Abstract
Human fossils and the genetics of extant human populations indicate that living people derive primarily from an African population that lived within the last 200,000 years. Yet it was only ≈50,000 years ago that the descendants of this population spread to Eurasia, where they swamped or replaced the Neanderthals and other nonmodern Eurasians. Based on archaeological observations, the most plausible hypothesis for the delay is that Africans and Eurasians were behaviorally similar until 50,000 years ago, and it was only at this time that Africans developed a behavioral advantage. The archaeological findings come primarily from South Africa, where they suggest that the advantage involved much more effective use of coastal resources. Until now, the evidence has come mostly from deeply stratified caves on the south (Indian Ocean) coast. Here, we summarize results from recent excavations at Ysterfontein 1, a deeply stratified shelter in a contrasting environment on the west (Atlantic) coast. The Ysterfontein 1 samples of human food debris must be enlarged for a full comparison to samples from other relevant sites, but they already corroborate two inferences drawn from south coast sites: (i) coastal foragers before 50,000 years ago did not fish routinely, probably for lack of appropriate technology, and (ii) they collected tortoises and shellfish less intensively than later people, probably because their populations were smaller.
It is uncertain when people first exploited coastal resources because there are few coastal archaeological sites that antedate the beginning of global oxygen-isotope stage 5 (the Last Interglacial period) ≈127,000 years ago (127 kya). The most promising sites would be coastal caves, but most known caves formed only after 127 kya or were subsequently flushed of older deposits by wave action or other erosion. At the moment, the oldest firm evidence for human coastal adaptation comes from deposits that formed sometime between 127 and perhaps 50 kya in a handful of caves around the Mediterranean Sea, in Italy (1), Gibraltar (2–4), Morocco (5, 6), and Libya (7), and on the western and southern coasts of South Africa (8, 9).
Archaeologists assign the artifacts from the Mediterranean sites to the Mousterian or Middle Paleolithic cultural complex, which spanned the interval from 250–200 to 40–35 kya in western Eurasia and northern Africa (10). They assign the artifacts from the South African caves to the Middle Stone Age (MSA), which covered roughly the same period in sub-Saharan Africa. The difference in names is a matter of geographic distance and scholarly tradition, and there is more artifactual variability within the Mousterian and the MSA than there is between them. MSA and Mousterian sites are also similar in what they mainly lack: unequivocal art objects; finely crafted artifacts in bone, ivory, antler, or shell; graves with irrefutable evidence for burial ritual or ceremony; and unequivocal remnants of structures. Singly or in combination, these items became common only after 40 kya, when the Later Stone Age (LSA) appeared south of the Sahara and the Upper Paleolithic spread through Europe and northern Africa. The LSA and Upper Paleolithic also varied far more through time and space than the MSA/Mousterian, and they are the oldest culture complexes to signal the fully modern human ability to innovate.
Despite the artifactual similarities, the people who made Mousterian tools in Europe were Neanderthals, whereas the people who made MSA/Mousterian artifacts in Africa were anatomically modern or near-modern humans (10). In contrast, both Upper Paleolithic Europeans and LSA Africans were fully modern, and even the oldest known Upper Paleolithic people lack any specialized Neanderthal features (11). The implication is that LSA and Upper Paleolithic populations shared a common MSA/Mousterian African ancestor. Early LSA people expanding northwards probably invented the Upper Paleolithic in northern Africa or southwest Asia, and Upper Paleolithic people then extinguished the Neanderthals when they spread through Europe, beginning ≈40 kya. The genetics of both living humans (12) and Neanderthals (13, 14) argue that Neanderthals contributed few, if any, genes to their successors.
Enhanced inventive ability implies that fully modern LSA/Upper Paleolithic people could extract more energy from nature, and this would explain how they came to dominate both Africa and Europe (10). More effective LSA/Upper Paleolithic exploitation of coastal resources is likely, and South African coastal MSA and LSA sites allow a direct check of this hypothesis. The MSA sites are far richer in coastal food debris than their Mediterranean counterparts, and they are so far the only sites of such antiquity to provide true shell middens. LSA sites abound on the same South African coasts, and the contents of MSA and LSA sites have been excavated and analyzed by using the same methods. Until now, MSA/LSA comparisons have been based mainly on three deeply stratified MSA sites on the south (Indian Ocean) coast: Die Kelders Cave 1 (15–17), Blombos Cave (18, 19), and the multicave complex at Klasies River Main (20, 21). The Klasies River caves are particularly famous for their fossils of anatomically modern or near modern humans, dated between ≈115 and 60 kya (22, 23). Supported by findings from Die Kelders 1 and Blombos, the Klasies River shells and marine vertebrate remains suggest that MSA people exploited coastal resources less effectively than their LSA successors (9), but the finding could be strictly local and might not pertain in a different environmental zone.
In this report, we present recent findings from the South African west (Atlantic) coast, where terrestrial foods are significantly sparser than on the south coast, owing to lower rainfall, and marine foods are more abundant, because of persistent offshore upwelling of nutrient-rich waters. We summarize results from various west coast sites, but we focus on those from the 2002 and 2003 excavations at Ysterfontein 1 (YFT1). Like the well known south coast sites, YFT1 is a deeply stratified MSA rockshelter (24), and it is the first west coast site to provide large samples of MSA artifacts and food debris in place, before they had eroded out. We conclude that YFT1 and other west coast sites support south coast evidence for more limited, less intensive MSA coastal exploitation. In general, unlike their LSA successors, MSA Africans seem to have been behaviorally similar to their European Neanderthal contemporaries, and this helps to explain why MSA people remained confined to Africa.
