Abstract
Background
Setting a target quit date (TQD) is often an important component in smoking cessation treatment, but ambiguity remains concerning the optimal timing (ie, quitting spontaneously versus delaying to prepare).
Objective
We examined four questions about the timing of TQDs and smoking outcomes in secondary analyses of The iQUITT Study, a randomized trial of Internet and telephone treatment for cessation: (1) What are the characteristics of TQDs set using an online interactive quit date tool?, (2) What are the characteristics of individuals who use a quit date tool and do they differ from those who do not?, (3) Are there differences in smoker characteristics, treatment utilization, and cessation outcomes based TQD timing?, and (4) Is maintenance of an initial TQD predictive of abstinence or do changes to TQDs lead to cessation?
Methods
A total of 825 adult current cigarette smokers were randomized to enhanced Internet or enhanced Internet plus telephone counseling. Latency to TQD in days was calculated as the date difference between the initial TQD and enhanced Internet registration; prospective TQD setters were stratified into four latency groups (0, 1-14, 15-28, 29+ days). Baseline variables, website utilization, and 3-month cessation outcomes were examined between prospective TQD groups. Desire and confidence to quit, number of TQDs, and website logins were tested as predictors of 30-day point prevalence abstinence (ppa) at 3 months (responder-only analyses). Classification and regression tree (CART) analysis explored interactions among baseline variables, website utilization, and latency to TQD as predictors of 30-day ppa.
Results
There were few baseline differences between individuals who used the quit date tool and those who did not. Prospective TQDs were set as follows: registration day was 17.1% (73/427), 1-14 days was 37.7% (161/427), 15-28 days was 18.5% (79/427), and 29+ days was 26.7% (114/427). Participants with a TQD within 2 weeks had higher baseline self-efficacy scores but did not differ on smoking variables. Individuals whose TQD was the same day as registration had the highest logins, page views, number of TQDs set using the tool, and messages sent to other members. Logistic regression revealed a significant interaction between number of TQDs and website logins for 30-day ppa (P=.005). Among those with high logins, 41.8% (33/79) with 1 TQD were abstinent versus 25.9% (35/135) with 2+TQDs. Logins and self-efficacy predicted 30-day ppa in the CART model.
Conclusions
TQD timing did not predict cessation outcomes in standard or exploratory analyses. Self-efficacy and an apparent commitment to an initial TQD were the components most highly related to abstinence but only via interactions with website utilization. Findings highlight the importance of feeling efficacious about handling specific smoking situations and engaging with treatment. Additional research focused on increasing engagement in Web-based cessation studies is needed.
Trial Registration
ClinicalTrials.gov: NCT00282009; http://clinicaltrials.gov/show/NCT00282009 (Archived by WebCite at http://www.webcitation.org/6Kt7NrXDl).
Keywords: smoking cessation, Internet, quit date, tobacco dependence
Introduction
Setting a quit date is often a central element of tobacco dependence treatment [1-4]. Establishing a target quit date (TQD) may increase the likelihood of success for several reasons. The public commitment often involved in setting a quit date may increase or solidify a smoker’s motivation to quit [5] and the probability that they will follow through with intentions to quit [6]. Setting a TQD may also provide time for the smoker to develop relevant coping skills [6,7] and to obtain and initiate medication use, which can increase the likelihood of abstinence [2,8].
However, there is mixed evidence regarding the importance of the nature (ie, planned vs unplanned) and timing (ie, sooner vs later) of quit dates. Some evidence suggests that setting a TQD is associated with a greater likelihood of making a quit attempt [9] and is a predictor of abstinence [10,11]. Other studies indicate that roughly half of smokers prefer to quit abruptly [12] and do not plan a quit attempt [13-16] and that unplanned or spontaneous quit attempts are more likely to be successful than those involving a TQD [13-17]. It is also unclear whether the timing of a quit date matters. A recent randomized controlled trial by Hughes et al [18] in which smokers were prompted to select a quit date found that those who selected a later quit date or delayed a planned quit attempt were less likely to quit smoking compared to participants who selected an early quit date or adhered to their original date. Similarly, in a trial of varenicline versus placebo for smoking cessation in which smokers chose their own quit dates (within a 5-week time frame), smokers who selected later quit dates (particularly in the last week) were less likely to achieve abstinence in both treatment arms [19]. In contrast, among smokers who planned to quit within a month, proximity of the quit date did not predict abstinence [9]. Similarly, in a Web-based trial by Etter et al [12], smokers randomized to abrupt versus gradual quitting had equivalent quit rates at all follow-ups.
This ambiguity regarding quit dates is reflected in the varying recommendations found on smoking cessation websites. For example, the instructions on the American Cancer Society’s website state “Once you’ve decided to quit, you’re ready to pick a quit date. This is a very important step. Pick a day within the next month as your Quit Day” [20]. The American Legacy Foundation’s BecomeAnEX website instructs smokers “Don’t pick tomorrow as your quit date… Don’t set your date too far off in the future… We recommend a day that’s about 2-4 weeks away” [21]. The National Cancer Institute’s cessation website tells smokers who are preparing to quit to “Pick a date within the next 2 weeks to quit” [22]. QuitAssist, a free website provided by the tobacco company, Altria, simply encourages smokers to “get ready” and “choose a specific quit date” with no specific timeline [23]. For the millions of smokers who search online for assistance quitting smoking [24-26], these mixed messages may be confusing.
Most studies that have examined the timing of a quit date have used retrospective, cross-sectional population-based survey data [13-17] or data gathered in the context of randomized controlled trials in which participants were required to set a quit date or adhere to a researcher-defined date [27,28]. Each of these approaches has limitations. Retrospective reports are subject to recall bias skewed toward remembering more planned quit attempts [29], and required quit dates may not be representative of actual quitting behavior. Prospective research is needed that uses objective methods for measuring the timing of quit dates that occur naturally during the course of quitting.
Web-based cessation programs represent both an effective intervention approach to help smokers quit and a means to address some of the limitations of previous analyses of quitting behavior. Sites that offer interactive tools to assist users in choosing and/or documenting a quit date [30] can yield prospective, naturalistic, and objective measures of quitting behavior with regard to the nature and timing of quit dates. We are aware of only one study that has examined the use of an online quit date tool and its association with abstinence [31].
