MDSC enhance mast cell inflammatory responses that can be altered by an existing chemotherapeutic gemcitabine.
Keywords: Nippostrongylus, Trichinella, asthma, allergy, inflammation
Abstract
Mast cells and MDSCs are increased by parasitic infection and tumor growth. We previously demonstrated that enhanced MDSC development in ADAM10 transgenic mice yielded resistance to Nb infection and that coculturing MDSCs and mast cells enhanced cytokine production. In the current work, we show that MDSC-mast cell coculture selectively enhances IgE-mediated cytokine secretion among mast cells, without increasing MDSC cytokine production. This effect was independent of cell contact and elicited by Ly6C+ and Ly6C/G+ MDSC subsets. These interactions were functionally important. MDSC depletion with the FDA-approved drug gemcitabine exacerbated Nb or Trichinella spiralis infection and reduced mast cell-dependent AHR and lung inflammation. Adoptive transfer of MDSC worsened AHR in WT but not mast cell-deficient Wsh/Wsh mice. These data support the hypothesis that MDSCs enhance mast cell inflammatory responses and demonstrate that this interaction can be altered by an existing chemotherapeutic.
Introduction
The role of mast cells in allergic disease and asthma is well-established [1, 2]. Antigen binding to surface-bound IgE molecules on mast cells causes the release of preformed mediators that initiates an inflammatory response marked by vasodilatation, endothelial cell activation, recruitment of other immune cells, including eosinophils and lymphocytes, and release of neurogenic peptides that can cause itching and pain. Activation by antigen:IgE is best-characterized via signaling through the high-affinity IgER, FcεRI [3]. Chronic mast cell activation contributes to the hyper-responsiveness embodying the pathology of asthma [1].
Mast cells evolved to protect the host from infection by multicellular parasites. Although present in nearly every tissue, mast cells are most prevalent at interfaces with the environment, such as in the skin and gut epithelium. In the gut, mast cell proteases degrade tight junctions between epithelial cells, allowing fluid escape. Mast cells also secrete mediators, such as VEGF, MCP-1, MCP-2, MIP-1α, IL-4, IL-13, IL-1β, GM-CSF, G-CSF, IFN-γ, and TNF [1]. Whereas these inflammatory mediators are important for parasite expulsion, they are potentially damaging to host cells and tissues. Therefore, mast cell responses to parasites frequently result in a state of low-grade inflammation, allowing host and parasite to coexist in a form of homeostasis that minimizes damage to the host tissues. In fact, helminth infections are linked to the expansion of several populations of immune regulatory cells, causing an overall immunosuppressive state [4].
One of the immune regulatory populations expanded in helminth-infected individuals is MDSCs. Although MDSCs have been linked recently to T cell suppression in various models of parasitic infection, including Trypanosoma cruzi, Leishmania major, and Toxoplasmosis gondii [5, 6], they are best known for their role in tumor promotion. In tumor-bearing animals, MDSCs suppress CD4 and CD8 T, B, and NK cells [7, 8]. Much attention has been given to the diverse set of suppressive mechanisms attributed to MDSCs, including the secretion of NO, the depletion of L-arginine via arginase-1, production of oxygen-free radicals, and production of regulatory cytokines, such as TGF-β (reviewed in refs. [7, 9, 10].
Eliminating MDSCs has been an attractive therapeutic approach to cancer therapy that has shown promise. Gemcitabine is a deoxycytidine analog that incorporates into DNA after conversion to its active triphosphate form by deoxycytidine kinase. The diphosphate form of gemcitabine also targets the active site of ribonucleotide reductase, reducing DNA synthesis. Interestingly, reports have found that gemcitabine selectively eliminates MDSCs [11]. The lack of broad immunosuppression has made gemcitabine, which is already commonly used as a cancer chemotherapeutic, a preferred candidate for combination with immunotherapy [12, 13].
Whereas interactions between mast cells and MDSCs have been suggested recently in tumor models [14], the presence and importance of MDSCs have just begun to be recognized in inflammatory conditions, such as allergy and asthma [15–17]. We reported recently that mast cells augment MDSC immunosuppressive activities and that mast cells are required for MDSCs to enhance tumor metastasis [18]. Given the presence of mast cells and MDSCs in parasitic infection and AHR, mast cell-MDSC interplay is functionally relevant and could shed light on a novel role for MDSCs. In this report, we demonstrate that MDSCs enhance IgE-mediated mast cell cytokine production and are required for resistance to the helminthic parasites Nb and T. spiralis. Conversely, MDSC depletion reduced AHR in a chronic allergic model, whereas MDSCs transfer exacerbated AHR. MDSCs appear to be capable of not only suppressive but also stimulatory actions that alter the outcome of protective and pathological inflammatory responses.