Origin and Stratigraphy of the YFT1 Deposits
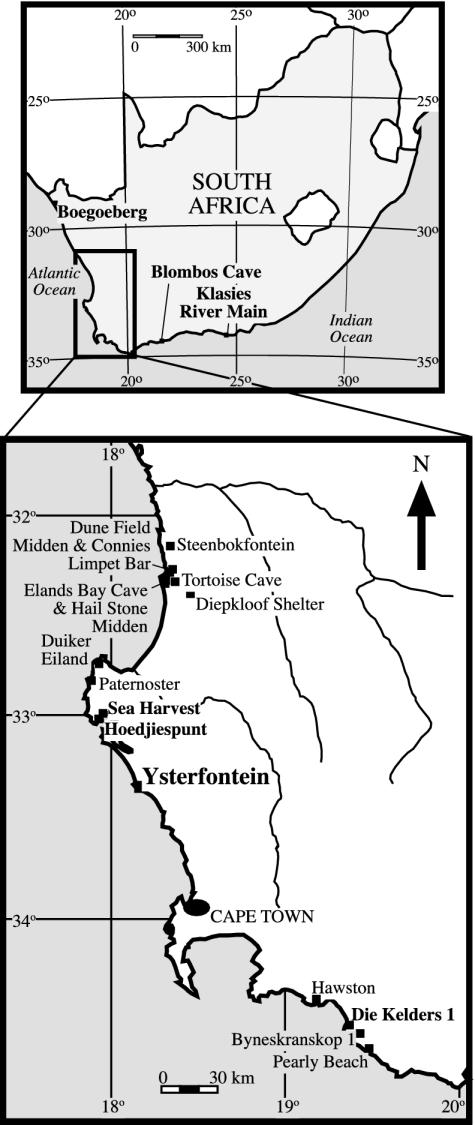
YFT1 (33.20°S, 18.09°E) is a crevice-like rockshelter adjacent to the small boat harbor at Ysterfontein (= Yzerfontein), ≈70 km north-northwest of Cape Town (Fig. 1). A diorite platform rising to ≈7 m above mean sea level floors the shelter, and calcrete forms its walls and roof. The fill, which is 3–3.5 m thick, comprises weakly cemented, highly calcareous, yellowish sands. In addition to artifacts, numerous shells, and rarer bones, the sands sporadically enclose calcareous root casts and blocks of calcrete, diorite, and aeolianite (dune rock). A calcrete “shelf” that probably collapsed from the roof divides the fill in two. Artifacts and faunal remains tend to occur in lenses or discrete horizons that dip into the shelter, suggesting that the enclosing sands slid down the rear face of a dune that formerly stood between the shelter and the sea. If this suggestion is correct, then many of the artifacts and associated faunal remains could have originated outside the shelter, perhaps from camps on the fronting dune crest. However, carbonaceous patches that almost certainly mark fireplaces indicate that people also camped inside. In one small area, the patches are stacked to form a multistoried hearth feature resembling the ones that have been described from Klasies River Main (21).
Fig. 1.
The approximate locations of the MSA and LSA sites discussed in the text. Boldface marks the MSA sites, three of which (Die Kelders 1, Blombos Cave, and Klasies River Main) also have LSA occupations.
In general, the individual YFT1 lenses and layers recognized in the field contain too few cultural items for meaningful analysis, and we have therefore lumped the field units into groups: CEN02, DH021, DH022, HY033, HY034, and HY035 above the calcrete “shelf” that divides the deposit in two and RY021, RY022, and RY023 below it. The numbering within the DH, HY, and RY groups indicates stratigraphic order from top to bottom, but the CEN, DH, and HY groups come from separate parts of the excavation, and their precise stratigraphic relationship to one another is presently uncertain. Even when considered by stratigraphic group, some items [artifacts, bones, and measurable (i.e., intact) limpet shells] are mainly too rare to detect significant numeric differences between groups, and we present them here either for the site as a whole or divided between only two units: “Upper” for the CEN, DH, and HY material above the shelf and “Lower” for the RY material below it.
Antiquity of the YFT1 Deposits
The organic (protein) component of ostrich eggshell from a unit within DH022 (above the shelf) has provided an accelerator radiocarbon (AMS) age of 33,470 ± 510 years B.P. (Beta-169978). The inorganic carbonate fraction produced an AMS age of >46,400 years B.P. (Beta-171202). The amount of undetectable, recent carbon contaminant required to make the organic component appear “too young” is far smaller than the amount of ancient contaminant necessary to make the inorganic component appear “too old,” and we conclude that the infinite, inorganic determination is closer to the actual age. This means that the deposits lie beyond the effective range of the radiocarbon method, as they do at all other known coastal MSA sites (19, 21, 25–27).
At Die Kelders Cave 1, MSA artifacts that closely resemble those at YFT1 have been dated to 70–60 kya by electron-spin-resonance analysis of associated ungulate teeth (28) and optically stimulated luminescence (OSL) analysis of the enclosing sands (29). OSL dating may suggest a similar age for YFT1. We argue below that the deposits probably span many thousands of years, because they record shifts in the limpet-to-mussel ratio that are likely to reflect significant fluctuations in sea level. If we combine the offshore topography with global sea-level oscillations inferred mainly from the deep-sea oxygen-isotope record (30, 31) and we assume that coastal foragers would have mostly abandoned YFT1 when the coastline was displaced >10 km seawards, then the occupation is unlikely to date from the interval of especially low sea level corresponding to global isotope stage 4, between ≈71 and 57 kya. A time within the early part of stage 3, between 57 and 46 kya, would be more probable, but the infinite radiocarbon date also allows a period within substages d to a of stage 5, between ≈115 and 71 kya. A yet earlier time is precluded, because waves associated with the high sea level of substage 5e would have flushed the shelter, if it existed.
YFT1 Artifacts
Among the YFT1 stone artifacts, radial cores and flakes or flake-blades with faceted butts establish the MSA character of the assemblage (illustrations in ref. 24). As in other MSA assemblages, typical LSA stone artifacts, including bladelets, small scrapers, and tiny backed pieces, are totally absent. Like most other MSA sites, YFT1 also lacks shaped bone or ostrich eggshell artifacts. The bone sample remains small, and the expanding excavation might yet produce a bone “point” or “awl” like those recovered in the MSA layers of Blombos Cave (32), but the ostrich eggshell sample is large, particularly from above the shelf, and it implies that the MSA inhabitants rarely if ever worked eggshell. The difference from the LSA is striking, because LSA sites routinely provide well made bone and ostrich eggshell artifacts, even when the bone sample is no larger than the one from YFT1 and the ostrich eggshell sample is much smaller.
