Abstract
Triple A syndrome is a rare, autosomal recessive cause of adrenal failure. Additional features include alacrima, achalasia of the esophageal cardia, and progressive neurodegenerative disease. The AAAS gene product is the nuclear pore complex protein alacrima-achalasia-adrenal insufficiency neurological disorder (ALADIN), of unknown function. Triple A syndrome patient dermal fibroblasts appear to be more sensitive to oxidative stress than wild-type fibroblasts. To provide an adrenal and neuronal-specific disease model, we established AAAS-gene knockdown in H295R human adrenocortical tumor cells and SH-SY5Y human neuroblastoma cells by lentiviral short hairpin RNA transduction. AAAS-knockdown significantly reduced cell viability in H295R cells. This effect was exacerbated by hydrogen peroxide treatment and improved by application of the antioxidant N-acetylcysteine. An imbalance in redox homeostasis after AAAS knockdown was further suggested in the H295R cells by a decrease in the ratio of reduced to oxidized glutathione. AAAS-knockdown SH-SY5Y cells were also hypersensitive to oxidative stress and responded to antioxidant treatment. A further impact on function was observed in the AAAS-knockdown H295R cells with reduced expression of key components of the steroidogenic pathway, including steroidogenic acute regulatory and P450c11β protein expression. Importantly a significant reduction in cortisol production was demonstrated with AAAS knockdown, which was partially reversed with N-acetylcysteine treatment. Conclusion: Our in vitro data in AAAS-knockdown adrenal and neuronal cells not only corroborates previous studies implicating oxidative stress in this disorder but also provides further insights into the pathogenic mechanisms in triple A syndrome.
Triple A syndrome (Allgrove syndrome, online mendelian inheritance in man number 231550) is a rare autosomal recessive disease characterized by alacrima, achalasia of the esophageal cardia, and adrenal failure (1, 2). In approximately 60% of patients, a highly disabling neurodegenerative process ensues. Features include peripheral neuropathy, autonomic impairment, pyramidal and bulbar dysfunction, and cerebellar and neuroophthamological signs (3–5). The phenotype is variable, even within members of the same kindred, and no genotype-phenotype correlation has been identified (6, 7). The pathogenic mechanism has yet to be clarified and therapeutic management is limited to symptom relief. In more than 90% of cases, the defect is due to mutations in the AAAS gene (8).
The AAAS gene product is the 60-kDa nuclear pore complex (NPC) protein alacrima-achalasia-adrenal insufficiency neurological disorder (ALADIN). AAAS mRNA and the ALADIN protein are ubiquitously expressed with predominance in the adrenal and central nervous system structures in the human (2, 9) and the rat (10). ALADIN, a Tryptophan-Aspartic acid (WD) repeat containing protein, was the first nuclear pore complex protein to be associated with hereditary neurodegenerative disease and the only nuclear pore complex protein to be associated with hereditary adrenal disease (2, 11). Central nervous system disorders have subsequently been described in 2 other nucleoporinopathies, resulting from mutations in Nup62 and RanBP2/Nup358, although the precise pathogenic mechanisms for these are unclear (12–14). ALADIN's precise function at the nuclear pore complex is unknown. Most naturally occurring AAAS mutations result in mislocalization of the abnormal ALADIN protein (mainly into the cytoplasm), implying that correct NPC targeting is vital for its function (8, 15, 16).
The phenotype in triple A syndrome is complex and all the clinical features are progressive, suggesting a degenerative process. Oxidative stress may play a role in the pathogenesis of this disease. Triple A patient dermal fibroblasts have higher basal intracellular reactive oxygen species (ROS) and are more sensitive to oxidative stress than wild-type fibroblasts (17–19). Additionally, in the dermal fibroblast model, failure of nuclear import of 2 DNA repair proteins is described (17, 18). We have previously identified full-length human ferritin heavy chain protein (FTH1), which has a DNA-protective role in the nucleus, as an interacting protein partner for ALADIN in vitro (20). Unlike control cells, no nuclear FTH1 is apparent in triple A patient fibroblasts or lymphocytes implicating ALADIN in the nuclear localization of FTH1 (20). Apoptosis of neuronal cells induced by hydrogen peroxide is significantly reduced by transfection of AAAS or FTH1 and maximally by both genes together (20). These findings provide further compelling evidence that oxidative stress is involved in disease progression and that nuclear import of specific cargo(es) may be defective. How these nuclear import defects lead to an increase in intracellular ROS remains unclear. Current models of the disease, that is, dermal fibroblasts derived from triple A patients and the AAAS−/− mouse, which does not lead to a triple A syndrome-like phenotype, have significant limitations. Therefore, to further understand the functional role of ALADIN in the pathogenesis of triple A syndrome, a better model of this complex disease is necessary.
In the present study, we aimed to establish for the first time novel in vitro models of the disease by inducing AAAS knockdown in H295R human adrenocortical tumor cells and SH-SY5Y human neuroblastoma cells, chosen as representative of the predominant cell types affected by the disease. We investigated the potential role of oxidative stress in the pathogenesis of triple A syndrome and antioxidant treatment in recovery. These studies provide a better understanding of the pathogenic mechanisms of triple A syndrome looking specifically at affected tissue types.
Materials and Methods
Cell culture
H295R adrenocortical tumor cells were cultured in Gibco DMEM/ F12-Ham (1:1) + GlutaMAX-I (GIBCO, Life Technologies, Paisley, United Kingdom), supplemented with 5% NuSerum, 1% penicillin/streptomycin solution, and insulin-transferrin-selenium. SH-SY5Y neuroblastoma cells were cultured in DMEM/F12-Ham (1:1), supplemented with 10% fetal calf serum and 1% penicillin/ streptomycin solution. Human embryonic kidney (HEK)-293T cells were maintained in DMEM with 10% fetal calf serum and 1% penicillin/streptomycin. All cells were incubated in a humidified incubator at 37°C and 5% CO2.
Short hairpin RNA (shRNA) lentiviral transduction
HEK293T cells were used at 60% confluency on the day of transfection in 6-well plates. In each well, packaging vectors PMDG.2 plasmid (0.75 μg) and the pCMV 8.74 plasmid (1.0 μg) together with a combination of 2 commercially produced puromycin-resistant synthetic shRNA (0.625 μg each) (Sigma-Aldrich, Poole, United Kingdom; numbers TRCN0000118924 and TRCN0000118926) were transfected using Lipofectamine 2000 (Invitrogen, Life Technologies, Paisley, United Kingdom) as per the manufacturer's guidelines. A commercially available lentivirus plasmid vector containing an shRNA insert that does not target human and mouse genes was used to generate controls (Sigma-Aldrich). A total of 3 μg of DNA was transfected into each well of HEK293T cells in 1.5 mL of media. Cells were incubated for 48 hours in a humidified incubator at 37°C and 5% CO2. Forty-eight hours after transfection, 1.5 mL of viral media from each well of the HEK293T cells was filtered using 0.22 μM Millex-GP filter units (Merck Millipore, Cork, Ireland) and used to transduce 1 well of H295R or SH-SY5Y cells. Viral media was left on the cells for 24 hours and then replaced with fresh media. Cells were left to grow for 5 days before application of a selection antibiotic, puromycin, at a concentration of 5 μg/mL. AAAS-knockdown cell lines were generated in 3 biological replicates. All subsequent experiments were conducted on cultured cells in which AAAS knockdown was achieved by the combination of the 2 shRNAs together and after a minimum of 10 days of the puromycin selection.