MATERIALS AND METHODS
Animals
BALB/c, C57BL/6, and KitW-sh (hence, referred to as Wsh) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and were used at a minimum of 9 weeks old. A strain of ADAM10 transgenic mice, overexpressing ADAM10 at early stages of lympho- and myelopoiesis, through use of the H-2Kb promoter and IgH enhancer regulatory elements, has been described previously [19]. All studies were done with approval from the Virginia Commonwealth University Institutional Animal Use and Care Committee.
Mouse BMMC cultures
Mouse BMMCs were generated from mice through isolation and subsequent culture of bone marrow cells in cRPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA, USA), containing 10% FBS, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM HEPES (Quality Biological, Gaithersburg, MD, USA) and 1 mM sodium pyruvate (Cellgro; Mediatch, Manassas, VA, USA). Cultures were supplemented with IL-3-containing supernatant from WEHI-3 cells and SCF-containing supernatant from BHK-MKL cells. The final concentrations of IL-3 and SCF were adjusted to 1 ng/ml and 10 ng/ml for IL-3 and SCF, respectively. Mature BMMCs were used after 21 days of culture.
Generation and isolation of MDSCs
BALB/c mice were injected s.c. in the flank with 5 × 105 syngeneic 4T1 breast carcinoma cells. Tumors were allowed to grow for 14–17 days, after which, mice were killed, and spleens were harvested. RBCs were lysed, and white blood cells were blocked with anti-FcγRII/RIII (Clone 2.4G2) and labeled with antibodies against Gr1 and CD11b (FITC-Gr1, Clone RB6-8C5; PE/CY5-CD11b, Clone M1/70; BioLegend, San Diego, CA, USA). Cells coexpressing CD11b and Gr1 were sorted with doublette discrimination on a FACSAria II to >96% purity. For some experiments, Gr1+ cells were sorted from the spleens of tumor-bearing mice using the EasySep PE-Selection Kit from StemCell Technologies (Vancouver, BC, Canada), along with PE-Gr1 antibody (RB6-8C5), following the manufacturer's protocol. These Gr1+ cells coexpressed CD11b and yielded results similar to experiments using FACS-sorted cells.
BMMC coculture with MDSCs
BMMCs were washed and resuspended at 5 × 105 cell/ml in 10 ng/ml rIL-3 with 0.5 μg/ml anti-DNP mouse IgE (BD PharMingen, San Diego, CA, USA) for 24 h. BMMCs were washed twice and cultured at a 1:1 ratio with MDSCs in cRPMI with 10 ng/ml IL-3 and 10 ng/ml GM-CSF (PeproTech, Rocky Hill, NJ, USA) overnight. DNP-HSA was added to all wells at a concentration determined to be optimal for that lot of reagent (20–100 ng/ml). Supernatants were collected 6 h or 18 h after cross-linking and kept at −80°C until analysis by ELISA.
ELISA analysis
All cytokine ELISAs were obtained from PeproTech and were used per the manufacturer's instructions. Supernatants, collected after 6 h, were analyzed for TNF; supernatants collected after 18 h were analyzed for additional cytokines and chemokines. IgE ELISA measurements were performed as described previously [20].
Flow cytometric analysis
Splenocytes were homogenized and RBCs lysed using ammonium-chloride-potassium lysing buffer (Quality Biological). Nonspecific binding was blocked using 2.4G2, followed by staining with the following antibodies: FITC-CD4, PE-CD8, PE/Cy5 B220, or FITC-Gr1 and PE/Cy5-CD11b (BioLegend) at a final concentration of 10 μg/ml for FITC staining and 2 μg/ml for PE and PE/Cy5 staining. Cells were incubated in the dark for 30 min and washed twice in staining buffer (PBS/3% FBS/0.1% NaN3) for flow cytometric analysis. For analysis of intracellular cytokines, BMMCs and sorted MDSCs were cocultured overnight as above. Ninety minutes after addition of DNP-HSA, GolgiStop (BD Biosciences, San Jose, CA, USA) was added, and cells were cultured for an additional 5 h. Cells were harvested, surface FcRs were blocked with 2.4G2, and surface staining for PE-Gr1 (BioLegend) was performed for 30 min at 4°C in the dark in staining buffer. Cells were washed twice after surface staining and fixed with 4% PFA. Cells were permeabilized using staining buffer with 0.1% saponin. Allophycocyanin-conjugated rat anti-mouse TNF or IL-6 (BD PharMingen) was added at the manufacturer's recommended concentration prior to analysis on a BD FACSCalibur flow cytometer.
