Abstract
Among patients with chronic cardiopulmonary disease, increasing healthy behaviors improves outcomes, but such behavior changes are difficult for patients to make and sustain over time. This study aims to demonstrate how positive affect and self-affirmation improve health behaviors compared with a patient education control group. The patient education (PE control) patients completed a behavioral contract, promising to increase their physical activity or their medication adherence and received an educational guide. In addition to the contract and guide, the positive affect/self-affirmation intervention (PA intervention) patients also learned to use positive affect and self-affirmation to facilitate behavior change. Follow-up was identical. In 756 patients, enrolled in three randomized trials, the PA intervention resulted in increased positive affect and more success in behavior change than the PE control (p < .01). Behavior-specific self-efficacy also predicted success (p < .01). Induction of positive affect played a critical role in buffering against the adverse behavioral consequences of stress. Patients who experienced either negative psychosocial changes (p < .05) or interval negative life events (p < .05) fared better with the PA intervention than without it. The PA intervention increased self-efficacy and promoted success in behavior change by buffering stress.
KEYWORDS: Behavior change, Self-efficacy, Stress, Positive affect, Self-affirmation
INTRODUCTION
Patients with coronary artery disease, hypertension, and asthma frequently experience adverse events that could be prevented by adopting new health behaviors, such as increasing physical activity or improving medication adherence. However, engaging in such behavior change and sustaining it over time are difficult for most patients with cardiopulmonary disease. For example, among angioplasty patients, increasing physical activity reduces all-cause mortality by 25 % over 1–2 years [1, 2], but 60 % of patients fail to achieve recommended physical activity levels [3]. Among patients with mild-moderate asthma, increased lifestyle physical activity can yield cardiovascular and pulmonary benefits [4, 5], but most asthmatics avoid exercise because it may induce bronchoconstriction [6]. Among African-American hypertensives, lack of medication adherence is a major contributor to uncontrolled blood pressure control [7] that results in significantly increased mortality [8]. Thus, these three groups of patients with chronic cardiopulmonary disease often experience adverse sequelae because they cannot make and sustain behavior change. To address this dilemma and develop innovative new approaches to behavior change, the National Heart, Lung, and Blood Institute designed an initiative to evaluate promising approaches from basic behavioral research that had potential to apply to clinical practice [9].
Drawing upon on a large body of basic behavioral research about positive affect and self-affirmation, Cornell Translational Behavioral Science Research Consortium evaluated an intervention that combined positive affect and self-affirmation to motivate health behavior change in these patients with chronic cardiopulmonary disease [10–12]. Three randomized trials were intentionally designed to simultaneously test an identical positive affect/self-affirmation intervention in different populations with the goal of behavior change, albeit with different outcomes (i.e., physical activity and medication adherence). This was the first time ever that the same behavioral intervention was evaluated synchronously in parallel randomized trials using the same methods, and common clinical and psychosocial measures, including measures of affect, stress, social support, and self-efficacy. Thus, by design, the three randomized trials had a common theoretical perspective and substantial cross-linking themes woven through their objectives and methodology [13].
We previously reported the results of these randomized trials in angioplasty patients, mild-moderate asthma patients, and African-American hypertensives that evaluated a positive affect/self-affirmation intervention [13–16]. In the angioplasty and hypertension trials, the positive affect intervention resulted in significant improvement in outcomes vs. the control [15, 16]; in the asthmatics, there was no difference between treatment and control [14]. The overall goal of this preplanned cross-trial analysis is to elucidate the psychosocial mediators and moderators of success in behavior change, and specifically the impact of induced positive affect, across multiple populations.
Positive affect has been shown to influence health in multiple ways [17]. Positive affect interventions have had marked effects on people’s cognitive processes, social behavior, motivation, self-efficacy, and ability to cope with stressful life events. People with positive affect have felt more intrinsically motivated and were more able to try new things [18–20]. Positive affect also increased an individual’s perception of the value of the ultimate goal [21], their belief that the immediate behavioral goal can be achieved, and their expectation that reaching the behavioral goal will lead to the better outcomes [22]. Self-affirmation interventions have enhanced people’s ability to overcome negative expectations of their own ability to stay resolved to avoid unhealthy behaviors and to practice positive ones [10–12].
We hypothesized that the positive affect/self-affirmation intervention would increase self-efficacy for behavior change. We also hypothesized that positive affect/self-affirmation would also buffer against an adverse behavioral impact of negative psychosocial changes including increased perceived stress and depressive symptoms. The objective of this analysis of 756 patients enrolled in the three trials is to elucidate the mechanism whereby the positive affect and self-affirmation intervention resulted in significant improvements in health behaviors in comparison to the PE control group and to explore the similarities and differences between the trials.
METHODS
Population
The 242 coronary artery disease patients had just undergone angioplasty and had been cleared by their cardiologists to resume physical activity [16]. The 252 asthma patients had mild-moderate disease [14], and more than two thirds of the 256 African-American hypertensives had uncontrolled hypertension [15]. Patients with either asthma or hypertension were recruited through an academic primary care practice. The detailed common methods of the three trials have been published [13].
Measures
All three trials employed the Charlson Comorbidity Index [23], the Centers for Epidemiologic Studies Depression Scale 10-item version [24], the Perceived Stress Scale, [25], the Positive and Negative Affect scale [26, 27], the MOS social support scale (which measures positive social support) [28], the Morisky adherence scale [29], and the Paffenbarger Activity and Exercise Index [30, 31].
All patients completed contracts specifying the behavior that they would adopt [13], what they would do, when, how often, and how much they would do [32, 33]. In order to evaluate their self-efficacy for the behavior, patients were asked to rate how confident they were that they would be able to fulfill the contract. Patients rated themselves on a scale of 0–10, with 0 indicating no confidence and 10 indicating complete confidence. [32, 33]
All psychosocial measures were obtained at baseline and at 12 months. For depressive symptoms, the usual cutoff of 10 on the CES-D was used to define patients who had increased depressive symptoms. To define change in other psychosocial measures such as perceived stress and social support, the 75th and 25th percentiles for within-patient change were first defined, with the cutoffs generated from the distribution of the patients’ scores from all three trials. Disease-specific severity measures included the Seattle angina questionnaire [34], stage of hypertension [35], and the Severity of Asthma scale [36]. For the angioplasty trial, patients with an anginal frequency under 50 from the Seattle angina questionnaire were classified as having severe disease, those rating 50–74 were classified as moderate, and those rating 75 or higher were classified as mild [34]. For the hypertension trial, patients with blood pressures >160/100 were classified as severe, 140–159/90–99 as moderate, and <140/90 as mild [35]. For the asthma trial, patients with a Severity of Asthma [37] score >10 were classified as severe disease, those with 5–10 as moderate, and those <5 as mild.
