Abstract
BACKGROUND
Obesity is linked to cardiovascular disease, stroke, increased mortality and vascular remodeling. Although increased arterial diameter is associated with multiple cardiovascular risk factors and obesity, it is unknown whether lumen enlargement is accompanied by unfavorable vascular changes in young and otherwise healthy obese individuals. The purpose of this study was to compare carotid and brachial artery diameter, blood pressure, arterial stiffness, and endothelial function in young, apparently healthy, normal-weight, overweight, and obese male subjects.
METHODS
One hundred sixty-five male subjects (27.39±0.59 years) were divided into 3 groups (normal weight, overweight, and obese) according to body mass index. Subjects underwent cardiovascular measurements to determine arterial diameter, function, and stiffness.
RESULTS
After adjusting for age, the obese group had significantly greater brachial, carotid, and aortic pressures, brachial pulse wave velocity, carotid intima media thickness, and carotid arterial diameter compared with both the overweight and normal-weight groups.
CONCLUSIONS
Obesity is associated with a much worse arterial profile, as an increased carotid lumen size was accompanied by higher blood pressure, greater arterial stiffness, and greater carotid intima media thickness in obese compared with overweight or normal-weight individuals. These data suggest that although obesity may be a factor in arterial remodeling, such remodeling is also accompanied by other hemodynamic and arterial changes consistent with reduced arterial function and increased cardiovascular risk.
Keywords: arterial diameter, arterial remodeling, arterial stiffness, blood pressure, hypertension, obesity.
Obesity is associated with metabolic disorders and an increased risk of mortality, cardiovascular disease, and stroke1,2 and can lead to vascular remodeling.3,4 It is unclear whether arterial enlargement in obesity is an adaptive mechanism due to a greater demand for oxygen and nutrients because of the increased tissue mass3 or a maladaptive mechanism in which there is a change in diameter without an accompanying increase in shear stress.4
Increased carotid artery lumen diameter is related to numerous cardiovascular risk factors.5 There is a positive association of lumen diameter with acute myocardial infarction, and a larger lumen in diastole might reflect a stiffer vessel due to decreased intrinsic vessel elasticity.6 Lumen diameter is also influenced by carotid intima-media thickness (IMT), an early marker of the atherosclerotic process predictive of cardiovascular events that is associated with visceral fat.7 Furthermore, obesity and fat distribution have also been linked to arterial stiffness in both central and peripheral arteries.8,9 Arterial stiffness is a significant risk factor for hypertension and cardiovascular mortality.10,11 Obesity is also often,12,13 but not always,14,15 associated with reduced endothelial function, another arterial marker of cardiovascular risk.16 It is not known if increases in lumen diameter observed in young and otherwise healthy obese individuals are also accompanied by other vascular changes, such as increases in arterial stiffness and IMT, or reduced endothelial function. Obesity and arterial remodeling, even at a young age, could be predictive of future cardiovascular risk, especially if this occurs in the presence of vascular dysfunction.
Chung et al. demonstrated that brachial artery remodeling in overweight individuals occurs in conjunction with the maintenance of shear stress, suggesting that arterial remodeling in obesity is an adaptive process.5 However, because obesity is sometimes, but not always,14,15 associated with reduced endothelial function12,13 and large artery stiffness,17 it is clinically relevant to determine whehter arterial remodeling in the presence of obesity is associated with concomitant changes in arterial function. However, it is currently not known whether arterial remodeling in obesity is concurrent with changes in arterial function. Furthermore, it is also unknown whether obesity affects arterial remodeling differentially at different arterial sites, as studies to date have mostly evaluated brachial or carotid remodeling but not both. Therefore, the purpose of this study was to examine differences between normal-weight, overweight, and obese young, healthy men in carotid and brachial arterial size, blood pressure (BP), central and peripheral arterial stiffness, and endothelial function. We hypothesized that obesity would be associated with increased brachial and carotid artery diameter concomitant with decreased arterial function shown in both the conduit and resistance arteries.