Reverse transcription and quantitative real-time PCR
Total RNA was extracted from cultured H295R and SH-SY5Y cells 10 days after puromycin selection using the RNeasy kit (QIAGEN, Crawley, United Kingdom) according to the manufacturer's protocol. Cell lysates from individual wells of a 6-well plate were used for RNA extraction. RNA samples were quantified with a spectrophotometer, and 1.0 μg of RNA from each sample was reverse transcribed after deoxyribonuclease treatment. Quantitative RT-PCR (qPCR) was set up in triplicate (per sample) on a Stratagene Mx3000P thermocycler (La Jolla, California) using KAPA SYBR Fast quantitative PCR master mix with 200 nM forward and reverse primers targeted to AAAS, GAPDH, and STAR (AAAS forward primer: GCCAGCAGCACCATAGTC; AAAS reverse primer: CAGGGTGAGACAGCACTTG; GAPDH forward primer: GAAGGTGAAGGTCGGAGTC; GAPDH reverse primer: GAAGATGGTGATGGGATTTC; STAR forward primer: GACATTCAAGCTGTGCGCTG; STAR reverse primer: TGTAGAGAGTCTCTTCTAGCCGA), giving a total volume of 10 μL. After an initial denaturation step of 3 minutes at 95°C, real-time qPCR cycling was performed for 40 cycles of 95°C for 3 seconds, 55°C for 20 seconds, and 72°C for 1 second, followed by 1 cycle of 1 minute at 95°C, 55°C for 30 seconds, and 95°C for 30 seconds.
Western blotting
Cells were lysed with radioimmunoprecipitation assay (RIPA) buffer [50 mM Tris-HCL (pH 8.0), with 150 mM sodium chloride, 1% IGEPAL CA-630 (Nonidet P-40), 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate], supplemented with complete, Mini, EDTA-free protease inhibitor cocktail tablets (Roche, Stockholm, Sweden) and phosphatase inhibitors (Sigma), placed on ice for 30 minutes and centrifuged at 15 000 × g for 12 minutes at 4°C. The supernatant was subsequently added to an equal volume of Laemmli loading buffer. Samples were heated to 95°C-100°C for 5 minutes and 10 μg of protein loaded on 4%–12% SDS-PAGE gels. The proteins were transferred for 30 minutes at 15 V to a nitrocellulose membrane using semidry transfer blot (Bio-Rad Laboratories, Hemel Hempstead, United Kingdom). Blots were blocked with 10 mM Tris buffer (pH 7.5), containing 100 mM NaCl, 0.1% Tween 20, and 5% nonfat dry milk). All incubations, unless otherwise stated, were performed for 1 hour at room temperature in blocking buffer. The membranes were subsequently incubated in a 1:500 dilution of mouse monoclonal antihuman ALADIN antibody (Abcam, Cambridge, United Kingdom) for 72 hours (the optimal duration for immunoblotting studies with this antibody). For normalization, rabbit polyclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Abcam) at 1:5000 dilution was used. Protein analysis for steroidogenic acute regulatory protein (StAR) expression was conducted as above with incubation in a 1:1000 dilution of mouse monoclonal antihuman StAR antibody (Abcam) overnight. For normalization, mouse monoclonal GAPDH antibody (Abcam) at 1:5000 dilution was used. Rabbit polyclonal antihuman CYP11A1 [P450 side chain cleavage (P450scc)] antibody (Sigma) was used at a dilution of 1:500 and rabbit polyclonal antihuman CYP11B1 (P450c11β) antibody (Sigma) at a dilution of 1:200; both with overnight incubation.
Cell counting using hemocytometer
Cells were plated at a seeding density of 2 × 104 cells/well in a 12-well plate. Prior to trypsinizing, cells were washed with Dulbecco's PBS, allowing removal of any dead cells. After trypsinization on day 5, a 50 μL sample of cells was removed and injected into the hemocytometer channel. Cells were counted in all 4 corners (each corresponding to an area of 1.0 mm2 and a volume of 100 nL) of one of the grids using the Leica DMIL light microscope with ×10 objective (Leica, Heidelberg, Germany). If more than 500 cells were counted, the cell stock was diluted and another sample taken. If there were fewer than 200 cells, all 4 corners of both grids were counted. The total number of cells in the cell suspension corresponded to the number of cells per milliliter (number of cells per 100 nL × 104) multiplied by the milliliters of media in which the cells were diluted. Results for cell numbers are comprised of 5 representative experiments and statistical analysis undertaken was the Student's t test.
Cell viability assay
The CellTiter96 Aqueous nonradioactive cell proliferation assay [MTS (3-[4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay; Promega, Southampton, United Kingdom] was used to assess cell viability. Cells were plated at a density of 5000 cells/well in a 96-well plate on day 0. Six wells were plated for each experimental condition required. Cells were incubated for 48 hours in normal media or media treated with 100 μM H2O2. Media were then removed and replaced with 100 μL fresh normal media. In each 96-well plate, standards were established by plating H295R cells at densities between 1000 and 35 000 cells per well, in triplicate. Cells were then incubated for 2 hours in a humidified incubator at 37°C. The CellTiter96 kit was used according to the manufacturer's protocol (Promega), and the absorbance of the wells at 490 nm was read at 2.5 hours using an ELISA plate reader. The mean absorbance for each set of cells was representative of the 6 readings. Results are comprised of representatives from 3–4 separate experiments with statistical analysis using the Student's t test.
Cell cycle analysis by flow cytometry
Cell cycle analysis of AAAS-knockdown cells compared with controls was conducted using flow cytometry following propidium iodide treatment of the cells at 50% confluency, on the FACSCalibur cytometer (Becton Dickinson, Lincoln Park, New Jersey). Briefly, for cell fixation, cells were suspended in 0.5 mL of phosphate-buffered saline (PBS) and the cell suspension was added to 5 mL of 1% (wt/vol) paraformaldehyde in PBS and placed on ice for 15 minutes. Cells were centrifuged for 5 minutes at 300 × g and the supernatant discarded. Cells were washed with PBS twice and then resuspended in 0.5 mL of PBS, which was added to 5 mL of ice-cold 70% (vol/vol) ethanol and left to stand for 30 minutes on ice. Cells were subsequently spun and the pellet washed with PBS and then resuspended with 0.5 mL of propidium iodide/ribonuclease A staining buffer (Invitrogen Life Technologies). Samples were incubated for 30 minutes at room temperature prior to fluorescence-activated cell sorter analysis. The CellQuestPro version 6.0 software program (Becton Dickinson) was used to deconvolute the cellular DNA content frequency histograms to obtain the percentage of cells in the 3 major phases of the cell cycle (G0/1, S, and G2/mitosis). For representative images of the DNA content frequency histograms for control and AAAS-knockdown H295R cells, see Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Poly-ADP-ribose polymerase (PARP) cleavage
Cells (80% confluency in 6 well plates) were incubated in serum-free medium containing 1 mM H2O2 for 30 minutes before the medium was replaced with fresh serum-containing medium in all cells as previously described (20). After overnight recovery at 37°C, cells were lysed and analyzed via Western analysis, using PARP rabbit polyclonal antibody at 1:1000 (Cell Signaling Technology, Beverly, Massachusetts) and mouse monoclonal GAPDH antibody at 1:5000 (Abcam). Cleaved PARP was quantified using densitometric analysis from 3 representative experiments relative to total PARP. A Student's t test was used for statistical analysis.
Analysis of cell glutathione (GSH) to oxidized glutathione measurement (GSSG) ratio
AAAS-knockdown and control cells were plated at a seeding density of 1 × 105 in duplicate in a luminometer compatible 96-well plate. At 48 hours GSH/GSSG Glo Assay (Promega) was used according to the manufacturer's guidelines, using a total glutathione lysis reagent or oxidized glutathione lysis reagent for each set of cells for the desired end point. As a positive control, control cells were treated with 40 μM menadione for 1 hour prior to the assay. After the application of a luciferin generation reagent and subsequent luciferin detection reagent in each well, luminescence was read. The total glutathione measurement and the GSSG were used to calculate the GSH to GSSG ratio, with a 2-fold adjustment made for GSSG because each mole of oxidized GSSG upon reduction in the assay produces 2 mol of reduced GSH. Results are comprised of representatives from 3 separate experiments with statistical analysis using the Student's t test.