Our study examined four key questions: (1) What are the characteristics of quit dates that are set using an online interactive quit date tool?, (2) What are the characteristics of individuals who use a quit date tool and do they differ from those who do not?, (3) Are there differences in smoker characteristics, treatment utilization, and cessation outcomes based on the timing of an initial (ie, first) TQD in relation to program initiation?, and (4) Is the maintenance of a TQD predictive of eventual abstinence, or are multiple changes of an online quit date more likely to lead to cessation? We approached these questions in secondary analyses of data from a pragmatic randomized trial of Internet and telephone treatment for smoking cessation [32]. Participants were not required to set a quit date and could use the website as they desired. We began with standard analytic methods to describe differences among those who used an online interactive quit date tool and those who did not. We then examined differences among prospective quit date setters based upon the latency to an initial TQD. We hypothesized that individuals whose target quit date was within 2 weeks of registration would be more motivated to quit, have higher indices of treatment utilization, and be more likely to maintain abstinence. We also hypothesized an interaction between the number of TQDs set and website utilization, such that the highest abstinence rates would be observed among participants with only one TQD (signaling unwavering commitment) and high levels of website utilization. To guide future studies, we employed an exploratory data analysis technique, classification and regression tree analysis (CART) [33], to examine the interactive nature of various predictors on abstinence. This exploratory method can augment traditional analytic approaches to identify unique combinations of variables related to tobacco use behavior patterns [34,35].
Methods
Participants
Participants in The iQUITT Study [32,36] were smokers aged 18 and older in the United States who smoked 5 or more cigarettes per day. To maximize generalizability of study findings, motivation to quit and willingness to set a quit date were not included as eligibility criteria. Active user interception sampling was used to recruit smokers who used the terms “quit(ting) smoking”, “stop(ping) smoking”, or “smoking” in a major Internet search engine and who clicked on a link to QuitNet, the cessation treatment website being evaluated [37]. Following online informed consent and a baseline telephone assessment, participants were randomized to basic Internet, enhanced Internet, or enhanced Internet plus telephone counseling in the parent trial. Follow-up assessments were conducted by phone or online for telephone non-responders at 3, 6, 12, and 18 months. These analyses focus on participants with complete 3-month follow-up data in the two enhanced Internet arms, which included an interactive tool to assist users in setting a quit date (“Quit Date Wizard”). The basic Internet intervention did not include the Quit Date Wizard. Across both enhanced Internet arms, 75% (995/1326) of participants were reached at 3 months. Due to a technical issue early in the trial, data on use of the Quit Date Wizard were not stored for 170 participants. Thus, the final sample for these analyses focused on 825 participants (412 enhanced Internet, 413 enhanced Internet plus telephone counseling).
Interventions
Participants randomized to enhanced Internet were given 6 months of free access to the premium service of the QuitNet website. QuitNet is a widely used Internet cessation program that incorporates evidence-based elements of tobacco dependence treatment [2] including practical counseling and tailored information for cessation, recommendations and support for approved pharmacotherapy, and intra-treatment social support through a large online social network [36,38,39].
The Quit Date Wizard is a central feature of QuitNet. It explains the importance of setting a quit date and prompts users to think about a realistic time frame for quitting (“To choose a timeframe, think about approximately when you will be ready to quit”) with options ranging from “In a week” to “In more than 2 months”. The Wizard also encourages users to consider potential triggers, steps to prepare to quit, and pharmacotherapy use. The Quit Date Wizard does not specify an optimal timeframe for quitting but encourages users to consider whether they feel prepared and if not “to spend a few weeks getting to the point where you are comfortable with the idea of ‘jumping in’ [to quitting]”. Users can enter their own date or select a Wizard-generated quit date. Users can also make their quit date visible to other members for support and can sign up for quit support emails timed around their quit date. Repeated reminders to set a quit date using the Quit Date Wizard or to confirm a previously set quit date are featured prominently throughout QuitNet. Users can update their quit date at any time. These analyses focus on the initial TQD, measured as the number of days between website registration and the first TQD that the user set in the Quit Date Wizard. We elected to examine this TQD versus subsequent updates or changes to a quit date to inform recommendations provided by Internet smoking cessation programs. These analyses are not designed to address the timing of a quit date subsequent to a slip or relapse.
Participants randomized to enhanced Internet plus telephone counseling were offered 5 calls in a relapse-sensitive schedule [40]. Counselors had real-time access to summary data regarding a participant’s use of the QuitNet site, which enabled them to prompt and reinforce use of QuitNet (including the Quit Date Wizard) during each call.
Data Collection and Measures
Summary
The three sources of data are described below. These analyses focus on 3-month data since study questions addressed initial quitting behavior, and this is typically where treatment utilization and intervention effects are the strongest.
Baseline Assessment
Age, gender, race, ethnicity, education, employment, and household income were assessed. We also assessed self-rated health status [41], history of smoking-related illness, body mass index, and whether they had spoken to a doctor about their smoking. Smoking variables included cigarettes per day, the time to first cigarette item from the Fagerström Test for Nicotine Dependence [42], duration of last quit attempt (days), desire to quit and confidence in quitting (scale=1-10), spouse smoking status, and number of smokers in the home. Psychosocial items included the Smoking Situations Confidence Inventory and the Smoking Temptations Inventory (short-form) [43] as measures of self-efficacy, the Perceived Stress Scale [44], the Center for Epidemiologic Studies-Depression (CES-D) Scale [45], Weight Concern Scale [46], the Social Network Index [47], an abbreviated version of the Partner Interaction Questionnaire [48,49], and an item from the Two-Item Conjoint Screen [50] assessing alcohol consumption.
Three-Month Follow-Up Assessment
Smoking outcomes included number of intentional quit attempts and 30-day point prevalence abstinence (ppa; primary outcome of the parent trial) calculated using responder-only analyses. Participants also reported use of other quit methods since enrolling in the trial, including nicotine replacement therapy, behavioral treatment (eg, self-help materials, individual counseling), and prescription medication use (eg, bupropion).
Treatment Utilization
Website utilization metrics included date of QuitNet registration, date of initial TQD, total number of quit dates set using the Quit Date Wizard, website logins, page views, total time online, exchange of messages with other QuitNet members (yes/no), and use of an interactive Medication Wizard (yes/no). Number of calls completed was examined among individuals randomized to enhanced Internet plus telephone counseling.
Statistical Analyses
For Study Question 1, frequency counts were used to characterize use of the Quit Date Wizard. Latency to TQD (days) was calculated as the difference between the first date designated using the Quit Date Wizard and the website registration date. To anchor our analyses to common recommendations provided to smokers in Web-based cessation programs, we categorized this variable as 0 days (registration day), 1-14 days (within 2 weeks), 15-28 days (2-4 weeks), and 29+ days (more than 4 weeks). For Study Question 2, selected baseline characteristics of QuitNet registrants were compared between those who set a quit date using the Quit Date Wizard and those who never set a quit date. For Study Question 3, selected baseline characteristics, treatment utilization metrics, and smoking outcomes were examined by latency to TQD using the categories described above: 0 days, 1-14 days, 15-28 days, and 29+ days. We report the median and interquartile range for skewed variables. Between-group comparisons of categorical items and skewed variables were analyzed using nonparametric statistics, and continuous items were analyzed with analysis of variance (ANOVA) using IBM SPSS (version 21.0). For Study Question 4, a logistic regression model examined 30-day ppa as the primary outcome, number of quit dates set using the Quit Date Wizard, number of logins, and the interaction term (centered at the mean) as predictors, and treatment group, desire to quit, and confidence in quitting as covariates using JMP (version 10.02). We examined Study Questions 1-3 by treatment arm and found no between group differences on likelihood of use of the Quit Date Wizard, latency to TQD, baseline characteristics, or website utilization metrics. Therefore, we combined participants from both treatment arms and report the results for the combined sample.
