Highlights
-
•
Self-renewal of common marmoset embryonic stem cells (CM ESCs) is promoted by bFGF.
-
•
bFGF activates the PI3K-AKT pathway in CM ESCs on feeder cells.
-
•
bFGF and TGFβ in combination support culture of CM ESCs without feeder cells.
-
•
CM ESCs show phenotypes similar to those of human ESCs and mouse epiblast SCs.
Abbreviations: AKT, protein kinase B; bFGF, basic fibroblast growth factor; CM, common marmoset; EB, embryoid body; EpiSCs, epiblast stem cells; ERK, extracellular signal-regulated kinase; ESCs, embryonic stem cells; FCM, flow cytometry; iPSCs, induced pluripotent stem cells; JAK, janus kinase; KSR, knockout serum replacement; LIF, leukemia inhibitory factor; MEFs, mouse embryonic fibroblasts; MEK, mitogen-activated protein/extracellular signal-regulated kinase kinase; PI3K, phosphatidylinositol-3-kinase; RT-PCR, reverse transcription-polymerase chain reaction; SMAD2/3, mothers against decapentaplegic homolog 2/3; STAT3, signal transducer and activator of transcription 3; TGFβ, transforming growth factor β
Keywords: Embryonic stem cells, Common marmoset, bFGF, TGFβ, Self-renewal
Abstract
Common marmoset (CM) is widely recognized as a useful non-human primate for disease modeling and preclinical studies. Thus, embryonic stem cells (ESCs) derived from CM have potential as an appropriate cell source to test human regenerative medicine using human ESCs. CM ESCs have been established by us and other groups, and can be cultured in vitro. However, the growth factors and downstream pathways for self-renewal of CM ESCs are largely unknown. In this study, we found that basic fibroblast growth factor (bFGF) rather than leukemia inhibitory factor (LIF) promoted CM ESC self-renewal via the activation of phosphatidylinositol-3-kinase (PI3K)-protein kinase B (AKT) pathway on mouse embryonic fibroblast (MEF) feeders. Moreover, bFGF and transforming growth factor β (TGFβ) signaling pathways cooperatively maintained the undifferentiated state of CM ESCs under feeder-free condition. Our findings may improve the culture techniques of CM ESCs and facilitate their use as a preclinical experimental resource for human regenerative medicine.
1. Introduction
Human regenerative medicine, including transplantation of various functional cells differentiated from embryonic stem cells (ESCs) or induced pluripotent stem cells (iPSCs), is considered to have great potential for treating various incurable diseases, and has thus attracted much public attention. However, preclinical studies using animal disease models are required to evaluate the efficacy and safety of ESC/iPSC-derived cells prior to their clinical application. Common marmoset (CM, Callithrixjacchus) has recently been recognized as a useful non-human primate for such studies, because of its small size, high reproductive capacity, and genetic similarity to humans [1].
Understanding the molecular mechanisms governing the self-renewal of ESCs is important for the development of technologies to differentiate them into functional cells. Although both human and mouse ESCs are able to self-renew on feeder cells in vitro, their growth factor requirements for self-renewal are different. Basic fibroblast growth factor (bFGF), which activates phosphatidylinositol-3-kinase (PI3K)-protein kinase B (AKT) [2,3] and mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK)-extracellular signal-regulated kinase (ERK) pathways [2–8], and transforming growth factor β (TGFβ) leading to the activation of mothers against decapentaplegic homolog 2/3 (SMAD2/3) [2,6–11], maintain the self-renewal of human ESCs and mouse epiblast stem cells (EpiSCs). Conversely, in mouse ESCs, leukemia inhibitory factor (LIF), which activates janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) and PI3K-AKT pathways, is known to play important roles in maintaining self-renewal [12–14].
ESCs derived from CM have been established by us and others [15–17]. However, the growth factors used in the culture medium are different among reports [15,17–21]. Thus, the most appropriate growth factor and its downstream pathway for maintaining the self-renewal of CM ESCs still remain to be determined.
In the present study, we characterized two CM ESC cell lines, Cj11 and CM40, and found that CM ESCs were more similar to human ESCs rather than mouse ESCs in terms of their growth factor requirement and molecular signaling pathways for self-renewal.
2. Materials and methods
2.1. CM ESC culture on mouse embryonic fibroblasts (MEFs)
CM ESC lines, CM40 and Cj11, were maintained in CM ESC medium as described before [15] with or without 1:1000 LIF (Wako, Osaka, Japan), 5 ng/ml bFGF (PeproTech, NJ, USA), 5 μM PD0325901 (MEK inhibitor, Wako) or 10 μM LY294002 (PI3K inhibitor, Santa Cruz Biotechnology, CA, USA). CM40 cell line was established in our laboratory [15], and Cj11 cell line was obtained from WiCell Research Institute [16]. MEFs were prepared from 13.5 dpc embryos from ICR mice (Charles River, Japan) using established procedures [22].