The stone artifact assemblages from the Upper and Lower units are similar, although the Upper one is richer in retouched (secondarily modified) pieces and in raw materials other than silcrete (Table 1). The difference could reflect a change in raw material availability through time, but its meaning is otherwise unclear. Unretouched flakes and flaking debris dominate throughout, but there are also a small number of pieces with continuous scraper-like retouch and a larger number with deliberately denticulated (serrated) edges. There are no backed elements (ones on which one edge has been deliberately dulled) like those that typify the Howieson's Poort variant of the MSA or bifacial points (or point fragments) like those that characterize the contemporaneous or somewhat older Still Bay variant. Still Bay and Howieson's Poort people inhabited the Western Cape between ≈80 and 60 kya (21, 32, 33), which supports an age of <60 kya for YFT1. However, the MSA artifacts from Die Kelders Cave also include numerous denticulates and no Howieson's Poort or Still Bay markers (16, 34), and the electron-spin-resonance and OSL ages we cited above suggest that the Die Kelders assemblages accumulated between 70 and 60 kya. If the Die Kelders dating is reliable, strictly local circumstances could explain the composition of the Die Kelders and YFT1 artifacts, and the people who occupied both sites might have left Howieson's Poort or Still Bay artifacts elsewhere.
Table 1. Stone artifact inventory from the Lower and Upper units at YFT1.
| Upper unit | Lower unit | |
|---|---|---|
| Raw materials | ||
| Silcrete | 410 | 458 |
| Other | 1438 | 139 |
| Artifact types | ||
| Denticulates | 25 | 5 |
| Other retouched pieces | 13 | 4 |
| Pigment lumps | 23 | 6 |
| Other | 1787 | 582 |
Raw materials besides silcrete include calcrete, quartz, diorite, shale, quartzite, sandstone, and quartz porphyry. Artifact types besides denticulates, other retouched pieces, and pigment lumps include unretouched flakes and flake fragments, chips (mostly tiny flakes), cores, angular chunks, and manuports (unmodified, humanly introduced stones).
In common with other MSA sites (8, 19, 20, 35), YFT1 contains fragments of humanly introduced ochreous (red ferruginous) pigment. YFT1 may be the first to have also produced pieces of black (manganiferous) pigment. Scratches from rubbing or grinding are conspicuous on one red and one black pigment lump. Two diorite chunks and one diorite cobble also exhibit ochreous smears from rubbing or grinding. European Mousterians (Neanderthals) likewise collected and modified naturally occurring pigments (36, 37), and recent discoveries (38–40) suggest that an interest in pigments extends back into the Earlier Stone Age/Lower Paleolithic. It is not known whether MSA/Mousterian people used pigment for decorative or ritual purposes, and they may have used it more prosaically, perhaps as an ingredient in the glue used to fix stone artifacts to wooden handles or shafts (41). Even in this instance, however, together with a sophisticated ability to flake stone and control over fire, pigment use would imply that MSA/Mousterian people were behaviorally advanced in the direction of modern humans.
YFT1 Vertebrates
The YFT1 vertebrate fossils come from snakes, tortoises, mammals, and birds. Two fragmentary hyena coprolites show that hyenas occasionally occupied the shelter, but the abundant artifacts and intertidal shells pinpoint MSA people as the principal bone collectors. So far, conspicuous surface damage is limited to occasional tooth marks from small carnivores and small rodents. These animals might have introduced some of the smallest bones, but they could equally have been attracted by bones that were already present.
In overall composition, the YFT1 fauna recalls the faunas from other coastal MSA and LSA sites. However, it differs from coastal LSA faunas in the absence of fish bones and from LSA faunas and south coast MSA faunas in the profusion of ostrich eggshell fragments. Other coastal MSA sites also tend to lack fish bones, and where fish bones occur, mainly at Klasies River Main (20) and Blombos Cave (18), they are rare compared to mammal bones. The opposite is usually true in coastal LSA sites, where fish bones typically outnumber mammal bones by an order of magnitude. Only the LSA sites have provided artifacts that anticipate historic fishing implements (42), and the sum suggests that only LSA people fished routinely.
Relative to other vertebrate remains, ostrich eggshell fragments also abound in the west coast MSA deposits at Sea Harvest (43), Hoedjiespunt (44, 45), and Boegoeberg 2 (27), and this feature thus distinguishes west coast MSA sites as a group. A difference in environment could explain the much lower frequency of eggshell in south coast MSA sites, and environmental change could account for some variation in eggshell abundance through the YFT1 sequence. We document this variation below. However, environment seems unlikely to explain the contrast with LSA sites wherever they are located, and the only obvious, if so far untestable, alternative is a special west coast MSA preference or custom.
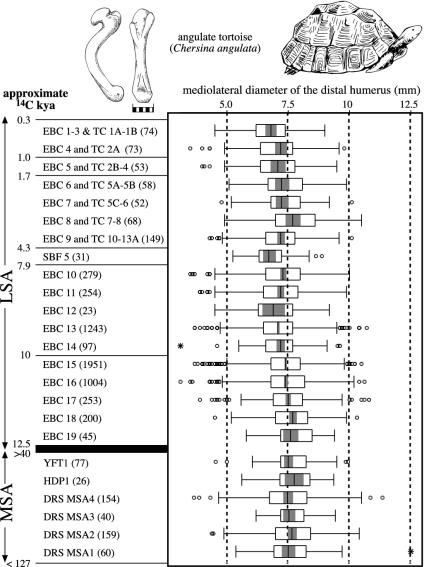
The YFT1 snake bones comprise 136 vertebrae that could not be identified to species and that do not presently inform on MSA behavior or environment. Fragments of carapace and plastron show that the tortoise elements come largely, if not entirely, from the angulate (or bowsprit) species (Chersina angulata), and limb bones indicate that at least 38 individuals are represented. The angulate tortoise was by far the most common species in the historic environment. It also dominates all other regional MSA and LSA samples, but average individual size varies significantly from site to site and time to time (17, 18, 46, 47). Fig. 2 illustrates the range of variation at west coast sites, based on box plots that summarize the mediolateral diameter of tortoise distal humeri from YFT1, the MSA layers of Hoedjiespunt 1 (HDP1) (44, 45), four successive MSA units at Diepkloof Rockshelter (DRS) (48), and LSA units spanning the last 12.5 ky at Elands Bay Cave (EBC) (49), Steenbokfontein (SBF) (50, 51), and Tortoise Cave (TC) (52, 53). The relative ages of the MSA sites are uncertain, but Howieson's Poort-backed elements occur only at Diepkloof, which may mean that it mainly antedates YFT1 and Hoedjiespunt 1, both of which lack them.
Fig. 2.