Classification and regression trees (CART) analysis was performed in JMP (version 10.02) to explore the effects of study condition, all baseline variables, and selected treatment utilization measures (logins, number of quit dates set using the Quit Date Wizard, Medication Wizard use, latency to TQD, behavioral treatment use, and pharmacotherapy use) on 30-day ppa, the main outcome of the parent trial [32]. CART analysis allows for a flexible format in terms of allowable response and predictor variables, and handling of missing data [33]. CART is a machine-learning approach that utilizes a classification algorithm to split data into binary subgroups (branches) based upon predictor variables in order to maximize the homogeneity of the two samples for the outcome of interest. In JMP, binary splits for a categorical dependent variable (Y) like abstinence (yes, no) are determined by maximizing the LogWorth statistic ((-log10(P value)) [51]. The factors (X; predictors) can be either continuous or categorical (nominal or ordinal). If X is continuous, then the partition is done according to a splitting “cut” value for X. If X is categorical, then it divides the X categories into two groups of levels and considers all possible groupings in two levels. Our CART model included all predictor variables entered simultaneously. To gauge the reliability of our CART analyses, we utilized a k-fold cross-validation procedure that divides the data into k subsets (in this case k=5) that are used to validate the model fit on the rest of the data, fitting a total of K models. The model giving the best validation statistic (-2LogLikelihood) is chosen as the final model.
Results
Study Question 1
Among all participants, 77.3% (638/825) registered on QuitNet following randomization and 22.7% (187/825) never registered (Figure 1). Among QuitNet registrants, 66.9% (427/638) used the Quit Date Wizard to prospectively set a TQD, 12.9% (82/638) used it to record a TQD that occurred prior to registration (retrospective), and 9.7% (62/638) did not use the tool at all. For 10.5% (67/638) of registered participants, use of the Quit Date Wizard was documented but TQDs were not stored due to a database error.
Figure 1.
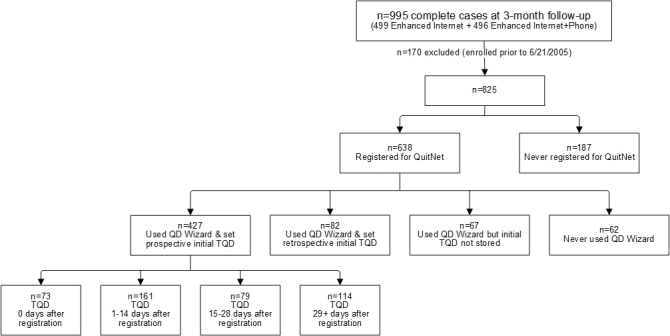
Diagram of data flow from complete cases randomized to the enhanced Internet and enhanced Internet plus telephone counseling arms based on QuitNet registration status, Quit Date (QD) Wizard use, and initial target quit date (TQD) status, and latency to TQD relative to registration date (among prospective quit date setters).
Study Question 2
Compared to those who used the Quit Date Wizard (n=576), those who did not (n=62) were more likely to be male (67.7%, 42/62 vs 49.3%, 284/576, P=.009) and to have a spouse who smokes (64.9%, 24/37 vs 45.5%, 157/345, P=.039). There were no differences on smoking variables, including smoking rate, desire to quit, or confidence in quitting (Multimedia Appendix 1).
Study Question 3
Among those who set a prospective TQD (n=427), 17.1% (73/427) picked the same day as registration, 37.7% (161/427) picked a date 1-14 days later, 18.5% (79/427) picked a date 15-28 days later, and 26.7% (114/427) picked a date 29 or more days later (see Figure 1). There were differences between prospective TQD groups on education (P=.040) and the Confidence Inventory (P=.045) (Table 1). There were also differences between groups on treatment utilization metrics. Individuals whose TQD was the same day as registration had the highest number of logins, viewed more webpages, and set more TQDs using the Quit Date Wizard relative to other groups. This group was also the most likely to use one-to-one messaging (31.5%, 23/73) and the least likely to use the Medication Wizard (16.4%, 12/73). Among those who reported at least one quit attempt at the 3-month follow-up (total 389/425; 2 missing cases), there were no differences in use of behavioral quit methods, pharmacotherapy, or telephone counseling calls completed based upon latency to TQD. There were no differences based on latency to TQD on cessation outcomes (Table 2). Overall, 30-day ppa was 21.1% (90/426), and 91.5% (389/425) reported at least one quit attempt.
Table 1.
Baseline characteristics by latency to target quit date (TQD) relative to website registration date.
| Baseline variable | TQD, 0 days | TQD, 1-14 days | TQD, 15-28 days | TQD, 29+ days | P valuea | |||
| n=73 | n=161 | n=79 | n=114 | |||||
| Demographic variables b | ||||||||
|
|
Age, (years), mean (SD) | 34.22 (10.37) | 36.99 (10.97) | 36.90 (9.98) | 38.61 (11.49) | .064 | ||
|
|
Gender (Female), n (%) | 36 (49.3) | 84 (52.2) | 37 (46.8) | 53 (46.5) |
|
||
|
|
Race, n (%) |
|
|
|
|
.328 | ||
|
|
|
White | 66 (90.4) | 145 (90.1) | 70 (88.6) | 95 (83.3) |
|
|
|
|
|
Non-white | 7 (9.6) | 16 (9.9) | 9 (11.4) | 19 (16.7) |
|
|
|
|
Ethnicity (Hispanic), n (%) | 1 (1.4) | 8 (5.0) | 3 (3.8) | 2 (1.8) | .363 | ||
|
|
Education, n (%) |
|
|
|
|
.040 | ||
|
|
|
High school or less | 9 (12.3) | 33 (20.5) | 22 (27.8) | 37 (32.5) |
|
|
|
|
|
Some college | 42 (57.5) | 76 (47.2) | 34 (43.0) | 43 (37.7) |
|
|
|
|
|
College 4+ yrs | 22 (30.1) | 52 (32.3) | 23 (29.1) | 34 (29.8) |
|
|
|
|
Employment, n (%) |
|
|