Patient education and positive affect/self-affirmation intervention groups
The PE control group was not a usual-care group. Patients in both the intervention and control groups in all three trials received a disease-specific workbook that explained the importance of the behavior (e.g., increasing physical activity or taking medications). The workbooks had stories about patients who successfully overcame obstacles to behavior change [38–40]. Patients were asked to select a behavior for which they had a high self-efficacy (≥8); for the selected behavior, all patients also completed a contract that stated exactly what behavior they would do, when, how often, and for how long they would do it. Patients were required to select behaviors for which they had a high self-efficacy because to do otherwise would increase the failure rate. All patients were also given a pedometer in order to insure uniformity of the intervention, although the outcome was physical activity in two of the trials and medication adherence in hypertensives. All patients in both arms of the three trials received telephone follow-up every 2 months for 1 year, and, at each follow-up, patients were asked about their physical activity or medication adherence behavior and their self-efficacy on a scale of 0–10 for continuing the behavior. Thus, the PE control group received equal attention including workbooks, behavioral contracts, pedometers, and follow-up calls; the only difference was they did not receive the positive affect/self-affirmation intervention.
Patients in the positive affect/self-affirmation (PA intervention) [41] group also received the workbook, the contract, a pedometer, and telephone follow-ups at 2-month intervals. In addition, the positive affect/self-affirmation group was taught how to use positive affect and self-affirmation [13]. At enrollment, patients in the PA intervention group were asked to identify small things that make them feel good and were told that, “Thinking about these small things that make you feel good may help you to overcome challenges and improve your health” [13]. They were asked to think about these things when they first wake up in the morning and throughout their day. For example, some patients induced their own positive affect every day by taking a moment to enjoy beautiful things, such as a sunrise or sunset. In addition, the PA intervention group was sent a gift prior to each follow-up call (i.e., an umbrella, desk clock, fleece blanket, stainless steel coffee mug, pen and key chain, or duffel bag). For the self-affirmation component of the PA intervention, patients were also asked to think of some proud moments in their lives or something they had done that they were proud of and were told that thinking about these things could help them overcome challenges in carrying out their new health behavior. For example, patients used self-affirmation to overcome obstacles by remembering how they had reached their own goals in the past [13]. At each follow-up, patients’ use of positive affect and self-affirmation intervention were assessed and the positive affect/self-affirmation script intervention was repeated.
Treatment fidelity
The research assistants involved in the trials received the same training and used identical scripts for the PA intervention and PE control groups, respectively [13]. To ensure that the interventions were applied equally in the different trials, we evaluated the elements that were common to the PA intervention and the PE control groups, as well as the elements of the PA intervention. One difference was that only asthma patients were instructed in the use of the pedometer at the time of enrollment. Likely a result of this difference, a greater percentage of patients in the asthma trial [42] than in the angioplasty trial or hypertension trial (52 vs. 25 %, P < .0001) thought the pedometer was helpful.
Overall, with regard to the PA intervention check, 70 % of the patients in all trials reported that they were completely or mostly able to think about things that made them feel good at each interval of follow-up, whereas 50–60 % of patients said that they were completely or mostly able to use self-affirmation at each follow-up interval. At the end of the trial, 60–70 % of patients said that they had employed positive thoughts daily, whereas 84–88 % said they used positive thoughts either daily or weekly. Overall, about 50 % of patients used self-affirmation daily, whereas 80–88 % used it either daily or weekly. About half of the patients thought that using positive thoughts had helped them change their behavior and roughly half of the patients thought that self-affirmation had been helpful in overcoming barriers to behavior change.
Outcomes for this cross-study analysis
The asthma and angioplasty trials were designed to increase physical activity, whereas the hypertension trial was designed to increase medication adherence and blood pressure control. To conduct a cross-study analysis of patients in all three trials, we needed to create a common outcome (i.e., “success”). In the angioplasty and asthma trials, patients who increased their physical activity by more than the prespecified clinically important difference (336 kcal/week) were classified as a success. For the hypertension trial, the JNC VII criteria were used; patients whose blood pressure was <140/90 (or <130/80 if they had diabetes or renal disease) were classified as a success [13, 35].
Statistical analysis
Endpoints were available in 95 % of patients randomized. Statistical modeling of associations among multiple continuous outcomes, such as path and structural equation models has become routine [43]. In contrast, trials such as these whose mixed outcomes include both discrete and continuous outcomes pose challenging issues in data analysis. To address this, we used a general framework to model directed association between variables representing binary and continuous outcomes. We treated discrete outcomes as observed hidden continuous variables and used a latent path model to structurally construct directed association between continuous variables, using an extension of the class of models developed by Amemiya [44] and Gueorguieva and Agresti [45]. To fit this mixed-data structural equation model, we used the full-information maximum likelihood Monte Carlo EM estimation procedure developed by Zhang and Wells [46]. The estimates are calculated using a generalized EM algorithm constructed by maximizing the expected complete-data log-likelihood function (M-step) and using a Monte Carlo approach to evaluate the conditional expectations (E-step). For the analysis, the self-efficacy variable was a discrete version of the highly skewed continuous self-efficacy scale. Self-efficacy was coded as 1 if it was above the median (>8) and 0 if it was below the median self-efficacy score. Several other categorizations for self-efficacy were fit, but the results did not substantively differ from those obtained using the median split.
Mediated moderator analyses were performed to assess the role of other mediating variables (i.e., stress and self-efficacy) in the link between psychosocial changes and the ultimate behavior change. One common approach to combine moderation and mediation involves analyzing moderation and mediation in piecemeal fashion and interpreting their results jointly. With this approach, moderation is usually tested with classical regression analysis, in which the dependent variable is regressed on the independent variable, the moderator variable, and their product [47]. Mediation is typically tested separately using the causal steps procedure [48]; however, the sequential Baron and Kenny methodology cannot simultaneously assess mediation and moderation [49, 50]. Structural equation models provide a more coherent approach for addressing mediation and moderation; this approach, as in testing mediation, characterizes the impact of moderation on the direct and indirect effects. Using bootstrapping resampling techniques [51], we constructed critical values needed for significance tests of the product of coefficients to assess the direct and indirect effects, as well as the moderation effects. The analysis controlled for age, gender, race, disease severity (measured as mild, moderate, or severe for each disease), body mass index, and trial clustering.
Classical path coefficients are the partial regression coefficients that measure the extent of one variable’s effect on another in the path model, controlling for other prior variables. In our analysis, however, we have a mix of binary and continuous outcomes with no easy interpretation because the probit and traditional continuous path coefficients are on different scales. Consequently, to prevent misinterpretation of our results, the mixed path coefficients are not shown.