METHODS
Participants
One hundred sixty-five apparently healthy young men (mean age = 27.39±0.59 years) participated in this study. Participants were recruited from the local university population, the greater Urbana-Champaign area, and the firefighter population in central Illinois. All testing was conducted in the same lab at the University of Illinois at Urbana-Champaign. Subjects were divided into groups according to body mass index (BMI): obese (OB; n = 51; body mass index (BMI) > 30kg/m2), overweight (OW; n = 62; BMI = 25–30kg/m2), and normal weight (NW; n = 52; BMI < 25kg/m2). Participants completed a health history questionnaire and were free of any cardiovascular disease, hypertension, or hyperlipidemia. The participants were excluded if they were taking any prescription medication influencing heart rate, BP, or blood flow, or if they had taken any over-the-counter medication in the past 7 days. Any subjects that were current smokers were asked not to smoke 12 hours before testing. The study followed the procedures for protection of human participants as provided in the 1975 Declaration of Helsinki. Before any data collection, all participants signed informed consent, and the study was approved by the University of Illinois at Urbana-Champaign Institutional Review Board.
Subjects reported to the lab for 1 visit. Subjects were instructed to be 4 hours postprandial and to abstain from caffeine, alcohol, and exercise for at least 24 hours before testing. Measurements of height were taken using a stadiometer to the nearest 0.5, cm and body weight was obtained using a beam balance platform scale to the nearest 0.5kg. BMI was calculated as the weight in kilograms divided by the height in meters squared.
Subjects then assumed a supine position and rested quietly for 5 minutes in a temperature-controlled room before systolic and diastolic BP measurements were obtained using an automated oscillometric cuff (HEM-907 XL; Omron, Kyoto, Japan). Brachial BP measurements were repeated, and if values were within 5mm Hg of each other, the average of the 2 values was used for analysis. If measurements were not within 5mm Hg, readings were taken until 2 values within 5mm Hg were obtained. Heart rate was also recorded during this time. Subjects remained in a supine position for the remainder of the measurements.
Aortic and brachial pulse wave velocity (PWV)
Using a high-fidelity strain-gauge transducer (SphygmoCor; AtCor Medical, Sydney, Australia), central pressure waveforms were taken first at the right common carotid artery and then at the right femoral artery, and peripheral pressure waveforms were taken at the right common carotid artery and then at the right radial artery. Distances from the carotid artery to the suprasternal notch and from the suprasternal notch to the femoral artery or radial artery were measured as straight lines with a tape measure and recorded to the nearest millimeter. The distance from the carotid artery to the suprasternal notch was subtracted from the distance from the suprasternal notch to the femoral or radial artery to account for the differences in the direction of the pulse wave propagation. Aortic and brachial PWV was then calculated from the distances between measurement points and the measured time delay between 10 proximal and distal waveforms. All measurements were conducted according to the guidelines of the Clinical Application of Arterial Stiffness, Task Force III.18 Test–retest repeatability (intra-class correlation) for resting PWV in a subset of subjects from this investigation calculated on 2 separate days was greater than 0.90 (P < 0.01).
Aortic and carotid BP
Applanation tonometry was performed using a high- fidelity strain-gauge transducer (SphygmoCor; AtCor Medical) on the radial artery to obtain pressure waveforms. A central aortic pressure waveform was reconstructed from the radial artery pressure waveforms using a generalized validated transfer function.19 Carotid artery pressure waveforms were taken using applanation tonometry and calibrated against brachial mean arterial and diastolic pressure to determine carotid BP. The intraclass correlation coefficient for all variables derived from the radial pulse contour in a subset of subjects from this investigation, collected on 2 separate days, was >0.85 (P < 0.01).
Reactive hyperemia (RH)
Forearm RH, an index of endothelial function, was measured using strain gauge plethysmography (EC-4; D E Hokanson, Bellevue, WA) immediately after obtaining resting forearm blood flow. The BP cuff on the upper arm was inflated to 250mm Hg for 5 minutes to occlude arm blood flow. One minute before the release of the upper arm cuff, the wrist cuff was inflated to 250mm Hg. The upper arm cuff was rapidly released, and changes in forearm volume were measured in 15-second cycles for 13 readings (3 minutes). The peak blood flow was the highest reading recorded (RH peak), and all 13 measurements were plotted into a curve, and the area under the curve was taken as a measure of total RH. The intraclass correlation coefficient for peak forearm blood flow after RH in a subset of subjects from this investigation, collected on 2 separate days, was 0.90 (P < 0.01).