2.2. CM ESC culture under feeder-free conditions
CM40 and Cj11 ESC lines were cultured on Matrigel (BD Biosciences, CA, USA)-coated dishes in Essential 8 medium (Life Technologies, NY, USA) or Essential 6 medium (Life Technologies) with or without 1:1000 LIF (Wako), 100 ng/ml bFGF (PeproTech), 2 ng/ml TGFβ (PeproTech), 5 μM PD0325901 (MEK inhibitor, Wako), 10 μM LY294002 (Santa Cruz Biotechnology).
2.3. CM ESC differentiation
Undifferentiated ESCs were detached from the feeder cells by treatment with 0.25% trypsin (NacalaiTesque, Kyoto, Japan) for 1 min. The collected colonies were processed for embryoid body (EB) formation assay in CM ESC medium on low cell-binding 12-well plates (Nalge Nunc International KK, Japan) for 4 or 8 days. Detailed protocols to differentiate CM ESCs into three germ layers are described in “Supplementary Materials and Methods”.
2.4. Immunocytochemistry
Cells were fixed in 4% paraformaldehyde (PFA)/phosphate-buffered saline (PBS) (NacalaiTesque), permeabilized with 0.3% Triton X-100/PBS, blocked with staining buffer (2% fetal bovine serum (FBS)/PBS). The primary antibodies used are shown in Supplementary Table 1. Nuclei were counterstained with DAPI. Images were obtained under a fluorescence microscope (Axiovert 135M; Carl Zeiss, Germany, or BZ-9000; Keyence) and then analyzed by Axiovert software (Carl Zeiss) or BZ-Analyzer software (Keyence).
2.5. Flow cytometry (FCM)
CM ESCs were fixed in 4% PFA/PBS, permeabilized with 0.3% Triton X-100/PBS, blocked with staining buffer (2% FBS/PBS), and then incubated with an anti-OCT3/4 antibody (Santa Cruz Biotechnology, sc-5279 or sc-8628). The cells were detected on a FACSVerse flow cytometer (Becton Dickinson, USA), followed by data analysis using FlowJo software (Tomy Digital Biology, Japan).
2.6. Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using an RNeasy Mini Kit (Qiagen, USA), and cDNA was synthesized using Superscript III reverse transcriptase (Life Technologies). Then PCR was carried out using the synthesized cDNA as templates and gene-specific primers (see Supplemental Table 2). The primers were designed based on different exons to span the intervening intron and avoid amplification of contaminating genomic DNA.
2.7. Western blotting
Cells were incubated on ice with RIPA buffer containing protease inhibitors (Complete Mini, EDTA-free; Roche, Basel, Switzerland) and a phosphatase inhibitor cocktail (NacalaiTesque). The cell lysates were then resolved by SDS-polyacrylamide gel electrophoresis, followed by immunoblotting. The primary antibodies used are shown in Supplementary Table 3. The signals were detected using a LAS3000 (Fujifilm, Japan). Band intensities were measured by ImageJ software (NIH).
2.8. Statistical analysis
Unless otherwise noted, inter-group differences were analyzed using analysis of variance (ANOVA) followed by the Tukey’s post-hoc test with GraphPad Prism 5 (GraphPad Software, CA, USA).
3. Results
3.1. bFGF promotes self-renewal of CM ESCs on feeder cells
bFGF and LIF have been reported to be essential for the maintenance of human and mouse ESCs, respectively [3,12–14,23–27], and either or both of these growth factors were considered to be required for the maintenance of CM ESCs. To determine the optimal condition for culturing CM ESCs, we first examined the expression of receptors for bFGF (FGFR1, FGFR2, FGFR3, and FGFR4) and LIF (LIFR and gp130). RT-PCR analysis demonstrated that all of these receptors were expressed in the CM ESCs (Fig. 1A), suggesting that both growth factors play important roles in the biology of CM ESCs.
Fig. 1.