Cortisol analysis
AAAS-knockdown and control cells were cultured in serum-free media. Media were extracted when cells were at 90% confluency following 24 hours of stimulation with 10 μM forskolin. Cortisol levels were analyzed on a Roche Modular E170 automated immunoassay analyzer using electrochemiluminescent detection; the assay imprecision has been described as less than 6%. Levels were subsequently normalized to 1 mg of protein after Bradford assays of the corresponding cell lysates. For N-acetylcysteine treatment, media were supplemented with 1 mM N-acetylcysteine for the 24-hour period.
Results
AAAS knockdown in H295R adrenocortical tumor cells and SH-SY5Y neuroblastoma cells
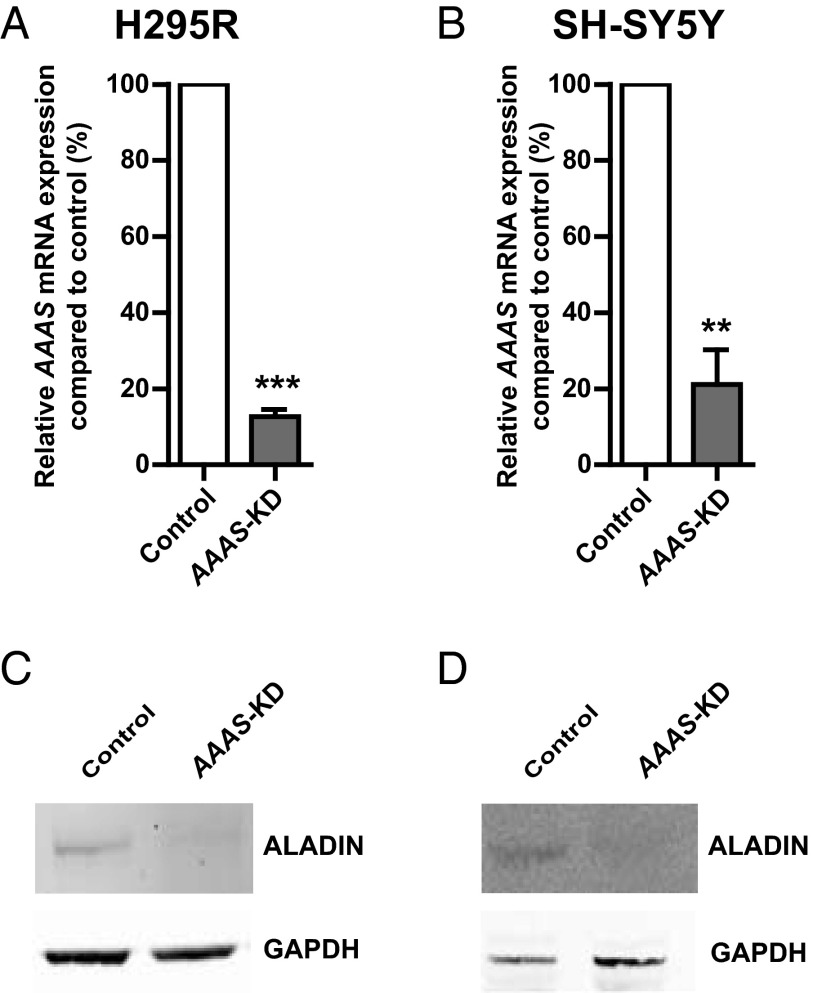
A combination of 2 commercially available lentiviral shRNA, targeting overlapping regions of exon 5 in the AAAS gene, was used together to achieve AAAS knockdown (AAAS-KD). Greater than 70% AAAS knockdown was established in both cell lines, quantified by real-time quantitative PCR (Figure 1, A and B). AAAS mRNA expression was reduced to 12.6% ± 1.6% in AAAS-KD H295R cells and to 21.1% ± 7.4% in AAAS-KD SH-SY5Y cells compared with controls. In both cell lines, a reduction in ALADIN protein expression was observed by immunoblotting (Figure 1, C and D).
Figure 1.
Lentiviral shRNA knockdown of the AAAS gene in H295R (A) and SH-SY5Y (B) cells. AAAS mRNA expression was quantified using real-time qPCR and normalized to GAPDH (n = 3). Control represents transfection with scrambled shRNA. Reduced expression of the protein ALADIN is seen in AAAS-knockdown (AAAS-KD) H295R (C) and SH-SY5Y (D) cells. Data represents mean ± SD. **, P < .01; ***, P < .001.
AAAS knockdown results in a reduction in cell number and viability in H295R adrenocortical cells
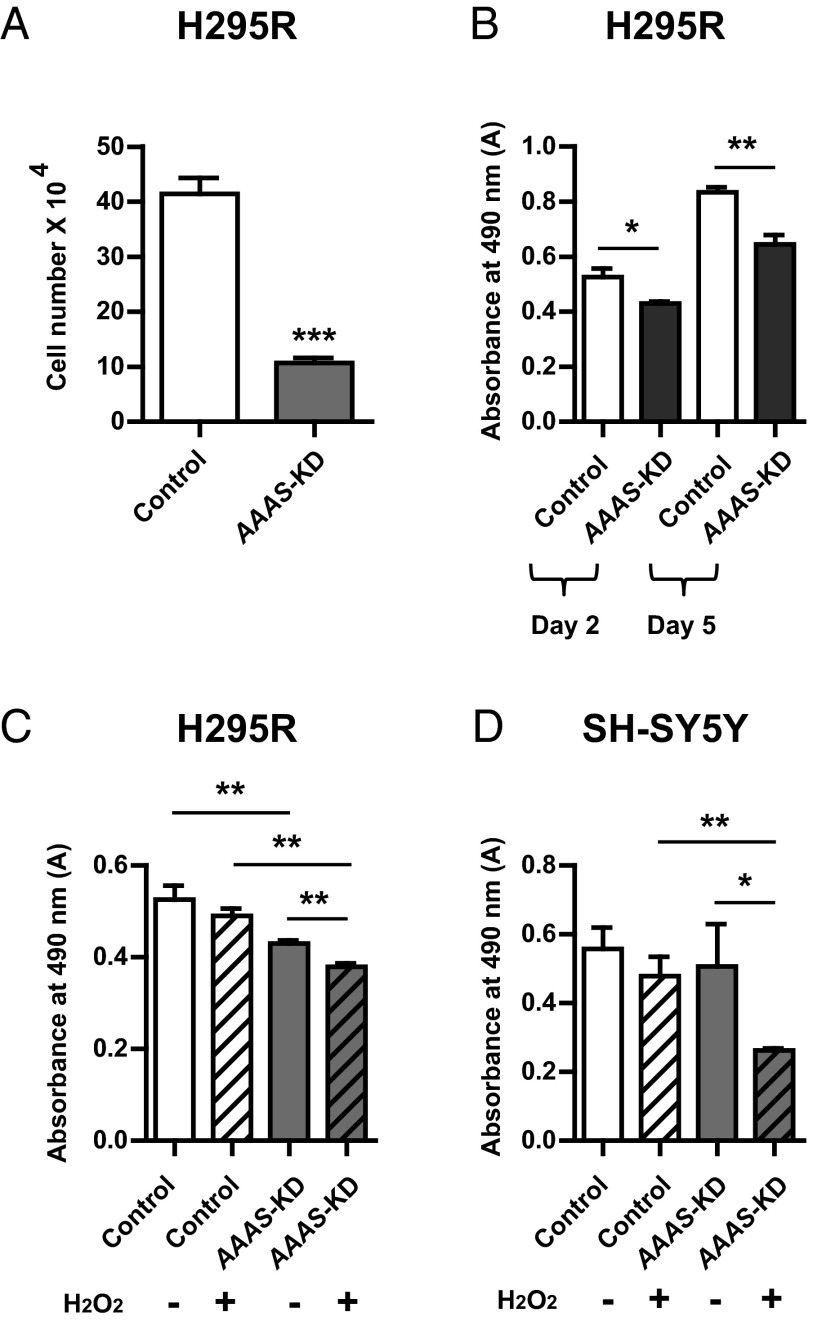
Cell number assessed by cell counting was significantly reduced on day 5 after plating AAAS-KD H295R cells (10.73 × 104 ± 1.59 × 104) (mean ± SD), compared with controls (41.45 × 104 ± 4.97 × 104) (P < .0001, n = 4; Figure 2A). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) assays were used to assess cell viability in which the absorbance readings are proportional to cellular metabolic activity. In AAAS-KD H295R cells, there was a significant reduction in cell viability compared with the controls; measured absorbance 0.43 ± 0.01 vs 0.53 ± 0.04 on day 2 after plating (P < .05, n = 3) and 0.64 ± 0.05 vs 0.83 ± 0.03 on day 5 (P < .01, n = 3), respectively (Figure 2B).