Intima-media thickness (IMT)
The IMT of the common carotid artery was measured by ultrasonography (SSD-5500; Aloka, Tokyo, Japan) using a high-frequency (7.5-MHz) linear array probe and was defined as the distance between the leading edge of the lumen–intima interface to the leading edge of the media adventitia interface of the far wall. All measurements for the carotid IMT were made at end-diastole from an average of 5 measurements of a 10-mm segment (separated by 2-mm intervals) obtained 20mm proximal to the carotid bifurcation.
Carotid arterial stiffness measurements
Using ultrasound (SSD-5500; Aloka) echo-tracking technology, indices of arterial stiffness and compliance, such as B-stiffness, arterial compliance, and elastic modulus measurements, were obtained from the common carotid artery using a high-frequency (7.5-MHz) linear array probe. The cephalic portion of the carotid artery was imaged in a longitudinal section, 10–20mm proximal to the bifurcation. Echo-tracking gates were manually set just outside the intima-media complex, near the edge of the adventitia side. β-Stiffness index, one of the most commonly used clinical markers20 and an independent risk factor for future vascular events21 was calculated as a means of adjusting arterial compliance for changes in distending pressure using the equation:
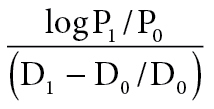 |
where P1 and P0 are the highest (systolic) and lowest (diastolic) carotid pressures and D1 and D0 are the maximum (systolic) and minimum (diastolic) diameters. The intraclass correlation coefficient for measures of carotid stiffness in a subset of subjects from this investigation, collected on 2 separate days, was 0.94 for β-stiffness and 0.86 for PWV. Arterial compliance was calculated as an absolute change in carotid lumen area for a given increase in pressure. Pressure-strain elastic modulus was determined by the change in carotid artery diameter for a change in arterial BP relative to the average diameter.22
Brachial artery diameter
The brachial artery diameter was imaged 5–10cm above the antecubital fossa using an SSD-5500 ultrasound (Aloka) with a high-frequency (7.5-MHz) linear array probe. A baseline diameter was recorded during a 10-second interval during diastole only. One experienced sonographer collected all images. The images were digitized from the video output of the ultrasound machine using a frame grabber under control of custom software (Vascular Research Tools 5; Medical Imaging Applications, Corallvile, Iowa). Image acquisition was gated with an electrocardiogram signal so that the images were captured at end-diastole in each cardiac cycle.
Carotid tensile wall stress
Carotid circumferential wall stress was calculated using the equation23
 |
where diameter is the carotid artery diameter in centimeters, IMT is the intima-media thickness in centimeters, and MAP is the mean arterial pressure in dynes/cm2.
Statistical analysis
All data are presented as the mean ± SE. All analyses were adjusted for age. Group differences in hemodynamics, anthropometrics, microvascular function, and macrovascular structure and function were tested separately with a multivariate analysis of variance followed by Tukey–Kramer post hoc tests. A stepwise multiple linear regression was performed with carotid artery diameter as the dependent variable using age, BMI, brachial systolic and diastolic BP, IMT, β stiffness, aortic pulse wave velocity, and endothelial function as potential predictors. Statistical significance was set at P < 0.05. All analyses were conducted using Statistical Package for Social Sciences software, version 18.0 (SPSS, Chicago, IL).
RESULTS
Table 1 shows the descriptive statistics of the study population divided into NW, OW, and OB groups based on BMI. The OW and OB groups were both significantly older than the NW group. All groups were significantly different from each other in both BMI and weight, with both measures increasing significantly from NW to OW and OW to OB. The OB group had significantly higher brachial, carotid, and aortic BPs when compared with both the NW and OW group. There were no significant differences in heart rate.
Table 1.