|
|
.616 | ||
|
|
|
Employed fulltime | 50 (68.5) | 114 (70.8) | 55 (69.6) | 87 (76.3) |
|
|
|
|
|
Otherc | 23 (31.5) | 47 (29.2) | 24 (30.4) | 27 (23.7) |
|
|
|
|
Income, n (%) |
|
|
|
|
.409 | ||
|
|
|
Low income (≤$40,000) | 33 (45.2) | 69 (42.9) | 36 (47.4) | 60 (53.1) |
|
|
|
|
|
High income (>$40,000) | 40 (54.8) | 92 (57.1) | 40 (52.6) | 53 (46.9) |
|
|
| Smoking variables | ||||||||
|
|
Cigarettes per day, mean (SD) | 20.26 (10.15) | 18.75 (7.90) | 20.80 (9.32) | 19.61 (9.32) | .356 | ||
|
|
Time to first cigarette, n (%) |
|
|
|
|
.825 | ||
|
|
|
Within 30 minutes | 57 (78.1) | 122 (75.8) | 57 (72.2) | 88 (77.2) |
|
|
|
|
|
After 30 minutes | 16 (21.9) | 39 (24.2) | 22 (27.8) | 26 (22.8) |
|
|
|
|
Duration of last quit attempt, n (%) |
|
|
|
|
.497 | ||
|
|
|
≤3 days | 34 (49.3) | 82 (55.0) | 44 (59.5) | 59 (60.2) |
|
|
|
|
|
4+ days | 35 (50.7) | 67 (45.0) | 30 (40.5) | 39 (39.8) |
|
|
|
|
Desire to quit, mean (SD) | 9.25 (1.08) | 9.07 (1.24) | 8.87 (1.25) | 8.95 (1.43) | .263 | ||
|
|
Confidence in quitting, mean (SD) | 6.49 (2.09) | 6.48 (2.13) | 5.72 (2.28) | 6.16 (2.08) | .052 | ||
| Psychosocial variables | ||||||||
|
|
Health status, n (%) |
|
|
|
|
.558 | ||
|
|
|
Excellent | 12 (16.4) | 13 (8.1) | 7 (8.9) | 11 (9.7) |
|
|
|
|
|
Very good | 26 (35.6) | 63 (39.1) | 26 (32.9) | 40 (35.4) |
|
|
|
|
|
Good | 20 (27.4) | 57 (35.4) | 31 (39.2) | 35 (31.0) |
|
|
|
|
|
Fair/Poord | 15 (20.5) | 28 (17.4) | 15 (19.0) | 27 (23.9) |
|
|
|
|
Illness caused by smoking, n (%) | 45 (61.6) | 105 (65.2) | 40 (51.3) | 59 (51.8) | .069 | ||
|
|
Spouse smokes, n (%) | 20 (51.3) | 40 (42.6) | 23 (45.1) | 31 (48.4) | .787 | ||
|
|
1+ smokers in house, n (%) | 17 (23.3) | 23 (14.3) | 11 (13.9) | 27 (23.7) | .103 | ||
|
|
Temptations Inventory, mean (SD) | 4.00 (0.47) | 3.90 (0.49) | 3.90 (0.59) | 3.92 (0.52) | .567 | ||
|
|
Confidence Inventory, mean (SD) | 2.88 (0.60) | 2.82 (0.57) | 2.67 (0.48) | 2.71 (0.57) | .045 | ||
|
|
Perceived Stress Scale, mean (SD) | 6.10 (3.11) | 5.90 (2.91) | 6.25 (3.26) | 6.89 (3.18) | .067 | ||
|
|
CES-D Scale, mean (SD) | 8.79 (5.78) | 8.73 (5.27) | 9.96 (6.12) | 10.31 (5.81) | .082 | ||
aNonparametric test (categorical) or ANOVA used.
bParticipants could refuse to answer a question or respond “I don’t know”. Income, n=423; duration of last quit attempt, n=390; health status, n=426; illness caused by smoking, n=426; spouse smokes, n=248 (asked only among individuals with spouse).
cIncludes part-time employment, retired, student, homemaker, and unemployed.
dCollapsed “Fair” and “Poor” categories due to small cell counts.
Table 2.
Treatment utilization and smoking outcomes at 3 months by latency to target quit date (TQD) relative to website registration date.
| Dependent measure | TQD, 0 days | TQD, 1-14 days | TQD, 15-28 days | TQD, 29+ days | P valuea | ||
| n=73 | n=161 | n=79 | n=114 | ||||
| Website utilization | |||||||
|
|
Logins, n (%) |
|
|
|
|
.024 | |
|
|
|
1-2 | 14 (19.2) | 51 (31.7) | 17 (21.5) | 37 (32.5) |
|
|
|
|
3-5 | 11 (15.1) | 29 (18.0) | 26 (32.9) | 28 (24.6) |
|
|
|
|
6-10 | 18 (24.7) | 28 (17.4) | 9 (11.4) | 19 (16.7) |
|
|
|
|
More than 10 | 30 (41.1) | 53 (32.9) | 27 (34.2) | 30 (26.3) |
|
|
|
Page views, median (interquartile range) | 138 (362) | 102 (198) | 98 (256) | 59.50 (158) | .016 | |
|
|
Total number minutes online, median (interquartile range) | 88 (237) | 62 (157) | 54 (150) | 43 (119) | .212 | |
|
|
Number of quit dates set using Quit Date Wizard, mean (SD) | 2.44 (1.73) | 1.95 (1.42) | 1.72 (0.97) | 1.57 (1.40) | .002 | |
|
|
Used one-to-one messaging, n (%) | 23 (31.5) | 38 (23.6) | 16 (20.3) | 15 (13.2) | .023 | |
|
|
Used Medication Wizard, n (%) | 12 (16.4) | 53 (32.9) | 30 (38.0) | 35 (30.7) | .025 | |
| Other treatment utilization at 3 months (among those who made a quit attempt, n=389) b | |||||||
|
|
Used pharmacotherapy, n (%)c | 38 (55.9) | 93 (63.7) | 47 (61.8) | 52 (53.1) | .350 | |
|
|
Used behavioral treatment, n (%)d | 10 (14.7) | 33 (22.6) | 23 (30.3) | 21 (21.4) | .167 | |
|
|
No. counseling calls completed, mean (SD)e | 3.39 (2.72) | 4.43 (2.87) | 4.47 (2.58) | 4.83 (3.03) | .159 | |
| Smoking outcomes f | |||||||
|
|
30-day ppa, n (%) | 19 (26.0) | 33 (20.6) | 18 (22.8) | 20 (17.5) | .555 | |
|
|
No. quit attempts, n (%) |
|
|
|
|
.158 | |
|
|
|
0 | 4 (5.5) | 14 (8.8) | 3 (3.8) | 15 (13.3) |
|
|
|
|
1 | 22 (30.1) | 50 (31.3) | 29 (36.7) | 30 (26.5) |
|
|
|
|
2 | 24 (32.9) | 33 (20.6) | 19 (24.1) | 21 (18.6) |
|
|
|
|
3 | 8 (11.0) | 26 (16.3) | 10 (12.7) | 25 (22.1) |
|
|
|
|
4+ | 15 (20.5) | 37 (23.1) | 18 (22.8) | 22 (19.5) |
|
aNonparametric test (median; categorical) or ANOVA used.
bParticipants could refuse to answer a question or respond “I don’t know”. Pharmacotherapy, n=388; used behavioral treatment, n=388.
cNRT, Zyban, Chantix.
dIndividual counseling, group counseling, pamphlet/books, telephone counseling not through the study.
eAmong those randomized to enhanced Internet plus telephone counseling (n=33 among TQD 0 days; n=77 among TQD 1-14 days, n=38 among TQD 15-28 days, and n=47 among TQD 29+ days).
fParticipants were able to refuse answering a question or respond “I don’t know”. Sample sizes are follows: 30-day ppa, 426; no. quit attempts, 425.