Figure 2.
A reduction in cell viability of AAAS-KD cells. A, There was a significant difference in the total number of cells (by cell counting) on day 5 in the AAAS-KD H295R cells (n = 4) compared with controls after seeding at 2 × 104 cells/well. B, MTS assays on days 2 and 5 with initial seeding density of 5 × 103 cells/well on day 0. There was a significant reduction in absorbance readings of AAAS-KD H295R cells in comparison with controls on day 2 (n = 3) and day 5 (n = 3). After treatment with 100 μM H2O2, a significant reduction in absorbance readings is seen in AAAS-KD H295R cells (n = 3) (C) and AAAS-KD SH-SY5Y cells (n = 4) (D) using MTS assays. There was no reduction in absorbance in untreated AAAS-knockdown SH-SY5Y cells compared with controls. Data represent mean ± SD. *, P < .05; **, P < .01; ***, P < .001.
AAAS-knockdown cells are hypersensitive to oxidative stress
In addition to the reduction in cell viability seen at baseline, application of oxidative stress in the form of 100 μM hydrogen peroxide for 48 hours resulted in a further significant decrease in cell viability during the MTS assay in AAAS-KD H295R cells (absorbance readings 0.38 ± 0.01; mean ± SD) compared with untreated knockdown cells (0.43 ± 0.01) (P < .01, n = 3) (Figure 2C). A concentration of 100 μM hydrogen peroxide was used for these assays as higher concentrations proved toxic to the cells for this duration of treatment. No significant difference in absorbance was seen between the untreated and treated control H295R cells (Figure 2C). There was no reduction in measured absorbance in AAAS-knockdown SH-SY5Y cells compared with controls, day 2 after plating (Figure 2D). However, treatment of AAAS-KD SH-SY5Y cells with 100 μM hydrogen peroxide resulted in a significant reduction in absorbance readings (0.26 ± 0.01), compared with treated controls (0.48 ± 0.05; P < .01, n = 4) (Figure 2D).
AAAS knockdown in H295R adrenocortical cells results in cell cycle arrest and an increase in cell death by apoptosis
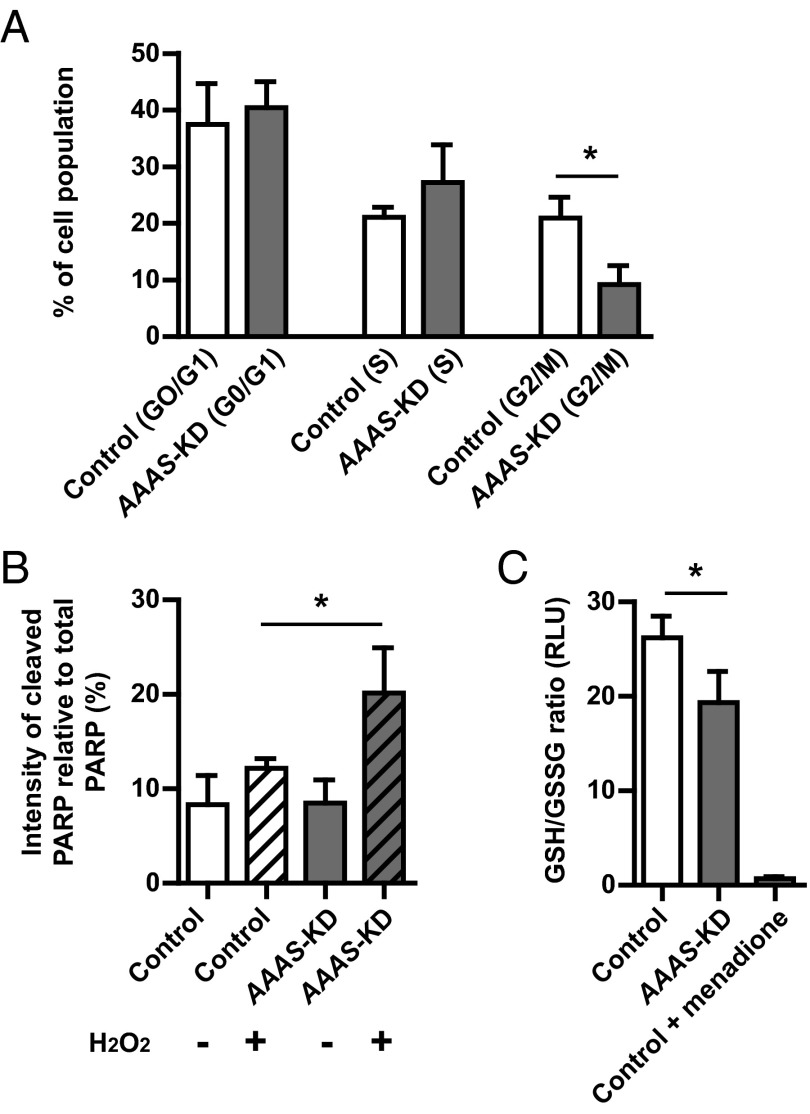
To further investigate the finding of reduced cell viability of the AAAS-KD H295R cells at baseline, cell cycle analysis of AAAS-KD cells compared with controls was carried out using flow cytometry after propidium iodide treatment. In flow cytometry, propidium iodide is used as a DNA stain to allow the evaluation of DNA content during cell cycle analysis. There was a significant reduction in the proportion of cells in the G2/mitotic phase of the cell cycle in the AAAS-KD H295R cell population (9.2% ± 2.7%), compared with controls (21.0% ± 3.0%; P < .05, n = 3). This suggests that ALADIN deficiency causes a reduction in cellular proliferation secondary to cell cycle arrest prior to the G2/mitotic phase (Figure 3A and Supplemental Figure 1).
Figure 3.
Cell cycle arrest, increased apoptosis, and an imbalance of redox homeostasis is observed in AAAS-KD H295R adrenal cells. A, A significant reduction in the percentage cell population in the G2/mitotic phase of the cell cycle is seen in AAAS-KD H295R cells compared with controls, suggesting cell cycle arrest (n = 3). B, An increase in cleaved relative to total PARP, measured by densitometric analysis after immunoblotting, is observed in H2O2-treated AAAS-KD cells compared with controls (n = 3). C, The GSH to GSSG ratio was lower in AAAS-KD cells relative to controls (n = 3). Oxidative stress induced by treatment with 40 μM menadione to control cells further reduces the ratio. RLU, relative light units. Data represent mean ± SD. *, P < .05.