Subject descriptive statistics
| Variable | Normal weight (n = 52) | Overweight (n = 62) | Obese (n = 51) |
|---|---|---|---|
| BMI, kg/m2 | 23±0.2 | 27±0.2* | 33±0.4*,** |
| Age, y | 24±0.9 | 28±0.8* | 30±1.3* |
| Height, cm | 178±0.9 | 177±0.8 | 177±0.9 |
| Weight, kg | 72±1.4 | 86±1.3* | 104±1.4*,** |
| HR, bpm | 57±1.3 | 58±1.2 | 60±1.3 |
| Brachial SBP, mm Hg | 124±1.3 | 126±1.2 | 132±1.3*,** |
| Brachial DBP, mm Hg | 70±1.2 | 70±1.1 | 76±1.2*,** |
| Brachial MAP, mm Hg | 88±1.1 | 88±1.0 | 94±1.1*,** |
| Carotid SBP, mm Hg | 116±1.5 | 116±1.3 | 124±1.5*,** |
| Carotid DBP, mm Hg | 70±1.2 | 70±1.1 | 76±1.2*,** |
| Aortic SBP, mm Hg | 105±1.3 | 106±1.1 | 112±1.3*,** |
| Aortic DBP, mm Hg | 71±1.2 | 71±1.1 | 77±1.2*,** |
Data are mean ± SEM.
Abbreviations: NW: normal weight. BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
*P < 0.05 vs. normal weight; **P < 0.05 vs. overweight.
Table 2 shows subject arterial characteristics of the NW, OW, and OB groups. There were no significant differences between groups in measures of aortic PWV, RH peak, RH area under the curve, or arterial compliance. Brachial PWV and IMT were significantly higher in OB individuals compared with the OW and NW groups. Elastic modulus and carotid circumferential wall stress were significantly higher in the OB group compared with the NW group.
Table 2.
Subject arterial characteristics
| Variable | Normal weight (n = 52) | Overweight (n = 62) | Obese (n = 51) |
|---|---|---|---|
| Aortic PWV, m/s | 6.1±0.1 | 6.3±0.1 | 6.4±0.1 |
| Brachial PWV, m/s | 4.4±0.1 | 4.6±0.1 | 5.0±0.1*,** |
| RH peak, %/min – 1 | 27±1.2 | 29±1.1 | 25±1.3 |
| RH AUC, AU | 86±5.6 | 102±5.0 | 93±5.8 |
| Ep, kPa | 56±3.0 | 59±2.7 | 68±3.1* |
| AC, mm2/kPa | 1.3±0.1 | 1.3±0.0 | 1.4±0.1 |
| Carotid IMT, mm | 0.46±0.0 | 0.46±0.0 | 0.51±0.0*,** |
| Carotid CWS, dynes/cm2 × 104 | 86.47±2.4 | 91.28±2.2 | 96.44±2.4* |
Data are mean ± SEM.
Abbreviations: AC, arterial compliance; AU, arbitrary units; AUC, area under the curve; CWS, circumferential wall stress; Ep, elastic moduls; IMT, intima-media thickness; PWV, pulse wave velocity; RH, reactive hyperemia.
*P < 0.05 vs. normal weight; **P < 0.05 vs. overweight.
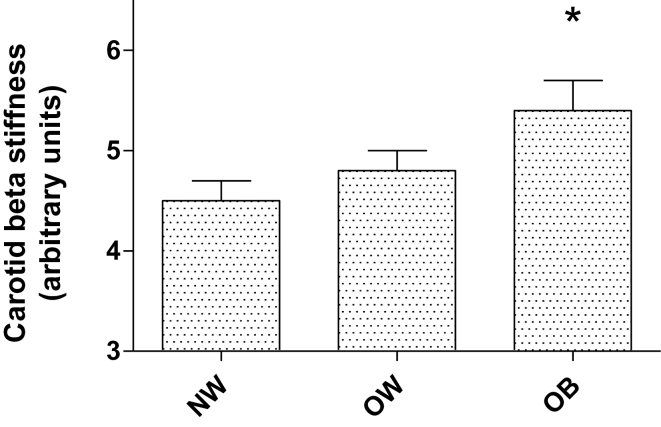
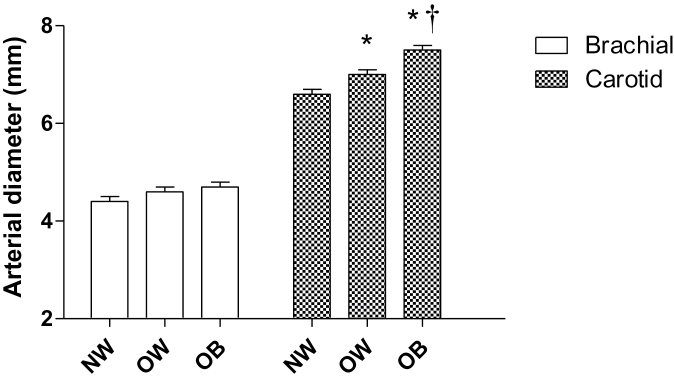
Figure 1 shows carotid β stiffness in the 3 groups. β Stiffness was significantly higher in the OB group compared with the NW group. Figure 2 shows that there was no difference in brachial diameter with increasing BMI; however, the carotid arterial diameter was significantly higher in the OB and OW groups compared with the NW group, and the OB group also exhibited significantly higher carotid diameter compared with the OW group.