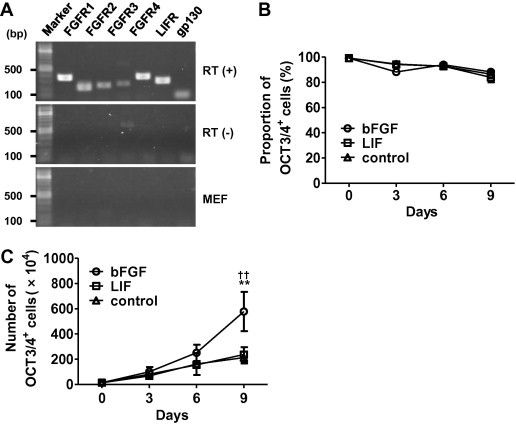
bFGF promotes self-renewal of CM ESCs in the presence of feeder support. (A) RT-PCR analysis showing the expression of FGFR1, FGFR2, FGFR3, FGFR4, LIFR, and gp130 genes in CM ESCs (CM40). (B) No effect of bFGF on the proportion of OCT3/4+ cells. CM ESCs (CM40; 1.4 × 105) were seeded on mitomycin C (MMC)-treated MEFs and cultured with LIF (open square), bFGF (open circle), or without growth factors (control; open triangle). The percentage of OCT3/4+ cells was determined by FCM. (C) Enhancement of undifferentiated CM ESC growth by bFGF. CM ESCs (CM40; 1.4 × 105) were seeded on mitomycin C (MMC)-treated MEFs and cultured with LIF (open square), bFGF (open circle), or without growth factors (control; open triangle). The number of cells was then counted by trypan blue exclusion. The number of OCT3/4+ cells was determined by multiplying the number of cells by the percentage of OCT3/4+ cells and the passage ratio together. Data are shown as the mean ± SD (n = 4). ∗∗P < 0.01 (bFGF vs. control) and ††P < 0.01 (bFGF vs. LIF). Note that all of PCR in (A) was performed with 30 cycles, and no bands were detected for FGFR expression in MEFs in (A), however, they were faintly done when PCR was performed with 40 cycles.
In culture, ESCs are generally known to spontaneously differentiate. However, the addition of appropriate growth factors inhibits such spontaneous differentiation. To evaluate the effects of bFGF and LIF on the proliferation and differentiation of CM ESCs in vitro, we passaged CM ESCs at a ratio of 1:3 every three days for three passages, and then counted the numbers of undifferentiated OCT3/4+ cells. We found that the proportion of OCT3/4+ cells was unchanged regardless of the addition of bFGF or LIF (Fig. 1B). However, the numbers of OCT3/4+ cells were significantly increased by the addition of bFGF, but not LIF, compared with those of controls cultured without bFGF and LIF (Fig. 1C). Similar results were obtained when the cells were cultured for more than ten passages (Supplementary Fig. S1 and data not shown). The above experiments were performed using CM40 cell line, and similar results were obtained with Cj11 cell line (Supplementary Fig. S2). These results strongly suggest that bFGF promotes the proliferation of CM ESCs rather than maintaining the undifferentiated state of CM ESCs.
3.2. bFGF-PI3K-AKT pathway supports self-renewal of CM ESCs on feeder cells
bFGF and its downstream PI3K-AKT and MEK-ERK pathways are important for the self-renewal of human ESCs [2,3,5,6]. We therefore examined whether these pathways were activated by bFGF for CM ESC self-renewal on feeder cells. CM ESCs were cultured overnight in medium lacking knockout serum replacement (KSR) and any growth factors. Then, we added bFGF (5 ng/ml), and examined the activation of AKT and ERK1/2 in the cells by Western blotting. The results showed that the band intensity of phosphorylated AKT was significantly increased after the treatment with bFGF, while that of phosphorylated ERK1/2 was not changed (Fig. 2A and B). These data suggested that PI3K-AKT, but not MEK-ERK, pathway was activated by bFGF in CM ESCs under feeder-dependent culture condition.
Fig. 2.
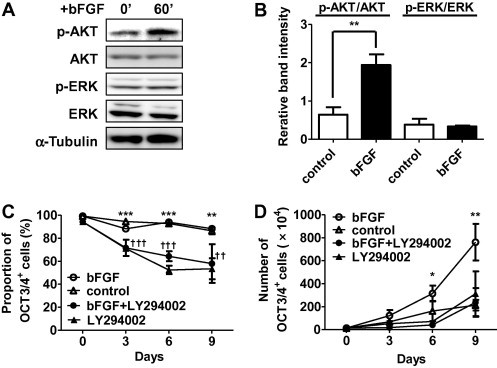
bFGF-PI3K-AKT pathway supports self-renewal of CM ESCs. (A) Western blot analysis showing the activation of AKT by bFGF in CM ESCs. CM40 cells were starved of bFGF and KSR overnight, and then stimulated with 5 ng/ml of bFGF for the indicated durations. AKT, ERK1/2 and α-Tubulin are shown as loading controls. The relative band intensities of p-AKT/AKT and p-ERK/ERK are shown in (B). Band intensities were measured by ImageJ software. Data are shown as the mean ± SD. The Student’s t-test was used to test inter-group differences. ∗∗P < 0.01. (C) Inhibition of self-renewal by LY294002. CM ESCs (CM40; 1.4 × 105) were seeded on MMC-treated MEFs and cultured in medium containing bFGF (open circle), control medium (open triangle), bFGF+LY294002 (closed circle) or LY294002 (closed triangle). The percentage of OCT3/4+ cells was then determined by FCM at the indicated day as shown in (C). The number of live cells was counted by trypan blue exclusion. Growth curves were generated by multiplying the number of live cells by the percentage of OCT3/4+ cells and passage ratio together as shown in (D). Data are shown as the mean ± SD. bFGF, n = 4; control, n = 4; bFGF+LY294002, n = 3; LY294002, n = 3; ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.005, bFGF vs. control; ††P < 0.01 and †††P < 0.005, bFGF+LY294002 or LY294002 vs. control.