Apoptosis was assessed by immunoblot analysis of cleaved PARP. The cleavage of full-length PARP (116 kDa) to 85- and 27-kDa fragments is a well-documented effect of cell death by apoptosis (21, 22). An increase in the ratio of cleaved PARP relative to total PARP was observed in AAAS-KD H295R cells treated with 1 mM hydrogen peroxide compared with controls (20.2% ± 3.9% vs 12.2% ± 0.8%, respectively; P < .05, n = 3) (Figure 3B).
AAAS knockdown in H295R adrenocortical cells results in an imbalance of redox homeostasis
Glutathione, a 3-amino acid peptide (gamma glutamyl-cysteinylglycine), is an abundant antioxidant found in eukaryotic cells, existing in reduced (GSH) and oxidized (GSSG) states. GSH is the predominant form and maintains strong reducing conditions in the cytosol. The ratio of oxidized to reduced glutathione is used as a measure of redox thiol status and therefore the intensity of oxidative stress. A significant reduction in the GSH to GSSG ratio is seen in AAAS-KD H295R cells (19.3 ± 2.7 relative light units) compared with controls (26.2 ± 1.9) (P < .05, n = 3), suggesting a baseline increase in oxidative stress in the knockdown cells (Figure 3C). As a positive control, a significant reduction in the GSH to GSSG ratio (0.7 ± 0.2 relative light units) was demonstrated by treatment of the control cells with 40 μM menadione, a potent inducer of oxidative stress.
Treatment with the antioxidant N-acetylcysteine improves cell viability in AAAS-knockdown cells
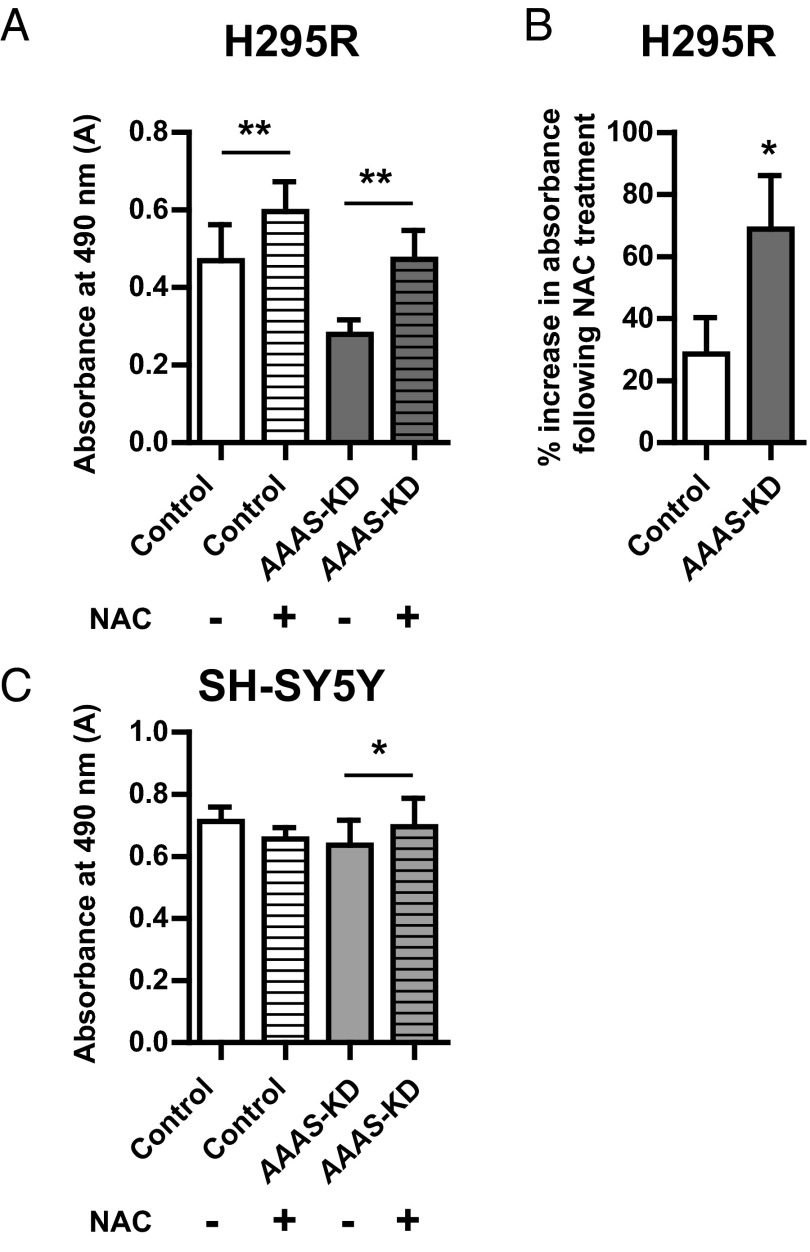
Application of the antioxidant N-acetylcysteine (NAC) for 48 hours at a dose of 1 mM significantly increased absorbance readings in the AAAS-KD H295R cells (0.47 ± 0.06; mean ± SD), compared with untreated knockdown cells (0.28 ± 0.03) (P < .01, n = 4). Higher doses of NAC were found to be toxic to all the cell lines. Absorbance readings were also increased in NAC-treated control cells (0.60 ± 0.07) compared with untreated controls (0.47 ± 0.08; P < .01, n = 4) (Figure 4A). However, the percentage increase in absorbance readings after NAC application was greater in the AAAS-knockdown cells (68.8% ± 14.9%) compared with controls (28.5% ± 10.1%; P < .05, n = 4) (Figure 4B). A dose of 10 mM NAC for 48 hours significantly increased absorbance readings in AAAS-KD SH-SY5Y cells (0.70 ± 0.08, mean ± SD) compared with untreated knockdown cells (0.64 ± 0.07) (P < .05, n = 4). However, no significant difference in cell viability was seen in the control SH-SY5Y cells with the same NAC treatment (Figure 4C).
Figure 4.
Treatment with the antioxidant NAC improves AAAS-KD cell viability. A, Treatment with 1 mM NAC for 48 hours significantly increases absorbance in both AAAS-KD and control H295R cells (n = 4). B, There is a significantly greater percentage increase in absorbance after treatment in the knockdown cells in comparison with the controls (n = 4). C, Treatment with 10 mM NAC significantly increases the absorbance in AAAS-KD SH-SY5Y cells but not controls (n = 4). Data represent mean ± SD. *, P < .05; **, P < .01.
AAAS knockdown affects key components of the steroidogenic pathway including a reduction of StAR and P450c11β protein expression
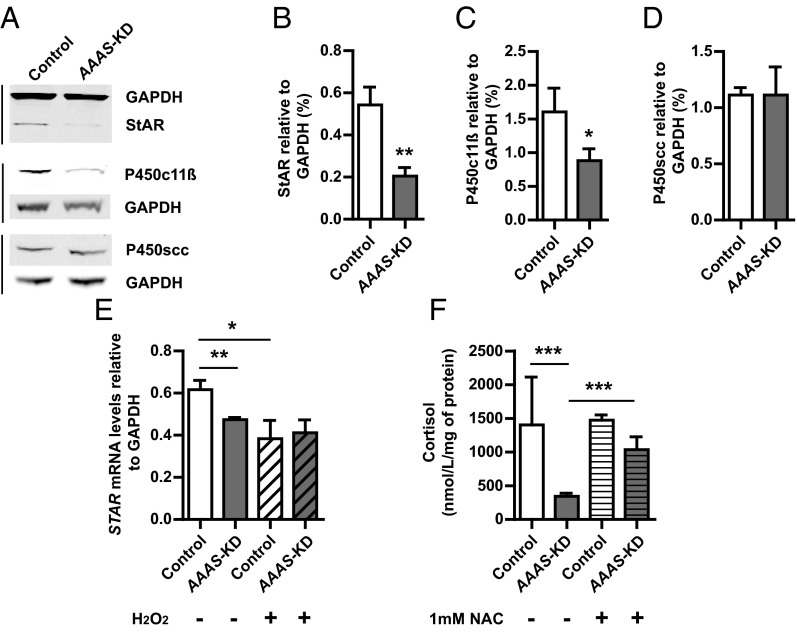
After immunoblot and densitometric analysis, we observed a significant reduction in the expression of 30 kDa StAR protein relative to GAPDH in AAAS-knockdown H295R cells compared with controls (P < .01, n = 4) (Figure 5, A and B). Furthermore, we demonstrate a significant reduction in protein expression of P450c11β (encoded by CYP11B1), responsible for the final step in cortisol production (P < .05, n = 3) (Figure 5, A and C). There was no significant difference in the expression of the other steroidogenic enzyme investigated, P450scc (encoded by CYP11A1) (n = 4) (Figure 5, A and D).