Figure 1.
Carotid beta stiffness. Mean ± SEM. *P < 0.05 vs. normal-weight group. Abbreviations: NW, normal weight; OB, obese; OW, overweight.
Figure 2.
Brachial and carotid arterial diameter. Mean ± SEM. *P < 0.05 vs. normal-weight group. †P < 0.05 vs. overweight group. Abbreviations: NW, normal weight; OB, obese; OW, overweight.
Results of the stepwise multiple linear regression demonstrated that although BMI is a predictor of carotid arterial diameter, other variables such as IMT, β stiffness, and aortic PWV are also associated with carotid diameter, collectively explaining 41% of the variance. Although there were 21 smokers (9 in the NW group and 6 in both the OW and OB groups), smoking was not included as a significant predictor in the linear regression. The equation developed was
Carotid Arterial Diameter (mm) = 3.043 + (0.052 × BMI) + (2.426 × IMT) + (0.096 × β stiffness) + (0.154 × aortic PWV); standard error of estimate = 0.621mm; R 2 = 0.412; p < 0.0001.
DISCUSSION
Our study provides several novel findings. First, although carotid diameter increased linearly with BMI, BMI did not impact brachial diameter in these young, otherwise healthy men, suggesting obesity has differential effects depending on arterial site. Obesity was also accompanied by higher carotid artery stiffness, but interestingly, resistance artery endothelial function was not reduced in the groups with higher BMI. Furthermore, obesity was associated with an impaired arterial profile, as evidenced by the increased carotid lumen size, higher BP, greater carotid artery stiffness, and greater carotid IMT in the OB group compared with the other groups. In addition, a stepwise multiple regression demonstrated that carotid artery diameter was positively associated with BMI, aortic PWV, carotid β stiffness, and carotid IMT. These data suggest that obesity in these young men is associated with arterial remodeling and is accompanied by other hemodynamic and arterial changes consistent with reduced arterial function and increased cardiovascular risk.
Arterial enlargement has been speculated to occur as a compensatory mechanism due to atherosclerosis, which leads to arterial wall thickening and an increase in lumen diameter.24–27 This occurs because of increases in shear stress at the intimal thickening of the stenotic site, which is thought to stimulate luminal enlargement to regulate wall shear stress.28 Chung et al. suggested that the arterial enlargement in obese subjects is an adaptive response due to proportionate changes in blood flow to maintain shear stress;3 therefore their study could not support measurement of the brachial artery lumen size in determination of cardiovascular risk.29,30 However, their study included individuals with BMIs <29.7kg/m2, and obesity is considered to occur at 30kg/m2. Therefore, their population might not have reached the point where arterial remodeling was associated with a decrease in shear stress. Conversely, recent data4 from a population with a mean BMI of 46kg/m2 found a larger brachial artery diameter was accompanied by a decrease in shear stress. This maladaptive remodeling is thought to be associated with chronic inflammation of the vascular wall, which leads to larger arteries with low shear stress.31 Our data support arterial remodeling of the carotid artery in obesity, but provide little evidence for a significant effect of obesity on brachial artery diameter, suggesting that obesity may have different effects on different arterial sites in young, relatively healthy subjects.