RESULTS
All three randomized trials were designed to achieve success in behavior change, although the behaviors targeted differed by trials. In angioplasty patients and in hypertensive patients, the positive affect [41] intervention resulted in a significant improvement in outcomes vs. the PE [21] control. For asthmatic patients, however, there was no difference in outcomes between the randomization groups [14]. This analysis, by design, combines the patients from the three trials in order to identify common mediators and moderators of success in behavior change.
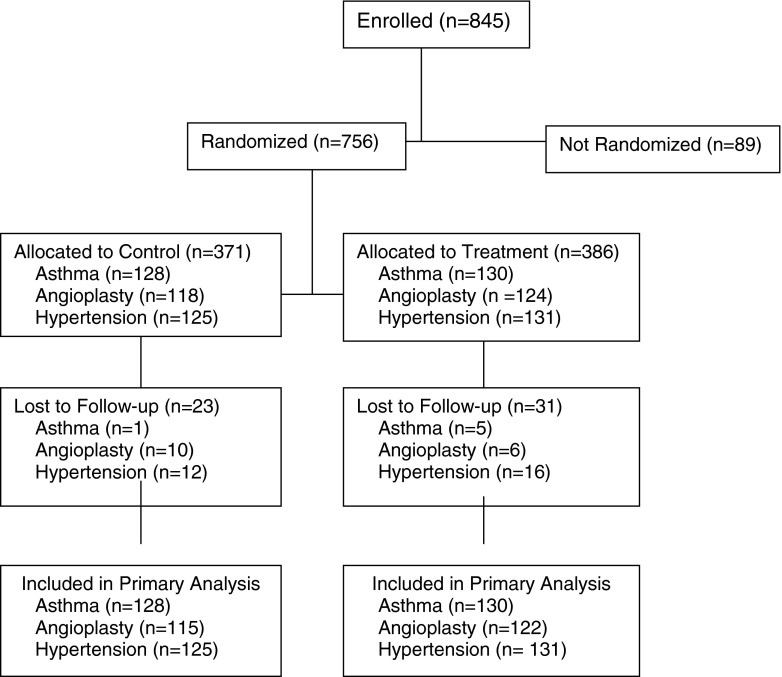
Figure 1 shows the CONSORT diagram for the three trials after enrollment; the CONSORT diagram for each trial has been published [14–16]. Table 1 provides a context for the cross-study analysis, showing that patients in the three trials differed significantly in their demographic and clinical characteristics. Angioplasty patients were predominantly male and often working. Patients in the hypertension trial were predominantly older African-American women, less than half of whom were working. Patients in the asthma trial were younger, working, single women. About 30 % of the patients in the angioplasty and hypertension trials had significant comorbidity, whereas the majority of asthma patients did not. Using disease-specific measures of severity, more patients in the angioplasty and hypertension study had severe disease than those in the asthma trial (P < .05). All analyses adjust for these demographic and clinical differences, as well as the specific trial.
Fig 1.
CONSORT Consolidated Standards of Reporting Trials diagram. Flow of participants from enrollment to completion of the final follow-up assessment
Table 1.
Demographic, clinical, and psychosocial characteristics at baseline in the three trial populations
| Angioplasty | Hypertension | Asthma | P value | |
|---|---|---|---|---|
| Demographic | ||||
| Age | 63 ± 11 | 58 ± 12 | 42 ± 12 | <.0001 |
| Women | 30 % | 80 % | 75 % | <.0001 |
| African American | 11 % | 100 % | 22 % | |
| Caucasian | 81 % | 0 % | 54 % | |
| Latino | 13 % | 3 % | 31 % | <.0001 |
| Married | 69 % | 25 % | 32 % | <.0001 |
| Never married | 11 % | 28 % | 42 % | <.0001 |
| Completed college | 55 % | 26 % | 61 % | <.0001 |
| Working | 56 % | 40 % | 73 % | <.0001 |
| Retired | 31 % | 27 % | 1 % | <.0001 |
| Clinical | ||||
| Comorbidity | <.0001 | |||
| 1-2 | 46 % | 41 % | 95 % | |
| > 3 | 29 % | 35 % | 5 % | |
| Diabetes | 25 % | 36 % | 7 % | <.0001 |
| Myocardial infarction | 28 % | 5 % | 0 % | <.0001 |
| Stroke | 7 % | 11 % | 0 % | <.0001 |
| Disease severity | <.05 | |||
| Mild | 47 % | 41 % | 45 % | |
| Moderate | 40 % | 46 % | 48 % | |
| Severe | 14 % | 13 % | 7 % | |
| Psychosocial | ||||
| PANAS positive | 33 | 35 | 35 | <.001 |
| PANAS negative | 23 | 19 | 20 | <.0001 |
| CES-D 10 | 9.2 | 8.5 | 8.7 | n.s. |
| Social support | 80 | 76 | 78 | n.s. |
| Perceived stress | 14 | 14 | 15 | n.s. |
| Self-efficacy for behavior change | 8.8 | 9.5 | 8.6 | <.05 |
Patients in all three trials had very similar psychosocial characteristics [42]. The patients scored around 33–35 for positive affect, ranking at about the 57th percentile for the general population. [26] The negative affect score was higher than in the general population, with patients scoring 19–23, at around the 75th–85th percentile for the general population [26]. About 40 % of patients in each trial had important depressive symptoms (CESD-10 ≥10), exhibiting a considerably higher rate than the 12 % rate reported in well, older adults [52]. In all three trials, the MOS social support was 78–80, indicating significant support [28]. In all three trials, the perceived stress scale was 14–15, indicating moderate stress [53]. At baseline, patients had a high self-efficacy for their selected behavior change averaging between 8.6 and 9.5; 81 % had a self-efficacy of ≥8. Almost 98 % of patients in all trials had high expectations that engaging in the behavior would be beneficial.