Box plots summarizing the mediolateral diameter of angulate tortoise distal humeri at YFT1 and other Stone Age sites on the west coast of South Africa. Numbers in parentheses after each site abbreviation indicate the number of humeri in the site sample. The key elements in each box plot are the median, represented by the vertical line near the center of each box plot; the open rectangle, which encloses the middle half of the data (between the 25th and 75th percentiles); the shaded rectangle, which designates the 95% confidence limits of the median; and the horizontal line bisecting each plot, which signifies the range of more or less continuous data. Starbursts and open circles mark outliers (values that are especially far from the median). When the 95% confidence limits for two sample medians do not overlap, the medians differ at or below the 0.05 significance level.
Changes in the intensity of collection provide the most obvious explanation for long-term shifts in average tortoise size, because collectors would tend to take the largest individuals first. Tortoise collection does not require special technology, and the intensity of MSA and LSA collection probably hinged mainly on the number of collectors. The number of collectors in turn probably depended mostly on environmental conditions and on the level of hunting–gathering technology. Assuming that average tortoise size mainly reflects collector numbers, Fig. 2 suggests that MSA and LSA numbers may sometimes have been similar, but that in general, LSA populations were larger. Paleoenvironmental indicators, especially associated mammal species that we present for YFT1 immediately below, imply that the surroundings of each MSA site were at least as rich as the surroundings of any LSA site, and the implication is that LSA populations were mostly larger for technological, not environmental, reasons. The technological innovations that allowed LSA fishing could by themselves explain larger LSA populations and smaller tortoises.
The mammal samples from the Upper and Lower Units at YFT1 include 299 and 127 bones, respectively, that could be identified to skeletal part and taxon below the family level. The equivalent numbers for birds are 64 and 5 bones. The identified mammal species are hare (Lepus sp.), Cape dune molerat (Bathyergus suillus), porcupine (Hystrix africaeaustralis), black-backed jackal (Canis mesomelas), honey badger (Mellivora capensis), wildcat (Felis libyca), caracal or serval (Felis caracal aut serval), Cape fur seal (Arctocephalus pusillus), zebra (Equus sp.), rhinoceros (Rhinocerotidae gen. et sp. indet.), steenbok (Raphicerus campestris), blue antelope (Hippotragus leucophaeus), southern reedbuck (Redunca arundinum), black wildebeest (Connochaetes gnou), eland (Taurotragus oryx), and long-horned buffalo (Pelorovis antiquus). The bird species are ostrich (Struthio camelus), African penguin (Spheniscus demersus), Cape gannet (Morus capensis), Cape cormorant (Phalacrocorax capensis), bank cormorant (Phalacrocorax neglectus), spur-winged goose (Plectopterus gambensis), red-knobbed coot (Fulica cristata), and kelp gull (Larus dominicanus). The mammal list should probably also include whale, because the shellfish sample includes a barnacle from a species that adheres exclusively to whales. Such barnacles have also been found at LSA sites (54), where they likewise imply that people brought home whale flesh, even when whale bones are absent.
The fur seal and sea birds occur on the coast today, and like the associated shells, they document the proximity of the shore when the deposits accumulated. In contrast, the zebra, blue antelope, southern reedbuck, black wildebeest, greater kudu, and long-horned buffalo were unknown nearby historically and have not been found in any regional site that postdates 10 kya. The long-horned buffalo became extinct ≈10 kya (55), but its habits can be broadly inferred from its anatomy and the habits of its surviving relatives, particularly the Cape buffalo (Syncerus caffer). Historically, the vegetation in the YFT1 region was dominated by sclerophyllous shrubs that supplied too little food to sustain the extralimital ungulates, and their occurrence implies a setting in which both grasses and broad-leaved browse were significantly more common. In addition, the reedbuck and probably also the spur-winged goose and the coot imply standing fresh water where none exists today. The sum suggests that, in general, the YFT1 MSA people enjoyed a richer terrestrial setting than the LSA people to whom we are comparing them.
In theory, YFT1 and other west coast sites could allow a test of the proposition based on south coast sites that more limited MSA technology (particularly the lack of evidence for projectile weapons) forced MSA people to focus mainly on the least dangerous terrestrial ungulates, especially eland, and on penguins as opposed to cormorants and other airborne seabirds (9, 56). South coast LSA sites that were occupied under similar environmental conditions are significantly richer in more dangerous ungulates, especially buffaloes, and in airborne birds. If it is assumed that MSA and LSA people obtained most birds by scavenging for beached individuals, the seeming MSA preference for penguins might indicate a different seasonal round, because the ratio of beached penguins to other species varies seasonally today (57, 58). Fur seal ages at death contrast strongly between the large MSA sample from Klasies River Main and equally large LSA samples, and the difference suggests that MSA coastal visits may have been more evenly spread through the year (59). The MSA emphasis on less dangerous ungulates persists from isotope stage 5 (Last Interglacial) into 4 (early Last Glacial), and the LSA focus on more dangerous ones crosses from stage 2 (end Last Glacial) to stage 1 (Present Interglacial), implicating human behavior rather than climate as the responsible factor.
However, the YFT1 mammal sample is much too small to determine whether less dangerous ungulates are significantly better represented than others. The bird sample hints at the anticipated predominance of penguins (37 bones versus 29 for gannet and cormorants), and future excavations may provide confirmation. Other west coast MSA sites have produced even fewer mammal and bird bones, and YFT1 is the only known site where excavation could provide much larger samples.
YFT1 Shellfish
Intertidal shellfish dominate the YFT1 fauna, and, based on minimum numbers of individuals and shell weights, Table 2 shows that granite limpets, granular limpets, Argenville's limpets, and black mussels dominate the shellfish in both major stratigraphic units. The table includes ostrich eggshell weights to document the remarkable abundance of eggshell that we stressed above.