Next, to examine whether bFGF-PI3K-AKT pathway plays any roles in the maintenance of self-renewal of CM ESCs, the cells were cultured in medium containing bFGF in the presence or absence of the PI3K inhibitor, LY294002. We found that the proportion of OCT3/4+ cells was maintained at approximately 90% for at least three passages when the cells were cultured without LY294002, whereas it was gradually decreased when the cells were cultured with LY294002 (day0, 96.75 ± 2.83% vs. day9, 57.97 ± 16.76%, Fig. 2C). In addition, OCT3/4+ cell proliferation was inhibited in the presence of LY294002 (day9, bFGF, 7.61 ± 1.59×106 cells vs. bFGF+LY294002, 2.36 ± 1.25×106 cells, Fig. 2D). Additionally, even when bFGF was not added, the proportion of OCT3/4+ cells was significantly reduced by the treatment with LY294002 (Fig. 2C), indicating that PI3K-AKT pathway is activated by unknown factors from MEFs and play roles for self-renewal of CM ESCs. Overall, these results strongly suggest that bFGF-PI3K-AKT pathway is essential for the self-renewal of CM ESCs under feeder-dependent culture condition.
To examine the expression of OCT3/4, we used an antibody against amino acids 1–134 of human OCT3/4 (monoclonal OCT3/4 antibody, sc-5279) that was known to be useful for detecting the expression of CM OCT3/4 [28]. And recent study reported that another antibody raised against amino acids 1–19 of human OCT3/4 (polyclonal OCT3/4 antibody, sc-8628) was more useful to detect ESC-specific OCT3/4 [29]. Thus we performed immunocytochemistry and FCM analysis using sc-8628, and obtained the similar results (Supplementary Fig. S3).
3.3. bFGF and TGFβ signaling cooperate to maintain the undifferentiated state of CM ESCs under feeder-free conditions
All of the experiments described above were performed with feeder support. Thus, the various secreted factors including cytokines and adhesion molecules might have affected the results. To examine the dependency of CM ESCs on feeder cells, CM ESCs were cultured on a high or low density of feeder cells, and then the undifferentiated state was examined by immunocytochemistry using an anti-NANOG antibody (Supplementary Fig. S1). We found that CM ESCs on low-density feeder cells lost their expression of NANOG after four passages, whereas those on high-density feeder cells maintained NANOG expression even after ten passages (Supplementary Fig. S1). Therefore, it is conceivable that the self-renewal of CM ESCs is maintained by unknown factors derived from feeder cells.
Chen et al. showed that Essential 8 medium (Dulbecco’s modified Eagle’s medium/F12 supplemented with l-ascorbic acid-2-phosphate magnesium, insulin, transferrin, sodium selenium, NaHCO3, bFGF, and TGFβ) supports the self-renewal of human ESCs and iPSCs under feeder-free conditions [30]. To clarify the essential growth factors required for maintaining the undifferentiated state of CM ESCs, CM ESCs were cultured under feeder-free condition. We found that CM ESCs could be cultured on Matrigel in Essential 8 medium without feeder support, although they could not be maintained for more than three passages (data not shown). Next, we cultured CM ESCs on Matrigel in Essential 6 medium lacking bFGF and TGFβ overnight, and then the activation of signaling pathways known to maintain mouse and human ESCs (bFGF-PI3K-AKT, bFGF-MEK-ERK, TGFβ-SMAD2/3, and LIF-JAK-STAT3 pathways) were analyzed by Western blotting after the addition of bFGF, TGFβ, or LIF to the medium. We found that phosphorylation of AKT and ERK was increased by the addition of bFGF, while it was decreased by the treatment with LY294002 or PD0325901, suggesting that both of AKT and ERK were activated downstream of bFGF under feeder-free condition (Fig. 3A and B). And the addition of TGFβ resulted in an increase of phosphorylated SMAD2/3 (Fig. 3A and B), suggesting that SMAD2/3 was activated downstream of TGFβ. Moreover, the addition of LIF resulted in an increase of phosphorylated STAT3, suggesting that STAT3 was activated downstream of LIF (Supplementary Figs. S4B and D). These results suggested that bFGF-PI3K-AKT, bFGF-MEK-ERK, TGFβ-SMAD2/3 and LIF-JAK-STAT3 pathways known to regulate self-renewal of human or mouse ESCs were activated in CM ESCs under feeder-free condition. It should be noted that ERK was not activated by 5 ng/ml of bFGF that was used for the culture on feeder cells as described in Fig. 2A and B (Supplementary Fig. S4B), but it was remarkably activated by 100 ng/ml of bFGF generally used for feeder-free culture of human ESCs (Fig. 3A and B) [30].