Nb infection and gemcitabine treatment
BALB/c mice were injected s.c. with 650 infective L3. On Days 4 and 8 after inoculation, mice were given i.p. injections of 1.2 mg gemcitabine or an equivalent volume (200 μl) of PBS. Feces were collected daily, starting on Day 5 and for the duration of the experiment for enumeration of eggs. Mice were killed on Day 11 after inoculation and the intestines removed for quantification of adult worms.
T. spiralis infection and gemcitabine treatment
BALB/c mice were inoculated orally with 150 muscle-stage T. spiralis L1 (a generous gift from Dr. D. Hill, U.S. Department of Agriculture/Agricultural Research Service, Beltsville, MD, USA) and then given i.p. injections of 1.2 mg gemcitabine or PBS on Days 1, 4, and 7 postinoculation. A group of mice was harvested on Day 9 to assess adult T. spiralis in the intestine. A second group continued to receive gemicitabine weekly, starting on Day 10 through Day 38, and was harvested on Day 41 to assess T spiralis L1 in muscle tissue. The protocol to assess T. spiralis infection has been described [21].
AHR model of established lung inflammation
Female BALB/c mice were sensitized with 10 μg OVA i.p. every other day for 13 days. Mice were challenged with i.n. OVA (200 μg) on Days 40, 43, and 46 and then given i.n. gemcitabine (1.2 mg) on Days 47, 50, 54, 57, 60, 63, 66, 69, 72, and 75. Mice were also challenged with OVA (200 μg i.n.) on days 66,69,72, and 75. Mice were anesthetized on Day 76 and cannulated for analysis of airway resistance in response to increasing methacholine concentration, as measured by Flexivent. Mice were killed after Flexivent analysis, and BAL and cardiac puncture was performed. Blood from cardiac puncture was centrifuged, and serum was stored at −80°C until used for analysis of IgE levels.
AHR model with MDSC transfer
Age-matched C57BL/6 and Wsh/Wsh mice received 50 mg OVA i.p. every 2 days for 6 days. Mice then received 1 × 107 MDSC by i.p. injection on Days 17, 20, 23, and 26, and i.n. challenge with OVA (200 μg) was performed on Days 21, 24, and 27. Methacholine challenge to measure AHR was performed on Day 28.
Statistics
Data shown in each figure are the mean and se of the indicated number of samples. For comparisons of two samples, Student's t-test was used.
RESULTS
MDSCs enhance IgE-mediated mast cell cytokine production
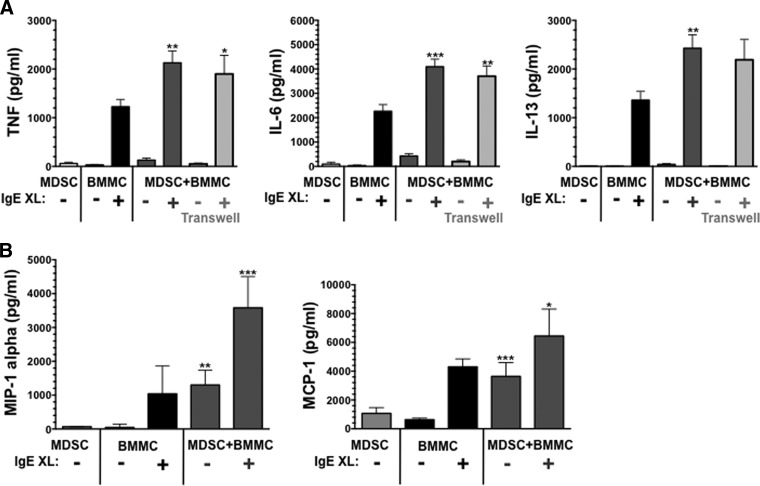
MDSCs and mast cells can promote the Th2 response, and both are elevated during tumor growth and parasite infection [6, 22–24]. We therefore determined the effect of mast cell-MDSC coculture on IgE-induced cytokine secretion. MDSCs sorted from the spleens of mice-bearing 4T1 breast carcinomas were cultured at a 1:1 ratio with IgE-sensitized BMMCs. After overnight coculture, DNP-HSA was added, and supernatants were collected. Consistent with our previous work using ADAM10 transgenic MDSCs [18], tumor-elicited MDSCs enhanced the production of TNF, IL-6, and IL-13 (Fig. 1A). Histamine secretion, assessed at 1-h postcrosslinkage, was not altered by coculture (not shown). We also noted a modest but consistent increase in cytokine production among cocultures that did not include IgE. The importance of cell contact was determined using a 0.4-μm membrane to separate the cell types. As shown in Fig. 1A, TNF and IL-6 production was still enhanced in the absence of cell contact, whereas IL-13 enhancement trended higher but did not reach statistical significance. In addition to cytokines, coculture enhanced IgE-mediated chemokine secretion, as noted for MIP-1α and MCP-1 (Fig. 1B).