Table 2. The minimum number of individuals (MNI) for each shellfish species and the weights for shellfish and ostrich eggshell in the Upper and Lower units at YFT1.
| Upper unit
|
Lower unit
|
|||
|---|---|---|---|---|
| Taxon | MNI | Weight, kg | MNI | Weight, kg |
| Granite limpet (Patella granatina) | 499 | 11.835 | 236 | 4.9135 |
| Granular limpet (Patella granularis) | 16 | 0.129 | 36 | 0.223 |
| Argenville's limpet (Patella argenvillei) | 83 | 4.701 | 19 | 0.613 |
| Bearded limpet (Patella barbara) | 1 | 0.001 | 1 | 0.002 |
| Pear limpet (Patella cochlear) | 7 | 0.016 | 0 | 0.000 |
| Pink-rayed limpet (Patella miniata) | 5 | 0.041 | 2 | 0.035 |
| Prickly limpet (Helcion sp[p.]) | 21 | 0.004 | 60 | 0.005 |
| Keyhole limpet(s) (Fissurellidae gen. et sp. indet.) | 1 | 0.000 | 3 | 0.000 |
| Slipper limpet (Crepidula sp[p.]) | 16 | 0.006 | 26 | 0.007 |
| Topshell(s) (Oxystele sp[p.]) | 3 | 0.003 | 2 | 0.001 |
| Pustular triton (Argobuccinum pustulorum) | 1 | 0.002 | 0 | 0.000 |
| Burnupena(s)/dogwhelk(s) (Burnupena sp[p.]/Nucella sp[p.]) | 16 | 0.047 | 12 | 0.029 |
| Plough shell(s) (Bullia sp[p.]) | 5 | 0.009 | 0 | 0.000 |
| Black mussel (Choromytilus meridionalis) | 1,657 | 26.959 | 1,661 | 25.172 |
| Ribbed mussel (Aulacomya ater) | 30 | 0.061 | 37 | 0.086 |
| White (sand) mussel (Donax serra) | 34 | 0.205 | 105 | 0.812 |
| Corrugated Venus (Venerupis corrugata) | 3 | 0.001 | 4 | 0.003 |
| Chiton (Polyplacophora gen. et sp. indet.) | 3 | 0.001 | 1 | 0.000 |
| Whale barnacle(s) (Coronula sp[p.]) | 1 | 0.009 | 0 | 0.000 |
| Other barnacle(s) (Buccinidae gen. et sp. indet.) | 5 | 0.033 | 3 | 0.024 |
| Ostrich eggshell (Struthio camelus) | - | 7.722 | - | 0.344 |
The three limpet species (hereafter lumped as “patellas”) and black mussels are the most abundant, most visible, and most easily collected intertidal shellfish on the South African west coast (60), and they dominate all west coast MSA and LSA samples. However, the ratio of patellas to black mussels varies from site to site and time to time (61, 62), probably depending mainly on differences or changes in the nature and extent of nearby rocky coastline and on the distance from site to rocks. Black mussels prefer rocks exposed to the open sea, whereas the patellas favor protected embayments and gullies, and people will carry mussels further, because mussels have much higher flesh weights relative to shell. Even small sea level changes could significantly affect the relative numbers of patellas and mussels locally (60), and the changes that occurred from late global isotope stage 2 through stage 1 help explain long-term variation in the relative numbers at LSA sites spanning the past 11 ky near Elands Bay (63). Table 2 and Fig. 3 document changes in the ratio among YFT1 units that broadly recall those from the Elands Bay sites and that thus seem likely to reflect much more ancient sea level fluctuations over a roughly similar period. We suggested above that this period was in the early part of isotope stage 3 or perhaps within stage 5.
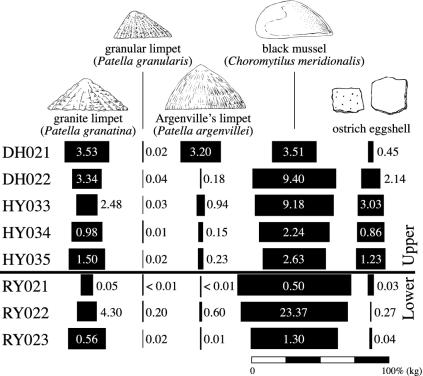
Fig. 3.
Limpet, black mussel, and ostrich eggshell weights (kg) in the main stratigraphic units at YFT1. The bars are proportional to the total weight of limpets, black mussels, and eggshell in each unit.
Table 2 and Fig. 3 indicate that ostrich eggshell abundance varied broadly in parallel with the ratio of patellas to black mussels, and this might mean that the global forces that drove sea level changes also affected local ostrich numbers. The point remains, however, that an environmental difference seems unlikely to explain why, relative to other faunal remains, YFT1 and other west coast MSA sites contain so much more eggshell than MSA sites elsewhere or LSA sites anywhere.
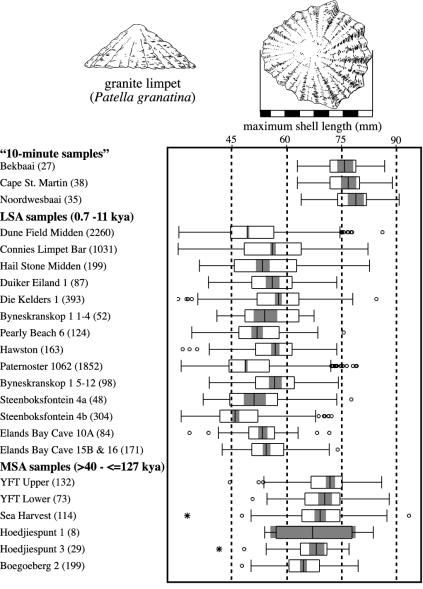
Despite a shared MSA and LSA emphasis on the same three patellas and black mussels, YFT1 and other west coast MSA sites differ from local LSA sites in four notable respects (8, 24): (i) the MSA assemblages are less diverse than LSA assemblages and are even more strongly dominated by patellas and black mussels; (ii) the MSA assemblages totally lack chelipeds of the crayfish or “rock lobster” (Jasus lalandii) that are a numerically variable but consistent feature of west coast LSA assemblages; (iii) the MSA assemblages are noticeably poorer in granular limpets relative to granite and Argenville's limpets; and (iv) on average, individuals of all three patellas tend to be significantly larger in MSA samples. Fig. 4 illustrates the contrast in average size for the granite limpet, which is the most numerous patella at YFT1 and most other west coast archaeological sites for which we have measurements.
Fig. 4.
Box plots summarizing granite limpet length at YFT1, other Stone Age sites on the west coast of South Africa, and three modern “10-minute samples.” The legend to Fig. 2 explains the box plot format. The LSA samples are arranged in rough chronological order from the youngest to oldest, beginning at the top. The relative ages of the MSA samples are uncertain.
South coast intertidal waters are dominated by different shellfish species, and in general, south coast MSA and LSA shellfish samples have not been analyzed as fully. However, measurements are available for two common species, the goat's eye limpet (Patella oculus) and the giant periwinkel (Turbo sarmaticus), and in both cases, the pattern is the same as for the west coast patellas: MSA specimens average significantly larger than their LSA counterparts (18, 64–66).