PATH MODELS: MEDIATORS AND MODERATORS OF SUCCESS ACROSS THE TRIALS
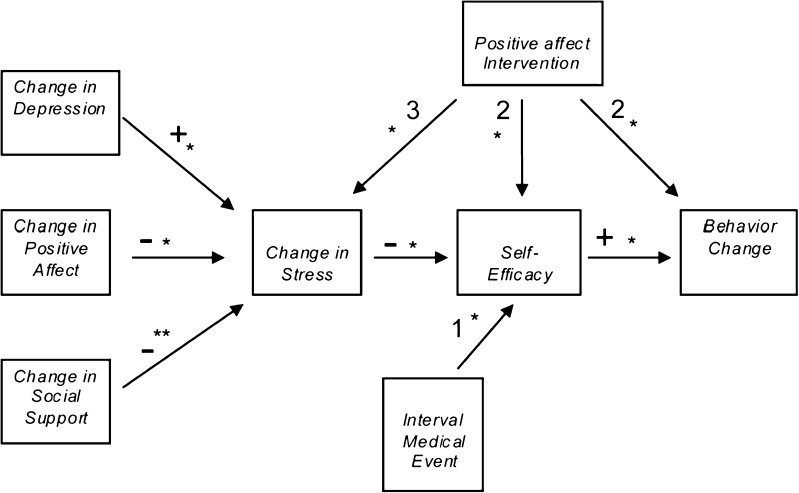
Induction of positive affect had a significant benefit on behavior change; overall, 53 % of those in the PA intervention, but only 41 % of those in the PE control had behavioral success (P < .001) [54]. A PATH model of the success across the trials was developed to evaluate mediators and moderators of successful behavior change. As shown in Fig. 2, the primary dependent variables are change in perceived stress, self-efficacy for behavior change, and success in behavior change. The continuous variables in the path models are baseline to closeout changes in perceived stress, positive affect), depression [52], and social support. The model controls for age, body mass index (BMI), disease severity, gender, race, and a trial level random effect.
Fig 2.
A PATH model: simultaneous mediation and moderation of the positive affect–self-affirmation intervention. Controls for age, BMI, disease severity, gender, race, and trial clustering. Asterisks indicate strength of association: *P < .01, **P < .05. Plus signs indicate direct relationship: + Increased depression leads to increased stress; decreased depression leads to decreased stress. + Increased positive affect leads to increased self-efficacy; decreased positive affect leads to decreased self-efficacy. + Increased self-efficacy leads to increased behavior change; decreased self-efficacy leads to decreased behavior change. Minus signs indicate inverse relationship: − Increased social support leads to decreased stress; decreased social support leads to increased stress. − Increased positive affect leads to decreased stress; decreased positive affect leads to increased stress. − Increased stress leads to decreased self-efficacy; decreased stress leads to increased self-efficacy. Numbers indicate the following: 1 Increased interval medical events leads to decreased self-efficacy. 2 Increased positive affect intervention leads to increased behavior change. 2 Increased positive affect intervention leads to increased self-efficacy. 3 Increased positive affect intervention leads to decreased stress
Figure 2 shows that PA intervention enabled patients to maintain success in behavior change in the face of increased perceived stress, new depressive symptoms, or decreased social support. Increases in positive affect were associated with decreased stress and increased success in behavior change (P < .01). The signs of the partial associations computed from our path analysis are reported in Fig. 2. With an increase in positive affect of 4 or more, perceived stress decreased by 4.2 points, and, with an increase in positive affect of 2–4, perceived stress dropped by 3.0 points. As the level of positive affect increased, perceived stress decreased to a greater degree.
Larger increases in Positive and Negative Affect Schedule (PANAS) positive affect in the PA intervention group were associated with greater reductions in perceived stress (P < .01). Increases in PANAS positive affect (P < .01), decreases in depressive symptoms (P < .01), and increases in social support (P < .05) are associated with reduction in stress, as expected. Conversely, decreased PANAS positive affect (P < .01), new depressive symptoms (P < .01), and decreased support (P < .01) were associated with increased stress.
Although the demographic characteristics did not identify patients with more or less success in behavior change, one clinical characteristic—disease severity—did. Patients with severe disease had less success overall, than those with mild-moderate disease but benefitted more from the PA intervention. For example, among patients with severe disease, 49 % of patients who received the PA intervention had success vs. 27 % of the PE control (P < .05). Among those with mild-moderate disease, only 53 % in the PA intervention vs. 43 % in the PE control achieved success (P < .01). Independently, none of the baseline psychosocial characteristics were predictors of success.
CLINICAL CONTEXT FOR THE PATH MODEL
To put the PATH model in context, Table 2 shows success in healthy behavior change according to the threshold definitions for psychosocial change cited in the table. These data are presented to help clinicians evaluate the potential treatment benefit for patients.
Table 2.
Success in behavior change according to randomization group combining the three trials according to changes in status between baseline and 12 months
| Patient education control (n = 354) | Positive affect/self-affirmation (n = 362) | ||
|---|---|---|---|
| Negative psychosocial changes | |||
| Increased perceived stress | 32 % (75) | 54 % (84) | <.001 |
| Newly depressed | 22 % (24) | 55 % (29) | <.01 |
| Decreased social support | 37 % (76) | 54 % (85) | <.05 |
| Total negative changes | |||
| 0 | 45 % (222) | 52 % (214) | n.s. |
| ≥1 | 36 % (133) | 63 % (14*) | <.05 |
| Positive psychosocial changes | |||
| Decreased stress | 48 % (79) | 63 % (89) | <.05 |
| Decreased depressive symptoms | 39 % (53) | 57 % (70) | <.05 |
| Increased social support | 47 % (85) | 65 % (90) | <.05 |
| Total positive changes | |||
| 0 | 38 % (211) | 45 % (187) | n.s. |
| ≥1 | 45 % (144) | 61 % (175) | <.001 |
| Life events | |||
| None | 44 % (227) | 53 % (234) | <.05 |
| Negative life events | 31 % (51) | 55 % (55) | <.05 |
| Positive life events | 38 % (77) | 51 % (73) | n.s. |
| Interval medical events | |||
| No | 43 % (299) | 55 % (302) | <.01 |
| Yes | 30 % (56) | 40 % (60) | n.s. |
For MOS social support, an increase in support was defined as an increase >7.8 (the 75th percentile for within-patient change); a decrease in support was defined as a fall of −6.5 (the 25th percentile for within-patient change). Using these cutoffs, patients with increased social support demonstrated a mean increase of 20 points and those with decreased support demonstrated a mean decrease of 20 points. For perceived stress, an increase in stress was defined as an increase greater than +2 (the 75th percentile for within-patient change), while a decrease in stress was defined as a fall of −7. Patients with decreased stress showed an average decrease of 12 points and those with increased stress showed an increase of 7 points. At 12 months, patients were also asked whether they had experienced any major negative or positive life events over the last year, and if so, what events occurred.
Mediators of success
In general, changes in perceived stress mediated success, regardless of intervention group. Successful behavior change was observed (P < .05) in 56 % of patients whose perceived stress decreased over follow-up, but only in 43 % of those whose perceived stress increased. Patients who had increased perceived stress were more likely to have success; however, if they received the positive affect/self-affirmation intervention (54 % vs. 32 %, P < .01). Behavior-specific self-efficacy over time was also a mediator of success (P < .01). Among those with high self-efficacy (i.e., ≥8), 44 % of those in the PE control and 55 % of those in the PA intervention group had success (P < .01), whereas among those with low self-efficacy (<8), 32 % of those in the PE control and 43 % of those in the PA intervention had success (P > .1).