Fig. 3.
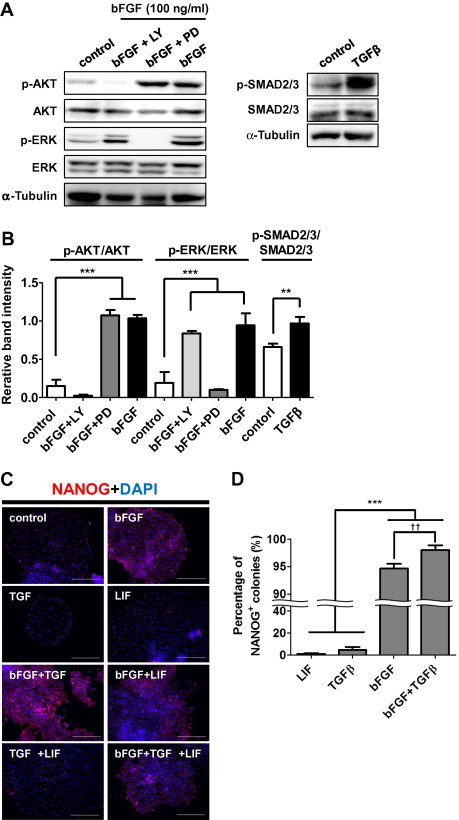
bFGF and TGFβ maintain the undifferentiated state of CM ESCs under feeder-free conditions. (A) Western blot showing the activation of AKT, ERK1/2 and SMAD2/3 by 100 ng/ml of bFGF or 2 ng/ml of TGFβ for 30 min in CM ESCs. CM40 were starved of growth factors overnight and then pre-treated with LY294002 or PD0325901for 1 h before stimulation with bFGF. AKT, ERK1/2, SMAD2/3, and α-Tubulin are shown as loading controls. The relative band intensities of p-AKT/AKT, p-ERK/ERK and p-SMAD2/3/SMAD2/3 are shown in (B). Band intensities were measured by ImageJ software. Data are shown as the mean±SD. One-way ANOVA followed by the Tukey’s post-hoc test was used to test inter-group differences. ∗∗P < 0.01 and ∗∗∗P < 0.005. (C) Immunocytochemical analyses of NANOG expression in CM ESCs (CM40) cultured with various growth factors for 4 days. Merged images of NANOG (red) and nuclei (DAPI; blue) are shown. Scale bars represent 200 μm. NANOG expression in cells cultured without any growth factors is shown as a control. (D) Proportion of NANOG+ colonies. The percentage of NANOG+ colonies cultured with various growth factors was analyzed by immunocytochemistry. For statistical analyses, 300 colonies were examined in each experiment (n = 3). Data are shown as the mean ± SD. One-way ANOVA followed by the Tukey’s post-hoc test was used to test inter-group differences. ∗∗∗P < 0.005. The difference between bFGF- and bFGF+TGFβ-treated cells was statistically analyzed using the Student’s t-test. ††P < 0.01.
Next, to determine the growth factors maintaining the undifferentiated state of CM ESCs under feeder-free condition, CM ESCs were cultured in the feeder-free system with various combinations of growth factors, followed by analysis of their undifferentiated state morphologically and immunocytochemically. Most of the colonies cultured in Essential 6 medium with bFGF, bFGF+TGFβ, bFGF+LIF, or bFGF+TGFβ+LIF showed a well-packed appearance, and a majority of the cells expressed NANOG (Fig. 3C). In contrast, most of the colonies cultured in Essential 6 medium with TGFβ, LIF, or TGFβ+LIF showed an unpacked appearance, and a majority of the cells did not express NANOG (Fig. 3C). Moreover, NANOG+ and well-packed colonies were found at the highest proportion (98.00 ± 0.88%) when the cells were cultured in the presence of TGFβ+bFGF (Fig. 3D). In addition, almost all of the colonies were positive for OCT3/4, SOX2, SSEA-4, TRA1-60 and TRA1-81 (Supplementary Fig. S3), and these colony forming cells kept the capability of differentiating three lineages (Supplementary Fig. S5). This observation indicates that the addition of both TGFβ and bFGF is the most appropriate growth factor combination for maintenance of the undifferentiated state of CM ESCs under feeder-free condition, which is similar to a characteristic of human ESCs [2,6,9–11].