Like the rarity or absence of fish in coastal MSA sites, all four contrasts between west coast MSA and LSA samples suggest that MSA people exploited coastal resources more selectively or less intensively. With regard to relative patella numbers, for example, the three principal species are about equally conspicuous in the intertidal zone, but adult granular limpets are especially accessible, because they tend to live higher on the shore than adults of the other two species. However, they are also smaller, and more selective collectors would thus be more likely to ignore them.
The link between less intensive collection and larger Patella size depends on the same logic we offered above for larger tortoises in Stone Age sites; collectors would naturally take the largest individuals first and more intensive collection would thus drive average size down. Arguably, spatial or chronological differences in patella growth rates could underlie some differences in average individual size like those observed in Fig. 4 (60), but a change in growth rate is unlikely to explain the consistency and magnitude of the difference between MSA and LSA patellas, because it would require that MSA intertidal waters uniformly favored patella growth far more than the waters near any sampled LSA site, and the difference would have to extend to MSA sites of various ages on the south coast. Moreover, the hypothesis that collection intensity played the major role can be tested in the present, because it predicts that unexploited rocks today should produce especially large patellas. Fig. 4 presents three relevant samples. Labeled “10-min samples” for the time each took to accumulate, they resulted when modern collectors scoured unexploited rocks for 10 min near the town of Paternoster (67). The figure shows that, on average, the unexploited granite limpets are significantly larger than those at either MSA or LSA sites, but the MSA granite limpets approach the unexploited ones much more closely. The unexploited granular limpets in modern “10-min samples” provide a similar result (8, 67).
Like angulate tortoises, the three patellas can be collected without special tools or risk, and more intensive collection is thus likely to signal a larger number of collectors. With this in mind, Fig. 4 suggests that both LSA and MSA collector numbers varied from site to site or time to time, but that MSA collectors were generally less numerous. Because we noted above that MSA people at YFT1 and other west coast sites enjoyed relatively rich terrestrial conditions, less effective hunting–gathering technology (as opposed to an impoverished environment) is likely to explain smaller MSA populations. The conclusion parallels the one we drew from MSA/LSA variation in tortoise size.
Unlike the three patellas, MSA black mussels do not tend to be especially large, and MSA and LSA mussels varied within a shared range (8, 24). The similarity in MSA and LSA black mussel sizes does not mean, however, that the people exploited black mussels with equal intensity. This is because, unlike the patellas, which are strictly intertidal, black mussels also thrive subtidally, and the subtidal populations provide a source for rapid intertidal recolonization. Black mussels also tend to grow much more quickly than limpets, and the sum means that average mussel size is less likely to reflect variation in Stone Age collection pressure.
Summary and Conclusion
Sites on the south coast of South Africa have suggested that MSA people exploited local resources less intensively than their local LSA successors. Excavation at YFT1 aims mainly to reconstruct west coast MSA ecology and to determine whether it contrasted with local LSA ecology in basically the same way. A geographically extended contrast would strengthen the claim that MSA hunting–gathering ability was more limited and it would help to explain why only LSA people expanded to Eurasia, where they swamped or replaced Neanderthals and other indigenous Eurasians after 50–40 kya.
The YFT1 MSA samples are smaller than their south coast counterparts, but they show that the YFT1 fauna differs from local LSA faunas in two highly conspicuous respects: (i) it lacks fish bones; and (ii) the YFT1 tortoises and patellas tend to be significantly larger than LSA specimens accumulated under similar or less favorable environmental conditions. The larger tortoises and patellas probably mean that MSA human populations were smaller, and a more limited MSA ability to fish could by itself explain the difference from the LSA. The mammals from south coast MSA sites suggest that MSA people were also less effective hunters, but the YFT1 mammal sample remains too small for an independent check.
In sum, like the south coast sites, YFT1 suggests that a major advance in hunting–gathering occurred near the MSA/LSA transition. The case for a similar contrast between the Middle and Upper Paleolithic in Eurasia is more equivocal, but stable carbon and nitrogen isotopes in human bones imply that Middle Paleolithic people (Neanderthals) did not exploit freshwater resources like fish and waterfowl, whereas at least some Upper Paleolithic people (modern humans) did (68). In addition, in the only instance for which tortoise measurements have been reported, they suggest that Upper Paleolithic people forced a reduction in average individual size (69, 70). Both the isotopic evidence for broadening of the resource base and smaller average tortoise size imply that Upper Paleolithic people were more numerous, probably because their technology allowed more intensive hunting and gathering.
If there is a problem with the YFT1 and other South African observations, it is that the MSA/LSA comparison is based on sites that are separated by 30 ky or more. The south coast MSA sites are all older than 60 kya, and the LSA sites with which they are being compared are all younger than 30 kya. YFT1 might help to fill the gap at the MSA end if OSL dating shows that it is younger than 60 kya, but the available radiocarbon dates indicate it cannot be much younger, and it would still be at least 20 ky older than the oldest well documented local LSA site. The gap will not be quickly filled, because southern Africa appears to have been widely depopulated between ≈60 and 30–20 kya (21, 71), probably owing to widespread hyperaridity during global isotope stage 3.
There is the additional problem that the oldest well documented southern African LSA sites, dating to after 30 kya, were occupied during isotope stage 2, when global sea level was at its nadir and the coastline was displaced far seawards. It was only after 12 kya that sea level recovered sufficiently for coastal foragers to occupy sites that remain on dry land, and the relatively advanced coastal adaptation that 12-ky-old sites imply need not extend back to the earliest LSA at 50–40 kya. The more fundamental point is that, for reasons of regional environmental history, southern Africa probably cannot document a shift in human ecology at the MSA/LSA interface. Archaeologists will have to investigate the likelihood of this elsewhere, perhaps in eastern Africa, where environmental conditions appear to have remained more favorable over the critical 60- to 30-ky interval and human populations thus maintained greater archaeological visibility.
Acknowledgments
We thank the Leakey Foundation for financial support; the South African Museum (Iziko Museums of Cape Town) for laboratory facilities; Cedric Poggenpoel and Antonieta Jerardino for access to faunal remains they excavated at Diepkloof and Steenbokfontein Shelters, respectively; and David DeGusta and Donald Grayson for helpful comments on the manuscript.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 29, 2003.