Another potential mechanism of arterial diameter remodeling is prolonged high BP. This produces mechanical stress and medial degeneration, leading to a decrease in arterial compliance and production of a large lumen diameter.32,33 This is considered maladaptive if this perpendicular tension to the arterial wall (circumferential wall stress) remains elevated despite lumen diameter remodeling. In our study, obese subjects had significantly higher carotid circumferential wall stress when compared with normal-weight individuals, in addition to greater carotid diameter, which might suggest maladaptive remodeling. We also observed increased aortic, carotid, and brachial pressures in obese individuals; however, our study design did not allow us to determine whether the higher BP had been sustained for a period of time. Interestingly, BP was not included as a significant predictor in the multiple regression analyses, suggesting that BP may not be a predictor of carotid lumen diameter, but carotid β stiffness and carotid IMT were, in addition to BMI. Furthermore, arterial stiffness and microvascular and endothelial function are predictive of future hypertension, suggesting that these variables precede increases in BP.34 These findings support our data, suggesting that structural changes in the arterial wall may be unrelated to BP, but are associated with increased lumen diameter in obesity. Our study confirms previous findings that obesity is related to arterial stiffness in multiple subject populations.35–38 In both young and older populations, body fat measures, including BMI, were among the strongest independent predictors of aortic stiffness, indicating that excess weight affects the vasculature at an early stage.8
An important finding, with clinical relevance, in our study was that arterial remodeling begins even at the overweight stage, with increasing arterial dysfunction developing as BMI increases. The Framingham Heart Study found that individuals who are overweight based on BMI standards exhibit increased risk for hypertension and cardiovascular risk factors.2 Coronary heart disease mortality has also been shown to increase 4%–5% for every BMI unit increase exceeding 22kg/m2.39 Furthermore, the increase in cardiovascular risk was exponential in the OB group because the OB group had many risk factors that were absent in the OW group, including higher carotid and aortic pressures, higher brachial PWV, higher carotid stiffness, and a larger carotid IMT. These are all considered risk factors for cardiovascular disease, even without the presence of obesity, and alterations in these measures are early markers of vascular disease and atherosclerosis.40–42 Our data also suggest that the OB group exhibited maladaptive carotid remodeling because circumferential wall stress was higher in this group, even with the increased carotid diameter. Further research needs to be done to determine whether weight reduction and decreased arterial diameter can also reverse other cardiovascular risk factors, such as those measured in this study.
Our study has several limitations. First, we used a cross-sectional design; thus only associations and no cause–effect can be inferred. Second, only men were included in our sample; thus it is unknown whether our findings also apply to women. Third, only young, apparently healthy men were included; thus our findings may not be generalized to other populations. We also did not perform blood analyses of traditional cardiovascular risk factors. However, all participants filled out a health history questionnaire and were free of diagnosed cardiovascular disease, hypertension, and hyperlipidemia; therefore we are classifying subjects as “apparently healthy.” Fourth, the age difference between our groups may not be completely accounted for by controlling for age in our multivariate analysis of variance. However, age was not included as a significant predictor in our regression model, suggesting that age was not a significant contributor to our findings. Finally, groups were defined based on BMI, which only takes into account height and weight. However, the definition of obesity is based on BMI, BMI is commonly used in epidemiologic studies and has been recommended for use in clinical practice,43 and BMI is widely used because of its simplicity.44
Our study demonstrates that obesity is associated with negative changes in the vasculature that can lead to increased cardiovascular risk, even in young, seemingly healthy individuals. These vascular changes occur when an individual is overweight, and then progress to further and more advanced arterial dysfunction in obese individuals. These vascular changes contribute to the increased risk of cariodvascular mortality, cardiovascular disease risk, and stroke associated with obesity. Consistent with prior data from our group,15 it appears that obesity affects arterial stiffness, IMT, and BP before a deterioration of endothelial function. Data also suggest that the arterial impact of obesity is not isolated to arterial size but is accompanied by a deterioration of other markers of vascular structure and function.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported in part by NHLBI 1R01HL093249-01A1 (to BF) and the Department of Homeland Security’s Fire Prevention and Safety Grant Program (EMW-2006-FP-02459).