Figure 5.
StAR and P450c11β (CYP11B1) expression are reduced in AAAS-KD H295R cells with a subsequent reduction in cortisol production. Immunoblot analysis (A) and densitometric analysis showing a reduction in StAR (B) (n = 4) and P450c11β protein expression (C) in AAAS-KD H295R cells compared with controls (n = 3), with no effect on P450scc levels (D) (n = 4). E, There is a significant reduction in StAR mRNA levels in AAAS-KD cells compared with controls, and hydrogen peroxide treatment of controls reduces levels to that seen with ALADIN deficiency (n = 3). F, There is a significant reduction in cortisol production by the untreated AAAS-KD H295R cells compared with controls (n = 9), with partial improvement after NAC treatment (n = 3). Data represent mean ± SD. *, P < .05; **, P < .01; ***, P < .001.
The effect on StAR is seen at the transcriptional level, with a significant reduction in STAR mRNA levels in AAAS-knockdown cells [0.47 ± 0.01 (mRNA levels relative to GAPDH)] compared with controls (0.62 ± 0.04) (P < .01, n = 3). One hundred micromoles of H2O2 treatment reduces the StAR mRNA levels (0.38 ± 0.07) in control cells to levels similar to the untreated AAAS-knockdown cells. In contrast, 100 μM H2O2 treatment does not reduce StAR mRNA expression further in the AAAS-knockdown cells (Figure 5E).
AAAS knockdown in H295R cells results in a significant reduction in cortisol production, which is partially rescued by N-acetylcysteine treatment
An impact on cell function is seen with a significant decrease in cortisol production after forskolin stimulation in the untreated AAAS-knockdown H295R cells (344.0 ± 44.4 nmol/L·mg of lysate protein) compared with controls (1404.4 ± 663.5) (P < .001, n = 9) (Figure 5F). Treatment for 24 hours with 1 mM N-acetylcysteine during forskolin stimulation (n = 3) significantly increases cortisol production in the AAAS-knockdown cells (1037.4 ± 154.7nmol/L·mg of lysate protein) (P < .001) (Figure 5F).
Discussion
The clinical manifestations of triple A syndrome are progressive and the pathogenic mechanisms involved in the disease process are unclear. Interestingly, despite ubiquitous expression of AAAS mRNA (2), tissue specificity is observed in this condition. In particular, the clinical features of adrenal insufficiency and neurodegeneration affecting the central, peripheral, and autonomic nervous systems are not present at birth but develop over time, implicating a degenerative process in the pathogenesis of this disorder (5–7, 23).
Although the AAAS−/− mouse does not exhibit a significant phenotype, suggesting functional redundancy for the AAAS gene product ALADIN in the mouse model (24), in vitro studies demonstrate that triple A patient dermal fibroblasts have increased susceptibility to oxidative stress (17–19). Additionally, a reduction in triple A fibroblast cell survival and an increase in DNA fragmentation are reported after exposure to the glutathione-depleting agent, L-buthionine-(S,R)-sulfoxamine (BS0) (17). Triple A patient dermal fibroblasts are also observed to have higher basal levels of ROS and more extensive mitochondrial networks (19). The precise mechanism(s) for these alterations remain unclear, and it is uncertain how reliably observations in dermal fibroblasts can be extrapolated to the tissues affected by triple A syndrome. Disease-causing mutations of the AAAS gene lead to either ALADIN deficiency or ALADIN mislocalization, suggesting correct targeting of ALADIN to the NPC is required (16). The failure of the nuclear accumulation of DNA repair proteins, aprataxin, and DNA ligase I together with the antioxidant protein ferritin heavy chain in triple A patient dermal fibroblasts may render these cells more susceptible to oxidative stress (17, 18, 20).
In this study, we observed a reduction in cell viability after AAAS knockdown in the adrenocortical H295R cell line, which is further exacerbated by exposure to artificial oxidative stress. Stress-induced premature senescence has been described in triple A patient fibroblasts (19). Cell cycle analysis of ALADIN-deficient adrenal cells suggests that a similar situation exists in adrenal cells secondary to cell cycle arrest, with a reduction of the proportion of cells progressing to the G2/mitotic phase. It is possible that ALADIN, as a member of the NPC, plays an important role during the cell cycle. Several nucleoporins (Nups) localize to kinetochores during mitosis, including members of the Nup 107–160 subcomplex and Nup358/RanBP2. These processes are thought to be integral for the cell to enter into mitosis (25). Depletion of certain Nups also leads to defects in spindle formation (26). This impact on cellular proliferation could adversely affect tissue remodeling in response to an insult, either as a result of or independent of ALADIN deficiency. The adult adrenal gland is capable of remodeling, as demonstrated by the regeneration of the adrenal cortex after enucleation of the rat adrenal gland and by compensatory adrenal growth in the contralateral adrenal gland after unilateral adrenalectomy (27).
We also observed an increase in apoptosis in AAAS-knockdown adrenal cells after exposure to artificial oxidative stress. Increased apoptosis combined with impaired cellular proliferation would be in keeping with histopathological findings in postmortem triple A syndrome adrenal glands. These reveal atrophy of the zona fasciculata, which would be in keeping with a progressive degenerative process (1). It is also possible that ALADIN deficiency affects cellular proliferation and apoptosis indirectly by impacting on the baseline levels of oxidative stress particularly in the adrenal gland. This may be a result of intracellular ROS interacting with critical cell signaling molecules, thus regulating several key signaling pathways critical in cellular proliferation and survival (28). A neurodegenerative process is present in a significant proportion of patients, and the other cardinal features of the disease, alacrima and achalasia, are also postulated to manifest secondary to neurological impairment. Autonomic dysfunction at the level of the lacrimal glands has been suggested as the cause of the failure of tear production (29), and a marked reduction of the number of myenteric ganglia and myenteric neurons suggests a lack of intrinsic innervation leading to achalasia (30). Although we do not see a difference in cell viability in untreated AAAS-knockdown neuronal cells, they appear hypersensitive to oxidative stress. The effects of reduction of AAAS expression are less remarkable in the SH-SY5Y cells, and this could be a feature of the lower efficacy of the knockdown in this cell line. Alternatively, ALADIN may have adrenal cell specific roles or compensatory mechanisms may exist in other cell types, and this warrants further investigation. Nevertheless, our preliminary data support the previous suggestions that oxidative stress is also involved in the neurodegenerative process.