Abbreviations: YFT1, Ysterfontein 1; MSA, Middle Stone Age; LSA, Later Stone Age; ky, thousand years; kya, thousand years ago; OSL, optically stimulated luminescence.
See accompanying Biography on page 5705.
References
- 1.Stiner, M. C. (1994) Honor Among Thieves: A Zooarchaeological Study of Neandertal Ecology (Princeton Univ. Press, Princeton).
- 2.Barton, R. N. E., Currant, A. P., Fernandez-Jalvo, Y., Finlayson, J. C., Goldberg, P., Macphail, R., Pettitt, P. B. & Stringer, C. B. (1999) Antiquity 73, 13–23. [Google Scholar]
- 3.Garrod, D. A. E., Buxton, L. H. D., Smith, G. E. & Bate, D. M. A. (1928) J. R. Anthropol. Inst. 58, 33–113. [Google Scholar]
- 4.Waechter, J. D. A. (1964) Bull. Inst. Archaeol. London 4, 189–213. [Google Scholar]
- 5.Howe, B. (1967) Bull. Am. Sch. Prehist. Res. 22, 1–200. [Google Scholar]
- 6.Roche, J. & Texier, J.-P. (1976) C. R. Acad. Sci. Paris Ser. D 282, 45–47. [Google Scholar]
- 7.McBurney, C. B. M. (1967) The Haua Fteah (Cyrenaica) and the Stone Age of the South-East Mediterranean (Cambridge Univ. Press, Cambridge, U.K.).
- 8.Parkington, J. E. (2003) S. Afr. J. Sci. 99, 243–247. [Google Scholar]
- 9.Klein, R. G. (2001) J. Anthropol. Res. 67, 1–16. [Google Scholar]
- 10.Klein, R. G. (1999) The Human Career: Human Biological and Cultural Origins (Univ. of Chicago Press, Chicago), 2nd Ed.
- 11.Bräuer, G. & Broeg, H. (1998) in The Origins and Past of Modern Humans–Towards Reconciliation, eds. Omoto, K. & Tobias, P. V. (World Scientific, Singapore), pp. 106–125.
- 12.Ingman, M., Kaessmann, H., Pääbo, S. & Gyllensten, U. (2000) Nature 408, 708–713. [DOI] [PubMed] [Google Scholar]
- 13.Krings, M., Capelli, C., Tschentscher, F., Geisert, H., Meyer, S., von Haeseler, A., Grossschmidt, K., Possnert, G., Paunovic, M. & Pääbo, S. (2000) Nat. Genet. 26, 144–146. [DOI] [PubMed] [Google Scholar]
- 14.Knight, A. (2003) J. Hum. Evol. 44, 627–632. [DOI] [PubMed] [Google Scholar]
- 15.Tankard, A. J. & Schweitzer, F. R. (1976) in Geoarchaeology, eds. Davidson, D. A. & Shackley, M. L. (Duckworth, London), pp. 289–316.
- 16.Grine, F. E., Klein, R. G. & Volman, T. P. (1991) J. Hum. Evol. 21, 363–395. [Google Scholar]
- 17.Klein, R. G. & Cruz-Uribe, K. (2000) J. Hum. Evol. 38, 168–195. [DOI] [PubMed] [Google Scholar]
- 18.Henshilwood, C. S., Sealy, J. C., Yates, R. J., Cruz-Uribe, K., Goldberg, P., Grine, F. E., Klein, R. G., Poggenpoel, C., Van Niekerk, K. L. & Watts, I. (2001) J. Archaeol. Sci. 28, 421–448. [Google Scholar]
- 19.Henshilwood, C. S., d'Errico, F., Yates, R. J., Jacobs, Z., Tribola, C., Duller, G. A. T., Mercier, N., Sealy, J. C., Valladas, H., Watts, I., et al. (2002) Science 295, 1278–1280. [DOI] [PubMed] [Google Scholar]
- 20.Singer, R. & Wymer, J. J. (1982) The Middle Stone Age at Klasies River Mouth in South Africa (Univ. of Chicago Press, Chicago).
- 21.Deacon, H. J. (1995) S. Afr. Archaeol. Bull. 50, 121–131. [Google Scholar]
- 22.Rightmire, G. P. & Deacon, H. J. (1991) J. Hum. Evol. 20, 131–156. [Google Scholar]
- 23.Rightmire, G. P. & Deacon, H. J. (2001) J. Hum. Evol. 41, 535–544. [DOI] [PubMed] [Google Scholar]
- 24.Halkett, D., Hart, T., Yates, R. J., Volman, T. P., Parkington, J. E., Orton, J., Klein, R. G., Cruz-Uribe, K. & Avery, G. (2003) J. Archaeol. Sci. 30, 955–971. [Google Scholar]
- 25.Schweitzer, F. R. (1979) Ann. S. Afr. Mus. 78, 101–233. [Google Scholar]
- 26.Grine, F. E. & Klein, R. G. (1993) S. Afr. J. Sci. 89, 145–152. [Google Scholar]
- 27.Klein, R. G., Cruz-Uribe, K., Halkett, D., Hart, T. & Parkington, J. E. (1999) Q. Res. 52, 393–403. [Google Scholar]
- 28.Schwarcz, H. P. & Rink, W. J. (2000) J. Hum. Evol. 38, 121–128. [DOI] [PubMed] [Google Scholar]
- 29.Feathers, J. K. & Bush, D. A. (2000) J. Hum. Evol. 38, 91–119. [DOI] [PubMed] [Google Scholar]
- 30.van Andel, T. H. (1989) J. Field Archaeol. 16, 133–155. [Google Scholar]
- 31.van Andel, T. H. (1989) Antiquity 63, 733–745. [Google Scholar]
- 32.Henshilwood, C. S., d'Errico, F., Marean, C. W., Milo, R. G. & Yates, R. J. (2001) J. Hum. Evol. 41, 631–678. [DOI] [PubMed] [Google Scholar]
- 33.Wurz, S. (2002) J. Archaeol. Sci. 29, 1001–1012. [Google Scholar]
- 34.Thackeray, A. I. (2000) J. Hum. Evol. 38, 147–168. [DOI] [PubMed] [Google Scholar]
- 35.Volman, T. P. (1984) in Southern African Prehistory and Paleoenvironments, ed. Klein, R. G. (A. A. Balkema, Rotterdam), pp. 169–220.