Negative psychosocial changes
Patients whose perceived stress increased were more likely to succeed with behavior change if they received the PA intervention vs. PE control (54 vs. 32 %, P < .01). Similarly, patients who became newly depressed were much more likely to maintain behavior change if they received the PA intervention vs. the PE control (56 vs. 25 %, P < .01). Those who had decreased social support were more likely to maintain behavior change if they received the PA intervention vs. PE control (54 vs. 37 %, P < .05). With one or more of these negative psychosocial challenges, patients in the PA intervention were much more successful in maintaining healthy behavior change than the PE control (53 vs. 34 %, P < .05). Thus, induction of positive affect buffered against the potential adverse behavioral impact of increased stress, new depressive symptoms, or decreased social support.
Positive psychosocial changes
Positive psychosocial events occurred significantly more frequently in the PA intervention group than in the PE control group (40 vs. 48 %, p < .05). Specifically, more patients in the PA intervention than in the PE control had decreased stress, decreased depressive symptoms, and increased social support (P < .05). Patients whose stress decreased were more likely to maintain healthy behavior change if they received the PA intervention vs. PE control (63 vs. 47 %, P < 0.05). Moreover, patients whose depressive symptoms abated or those whose support increased were more likely to maintain healthy behavior change if they received the PA intervention vs. PE control (P < .05 for both). Among patients who had positive psychosocial changes, Table 2), those in the PA intervention group were more likely to have success in behavior change than in the PE control group [54].
INTERVAL LIFE EVENTS OR INTERVAL MEDICAL EVENTS
During follow-up in all three trials, patients who experienced interval negative life events, such as deaths of relatives or friends, job loss, or financial or family crises, had 24 % more success with the PA intervention than did the PE control (55 vs. 31 %, P < .02). Patients who did not experience negative events had only about 10 % more success with the PA intervention than the PE control.
Overall, 16 % of patients experienced adverse clinical events during follow-up. Among patients who did not report adverse clinical events, those in the PA intervention fared better than those in the PE control in successful behavior change (55 vs. 43 %), (P < .01). Among those who did report interval medical events, those in the PA intervention fared better in successful behavior change than those in the PE control [55], but the difference was not significant.
Differences between the trials
Table 3 shows the similarities and differences in the success of behavior change in the three populations. The PA intervention helped patients in the angioplasty and hypertension trials, but did not help those in the asthma trial succeed with behavior change, despite increased stress [42]. Of note, angioplasty patients who received the PA intervention had about twice the increase in PANAS positive scores compared with asthma patients (5.1 vs. 2.9, P < .01) and a fivefold higher increase in PANAS scores compared with hypertension patients (5.1 vs. 0.9, P < .01). In all trials, the PA intervention helped patients who became depressed or who had decreased social support to maintain the behavior in comparison to the PE control group.
Table 3.
Similarities and differences in success in behavior change in the three populations according to negative and positive changes in psychosocial status
| Changes in psychosocial status | Angioplasty | Hypertension | Asthma |
|---|---|---|---|
| Negative changes | |||
| Increased stress | |||
| PE control | 18 % (28) | 30 % (27) | 55 % (20) |
| PA intervention | 40 % (20) | 59 % (29) | 57 % (35) |
| Newly depressed | |||
| PE control | 13 % (8) | 22 % (9) | 43 % (7) |
| PA intervention | 57 % (7) | 45 % (11) | 67 % (9) |
| Decreased support | |||
| PE control | 34 % (29) | 36 % (22) | 38 % (24) |
| PA intervention | 59 % (32) | 52 % (29) | 50 % (24) |
| Positive changes | |||
| Decreased stress | |||
| PE control | 38 % (24) | 44 % (18) | 56 % (36) |
| PA intervention | 74 % (34) | 63 % (24) | 52 % (31) |
| Decreased depressive symptoms | |||
| PE control | 19 % (16) | 38 % (8) | 50 % (26) |
| PA intervention | 50 % (32) | 75 % (12) | 60 % (25) |
| Increased support | |||
| PE control | 41 % (27) | 48 % (31) | 52 % (27) |
| PA intervention | 67 % (24) | 69 % (29) | 62 % (37) |
In each trial, when positive psychosocial events occurred, the PA intervention group demonstrated higher rates of success vs. the PE control (angioplasty, 53 vs. 42 %; hypertension, 41 vs. 37 %; asthma, 51 vs. 42 %). With decreased stress, angioplasty and hypertension patients in the PA intervention group had greater success than the PE control, whereas there was no difference between groups for asthma patients. In all three trials, the PA intervention group did better than the PE control if depressive symptoms abated.
Overall, 19 % of angioplasty, 9 % of hypertension, and 20 % of asthma patients experienced an interval medical event. The impact of interval medical events differed among the trials. In the hypertension and asthma trials, those with interval events fared better with the PA intervention; however, in the angioplasty, those with interval events fared worse with the PA intervention than PE controls. In angioplasty, patients with interval events exhibited a decrease in self-efficacy and positive affect. In asthma and hypertension patients, interval events did not decrease self-efficacy and positive affect patients did better, displaying the inverse of the trend observed in angioplasty patients. It should be noted that the medical events reported by angioplasty patients were often more serious than those reported by asthma and hypertensions patients.
Specificity of the intervention
It is important to note that the intervention impact was specific to the behavior cited in the behavioral contract, regardless of randomization group. For the angioplasty and asthma trials, the contract was based on physical activity; over the 12 months, 47 % of patients in the angioplasty trial and 46 % of those in the asthma trial increased their physical activity by more than 336 kcal, whereas only 23 % of those in the hypertension trial did so. The mean within-patient change in physical activity was 470 kcal in angioplasty, 405 kcal in asthma, but only 20 kcal in hypertension (P < .01). In hypertension, the contract was based on medication adherence. Using the Morisky measure for adherence, 38 % of hypertension patients were completely adherent at baseline, a rate that rose to 53 % by the end of 12 months. In contrast, 51 % of the angioplasty population reported adherence, both at baseline and at 12 months. Likewise, the asthma population reported nearly identical adherence at baseline and 12 months (i.e., 24 % at baseline and 29 % at 12 months (P < .01).