Figure 1. Mast cell-MDSC coculture enhances IgE-induced cytokine production.
MDSCs isolated from the spleens of 4T1 tumor-bearing BALB/c mice were cultured at a 1:1 ratio with BALB/c BMMCs that were presensitized with IgE. After overnight culture, cells were activated by antigen-induced IgE crosslinkage (XL). Supernatants were collected after 6 hours (TNF) or 18 h and analyzed by ELISA. Data are mean and sem from three to 34 measurements, obtained from two to four independent experiments. In the indicated samples, MDSCs and BMMCs were separated by 0.4 μm membranes (Transwells). *P < 0.05; **P < 0.01; ***P < 0.0001, when comparing the indicated samples with BMMCs or MDSCs alone.
These data demonstrated that the presence of MDSCs enhances IgE-mediated cytokine and chemokine secretion but did not reveal the cell lineage responsible for the enhanced production. To ascertain this, we performed similar cocultures, and examined the cells by flow cytometry for intracellular cytokine levels. As shown in Fig. 2, a small percentage of MDSCs produced TNF and IL-6. This fraction was unchanged when IgE-activated mast cells were added (Fig. 2A). By contrast, BMMCs demonstrated a consistent and statistically significant increase in the percentage of cytokine-positive cells when cocultured with MDSCs. The average per-cell fluorescence did not change (not shown). These data supported the theory that mast cell-MDSC coculture enhances IgE-induced cytokine production by selectively increasing the fraction of IgE-responding mast cells, without altering cytokine production among MDSCs.
Figure 2. Enhanced cytokine production in mast cell-MDSC cocultures is restricted to mast cells.

MDSCs derived from the spleens of 4T1 tumor-bearing BALB/c mice were cocultured at a 1:1 ratio with IgE-presensitized BALB/c BMMCs. After overnight coculture, BMMCs were activated by IgE crosslinkage, and cells were assessed for intracellular IL-6 or TNF content by flow cytometry, as described in Materials and Methods. (A) Representative flow cytometry analyses after gating on Gr1+ MDSCs or Gr1− mast cells (MC). (B) Summary of percent-positive cells for each population. Data are averages of five to six samples/point. FL4/2-H, Fluorescence 4/2-height.
MDSCs are categorized frequently by their expression of two surface markers, Ly6C and Ly6G [25]. Whereas all of the tumor-elicited MDSCs expressed Ly6C, only a subset expressed Ly6G. We isolated tumor-elicited MDSCs based on this Ly6C+ or Ly6C/G+ phenotype. These cells were then placed into cocultures with BMMCs. As shown in Fig. 3, both populations significantly increased IgE-mediated IL-6 secretion in cocultures. Ly6C+ MDSCs also showed significantly increased MIP-1α in cocultures. Unlike their Ly6C+ counterpart, Ly6C/G+ cells produced MIP-1α. In this case, the effects of mast cell coculture on MIP-1α production appeared to be additive. These data suggest that both of these MDSC subsets possess mast cell-stimulating capacity.
Figure 3. The ability to enhance mast cell cytokine production is not restricted by Ly6C/G subsets.
Splenocytes from 4T1 tumor-bearing BALB/c mice were sorted based on Ly6C+ or Ly6C/G+ expression patterns. These cells were cocultured as described in Fig. 1. Data shown are mean and sem of six samples from one of two experiments that gave similar results. ND, Not detectable within the limits of this assay. *P < 0.05; **P < 0.01; ***P < 0.0001.