3.4. CM ESCs show phenotypes similar to those of human ESCs and mouse EpiSCs
Human ESCs and mouse EpiSCs share a number of similar phenotypes as shown in Table 1 [7,8]. CM ESCs formed flattened colonies and expressed NANOG as well as markers for both mouse EpiSCs and human ESCs, such as T, CER1, EOMES, FOXA2, GATA6, and SOX17 (Supplementary Figs. S1 and S6A) [7]. Moreover, bFGF and TGFβ signalings play crucial roles in maintaining the undifferentiated state of human ESCs and mouse EpiSCs [2,7,8,11,30], and the same roles of these signaling pathways were also found in CM ESCs (Fig. 3C and D).
Table 1.
The characters of mouse EpiSCs and mouse, human and CM ESCs.
| Mouse ESCs | Mouse EpiSCs | Human ESCs | CM ESCs | ||
|---|---|---|---|---|---|
| Morphology of colony | Small, dome | Large, flat | Large, flat | Large, flat | |
| Growth factor dependency | LIF | + | − | − | − |
| bFGF | − | + | + | + | |
| TGFβ/activin | − | + | + | + | |
| Marker expression | NANOG | + | + | + | + |
| OCT3/4 | + | + | + | + | |
| T (brachyury) | − | + | + | + | |
| CER1 | − | + | + | + | |
| EOMES | − | + | + | + | |
| FOXA2 | − | + | + | + | |
| GATA6 | − | + | + | + | |
| SOX17 | − | + | + | + | |
| Tolerance to single cell dissociation | + | − | − | − | |
| Contribution in chimera | + | − | N/D | N/D |
N/D = not determined.
Previous reports have shown that apoptosis of human ESCs and mouse EpiSCs is induced by culturing after complete dissociation [31,32]. Watanabe et al. showed that dissociation-induced apoptosis of human ESCs is suppressed by treatment with the Rho-associated kinase (ROCK) inhibitor Y27632 [33]. To examine whether dissociation-induced apoptosis of human ESCs and mouse EpiSCs was similarly found in CM ESCs, colonies of CM ESCs were dissociated into single cells by trypsinization, and then the cells were plated on Matrigel-coated dishes with or without Y27632. Compared with untreated controls, we found that Y27632-treated CM ESCs produced significantly more colonies, suggesting that dissociation-induced apoptosis of CM ESCs occurred and was suppressed by Y27632 (Supplementary Figs. S6B and S6C). Thus, we concluded that CM ESCs are similar to human ESCs and mouse EpiSCs.
4. Discussion
Recent advances in the field of basic research for pluripotent stem cells such as the generation of ESCs, iPSCs and stimulus-triggered acquisition of pluripotency (STAP) cells have given us realistic expectations for human regenerative medicine [22,34–38]. And the need for the development of methods to test new therapeutic approach using such cells is increasing. CM is a useful experimental animal that can suit such needs, and therefore, the characterization of CM ESCs is important. In this study, we investigated essential signaling pathways for the self-renewal of CM ESCs under feeder-dependent and feeder-free culture conditions.
LIF has been widely used to establish and maintain non-human primate ESCs [15,17,18,39–42], although some researchers claim that LIF cannot maintain the self-renewal capacity of these cells [16,41–43]. We found that LIF did not affect the capacity for self-renewal of CM ESCs (Figs. 1 and 3), although it activated the JAK-STAT3 pathway (Supplementary Fig. S3). More extensive studies are needed to further explore the roles of the LIF-JAK-STAT3 pathway in CM ESCs. In our previous report, the expression of LIFR was not found in undifferentiated CM ESCs [15], but it was found in this study after repetitive experiments (Fig. 1A). This discrepancy was considered to be caused by the detection threshold of RT-PCR under different conditions, particularly PCR primers used in our previous report were human LIFR sequence-originated because there were no available marmoset genomic sequence data.
We also found that the self-renewal of CM ESCs cultured on feeder cells was remarkably promoted by bFGF, which is similar to the characteristic of human ESCs (Fig. 1). However, even in the absence of bFGF, most CM ESCs could be maintained in an undifferentiated state by culture on feeder cells, although they showed slower growth compared to those cultured in bFGF containing medium (Fig. 1B and C). This observation indicates that growth factors secreted from feeder cells such as activin, noggin and bFGF, maintain the undifferentiated state of CM ESCs [44,45]. Indeed, CM ESC colonies cultured on low-density feeder cells differentiated within four passages (Supplementary Fig. S1).