REFERENCES
- 1. Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med 2002; 162:2557–2562 [DOI] [PubMed] [Google Scholar]
- 2. Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002; 162:1867–1872 [DOI] [PubMed] [Google Scholar]
- 3. Chung WB, Hamburg NM, Holbrook M, Shenouda SM, Dohadwala MM, Terry DF, Gokce N, Vita JA. The brachial artery remodels to maintain local shear stress despite the presence of cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2009; 29:606–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hamburg NM, Mott MM, Bigornia SJ, Duess MA, Kluge MA, Hess DT, Apovian CM, Vita JA, Gokce N. Maladaptive enlargement of the brachial artery in severe obesity is reversed with weight loss. Vasc Med 2010; 15:215–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonithon-Kopp C, Touboul PJ, Berr C, Magne C, Ducimetiere P. Factors of carotid arterial enlargement in a population aged 59 to 71 years: the EVA study. Stroke 1996; 27:654–660 [DOI] [PubMed] [Google Scholar]
- 6. Bots ML, Grobbee DE, Hofman A, Witteman JC. Common carotid intima-media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke 2005; 36:762–767 [DOI] [PubMed] [Google Scholar]
- 7. Sturm W, Sandhofer A, Engl J, Laimer M, Molnar C, Kaser S, Weiss H, Tilg H, Ebenbichler CF, Patsch JR. Influence of visceral obesity and liver fat on vascular structure and function in obese subjects. Obesity (Silver Spring) 2009; 17:1783–1788 [DOI] [PubMed] [Google Scholar]
- 8. Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 2003; 42:468–473 [DOI] [PubMed] [Google Scholar]
- 9. Nemes A, Gavaller H, Csajbok E, Forster T, Csanady M. Obesity is associated with aortic enlargement and increased stiffness: an echocardiographic study. Int J Cardiovasc Imaging 2008; 24:165–171 [DOI] [PubMed] [Google Scholar]
- 10. Pannier B, Guerin AP, Marchais SJ, Safar ME, London GM. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension 2005; 45:592–596 [DOI] [PubMed] [Google Scholar]
- 11. Nemes A, Forster T, Csanady M. Impaired coronary flow velocity reserve and aortic distensibility in patients with untreated hypercholesterolemia—an echocardiographic study. Int J Cardiovasc Imaging 2007; 23:15–23 [DOI] [PubMed] [Google Scholar]
- 12. Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol 2012; 165:561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Guilder GP, Stauffer BL, Greiner JJ, Desouza CA. Impaired endothelium-dependent vasodilation in overweight and obese adult humans is not limited to muscarinic receptor agonists. Am J Physiol Heart Circ Physiol 2008; 294:H1685–H1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Biasucci LM, Graziani F, Rizzello V, Liuzzo G, Guidone C, De Caterina AR, Brugaletta S, Mingrone G, Crea F. Paradoxical preservation of vascular function in severe obesity. Am J Med 2010; 123:727–734 [DOI] [PubMed] [Google Scholar]
- 15. Fahs CA, Smith DL, Horn GP, Agiovlasitis S, Rossow LM, Echols G, Heffernan KS, Fernhall B. Impact of excess body weight on arterial structure, function, and blood pressure in firefighters. Am J Cardiol 2009; 104:1441–1445 [DOI] [PubMed] [Google Scholar]
- 16. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 2009; 120:502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizia-Stec K, Gasior Z, Zahorska-Markiewicz B, Holecki M, Haberka M, Mizia M, Gomulka S, Zak-Golab A, Goscinska A. The indexes of arterial structure and function in women with simple obesity: a preliminary study. Heart Vessels 2008; 23:224–229 [DOI] [PubMed] [Google Scholar]
- 18. Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15:445–452 [DOI] [PubMed] [Google Scholar]
- 19. Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension 2001; 38:932–937 [DOI] [PubMed] [Google Scholar]
- 20. Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res 1987; 21:678–687 [DOI] [PubMed] [Google Scholar]
- 21. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation 2003; 107:139–146 [DOI] [PubMed] [Google Scholar]