DISCUSSION
Although positive affect and self-affirmation have shown promise in small studies [10, 11, 56–62], the three recently published studies are the first randomized trials in patients with chronic disease that have assessed the efficacy of intervention based on positive affect and self-affirmation to motivate behavior change. The positive affect/self-affirmation intervention was identical in all three trials. In all trials, the two randomization groups received identical patient education, individually tailored behavioral contracts, and equal attention through follow-ups every 2 months. The only differences were that the PA group was taught a script about how to induce positive affect and how to use self-affirmation to overcome barriers to behavior change and they received small, unexpected gifts prior to their follow-ups. Across all three trials, patients reported using the positive affect/self-affirmation strategy to fulfill their behavioral contract, and their behavior changes were specific to the contract that they made. The final positive affect/self-affirmation intervention was adapted and tailored through the findings of parallel qualitative studies in the three different patient populations [38, 39, 63] and was pilot-tested and refined in parallel pilot studies.
These concurrent trials with parallel methods afford an unprecedented opportunity to elucidate the psychosocial mediators and moderators of success in behavior change and, specifically, the impact of induced positive affect. As hypothesized, self-efficacy over time predicted sustained success in behavior change [22, 64]. Self-efficacy, a key concept in Social Cognitive Theory, influences the direction, intensity, and persistence of behavior change [64].
A structural equation model (see Fig. 2) was used to assess the mechanism through which the positive affect/self-affirmation intervention worked to increase the success in behavior change. The PATH analysis shows that positive affect has mediation effects for behavior change for both self-efficacy and perceived stress. With regard to stress buffering, PA intervention patients were able to sustain behavior change when their perceived stress was increased, but the PE control patients were not. Thus, our findings support the hypothesis advanced by Pressman and Cohen that positive affect influences health, at least in part, through its ability to buffer the adverse consequences of increased perceived stress [65, 66]. The stress buffering was greater in angioplasty and hypertension patients, who were older and sicker, than in asthma patients, who were younger and less sick. The PA intervention was also effective in buffering against a potential adverse behavioral impact of depressive symptoms. Our findings also support the hypothesis [65, 66] that, despite increased depressive symptoms, the PA intervention patients were able to sustain their behaviors, but the PE control patients were not able to do so. Positive affect also appears to protect against the adverse behavioral impact of decreased social support. Thus, patients who received the PA intervention and who experienced a negative psychosocial change, whether the change involved increased perceived stress, new depressive symptoms, or decreased social support, did significantly better than patients who received only the PE controls in sustaining behavior change. In addition, patients who experienced negative life events during follow-up did significantly better with the PA intervention. Thus, positive affect provided a protection against the adverse behavioral influence of negative psychosocial stressors [67].
In addition, more of the patients in the PA intervention group experienced positive psychosocial changes. Success was higher in the PA intervention patients who had decreased perceived stress or whose depressive symptoms lessened when compared to the PE control patients. As hypothesized by Isen and Reeve, positive affect may foster more flexible thinking and increase problem solving abilities, including the ability to boost self-control [68, 69]. Positive affect leads to improved self-control by increasing the ability to consider future and present costs and benefits and to see the connection between their own efforts and their outcomes [70]. Cohen and colleagues have hypothesized that positive affect might increase social interactions, and have health benefits, by increasing social support and reducing the consequences of social isolation [67, 71]. However, in our data, similar proportions of patients in the two randomization groups had increased social support. The behavioral success rate of those with increased social support in the PA intervention group was higher than in the PE control. Thus, although the hypothesis of Cohen and colleagues may be true in some contexts, our data suggest that positive affect does not increase social interaction and support; instead, it enables patients to make the most of the benefits derived from increased social support and protects against the adverse behavioral impact of decreased support.
The difference between the results of the angioplasty and hypertension trials vs. the asthma trial still requires explanation. One answer may lie in the efficacy of the PA intervention for sicker patients, as more of the hypertension and angioplasty patients had severe disease. The impact of the PA intervention was greatest in angioplasty patients who had just faced a potentially life threatening event [39], in contrast to hypertension and asthma patients, who were enrolled from a practice where they received their ongoing care [38, 63]. Thus, the positive affect intervention may have had a greater impact in the patients who had more severe conditions. In particular, although there were no differences found in outcome expectations, angioplasty patients may have had a greater sense of urgency [39].
It is important to note that the dropout rate was extremely low in all of the trials; overall, the behavioral outcome was assessed in 95 % of patients. This is unusual for physical activity interventions, as previous studies have shown that 50 % will dropout within 3–6 months [72]. Factors that likely minimized dropout include patient-friendly educational workbooks, an individualized behavioral contract, follow-ups at reasonable intervals, and follow-up data collection that was concise and focused on providing support (rather than collecting data).
One limitation of the trials is that the different outcome measures—physical activity for angioplasty and asthma vs. medication adherence and blood pressure control—necessitated the creation of a common dichotomous measure of behavioral success for the cross-study analysis. Another possible limitation is that the PANAS measure of positive affect focuses on activated affect, such as feelings of alertness, determination, and enthusiasm, rather than on nonactivated positive affect, including feelings of calm, peacefulness, and contentment. Given this emphasis on activated affect, we may have missed opportunities to measure non-activated effects of the PA intervention. We also acknowledge the practical consideration that the small gifts, which have been found in many experiments to effectively induce positive affect, can raise the potential cost of the intervention. The small gifts were part of the positive affect intervention [13].
There is an enormous literature about the impact of self-efficacy on success in behavior change. Patients have the best chance of success if they have confidence that they can succeed; however, that confidence or self-efficacy is prerequisite but not a guarantee. These trials were specifically designed to evaluate the impact of positive affect intervention on behavioral changes that the patient selected because they had high self-efficacy for the specific contracted behaviors. Since behavior change is difficult for most patients, assessing the impact of any intervention for which patients had a low self-efficacy would be an exercise in futility. The study evaluated the impact of psychosocial, life and health stressors on the ability of patients to sustain behavior changes for which they had a high self-efficacy or confidence; it did not evaluate environmental, genetic, physiological, or neurological factors. Finally, research assistants who collected the outcome data were not universally blinded to the patients’ intervention status.
Many thoughtful analyses have evaluated the potential impact of positive affect on health outcomes and success [17, 69, 70, 73]. This is a unique NHLBI supported and partnered effort to build new interventions by translating findings in basic behavioral science to address the needs of patients with chronic illness. This study allowed us to create and rigorously evaluate the efficacy of an intervention designed to increase positive affect among patients with chronic cardiopulmonary disease.
The intervention created by this joint effort is published and has freely available and easy-to-use instructions for inducing everyday positive affect, as well as instructions for self-affirmation, when barriers to sustaining changes in behavior are encountered [13]. The positive affect- self-affirmation intervention builds on the knowledge from a workbook, a straightforward contract and regular follow-up, and can be employed to assist patients in changing their behavior.