Previous studies have demonstrated the critical roles of PI3K-AKT and MEK-ERK pathways in the self-renewal of human ESCs [2–6]. Our results showed that AKT, but not ERK1/2, was activated by the addition of bFGF (5 ng/ml), while ERK1/2 was continuously activated even in the absence of bFGF on feeder support (Fig. 2A). Moreover, inhibition of either MEK-ERK or PI3K-AKT pathways resulted in reduced self-renewal of CM ESCs (Fig. 2 and Supplementary Fig. S7). Therefore, activation of the PI3K-AKT pathway downstream of bFGF as well as the MEK-ERK pathway by unknown mechanisms is required for self-renewal of CM ESCs on feeder support. On the other hand, both AKT and ERK1/2 were activated by the addition of bFGF (100 ng/ml) under feeder-free condition (Fig. 3A and B). And treatment with LY294002 resulted in the elevated expression of endoderm and mesoderm markers, and treatment with PD0325901 caused the reduced expression of these markers, indicating that modulation of these pathways affects the differentiation process in CM ESCs (Supplementary Fig. S8). We are now extensively investigating the effect of these inhibitors on the differentiation process of CM ESCs induced by the treatment with specific cytokines and EB formation assay.
Several studies have demonstrated differences in the mechanisms of ESC self-renewal between mice and humans. Mouse ESCs require LIF for their self-renewal, whereas human ESCs require bFGF and TGFβ. Mouse EpiSCs originating from post-implantation embryos depend on bFGF and TGFβ, and show characteristics similar to those of human ESCs originating from the inner cells mass of blastocysts as shown in Table 1 [7,8,10]. Mouse EpiSCs are therefore considered to be the counterpart of human ESCs. In this study, we demonstrated that CM ESCs were very similar to human ESCs and mouse EpiSCs in terms of their morphology, gene expression, growth factor dependency for self-renewal, and vulnerability to single cell dissociation.
Our findings strongly suggest that CM ESCs are phenotypically similar to human ESCs. Therefore, CM ESCs may facilitate the development of valuable preclinical experimental systems to test new therapeutic modalities for incurable human diseases, particularly in the field of regenerative medicine.
Acknowledgments
We thank Michiko Ushijima for administrative assistance, Yoko Nagai and the members of Tani laboratory for their constructive criticisms and technical support. This work was supported by a grant from the Project for Realization of Regenerative Medicine (K. Tani, 08008010) and KAKENHI (T. Marumoto, 23590465) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Appendix A. Supplementary data
This document file contains supplementary figures and tables.
This document file contains supplementary data.
References
- 1.Hibino H. The common marmoset as a target preclinical primate model for cytokine and gene therapy studies. Blood. 1999;93:2839–2848. [PubMed] [Google Scholar]
- 2.Singh A.M. Signaling network crosstalk in human pluripotent cells: a Smad2/3-regulated switch that controls the balance between self-renewal and differentiation. Cell Stem Cell. 2012;10:312–326. doi: 10.1016/j.stem.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong L. The role of PI3K/AKT, MAPK/ERK and NFκβ signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006;15:1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 4.Li J. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 5.Na J., Furue M.K., Andrews P.W. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res. 2010;5:157–169. doi: 10.1016/j.scr.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 6.McLean A.B. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. doi: 10.1634/stemcells.2006-0219. [DOI] [PubMed] [Google Scholar]
- 7.Tesar P.J., Chenoweth J.G., Brook F.A., Davies T.J., Evans E.P., Mack D.L., Gardner R.L., McKay R.D.G. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 8.Brons I.G.M. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 9.James D., Levine A.J., Besser D., Hemmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 10.Greber B. Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells. Cell Stem Cell. 2010;6:215–226. doi: 10.1016/j.stem.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Vallier L., Alexander M., Pedersen R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 12.Williams R.L. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 13.Niwa H., Burdon T., Chambers I., Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takahashi K., Murakami M., Yamanaka S. Role of the phosphoinositide 3-kinase pathway in mouse embryonic stem (ES) cells. Biochem. Soc. Trans. 2005;33:1522. doi: 10.1042/BST0331522. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki E. Establishment of novel embryonic stem cell lines derived from the common marmoset (Callithrix jacchus) Stem Cells. 2005;23:1304–1313. doi: 10.1634/stemcells.2004-0366. [DOI] [PubMed] [Google Scholar]