Study Question 4
The final logistic regression model among prospective quit date setters did not include desire to quit and confidence in quitting measures as both were unrelated to 30-day ppa. For 30-day ppa, the interaction between number of quit dates set and logins was significant (parameter estimate=–0.003, standard error=0.001, P=.005). Among those with high levels of website utilization (n=214; median split), 41.8% (33/79) of those who set one quit date were abstinent compared to 25.9% (35/135) of those who changed their quit date one or more times. Among those with high logins who set only one quit date and who were abstinent, the majority (60.6%, 20/33) opted to quit within 2 weeks of website registration.
CART Analysis
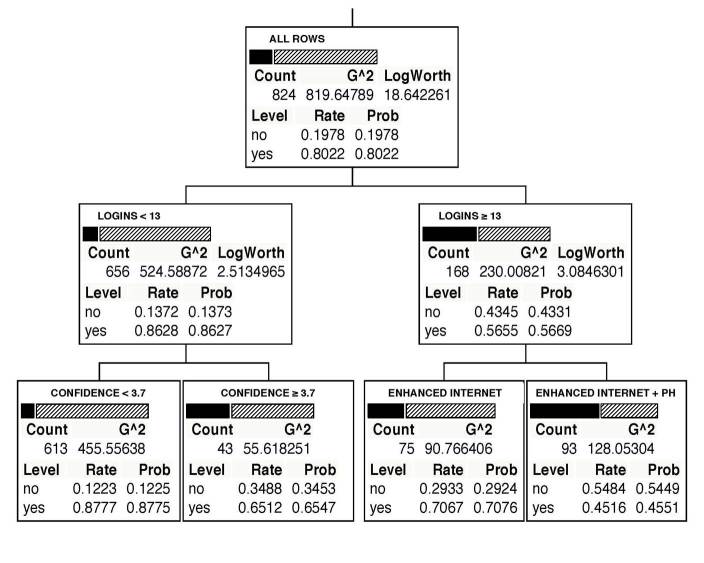
The CART model for 30-day ppa produced a tree with splits at three nodes (Figure 2), none of which were variables associated with quit date setting or timing. The first node, representing the total sample (n=824; 1 case missing outcome data), shows the overall proportion quit (19.8%, 163/824; Level=no) compared to the proportion smoking (80.2%, 661/824; Level=yes). The first split partitioned the total sample by logins (<13 logins, 79.6% of sample; ≥13 logins, 20.4% of sample). Among those who logged in <13 times, 13.7% (90/656) were abstinent, and for individuals who logged in ≥13 times, 43.5% (73/168) were abstinent. The second split occurred among individuals who logged in <13 times and was based on the Confidence Inventory scale score. Among those with a score <3.7, 12.2% (75/613) were abstinent compared to 34.9% (15/43) among those with a score ≥3.7. The third split occurred for those who logged in ≥13 times, where the sample was divided by treatment. Among enhanced Internet participants, 29.3% (22/75) were abstinent compared to 54.8% (51/93) of enhanced Internet plus telephone counseling participants. The k-fold cross-validation results showed good agreement (similar R squared values) between the folded and overall samples.
Figure 2.
CART model predicting 30-day point prevalence abstinence at 3 months (n=824). Bars correspond to smoking status: solid=abstinent; diagonal lines=not abstinent. Count=total number of participants in subset; Level=smoking abstinence status (no/yes); Rate=relative proportion of the count in each abstinence status group; Logins=frequency of website logins during first 3 months of study; enhanced Internet and enhanced Internet + ph (telephone counseling)=treatment arms; Confidence=Confidence Inventory score.
Discussion
Principal Findings
In these secondary analyses of The iQUITT Study, we explored the use of an online interactive tool to set a quit date and its relationship to smoking outcomes. The majority of study participants who used the website set a quit date using the Quit Date Wizard: most set a prospective quit date, but some used it to document a quit date that had already passed. Only 9.7% (62/638) of those who used the website did not use this tool. Our a priori hypotheses were only partially supported. We did not find evidence that individuals whose first TQD was set within 2 weeks of registration differed on baseline desire to quit or motivation to quit as hypothesized, but we did find that those who set a quit date within 2 weeks of registration had higher levels of baseline self-efficacy (Confidence Inventory score) and education compared to smokers who set later quit dates. We also found that participants whose TQD occurred within the first 2 weeks of website registration exhibited higher rates of website utilization than those with later quit dates. We did not find any differences in smoking outcomes based on latency to TQD. There was an interaction between website utilization and number of TQDs set on quit rates. At low levels of website utilization, there was no difference in abstinence rates based on number of quit dates set, but at high levels of website utilization, those who set only one quit date had significantly higher quit rates than those who changed their quit date.
Overall, the CART analysis was consistent with these findings. Latency to TQD did not predict abstinence, but website utilization (logins) and baseline self-efficacy did along with treatment group. Login frequency initially split the sample, and among individuals who logged into the website more frequently, the addition of telephone counseling appeared to increase abstinence relative to enhanced Internet alone. Self-efficacy appeared to be a key variable among those with lower levels of website utilization. Among this group, higher self-efficacy scores were associated with higher quit rates. This finding is consistent with a wealth of research demonstrating the importance of self-efficacy on smoking outcomes [52-55]. The importance of logins is consistent with other Web-based trials that have reported that website utilization is an important predictor of abstinence [31,56,57]. It should also be noted that none of the metrics of motivation to quit emerged in the CART, suggesting that website utilization was a stronger predictor of abstinence than motivation to quit.
In terms of the practical relevance of these results, both traditional and exploratory analyses both point to self-efficacy and website utilization as critical components of abstinence. Findings related to Study Questions 3 and 4 suggest that individuals who set a quit date early in the course of Web-based cessation treatment are more likely to be confident about their ability to achieve cessation and that setting a TQD early on and maintaining high levels of website utilization may incur an advantage for cessation. Taken together, these results suggest that Internet cessation programs should emphasize the importance of feeling efficacious about handling specific smoking situations and engaging with treatment at the highest level possible while potentially placing less emphasis on an absolute time frame for setting a TQD (ie, within 2 weeks versus 2-4 weeks). These results are consistent with a growing body of literature demonstrating the critical importance of engagement and adherence with regard to the effectiveness of Web-based health behavior change interventions [58-66].