Footnotes
Alice M. Isen (deceased)
Jared Jobe (retired)
Implications
Practice: A brief script that instructs patients about positive affect and self-affirmation can increase successful behavior change in patients with chronic cardiopulmonary disease.
Policy: Resources should be devoted to the dissemination of freely available, no cost interventions, which are not marketed, precisely because they are free.
Research: Research needs to be directed on how to harness positive affect to address a broader array of patient issues.
The author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by the National Heart, Lung and Blood Institute, contract 1NO1-HC-25096
The Hypertension Trial is registered in ClinicalTrials.gov (NCT00248872).
The Asthma Trial is registered in ClinicalTrials.gov [75].
The Angioplasty Trial is registered in ClinicalTrials.gov [76]).
Contributor Information
Mary E. Charlson, Phone: +1-212-7461684, FAX: +1-212-7467443, Email: mecharl@med.cornell.edu.
Martin T. Wells, Email: Mtw1@cornell.edu.
Janey C. Peterson, Email: jcpeters@med.cornell.edu.
Carla Boutin-Foster, Email: cboutin@med.cornell.edu.
Gbenga O. Ogedegbe, Email: olugbenga.ogedegbe@nyumc.org.
Carol A. Mancuso, Email: mancusoc@hss.edu.
James P. Hollenberg, Email: drjimhollenberg@aol.com.
John P. Allegrante, Email: Jpa1@columbia.edu.
Jared Jobe, Email: jljobe@hotmail.com.
Alice M. Isen, Email: ami4@cornell.edu.
References
- 1.Thompson PD, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011; 123(4): e18–e209. [DOI] [PMC free article] [PubMed]
- 3.Reid RD, et al. Determinants of physical activity after hospitalization for coronary artery disease: the Tracking Exercise After Cardiac Hospitalization (TEACH) Study. Eur J Cardiovasc Prev Rehabil. 2006;13(4):529–537. doi: 10.1097/01.hjr.0000201513.13343.97. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3 Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Dogra S, et al. Exercise is associated with improved asthma control in adults. Eur Respir J. 2011; 37(2): 318–323. [DOI] [PubMed]
- 6.Weiler JM, et al. American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol. 2007;119(6):1349–1358. doi: 10.1016/j.jaci.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 7.Bosworth HB, et al. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119(1):70.e9–70.e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Wong MD, et al. Contribution of major diseases to disparities in mortality. N Engl J Med. 2002;347(20):1585–1592. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 9.Institute, N.H.L.a.B. 2001; Available from: http://www.nhlbi.nih.gov/funding/inits/archive/baa02-07.htm.
- 10.Steele CM. The psychology of self-affirmation: Sustaining the integrity of self. In: Berkowitz L, editor. Advances in Experimental Social Psychology. New York: Academic; 1988. pp. 261–302. [Google Scholar]
- 11.Steele CM. A threat in the air: how stereotypes shape intellectual identity and performance. Am Psychol. 1997;52:613–629. doi: 10.1037/0003-066X.52.6.613. [DOI] [PubMed] [Google Scholar]
- 12.Steele CM, Liu T. Dissonance processes and self-affirmation. J Pers Soc Psychol. 1983; 45: 5–19.
- 13.Charlson ME, et al. Randomized controlled trials of positive affect and self-affirmation to facilitate healthy behaviors in patients with cardiopulmonary diseases: rationale, trial design, and methods. Contemp Clin Trials. 2007;28(6):748–762. doi: 10.1016/j.cct.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Mancuso CA, et al. Increasing physical activity in patients with asthma through positive affect and self-affirmation: a randomized trial. Arch Intern Med. 2012;172(4):337–343. doi: 10.1001/archinternmed.2011.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogedegbe GO, et al. A randomized controlled trial of positive-affect intervention and medication adherence in hypertensive african americans. Arch Intern Med. 2012;172(4):322–326. doi: 10.1001/archinternmed.2011.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson JC, et al. A randomized controlled trial of positive-affect induction to promote physical activity after percutaneous coronary intervention. Arch Intern Med. 2012;172(4):329–336. doi: 10.1001/archinternmed.2011.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131(6):925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- 18.Kahn B, Isen AM. The influence of positive affect on variety-seeking among safe, enjoyable products. J Consum Res. 1993;20:257–270. doi: 10.1086/209347. [DOI] [Google Scholar]
- 19.Isen AM. Positive affect and decision making. In: Lewis M, Haviland-Jones J, editors. Handbook of Emotions. New York: Guilford; 2000. pp. 417–435. [Google Scholar]
- 20.Isen A.M, Reeve JM. Positive affect promotes intrinsic motivation. Ithaca: Cornell University; 2002.