Intracellular redox homeostasis is maintained by several mechanisms involving both enzymatic and nonenzymatic antioxidant defenses. Oxidative stress refers to the imbalance of ROS and oxidants over the capability of the cell to mount an effective antioxidant response. A disruption in redox homeostasis is suggested in the ALADIN-deficient adrenal cells with a depletion of reduced GSH, a major endogenous antioxidant and a cofactor of the antioxidant enzyme glutathione peroxidase. We demonstrate an improvement in the viability of AAAS-knockdown adrenal and neuronal cells after treatment with the antioxidant N-acetylcyteine, which replenishes stores of reduced glutathione and acts as a direct scavenger of free radicals. Furthermore, we also demonstrate a significant improvement in cortisol production in the AAAS-knockdown adrenocortical cells after treatment with N-acetylcysteine. There are currently several ongoing clinical trials using N-acetylcysteine, both alone and in combination with other antioxidants, in the treatment of other neurodegenerative conditions such as Parkinson's disease, Alzheimer's disease, and adrenomyeloneuropathy (see http://www.clinicaltrials.gov). Our studies suggest the importance of the glutathione antioxidant pathway in the pathogenesis of triple A syndrome; hence, antioxidant treatments may prove to be a viable therapeutic strategy to slow or even prevent triple A syndrome disease progression.
We demonstrate that AAAS gene deficiency impacts on adrenocortical cell function with a significant reduction in cortisol production in the AAAS-knockdown H295R cells. StAR protein is fundamental for the transport of cholesterol across the mitochondrial membrane and is the rate-limiting step for steroidogenesis. There is evidence that StAR is particularly sensitive to ROS. In Leydig cells, oxidative stress inhibits the mitochondrial import and processing of StAR with a reduction in the 30-kDa intramitochondrial form of StAR, resulting in a reduction in steroidogenesis (31). Although several studies in Leydig cells demonstrate a clear reduction in 30-kDa StAR protein expression in response to ROS, it is unclear whether there is also an effect of ROS at a transcriptional level because current data are inconsistent (31, 32). Consistent with the findings of Shi et al (32), who demonstrate an effect on StAR promoter activity in Leydig cells, for the first time we demonstrate that an imbalance of redox homeostasis in AAAS-knockdown cells reduces both StAR mRNA and the expression of the 30-kDa StAR protein in adrenocortical cells with no effect on P450scc expression. Interestingly, we also see a significant reduction of P450c11β protein expression in the AAAS-knockdown cells. P450c11β catalyzes the final step in cortisol production and is cited as one of the most ROS-producing steps of steroidogenesis, whereas much smaller amounts of ROS are produced by the P450scc system (33).
A new model for H2O2-mediated physiological control of steroidogenesis in the adrenal has recently been described (34). H2O2 is produced as a byproduct of steroidogenesis. Specific mitochondrial antioxidant mechanisms exist in the adrenal cortex, and when the antioxidant capacity is exceeded, there is an accumulation of H2O2 in the mitochondria with overflow into the cytosol that triggers a reduction in StAR expression (34). It is possible that a similar mechanism causes a reduction in P450c11β (CYP11B1) expression, protecting the adrenal from further oxidative damage. This may explain the particular susceptibility of the zona fasciculata to disease, with predominance of glucocorticoid deficiency. Recently Korytowski et al (35) also described an additional mechanism by which steroidogenesis may be compromised by oxidative stress in Leydig cells. In this model, StAR-mediated trafficking of redox active cholesterol hydroperoxides results in mitochondrial toxicity, rendering these steroidogenic cells susceptible to further oxidative insult.
Although it is accepted that StAR plays a critical role in adrenal and gonadal steroidogenesis, STAR mutations can present with a spectrum of phenotypes ranging from severe congenital lipoid adrenal hyperplasia (CLAH) to milder atypical or nonclassic forms of CLAH (36, 37). Nonclassic CLAH is caused by StAR mutations that retain partial activity and may present with a phenotype analogous to familial glucocorticoid deficiency. We hypothesize that ALADIN deficiency leads to an imbalance in cellular redox homeostasis, resulting in a reduction in StAR and P450c11β and consequent impairment of cortisol and in approximately 15% cases, mineralocorticoid production (1). Preservation of mineralocorticoid synthesis may reflect not only the relative preservation of zona glomerulosa but also the lower production rate of aldosterone compared with cortisol. Interestingly, gonadal dysfunction is not reported in triple A syndrome. This suggests that the impairment of StAR is mild, similar to patients with R188C/R192C mutations in STAR in which the protein retains 25%–30% functionality or that other mechanisms also contribute to the adrenal phenotype (36).
Oxidative stress has been implicated in the pathogenesis of numerous other neurodegenerative diseases and in several adrenal conditions. One such disorder is X-linked adrenoleukodystrophy (ALD) in which mutations in ABCD1 (encoding the peroxisomal ABCD transporter) result in the accumulation of very long-chain fatty acids in the tissues and plasma. Interestingly, ALD has a similar phenotype to triple A syndrome, that is, the adrenal and central nervous system are most susceptible to the disease process (38). In ALD, the toxic effects of the excessive very long-chain fatty acids are thought to result from an increase in steady-state ROS production, depletion of GSH and dysregulation of the cell redox homeostasis (38, 39). More recently mutations in the antioxidant gene, NNT, encoding nicotinamide nucleotide transhydrogenase (responsible for producing the high concentrations of nicotinamide adenine dinucleotide phosphate oxidase (NADPH) necessary for the regeneration of reduced glutathione from oxidized glutathione), have been reported to cause familial glucocorticoid deficiency with ACTH-resistant adrenal failure (40). Thus, there is an emerging picture of defects in antioxidant defense, impacting specifically on the adrenal gland. It is possible that functional compensation by overlapping antioxidant defense mechanisms protects other cell types or tissues unaffected in these disorders.
The precise mechanisms by which antioxidant defenses are impaired in triple A syndrome need to be further characterized. However, using AAAS-knockdown adrenal and neuronal cells, we provide further compelling evidence that oxidative stress is involved in the progression of triple A syndrome. Because the adrenal cortex and neural tissue are highly oxidative environments, this may explain the susceptibility of these tissues in the absence of functional ALADIN.
Acknowledgments
This work was supported by a Clinical Training Fellowship from Barts and the London Charity and a Research Training Fellowship from the Wellcome Trust (WT095984AIA, to R.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALADIN
- alacrima-achalasia-adrenal insufficiency neurological disorder
- ALD
- adrenoleukodystrophy
- CLAH
- congenital lipoid adrenal hyperplasia
- FTH1
- ferritin heavy chain protein
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GSH
- glutathione
- GSSG
- oxidized glutathione measurement
- HEK
- human embryonic kidney
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt
- NAC
- N-acetylcysteine
- NPC
- nuclear pore complex
- Nup
- nucleoporin
- PARP
- poly-ADP-ribose polymerase
- P450scc
- P450 side chain cleavage
- qPCR
- quantitative RT-PCR
- ROS
- reactive oxygen species
- shRNA
- short hairpin RNA
- StAR
- steroidogenic acute regulatory protein
- WD
- Tryptophan-Aspartic acid.
