Kaplar ME, et al. Applying a stepped-care approach to the treatment of obesity. J Psychosom Res. 2005;59(6):375–383. doi: 10.1016/j.jpsychores.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 14.Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010;29(1):1–8. doi: 10.1037/a0016939. [DOI] [PubMed] [Google Scholar]
- 15.Rogers EM. Diffusion of innovations. 5. New York: Free; 2003. [Google Scholar]
- 16.Gaglio B, Glasgow RE. Evaluation approaches for dissemination and implementation research. In: Brownson R, Colditz G, Proctor E, editors. Dissemination and implementation research in health: translating science to practice. 1. New York: Oxford University Press; 2012. pp. 327–356. [Google Scholar]
- 17.Kessler RS, Purcell EP, Glasgow RE, Klesges LM, Benkeser RM, Peek CJ. What does it mean to “Employ” the RE-AIM model? Eval Health Prof. 2012;36(1):44–46. doi: 10.1177/0163278712446066. [DOI] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force (1998). Clinical Handbook of Preventive Services. 2nd ed. Washington, D.C.
- 19.NCQA, American Diabetes Association (2012). Diabetes Physician Recognition Program. Available at: http://www.ncqa.org/Programs/Recognition/ChangestoDRPandHSRP.aspx. Accessed 17 April 2013.
- 20.Glasgow RE, Nutting P, Toobert DJ, King DK, Strycker LA, Jex M, et al. Effects of a brief computer-assisted diabetes self-management intervention on dietary, biological, and quality-of-life outcomes. Chronic Illn. 2006;2(1):27–38. doi: 10.1177/17423953060020011001. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow RE, Kurz D, King D, Dickman JM, Faber AJ, Halterman E, et al. Outcomes of minimal and moderate support versions of an internet-based diabetes self-management support program. J Gen Intern Med. 2010;25(12):1315–1322. doi: 10.1007/s11606-010-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher L, Hessler D, Glasgow RE, Arean PA, Masharani U. Naranjo D et al. REDEEM: A pragmatic trial to reduce diabetes distress. Diabetes Care; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson RE, Kristal A, Tinker LF, Carter RA, Bolton MP, et al. Measurement characteristics of the Women's Health Initiative Food Frequency Questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/S1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 24.Paxton A, Strycker LA, Toobert DJ, Ammerman AS, Glasgow RE. Starting the conversation: performance of a brief dietary assessment and intervention tool for health professionals. Am J Prev Med. 2010;40(1):67–71. doi: 10.1016/j.amepre.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Thompson FE, Kipnis V, Subar AF, Schatzkin A, Potischman N, et al. Performance of a short instrument to estimate usual dietary intake of percent calories from fat. Euro J Clin Nutr. 1998;52:S63. [Google Scholar]
- 26.Patterson RE, Kristal AR, Biener L, Varnes J, Zeng Z, Glanz K, et al. Durability and diffusion of the nutrition intervention in the Working Well Trial. Prev Med. 1998;27:668–673. doi: 10.1006/pmed.1998.0342. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33(5):1034–1036. doi: 10.2337/dc09-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polonsky WH, Fisher L, Darles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 30.Thompson FE, Midthune D, Williams GC, Yaroch AL, Hurley TG, Resnicow K, et al. Evaluation of a short dietary assessment instrument for percentage energy from fat in an intervention study. J Nutr. 2008;138(1):193S–199S. doi: 10.1093/jn/138.1.193S. [DOI] [PubMed] [Google Scholar]
- 31.Krousel-Wood M, Munter P, Jannu A, Desalvo K, Re RN. Reliability of a medication adherence measure in an outpatient setting. Am J Med Sci. 2005;330:182–1336. doi: 10.1097/00000441-200509000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Glasgow RE, Strycker LA, Toobert DJ, Eakin EG. A social-ecologic approach to assessing support for disease self-management: the Chronic Illness Resources Survey. J Behav Med. 2000;23:559–583. doi: 10.1023/A:1005507603901. [DOI] [PubMed] [Google Scholar]
- 34.Glasgow RE, Wagner E, Schaefer J, Mahoney L, Reid R, Greene S. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43(5):436–444. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 35.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 36.Rabin BA, Purcell P, Naveed S, Moser RP, Henton MD, Proctor EK, et al. Advancing the application, quality and harmonization of implementation science measures. Implement Sci. 2012;7(1):119. doi: 10.1186/1748-5908-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan RM. Disease, diagnoses, and dollars: the ever-expanding market for medical care. New York: Copernicus Books; 2009. [Google Scholar]
- 38.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. doi: 10.1007/s10488-010-0319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