- 16.Thomson J.A., Kalishman J., Golos T.G., Durning M., Harris C.P., Hearn J.P. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- 17.Müller T., Fleischmann G., Eildermann K., Mätz-Rensing K., Horn P.A., Sasaki E., Behr R. A novel embryonic stem cell line derived from the common marmoset monkey (Callithrix jacchus) exhibiting germ cell-like characteristics. Hum. Reprod. 2009;24:1359–1372. doi: 10.1093/humrep/dep012. [DOI] [PubMed] [Google Scholar]
- 18.Shimada H. Efficient derivation of multipotent neural stem/progenitor cells from non-human primate embryonic stem cells. PLoS One. 2012;7:e49469. doi: 10.1371/journal.pone.0049469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda T., Kurita R., Yokoo T., Tani K., Makino N. Telomerase inhibition promotes an initial step of cell differentiation of primate embryonic stem cell. Biochem. Biophys. Res. Commun. 2011;407:491–494. doi: 10.1016/j.bbrc.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 20.Shimoji K. G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs. Cell Stem Cell. 2010;6:227–237. doi: 10.1016/j.stem.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Chen H. Common marmoset embryonic stem cell can differentiate into cardiomyocytes. Biochem. Biophys. Res. Commun. 2008;369:801–806. doi: 10.1016/j.bbrc.2008.02.141. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Niwa H., Ogawa K., Shimosato D., Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 24.Levenstein M.E., Ludwig T.E., Xu R.H., Llanas R.A., VanDenHeuvel-Kramer K., Manning D., Thomson J.A. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu R.H., Peck R.M., Li D.S., Feng X., Ludwig T., Thomson J.A. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods. 2005;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 26.Xu C. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- 27.Dahéron L., Opitz S.L., Zaehres H., Lensch W.M., Andrews P.W., Itskovitz-Eldor J., Daley G.Q. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778. doi: 10.1634/stemcells.22-5-770. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y., Zhang Y., Mishra A., Tardif S.D., Hornsby P.J. Generation of induced pluripotent stem cells from newborn marmoset skin fibroblasts. Stem Cell Res. 2010;4:180–188. doi: 10.1016/j.scr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warthemann R., Eildermann K., Debowski K., Behr R. False-positive antibody signals for the pluripotency factor OCT4A (POU5F1) in testis-derived cells may lead to erroneous data and misinterpretations. Mol. Hum. Reprod. 2012;18:605–612. doi: 10.1093/molehr/gas032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amit M., Carpenter M.K., Inokuma M.S., Chiu C.-P., Harris C.P., Waknitz M.A., Itskovitz-Eldor J., Thomson J.A. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev. Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- 32.Ohgushi M. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe K. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 35.Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 36.Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 37.Obokata H., Wakayama T., Sasai Y., Kojima K., Vacanti M.P., Niwa H., Yamato M., Vacanti C.A. Stimulus-triggered fate conversion of somatic cells into pluripotency. Nature. 2014;505:641–647. doi: 10.1038/nature12968. [DOI] [PubMed] [Google Scholar]
- 38.Obokata H. Bidirectional developmental potential in reprogrammed cells with acquired pluripotency. Nature. 2014;505:676–680. doi: 10.1038/nature12969. [DOI] [PubMed] [Google Scholar]
- 39.Shimozawa N., Nakamura S., Takahashi I., Hatori M., Sankai T. Characterization of a novel embryonic stem cell line from an ICSI-derived blastocyst in the African green monkey. Reproduction. 2010;139:565–573. doi: 10.1530/REP-09-0067. [DOI] [PubMed] [Google Scholar]
- 40.Simerly C.R. Establishment and characterization of baboon embryonic stem cell lines: An Old World Primate model for regeneration and transplantation research. Stem Cell Res. 2009;2:178–187. doi: 10.1016/j.scr.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suemori H. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev. Dyn. 2001;222:273–279. doi: 10.1002/dvdy.1191. [DOI] [PubMed] [Google Scholar]
- 42.Thomson J.A., Kalishman J., Golos T.G., Durning M., Harris C.P., Becker R.A., Hearn J.P. Isolation of a primate embryonic stem cell line. Proc. Nat. Acad. Sci. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitalipov S., Kuo H.C., Byrne J., Clepper L., Meisner L., Johnson J., Zeier R., Wolf D. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. doi: 10.1634/stemcells.2006-0125. [DOI] [PubMed] [Google Scholar]
- 44.Wang G. Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem. Biophys. Res. Commun. 2005;330:934–942. doi: 10.1016/j.bbrc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 45.Beattie G.M., Lopez A.D., Bucay N., Hinton A., Firpo M.T., King C.C., Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This document file contains supplementary figures and tables.
This document file contains supplementary data.