References
- 1. Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet. 1978;1:1284–1286 [DOI] [PubMed] [Google Scholar]
- 2. Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet. 2001;10:283–290 [DOI] [PubMed] [Google Scholar]
- 3. Gazarian M, Cowell CT, Bonney M, Grigor WG. The '4A' syndrome: adrenocortical insufficiency associated with achalasia, alacrima, autonomic and other neurological abnormalities. Eur J Pediatr. 1995;154:18–23 [DOI] [PubMed] [Google Scholar]
- 4. Huebner A, Elias LL, Clark AJ. ACTH resistant syndromes. J Pediatr Endocrinol Metab. 1999;12:277–293 [PubMed] [Google Scholar]
- 5. Vallet A-E, Verschueren A, Petiot P, et al. Neurological features in adult Triple-A (Allgrove) syndrome. J Neurol. 2012;259:39–46 [DOI] [PubMed] [Google Scholar]
- 6. Milenkovic T, Zdravkovic, Savic N, Todorovic S, Mitrovic K, Koehler K, Huebner A. Triple A syndrome: 32 years experience of a single centre (1977–2008). Eur J Pediatr. 2010;169:1323–1328 [DOI] [PubMed] [Google Scholar]
- 7. Houlden H, Smith S, De Carvalho M, et al. Clinical and genetic characterization of families with triple A (Allgrove) syndrome. Brain. 2002;125:2681–2690 [DOI] [PubMed] [Google Scholar]
- 8. Huebner A, Kaindl AM, Knobeloch KP, Petzold H, Mann P, Koehler K. The triple A syndrome is due to mutations in ALADIN, a novel member of the nuclear pore complex. Endocr Res. 2004;30:891–899 [DOI] [PubMed] [Google Scholar]
- 9. Cho AR, Yang KJ, Bae Y, et al. Tissue specific expression and subcellular localization of ALADIN, the absence of which causes human triple A syndrome. Exp Mol Med. 2009;41:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Storr HL, Clark AJ, Priestley JV, Michael GJ. Identification of the sites of expression of triple A syndrome mRNA in the rat using in situ hybridisation. Neuroscience. 2005;131:113–123 [DOI] [PubMed] [Google Scholar]
- 11. Cronshaw JM, Krutchinsky AN, Zhang W, Chait BT, Matunis MJ. Proteomic analysis of the mammalian nuclear pore complex. J Cell Biol. 2002;158:915–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neilson DE. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr Opin Pediatr. 2010;22:751–757 [DOI] [PubMed] [Google Scholar]
- 13. Basel-Vanagaite L, Muncher L, Straussberg R, et al. Mutated nup62 causes autosomal recessive infantile bilateral striatal necrosis. Ann Neurol. 2006;60:214–222 [DOI] [PubMed] [Google Scholar]
- 14. Neilson DE, Adams MD, Orr CM, et al. Infection-triggered familial or recurrent cases of acute necrotising encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84:44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cronshaw JM, Matunis MJ. The nuclear pore complex ALADIN is mislocalized in triple A syndrome. Proc Natl Acad Sci USA. 2003;100:5823–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krumbholz M, Koehler K, Huebner A. Cellular localization of 17 natural mutant variants of ALADIN protein in triple A syndrome: shedding light on an unexpected splice mutation. Biochem Cell Biol. 2006;84:243–249 [DOI] [PubMed] [Google Scholar]
- 17. Hirano M, Furiya Y, Asai H, Yasui A, Ueno S. ALADIN1482S causes selective failure of nuclear protein import and hypersensitivity to oxidative stress in triple A syndrome. Proc Natl Acad Sci USA. 2006;103:2298–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kiriyama T, Hirano M, Asai H, Ikeda M, Furiya Y, Ueno S. Restoration of nuclear-import failure caused by triple A syndrome and oxidative stress. Biochem Biophys Res Commun. 2008;374:631–634 [DOI] [PubMed] [Google Scholar]
- 19. Kind B, Koehler K, Krumbholz M, Landgraf D, Huebner A. Intracellular ROS level is increased in fibroblasts of triple A syndrome patients. J Mol Med. 2010;88:1233–1242 [DOI] [PubMed] [Google Scholar]
- 20. Storr HL, Kind B, Parfitt DA, et al. Deficiency of ferritin heavy-chain nuclear import in triple A syndrome implies nuclear oxidative damage as the primary disease mechanism. Mol Endocrinol. 2009;12:2086–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliver FJ, de la Rubia G, Rolli V, Ruiz-Ruiz MC, de Murcia G, Murcia JM. Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson from an uncleavable mutant. J Biol Chem. 1998;273:33533–33539 [DOI] [PubMed] [Google Scholar]
- 22. Nicholson DW, Ali A, Thornberry NA, et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43 [DOI] [PubMed] [Google Scholar]
- 23. Huebner A, Yoon SJ, Ozkinay F, et al. Triple A syndrome—clinical aspects and molecular genetics. Endocr Res. 2000;26:751–759 [DOI] [PubMed] [Google Scholar]
- 24. Huebner A, Mann P, Rohde E, et al. Mice lacking the nuclear pore complex protein ALADIN show female infertility but fail to develop a phenotype resembling human triple A syndrome. Mol Cell Biol. 2006;26:1879–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chatel G, Fahrenkrog B. Nucleoporins: leaving the nuclear pore complex for a successful mitosis. Cell Signal. 2011;23:1555–1562 [DOI] [PubMed] [Google Scholar]
- 26. Blower MD, Nachury M, Heald R, Weis K. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell. 2005;121:223–234 [DOI] [PubMed] [Google Scholar]
- 27. Bland ML, Desclozeaux M, Ingraham HA. Tissue growth and remodelling of the embryonic and adult adrenal gland. Ann NY Acad Sci. 2003;995:59–72 [DOI] [PubMed] [Google Scholar]
- 28. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brooks BP, Kleta R, Caruso RC, Stuart C, Ludlow J, Stratakis CA. Triple-A syndrome with prominent ophthalmic features and a novel mutation in the AAAS gene: a case report. BMC Ophthalmol. 2004;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khelif K, De Laet MH, Chaouachi B, Segers V, Vanderwinden JM. Achalasia of the cardia in Allgrove's (triple A) syndrome: histopathologic study of 10 cases. Am J Surg Pathol. 2003;27:667–672 [DOI] [PubMed] [Google Scholar]
- 31. Diemer T, Allen JA, Hales KH, Hales DB. Reactive oxygen disrupts mitochondria in MA-10 tumor Leydig cells and inhibits steroidogenic acute regulatory (StAR) protein and steroidogenesis. Endocrinology. 2003;144:2882–2891 [DOI] [PubMed] [Google Scholar]
- 32. Shi Z, Feng Y, Wang J, Zhang H, Ding L, Dai J. Perfluorododecanoic acid-induced steroidogenic inhibition is associated with steroidogenic acute regulatory protein and reactive oxygen species in cAMP-stimulated Leydig cells. Toxicol Sci. 2010;114:285–294 [DOI] [PubMed] [Google Scholar]
- 33. Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–196 [DOI] [PubMed] [Google Scholar]
- 34. Kil IS, Lee SK, Ryu KW, et al. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Mol Cell. 2012;46:584–594 [DOI] [PubMed] [Google Scholar]
- 35. Korytowski W, Pilat A, Schmitt JC, Girotti AW. Deleterious cholesterol hydroperoxide trafficking in steroidogenic acute regulatory (StAR) protein-expressing MA-10 Leydig cells: implications for oxidative stress-impaired steroidogenesis. J Biol Chem. 2013;288:11509–11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metherell LA, Naville D, Halaby G, et al. Nonclassic lipoid congenital adrenal hyperplasia masquerading as familial glucocorticoid deficiency. J Clin Endocrinol Metab. 2009;94:3865–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Flück CE, Pandey AV, Dick B, et al. Characterization of novel StAR (steroidogenic acute regulatory protein) mutations causing non-classic lipoid adrenal hyperplasia. PLoS One. 2011;6(5):e20178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kemp SJ, Berger J, Aubourg P. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta. 2012;1822:1465–1474 [DOI] [PubMed] [Google Scholar]
- 39. Galea E, Launay N, Portero-Otin M, et al. Oxidative stress underlying axonal degeneration in adrenoleukodystrophy: a paradigm for multifactorial neurodegenerative diseases? Biochim Biophys Acta. 2012;1822:1475–1488 [DOI] [PubMed] [Google Scholar]
- 40. Meimaridou E, Kowalczyk J, Guasti L, et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat Genet. 2012;44:740–742 [DOI] [PMC free article] [PubMed] [Google Scholar]