Strengths and Limitations
These findings should be considered in the context of several related strengths and limitations. First, the CART analysis is a novel contribution to the literature concerning predictors of smoking abstinence. It is a powerful exploratory technique that offers an unbiased assessment of a large set of predictors and requires little input from the analyst. However, inferences based upon these analyses should be tested and replicated under controlled conditions. Second, participants were not required to set a quit date and could use the website as they desired, resulting in relatively naturalistic observations of the use of a quit date tool. Future research should examine how the use of this tool corresponds to self-reported quit attempts using other assessment methods. Third, we are unclear what to make of retrospective TQDs since current smoking status was confirmed during the baseline telephone survey. We speculate that participants may have used the Quit Date Wizard to document their most recent quit attempt or perhaps entered an erroneous date. Relative to registration, 55% of retrospective dates occurred within the week prior to study randomization, which suggests that many smokers search for cessation assistance in the early days following a quit attempt when they have returned to smoking. Qualitative methods or formal usability testing may shed light on this finding. Fourth, while the use of responder-only analyses is less conservative than intention-to-treat analyses, we feel this approach was appropriate for these exploratory analyses since imputation of missing data using an intent-to-treat (missing=smoking) approach might have obscured results. Fifth, it was not feasible to biochemically verify self-reported abstinence outcomes since this was a national sample recruited entirely via the Internet. Self-reported abstinence is a commonly accepted outcome metric in Web-based cessation trials [67-71] where misreporting of abstinence is expected to be minimal [72]. Last, we cannot rule out the possibility that low levels of website utilization were a consequence (and not cause) of relapse [73]. Studies that establish a chronological sequence of patterns of treatment utilization and relapse are needed [74].
Conclusions
In the context of a pragmatic randomized trial of Internet and telephone treatment for cessation, the timing of a TQD was not a significant predictor of cessation outcomes. Self-efficacy and an apparent commitment to an initial TQD were the components most highly related to abstinence but only via interactions with website utilization. Increasing treatment engagement has been noted as an important area for future research in Web-based cessation studies [57,75].
Acknowledgments
Primary funding for this work was from the National Cancer Institute at the National Institutes of Health (R01 CA104836). The funding agency had no involvement in the conduct of the study or preparation of this manuscript. There are no contractual constraints on publishing imposed by the funder. We thank Dr Lorien Abroms for her comments on an earlier version of this manuscript.
Abbreviations
- ANOVA
analysis of variance
- CART
classification and regression tree
- CES-D
Center for Epidemiologic Studies-Depression Scale
- ph
telephone counseling
- ppa
point prevalence abstinence
- QD
quit date
- TQD
target quit date
Multimedia Appendix 1
Baseline characteristics of individuals who used the Quit Date Wizard (QD) (n=576) and those who did not (n=62).
Footnotes
Conflicts of Interest: All authors are employees of Legacy, a nonprofit public health foundation that runs the BecomeAnEX website, an online tobacco cessation intervention.
References
- 1.Abrams DB, Niaura R, Brown RA, Emmons KM, Goldstein MG, Monti PM. The tobacco dependence treatment handbook: A guide to best practices. New York, NY: The Guilford Press; 2003. [Google Scholar]
- 2.Fiore M, Jaén C, Baker T. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; 2008. May, [Google Scholar]
- 3.McEwen AHP, Hajek P, McRobbie H, West RH. Manual of Smoking Cessation: A Guide for Counsellors And Practitioners. Oxford, UK: Blackwell Publishers; 2006. [Google Scholar]
- 4.Perkins KA, Conklin CA, Levine MD. Cognitive-Behavioral Therapy for Smoking Cessation: A Practical Guidebook to the Most Effective Treatments. New York, NY: Routledge; 2007. [Google Scholar]
- 5.Hallaq JH. The pledge as an instrument of behavioral change. J Soc Psychol. 1976 Feb;98(First Half):147–8. doi: 10.1080/00224545.1976.9923382. [DOI] [PubMed] [Google Scholar]
- 6.Gollwitzer PM, Sheeran P. Implementation intentions and goal achievement: A meta-analysis of effects and processes. Adv Exp Soc Psychol. 2006;38:69–119. doi: 10.1016/S0065-2601(06)38002-1. [DOI] [Google Scholar]
- 7.Gollwitzer PM, Schaal B. Metacognition in action: The importance of implementation intentions. Pers Soc Psychol Rev. 1998;2(2):124–36. doi: 10.1207/s15327957pspr0202_5. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman S, Ferguson SG. Nicotine patch therapy prior to quitting smoking: A meta-analysis. Addiction. 2008 Apr;103(4):557–63. doi: 10.1111/j.1360-0443.2008.02138.x. [DOI] [PubMed] [Google Scholar]
- 9.Balmford J, Borland R, Burney S. The influence of having a quit date on prediction of smoking cessation outcome. Health Educ Res. 2010 Aug;25(4):698–706. doi: 10.1093/her/cyq013. http://her.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=20194359. [DOI] [PubMed] [Google Scholar]
- 10.Segan CJ, Borland R, Greenwood KM. Do transtheoretical model measures predict the transition from preparation to action in smoking cessation? Psychology & Health. 2002 Jan;17(4):417–435. doi: 10.1080/0887044022000004911. [DOI] [Google Scholar]
- 11.de Vries H, Eggers SM, Bolman C. The role of action planning and plan enactment for smoking cessation. BMC Public Health. 2013;13:393. doi: 10.1186/1471-2458-13-393. http://www.biomedcentral.com/1471-2458/13/393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etter JF. Comparing abrupt and gradual smoking cessation: A randomized trial. Drug Alcohol Depend. 2011 Nov 1;118(2-3):360–5. doi: 10.1016/j.drugalcdep.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson SG, Shiffman S, Gitchell JG, Sembower MA, West R. Unplanned quit attempts--results from a U.S. sample of smokers and ex-smokers. Nicotine Tob Res. 2009 Jul;11(7):827–32. doi: 10.1093/ntr/ntp072. [DOI] [PubMed] [Google Scholar]
- 14.Larabie LC. To what extent do smokers plan quit attempts? Tob Control. 2005 Dec;14(6):425–8. doi: 10.1136/tc.2005.013615. http://tobaccocontrol.bmj.com/cgi/pmidlookup?view=long&pmid=16319368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sendzik T, McDonald PW, Brown KS, Hammond D, Ferrence R. Planned quit attempts among Ontario smokers: impact on abstinence. Addiction. 2011 Nov;106(11):2005–13. doi: 10.1111/j.1360-0443.2011.03498.x. [DOI] [PubMed] [Google Scholar]
- 16.West R, Sohal T. "Catastrophic" pathways to smoking cessation: findings from national survey. BMJ. 2006 Feb 25;332(7539):458–60. doi: 10.1136/bmj.38723.573866.AE. http://www.bmj.com/cgi/pmidlookup?view=long&pmid=16443610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray RL, Lewis SA, Coleman T, Britton J, McNeill A. Unplanned attempts to quit smoking: missed opportunities for health promotion? Addiction. 2009 Nov;104(11):1901–9. doi: 10.1111/j.1360-0443.2009.02647.x. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JR, Callas PW. Is delaying a quit attempt associated with less success? Nicotine Tob Res. 2011 Dec;13(12):1228–32. doi: 10.1093/ntr/ntr207. http://europepmc.org/abstract/MED/21908460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes JR, Russ C, Messig MA. Association of deferring a quit attempt with smoking cessation success: a secondary analysis. J Subst Abuse Treat. 2014 Feb;46(2):264–7. doi: 10.1016/j.jsat.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 20.American Cancer Society. [2013-06-16]. How to quit http://www.cancer.org/healthy/stayawayfromtobacco/guidetoquittingsmoking/guide-to-quitting-smoking-how-to-quit.