- 22. O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990; 15:339–347 [DOI] [PubMed] [Google Scholar]
- 23. Carallo C, Irace C, Pujia A, De Franceschi MS, Crescenzo A, Motti C, Cortese C, Mattioli PL, Gnasso A. Evaluation of common carotid hemodynamic forces. Relations with wall thickening. Hypertension 1999; 34:217–221 [DOI] [PubMed] [Google Scholar]
- 24. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987; 316:1371–1375 [DOI] [PubMed] [Google Scholar]
- 25. Clarkson TB, Prichard RW, Morgan TM, Petrick GS, Klein KP. Remodeling of coronary arteries in human and nonhuman primates. JAMA 1994; 271:289–294 [PubMed] [Google Scholar]
- 26. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002; 105:297–303 [DOI] [PubMed] [Google Scholar]
- 27. Losordo DW, Rosenfield K, Kaufman J, Pieczek A, Isner JM. Focal compensatory enlargement of human arteries in response to progressive atherosclerosis. In vivo documentation using intravascular ultrasound. Circulation 1994; 89:2570–2577 [DOI] [PubMed] [Google Scholar]
- 28. Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med 1994; 330:1431–1438 [DOI] [PubMed] [Google Scholar]
- 29. Holubkov R, Karas RH, Pepine CJ, Rickens CR, Reichek N, Rogers WJ, Sharaf BL, Sopko G, Merz CN, Kelsey SF, McGorray SP, Reis SE. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J 2002; 143:802–807 [DOI] [PubMed] [Google Scholar]
- 30. Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 2007; 115:2390–2397 [DOI] [PubMed] [Google Scholar]
- 31. Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007; 49:2379–2393 [DOI] [PubMed] [Google Scholar]
- 32. McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension 1999; 33:1392–1398 [DOI] [PubMed] [Google Scholar]
- 33. Glagov S, Vito R, Giddens DP, Zarins CK. Micro-architecture and composition of artery walls: relationship to location, diameter and the distribution of mechanical stress. J Hypertens 1992; 10:S101–S104 [PubMed] [Google Scholar]
- 34. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012; 308:875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sutton-Tyrrell K, Mackey RH, Holubkov R, Vaitkevicius PV, Spurgeon HA, Lakatta EG. Measurement variation of aortic pulse wave velocity in the elderly. Am J Hypertens 2001; 14:463–468 [DOI] [PubMed] [Google Scholar]
- 36. Amar J, Ruidavets JB, Chamontin B, Drouet L, Ferrieres J. Arterial stiffness and cardiovascular risk factors in a population-based study. J Hypertens 2001; 19:381–387 [DOI] [PubMed] [Google Scholar]
- 37. Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension 1997; 30:654–659 [DOI] [PubMed] [Google Scholar]
- 38. Taquet A, Bonithon-Kopp C, Simon A, Levenson J, Scarabin Y, Malmejac A, Ducimetiere P, Guize L. Relations of cardiovascular risk factors to aortic pulse wave velocity in asymptomatic middle-aged women. Eur J Epidemiol 1993; 9:298–306 [DOI] [PubMed] [Google Scholar]
- 39. Jousilahti P, Tuomilehto J, Vartiainen E, Pekkanen J, Puska P. Body weight, cardiovascular risk factors, and coronary mortality. 15-year follow-up of middle-aged men and women in eastern Finland. Circulation 1996; 93:1372–1379 [DOI] [PubMed] [Google Scholar]
- 40. Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation 2004; 109:184–189 [DOI] [PubMed] [Google Scholar]
- 41. Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension 2007; 50:154–160 [DOI] [PubMed] [Google Scholar]
- 42. Pruissen DM, Gerritsen SA, Prinsen TJ, Dijk JM, Kappelle LJ, Algra A. Carotid intima-media thickness is different in large- and small-vessel ischemic stroke: the SMART study. Stroke 2007; 38:1371–1373 [DOI] [PubMed] [Google Scholar]
- 43. Seidell JC, Kahn HS, Williamson DF, Lissner L, Valdez R. Report from a Centers for Disease Control and Prevention Workshop on use of adult anthropometry for public health and primary health care. Am J Clin Nutr 2001; 73:123–126 [DOI] [PubMed] [Google Scholar]
- 44. Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis 1972; 25:329–343 [DOI] [PubMed] [Google Scholar]