- 36.Bordes, F. H. (1952) Bull. Soc. Préhist. Fr. 49, 169–171. [Google Scholar]
- 37.d'Errico, F. (2003) Evol. Anthropol. 12, 188–202. [Google Scholar]
- 38.Tryon, C. A. & McBrearty, S. (2002) J. Hum. Evol. 42, 211–235. [DOI] [PubMed] [Google Scholar]
- 39.Barham, L. S. (2002) Curr. Anthropol. 43, 181–190. [Google Scholar]
- 40.Cruz-Uribe, K., Klein, R. G., Avery, G., Avery, D. M., Halkett, D., Hart, T., Milo, R. G., Sampson, C. G. & Volman, T. P. (2003) J. Archaeol. Sci. 30, 559–575. [Google Scholar]
- 41.Wadley, L. (2003) S. Afr. J. Sci. 99, 247–250. [Google Scholar]
- 42.Deacon, J. (1984) in Southern African Prehistory and Paleoenvironments, ed. Klein, R. G. (A. A. Balkema, Rotterdam), pp. 221–328.
- 43.Volman, T. P. (1978) Science 201, 911–913. [DOI] [PubMed] [Google Scholar]
- 44.Berger, L. R. & Parkington, J. E. (1995) Am. J. Phys. Anthropol. 98, 601–609. [DOI] [PubMed] [Google Scholar]
- 45.Stynder, D. D., Moggi-Cecchi, J., Berger, L. R. & Parkington, J. E. (2001) J. Hum. Evol. 41, 369–383. [DOI] [PubMed] [Google Scholar]
- 46.Klein, R. G. & Cruz-Uribe, K. (1983) S. Afr. Archaeol. Bull. 38, 26–30. [Google Scholar]
- 47.Klein, R. G. & Cruz-Uribe, K. (1987) in Papers in the Prehistory of the Western Cape, South Africa, eds. Parkington, J. E. & Hall, M. (British Archaeological Reports, Oxford), pp. 132–163.
- 48.Parkington, J. E. & Poggenpoel, C. (1987) in Papers in the Prehistory of the Western Cape, South Africa, eds. Parkington, J. E. & Hall, M. (British Archaeological Reports, Oxford), pp. 269–293.
- 49.Parkington, J. E. (1988) in Prehistoric Cultures and Environments in the Late Quaternary of Africa, eds. Bower, J. & Lubell, D. (British Archaeological Reports, Oxford), pp. 197–206.
- 50.Jerardino, A. & Yates, R. (1996) S. Afr. Archaeol. Bull. 51, 7–16. [Google Scholar]
- 51.Jerardino, A. & Swanepoel, N. (1999) Curr. Anthropol. 40, 542–548. [Google Scholar]
- 52.Robey, T. S. (1987) in Papers in the Prehistory of the Western Cape, South Africa, eds. Parkington, J. E. & Hall, M. (British Archaeological Reports, Oxford), pp. 294–325.
- 53.Jerardino, A. (1995) S. Afr. Archaeol. Bull. 50, 21–27. [Google Scholar]
- 54.Jerardino, A. & Parkington, J. E. (1993) S. Afr. J. Sci. 89, 6–7. [Google Scholar]
- 55.Klein, R. G. (1994) J. Archaeol. Sci. 21, 725–733. [Google Scholar]
- 56.Klein, R. G. (1994) in Integrative Paths to the Past: Paleoanthropological Advances in Honor of F. Clark Howell, eds. Corruccini, R. S. & Ciochon, R. L. (Prentice–Hall, Englewood Cliffs, NJ), pp. 471–519.
- 57.Avery, G. (1987) in Papers in the Prehistory of the Western Cape, South Africa, eds. Parkington, J. E. & Hall, M. (British Archaeological Reports, Oxford), pp. 164–191.
- 58.Avery, G. & Underhill, L. G. (1986) J. Archaeol. Sci. 13, 339–360. [Google Scholar]
- 59.Klein, R. G., Cruz-Uribe, K. & Skinner, J. D. (1999) ArchaeoZoologia 10, 181–188. [Google Scholar]
- 60.Jerardino, A. (1997) J. Archaeol. Sci. 24, 1031–1044. [Google Scholar]
- 61.Buchanan, W. F., Parkington, J. E., Robey, T. S. & Vogel, J. C. (1984) in Frontiers: Southern African Archaeology Today, eds. Hall, M., Avery, G., Avery, D. M., Wilson, M. L. & Humphreys, A. J. B. (British Archaeological Reports, Oxford), pp. 121–130.
- 62.Buchanan, W. F. (1988) Shellfish in Prehistoric Diet: Elands Bay, S. W. Cape Coast, South Africa (British Archaeological Reports, Oxford).
- 63.Parkington, J. E. & Jerardino, A. (2004) in Elands Bay Cave: A View of the Past, ed. Parkington, J. E. (Univ. of Cape Town Press, Cape Town, South Africa), in press.
- 64.Voigt, E. A. (1982) in The Middle Stone Age at Klasies River Mouth in South Africa, eds. Singer, R. & Wymer, J. J. (Univ. of Chicago Press, Chicago), pp. 155–186.
- 65.Thackeray, J. F. (1988) S. Afr. Archaeol. Bull. 43, 27–32. [Google Scholar]
- 66.Klein, R. G. (2001) in Archaeology at the Millennium, eds. Feinman, G. M. & Price, T. D. (Plenum, New York), pp. 109–135.
- 67.Buchanan, W. F., Hall, S. L., Henderson, J., Olivier, A., Pettigrew, J. M., Parkington, J. E. & Robertshaw, P. T. (1978) S. Afr. Archaeol. Bull. 33, 89–93. [Google Scholar]
- 68.Richards, M. P., Pettitt, P. B., Stiner, M. C. & Trinkaus, E. (2001) Proc. Natl. Acad. Sci. USA 98, 6528–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Speth, J. D. & Tchernov, E. (2002) J. Archaeol. Sci. 29, 471–483. [Google Scholar]
- 70.Stiner, M. C., Munro, N. D., Surovell, T. A., Tchernov, E. & Bar-Yosef, O. (1999) Science 283, 190–194. [DOI] [PubMed] [Google Scholar]
- 71.Deacon, H. J. & Thackeray, J. F. (1984) in Late Cainozoic Palaeoclimates of the Southern Hemisphere, ed. Vogel, J. C. (A. A. Balkema, Rotterdam), pp. 375–390.