- 21.American Legacy Foundation. [2013-06-16]. Set your quit date http://www.becomeanex.org/set-your-quit-date.php.
- 22.Smokefree.gov. Quit guide: Preparing to quit http://www.smokefree.gov/qg-preparing-set.aspx.
- 23.Altria Client Services. [2013-06-16]. Get Ready http://www.quitassist.com/en/cms/five-keys-for-quitting/Get-Ready/default.aspx.
- 24.Cobb NK, Graham AL. Characterizing Internet searchers of smoking cessation information. J Med Internet Res. 2006;8(3):e17. doi: 10.2196/jmir.8.3.e17. http://www.jmir.org/2006/3/e17/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox S. Pew Research Center. 2005. Health information online http://www.pewinternet.org/~/media//Files/Reports/2005/PIP_Healthtopics_May05.pdf.pdf.
- 26.Fox S. 2006. [2013-08-19]. Online health search 2006 http://www.pewinternet.org/~/media//Files/Reports/2006/PIP_Online_Health_2006.pdf.pdf.
- 27.Rennard S, Hughes J, Cinciripini PM, Kralikova E, Raupach T, Arteaga C, St Aubin LB, Russ C, Flexible Quit Date Study Group A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012 Mar;14(3):343–50. doi: 10.1093/ntr/ntr220. http://europepmc.org/abstract/MED/22080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borrelli B, Papandonatos G, Spring B, Hitsman B, Niaura R. Experimenter-defined quit dates for smoking cessation: adherence improves outcomes for women but not for men. Addiction. 2004 Mar;99(3):378–85. doi: 10.1111/j.1360-0443.2004.00648.x. [DOI] [PubMed] [Google Scholar]
- 29.Borland R, Partos TR, Cummings KM. Systematic biases in cross-sectional community studies may underestimate the effectiveness of stop-smoking medications. Nicotine Tob Res. 2012 Dec;14(12):1483–7. doi: 10.1093/ntr/nts002. http://europepmc.org/abstract/MED/22318689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bock BC, Graham AL, Whiteley JA, Stoddard JL. A review of Web-assisted tobacco interventions (WATIs) J Med Internet Res. 2008;10(5):e39. doi: 10.2196/jmir.989. http://www.jmir.org/2008/5/e39/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An LC, Schillo BA, Saul JE, Wendling AH, Klatt CM, Berg CJ, Ahulwalia JS, Kavanaugh AM, Christenson M, Luxenberg MG. Utilization of smoking cessation informational, interactive, and online community resources as predictors of abstinence: cohort study. J Med Internet Res. 2008;10(5):e55. doi: 10.2196/jmir.1018. http://www.jmir.org/2008/5/e55/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham AL, Cobb NK, Papandonatos GD, Moreno JL, Kang H, Tinkelman DG, Bock BC, Niaura RS, Abrams DB. A randomized trial of Internet and telephone treatment for smoking cessation. Arch Intern Med. 2011 Jan 10;171(1):46–53. doi: 10.1001/archinternmed.2010.451. http://europepmc.org/abstract/MED/21220660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breiman L, Freideman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth International Group; 1984. [Google Scholar]
- 34.Kitsantas P, Moore TW, Sly DF. Using classification trees to profile adolescent smoking behaviors. Addict Behav. 2007 Jan;32(1):9–23. doi: 10.1016/j.addbeh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Zabor EC, Li Y, Thornton LM, Shuman MR, Bulik CM, Lichtenstein P, Pedersen NL, Sullivan PF, Furberg H. Initial reactions to tobacco use and risk of future regular use. Nicotine Tob Res. 2013 Feb;15(2):509–17. doi: 10.1093/ntr/nts180. http://europepmc.org/abstract/MED/22949572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham AL, Bock BC, Cobb NK, Niaura R, Abrams DB. Characteristics of smokers reached and recruited to an Internet smoking cessation trial: a case of denominators. Nicotine Tob Res. 2006 Dec;8 Suppl 1:S43–8. doi: 10.1080/14622200601042521. http://europepmc.org/abstract/MED/17491170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quitnet.com. [2013-11-04]. Quitnet homepage http://www.quitnet.com/qnhomepage.aspx.
- 38.Cobb NK, Graham AL, Bock BC, Papandonatos G, Abrams DB. Initial evaluation of a real-world Internet smoking cessation system. Nicotine Tob Res. 2005 Apr;7(2):207–16. doi: 10.1080/14622200500055319. http://europepmc.org/abstract/MED/16036277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cobb NK, Graham AL, Abrams DB. Social network structure of a large online community for smoking cessation. Am J Public Health. 2010 Jul;100(7):1282–9. doi: 10.2105/AJPH.2009.165449. http://europepmc.org/abstract/MED/20466971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. J Consult Clin Psychol. 1996 Feb;64(1):202–11. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 41.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–83. [PubMed] [Google Scholar]
- 42.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991 Sep;86(9):1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 43.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–83. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983 Dec;24(4):385–96. [PubMed] [Google Scholar]
- 45.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 46.Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addict Behav. 1998;23(5):609–22. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 47.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997 Jun 25;277(24):1940–4. [PubMed] [Google Scholar]
- 48.Graham AL, Papandonatos GD, Bock BC, Cobb NK, Baskin-Sommers A, Niaura R, Abrams DB. Internet- vs. telephone-administered questionnaires in a randomized trial of smoking cessation. Nicotine Tob Res. 2006 Dec;8 Suppl 1:S49–57. doi: 10.1080/14622200601045367. http://europepmc.org/abstract/MED/17491171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen S, Lichtenstein E. Partner behaviors that support quitting smoking. J Consult Clin Psychol. 1990 Jun;58(3):304–9. doi: 10.1037//0022-006x.58.3.304. [DOI] [PubMed] [Google Scholar]
- 50.Brown RL, Leonard T, Saunders LA, Papasouliotis O. A two-item conjoint screen for alcohol and other drug problems. J Am Board Fam Pract. 2001;14(2):95–106. http://www.jabfm.org/cgi/pmidlookup?view=long&pmid=11314930. [PubMed] [Google Scholar]
- 51.Gaudard M, Ramsey P, Stephens M. Interactive data mining and design of experiments: The JMP partition and custom design platforms. Brookline, New Hampshire: North Haven Group; 2006. Mar, [2014-02-11]. http://www.jmp.com/software/whitepapers/pdfs/372455_interactive_datamining.pdf. [Google Scholar]