- 21.Heurtin-Roberts S, Reisin E. The relation of culturally influenced lay models of hypertension to compliance with treatment. Am J Hypertens. 1992;5(11):787–792. doi: 10.1093/ajh/5.11.787. [DOI] [PubMed] [Google Scholar]
- 22.Erez A, Isen AM. The influence of positive affect on the components of expectancy motivation. J Appl Psychol. 2002;87(6):1055–1067. doi: 10.1037/0021-9010.87.6.1055. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Weissman MM, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 26.Crawford JR, Henry JD. The positive and negative affect schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. Br J Clin Psychol. 2004;43(Pt 3):245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- 27.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 28.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-B. [DOI] [PubMed] [Google Scholar]
- 29.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108(3):161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 31.Pereira MA, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–S205. [PubMed] [Google Scholar]
- 32.Lorig K, et al. Outcomes of self-help education for patients with arthritis. Arthritis Rheum. 1985;28(6):680–685. doi: 10.1002/art.1780280612. [DOI] [PubMed] [Google Scholar]
- 33.Lorig K, Ritter PL, Plant K. A disease-specific self-help program compared with a generalized chronic disease self-help program for arthritis patients. Arthritis Rheum. 2005;53(6):950–957. doi: 10.1002/art.21604. [DOI] [PubMed] [Google Scholar]
- 34.Spertus JA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 35.Seventh Report of the Joint National Committee on Prevention, detection, evaluation and treatment of high blood pressure. Washington: US Department of Health and Human Services; 2003. [DOI] [PubMed]
- 36.Eisner MD, et al. Assessment of asthma severity in adults with asthma treated by family practitioners, allergists, and pulmonologists. Med Care. 1998;36(11):1567–1577. doi: 10.1097/00005650-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso CA, et al. Patient-reported and physician-reported depressive conditions in relation to asthma severity and control. Chest. 2008;133(5):1142–1148. doi: 10.1378/chest.07-2243. [DOI] [PubMed] [Google Scholar]
- 38.Mancuso CA, et al. Novel use of patient-derived vignettes to foster self-efficacy in an asthma self-management workbook. Health Promot Pract. 2010; 11: 44–53. [DOI] [PubMed]
- 39.Peterson J, et al. Living with heart disease after angioplasty. Heart Lung. 2010;39:105–115. doi: 10.1016/j.hrtlng.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boutin-Foster C, et al. Applying qualitative methods in developing a culturally tailored workbook for black patients with hypertension. Patient Educ Couns. 2009;77(1):144–147. doi: 10.1016/j.pec.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. JAMA. 1982; 248(12): 1465–77. [PubMed]
- 42.Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994; 308(6921): 81–106. [PMC free article] [PubMed]
- 43.Bollen K. Structural Equations with Latent Variables. New York: Wiley; 1989. [Google Scholar]
- 44.Amemiya T. The estimation of a simultaneous equation generalized probit model. Econometrica. 1978;78:1193–1205. doi: 10.2307/1911443. [DOI] [Google Scholar]
- 45.Gueorguieva RV, Agresti A. A correlated probit model for joint modelling of clustered binary and continuous responses. J Am Stat Assoc. 2001;96:1102–1112. doi: 10.1198/016214501753208762. [DOI] [Google Scholar]
- 46.Zhang D, Wells MT. Graphical models for clustered binary and continuous responses. In: Wells MT, SenGupta A, eds. Advances in Directional and Linear Statistics. A Festschrift for Sreenivasa Rao Jammalamadaka. Berlin: Springer; 2009
- 47.Arora RR, et al. Restenosis after transluminal coronary angioplasty: a risk factor analysis. Cathet Cardiovasc Diagn. 1990;19(1):17–22. doi: 10.1002/ccd.1810190106. [DOI] [PubMed] [Google Scholar]
- 48.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 49.Sobel ME. An introduction to causal inference. Sociol Methods Res. 1996;24:353–379. doi: 10.1177/0049124196024003004. [DOI] [Google Scholar]
- 50.Edwards JR, Lambert LS. Methods for integrating moderation and mediation: a general analytic framework using moderated path analysis. Psychol Methods. 2007;12:1–22. doi: 10.1037/1082-989X.12.1.1. [DOI] [PubMed] [Google Scholar]
- 51.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman and Hall; 1993. [Google Scholar]
- 52.Andresen EM, et al. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 53.Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan SOS, editor. The social psychology of health. Newbury Park: Sage; 1988. [Google Scholar]
- 54.Five-year clinical and functional outcome comparing bypass surgery and angioplasty in patients with multivessel coronary disease. A multicenter randomized trial. Writing Group for the Bypass Angioplasty Revascularization Investigation (BARI) Investigators. JAMA. 1997; 277(9): 715–21. [PubMed]
- 55.Serruys PW, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994;331(8):489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 56.Isen AM, Nygren TE, Ashby FG. The influence of positive affect on the perceived utility of gains and losses. J Personal Soc Psychol. 1988;55:710–717. doi: 10.1037/0022-3514.55.5.710. [DOI] [PubMed] [Google Scholar]
- 57.Estrada CA, Isen AM, Young MJ. Positive affect facilitates integration of information and decreases anchoring in reasoning among physicians. Organ Behav Hum Decis Process. 1997;72(1):117–135. doi: 10.1006/obhd.1997.2734. [DOI] [Google Scholar]
- 58.Isen AM, Rosenzweig AS, Young MJ. The influence of positive affect on clinical problem solving. Med Decis Making. 1991;11(3):221–227. doi: 10.1177/0272989X9101100313. [DOI] [PubMed] [Google Scholar]
- 59.Rothman AJ, Salovey P. Shaping perceptions to motivate healthy behavior: the role of message framing. Psychol Bull. 1997;121:3–19. doi: 10.1037/0033-2909.121.1.3. [DOI] [PubMed] [Google Scholar]
- 60.Spencer SJ, Steele CM, Quinn DM. Stereotype threat and women’s math performance. J Exp Soc Psychol. 1999;35:4–28. doi: 10.1006/jesp.1998.1373. [DOI] [Google Scholar]
- 61.Sherman DAK, Enlson LD, Steele CD. Do messages about health risks threaten the self? Increasing the acceptance of threatening health messages via self affirmation. Personal Soc Psychol Bull. 2000;26:1046–1058. doi: 10.1177/01461672002611003. [DOI] [Google Scholar]
- 62.Reed MB, Aspinwall LG. Self affirmation reduces biased processing of health risk informatoin. Motiv Emot. 1998;22:99–132. doi: 10.1023/A:1021463221281. [DOI] [Google Scholar]
- 63.Boutin-Foster C, et al. Ascribing meaning to hypertension: a qualitative study among African Americans with uncontrolled hypertension. Ethn Dis. 2007;17(1):29–34. [PubMed] [Google Scholar]
- 64.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 65.Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. J. Appl Soc Psychol. 1983;13:99–125. doi: 10.1111/j.1559-1816.1983.tb02325.x. [DOI] [Google Scholar]
- 66.Cohen S, Rodriguez MS. Pathways linking affective disturbance and physical disorders. Health Psychol. 1995;14:374–380. doi: 10.1037/0278-6133.14.5.374. [DOI] [PubMed] [Google Scholar]
- 67.Cohen S, Pressman SD. Positive affect and health. Curr Dir Psychol Sci. 2006;15:122–125. doi: 10.1111/j.0963-7214.2006.00420.x. [DOI] [Google Scholar]
- 68.Gervey B, Igou ER, Trope Y. Positive mood and future-oreined self evaluation. Motiv Emot. 2005;29:269–296. doi: 10.1007/s11031-006-9011-3. [DOI] [Google Scholar]
- 69.Isen AM, Reeve J. The influence of positive affect on intrinsic and extrinsic motivation: facilitating enjoyment of play, responsible work behavior and self control. Motiv Emot. 2005;29:297–326. doi: 10.1007/s11031-006-9019-8. [DOI] [Google Scholar]
- 70.Isen AM. Positive affect, cognitive flexibility and self control. In: Shoda DCY, Downey G, editors. Persons in Context. New York: Guilford; 2007. pp. 130–147. [Google Scholar]
- 71.Cohen S, McKay G. Social support, stress and the buffering hypothesis: a theoretical analysis. In: Baum A, Singer JE, Taylor SE, eds. Handbook of Psychology and Health. Hillsdale: Erlbaum; 1984.
- 72.Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Med Sci Sports Exerc. 1996;28(6):706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 73.Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull. 2005;131(6):803–855. doi: 10.1037/0033-2909.131.6.803. [DOI] [PubMed] [Google Scholar]