- 52.O'Hea EL, Boudreaux ED, Jeffries SK, Carmack Taylor CL, Scarinci IC, Brantley PJ. Stage of change movement across three health behaviors: the role of self-efficacy. Am J Health Promot. 2004;19(2):94–102. doi: 10.4278/0890-1171-19.2.94. [DOI] [PubMed] [Google Scholar]
- 53.Prochaska JO, Crimi P, Lapsanski D, Martel L, Reid P. Self-change processes, self-efficacy and self-concept in relapse and maintenance of cessation of smoking. Psychol Rep. 1982 Dec;51(3 Pt 1):983–90. doi: 10.2466/pr0.1982.51.3.983. [DOI] [PubMed] [Google Scholar]
- 54.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977 Mar;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 55.Bandura A. Self-efficacy: The exercise of control. New York: W.H. Freeman; 1997. [Google Scholar]
- 56.Saul JE, Schillo BA, Evered S, Luxenberg MG, Kavanaugh A, Cobb N, An LC. Impact of a statewide Internet-based tobacco cessation intervention. J Med Internet Res. 2007;9(3):e28. doi: 10.2196/jmir.9.4.e28. http://www.jmir.org/2007/3/e28/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Richardson A, Graham AL, Cobb N, Xiao H, Mushro A, Abrams D, Vallone D. Engagement promotes abstinence in a Web-based cessation intervention: cohort study. J Med Internet Res. 2013;15(1):e14. doi: 10.2196/jmir.2277. http://www.jmir.org/2013/1/e14/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donkin L, Christensen H, Naismith SL, Neal B, Hickie IB, Glozier N. A systematic review of the impact of adherence on the effectiveness of e-therapies. J Med Internet Res. 2011;13(3):e52. doi: 10.2196/jmir.1772. http://www.jmir.org/2011/3/e52/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kohl LF, Crutzen R, de Vries NK. Online prevention aimed at lifestyle behaviors: a systematic review of reviews. J Med Internet Res. 2013;15(7):e146. doi: 10.2196/jmir.2665. http://www.jmir.org/2013/7/e146/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wangberg SC, Bergmo TS, Johnsen JA. Adherence in Internet-based interventions. Patient Prefer Adherence. 2008;2:57–65. http://www.dovepress.com/articles.php?article_id=2062. [PMC free article] [PubMed] [Google Scholar]
- 61.Crutzen R, de Nooijer J, Brouwer W, Oenema A, Brug J, de Vries NK. Strategies to facilitate exposure to Internet-delivered health behavior change interventions aimed at adolescents or young adults: a systematic review. Health Educ Behav. 2011 Feb;38(1):49–62. doi: 10.1177/1090198110372878. [DOI] [PubMed] [Google Scholar]
- 62.Kelders SM, Kok RN, Ossebaard HC, Van Gemert-Pijnen JE. Persuasive system design does matter: a systematic review of adherence to Web-based interventions. J Med Internet Res. 2012;14(6):e152. doi: 10.2196/jmir.2104. http://www.jmir.org/2012/6/e152/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hutton HE, Wilson LM, Apelberg BJ, Tang EA, Odelola O, Bass EB, Chander G. A systematic review of randomized controlled trials: Web-based interventions for smoking cessation among adolescents, college students, and adults. Nicotine Tob Res. 2011 Apr;13(4):227–38. doi: 10.1093/ntr/ntq252. [DOI] [PubMed] [Google Scholar]
- 64.Brouwer W, Kroeze W, Crutzen R, de Nooijer J, de Vries NK, Brug J, Oenema A. Which intervention characteristics are related to more exposure to Internet-delivered healthy lifestyle promotion interventions? A systematic review. J Med Internet Res. 2011;13(1):e2. doi: 10.2196/jmir.1639. http://www.jmir.org/2011/1/e2/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schubart JR, Stuckey HL, Ganeshamoorthy A, Sciamanna CN. Chronic health conditions and Internet behavioral interventions: a review of factors to enhance user engagement. Comput Inform Nurs. 2011 Feb;29(2 Suppl):TC9–20. doi: 10.1097/NCN.0b013e3182155274. [DOI] [PubMed] [Google Scholar]
- 66.Cugelman B, Thelwall M, Dawes P. Online interventions for social marketing health behavior change campaigns: a meta-analysis of psychological architectures and adherence factors. J Med Internet Res. 2011;13(1):e17. doi: 10.2196/jmir.1367. http://www.jmir.org/2011/1/e17/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wangberg SC, Nilsen O, Antypas K, Gram IT. Effect of tailoring in an Internet-based intervention for smoking cessation: randomized controlled trial. J Med Internet Res. 2011;13(4):e121. doi: 10.2196/jmir.1605. http://www.jmir.org/2011/4/e121/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leykin Y, Aguilera A, Torres LD, Pérez-Stable EJ, Muñoz RF. Interpreting the outcomes of automated Internet-based randomized trials: example of an international smoking cessation study. J Med Internet Res. 2012;14(1):e5. doi: 10.2196/jmir.1829. http://www.jmir.org/2012/1/e5/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, Greene SM, Collins LM, Carlier CC, Wiese CJ, Little RJ, Pomerleau CS, Pomerleau OF. Web-based smoking-cessation programs: results of a randomized trial. Am J Prev Med. 2008 May;34(5):373–81. doi: 10.1016/j.amepre.2007.12.024. http://europepmc.org/abstract/MED/18407003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing Internet assistance for smoking cessation: 13-month follow-up of a six-arm randomized controlled trial. J Med Internet Res. 2008;10(5):e45. doi: 10.2196/jmir.1008. http://www.jmir.org/2008/5/e45/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zbikowski SM, Hapgood J, Smucker Barnwell S, McAfee T. Phone and Web-based tobacco cessation treatment: real-world utilization patterns and outcomes for 11,000 tobacco users. J Med Internet Res. 2008;10(5):e41. doi: 10.2196/jmir.999. http://www.jmir.org/2008/5/e41/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002 May;4(2):149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 73.Raupach T, Brown J, Herbec A, Brose L, West R. A systematic review of studies assessing the association between adherence to smoking cessation medication and treatment success. Addiction. 2014 Jan;109(1):35–43. doi: 10.1111/add.12319. [DOI] [PubMed] [Google Scholar]
- 74.Borland R, Balmford J, Swift E. Effects of timing of initiation and planning on smoking cessation outcomes: study protocol for a randomised controlled trial. BMC Public Health. 2013;13:235. doi: 10.1186/1471-2458-13-235. http://www.biomedcentral.com/1471-2458/13/235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham AL, Cha S, Papandonatos GD, Cobb NK, Mushro A, Fang Y, Niaura RS, Abrams DB. Improving adherence to Web-based cessation programs: a randomized controlled trial study protocol. Trials. 2013;14:48. doi: 10.1186/1745-6215-14-48. http://www.trialsjournal.com/content/14//48. [DOI] [PMC free article] [PubMed] [Google Scholar]