MDSCs are required for effective clearance of Nb and T. spiralis
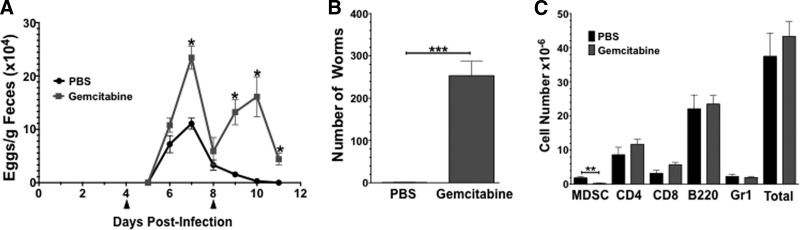
To determine the functional in vivo relevance of mast cell-MDSC interactions, we used the intestinal nematode Nb, as mice lacking mast cells have delayed Nb clearance [26]. The FDA-approved chemotherapeutic agent gemcitabine, which selectively ablates MDSCs [27, 28], was used to deplete MDSCs during Nb infection. BALB/c mice were inoculated with infective L3 larvae and given two doses (1.2 mg each in a 200-μl vol) of gemcitabine via i.p. injection, 4 and 8 days postinfection. Fecal egg counts were used to assess adult worm fecundity (Fig. 4A). Gemcitabine injection on Day 4 increased parasite egg production significantly on days 6 and 7. When egg production in this group began to decline, a second gemcitabine injection on Day 8 was associated with a rebound in egg numbers. The manufacturer's stated plasma half-life of gemcitabine is ∼60 min; hence, these biphasic data are likely be a result of a drop in circulating gemcitabine levels below an effective threshold.
Figure 4. MDSC depletion exacerbates Nb infection.
(A) BALB/c mice were inoculated with 650 infective Nb larvae and were subsequently treated with PBS or gemcitabine (1.2 mg i.p. injection; 200 μl vol) on Days 4 and 8 (indicated by arrowheads). Feces were collected daily and weighed and eggs counted and normalized/gram of feces. (B) Number of adult worms in the intestine on Day 11 postinoculation. (C) Flow cytometry of splenocytes on Day 11 for indicated surface markers. All data are mean and sem for five to six mice/group. *P < 0.05; **P < 0.01; ***P < 0.0001.
On Day 11, when egg counts in the PBS-treated mice reached zero, the mice were killed, and the number of adult worms in the intestine was counted. Mice treated with gemcitabine had ∼250 adult worms remaining in the intestine, whereas the PBS-treated group had a negligible number (average of 0.33 worms/mouse, n=6; Fig. 4B). Flow cytometry for splenic T cells (CD4+ and CD8+), B cells (B220), and neutrophils (Gr1+) showed no significant decreases in any of these cell types, concurrent with no significant change in the total number of splenocytes in the two groups. As expected, the number of CD11b+Gr1+ MDSCs was decreased significantly in the group treated with gemcitabine compared with the PBS-treated group (P=0.006; Fig. 4C).
As gemcitabine-mediated suppression of mast cell activity would also delay Nb clearance from the intestine, we examined its effects on the mast cell lineage in more detail. As shown in Supplemental Fig. 1, mast cell numbers in the mesenteric LN, spleen, and peritoneum were unaffected by gemcitabine treatment during Nb infection. Furthermore, the same timing of gemcitabine injections in naive mice (lacking MDSCs) did not alter subsequent IgE-induced passive systemic anaphylaxis, as measured by a drop in body temperature and plasma cytokine levels (Supplemental Fig. 1B and C). Peritoneal mast cell numbers were also unaffected (Supplemental Fig. 1D). These data demonstrate that gemcitabine has little impact on mast cell survival or function and supports the hypothesis that gemcitabine ablates MDSCs selectively, resulting in a reduced mast cell response to Nb infection.
To confirm independently the importance of mast cell-MDSC interactions during parasite clearance, we next tested the effects of gemcitabine on mast cell-dependent clearance of T. spiralis infection. Whereas Nb can be expelled in the absence of mast cells, the kinetics of adult T. spiralis expulsion from the intestines are mast cell-dependent [29]. BALB/c mice were inoculated with T. spiralis s.c. and then given gemcitabine or PBS on Days 1, 4, and 7 postinfection. A group of mice was harvested on Day 9 to assess adult T. spiralis in the intestine (Fig. 5A). A second group continued to receive gemicitabine weekly, starting on Day 10–38, and was harvested on Day 40 to assess T. spiralis larvae in muscle tissue (Fig. 5B). Gemcitabine treatment during T. spiralis infection greatly increased the number of intestinal adult worms, 9 days after inoculation of infective larvae, and subsequent development in the muscle of larvae derived from adult worms at 41 days postinoculation. Collectively, these data support the hypothesis that MDSCs are required for a competent mast cell response in vivo.
Figure 5. MDSC depletion exacerbates T. spiralis infection.
(A) BALB/c mice were inoculated with T. spiralis muscle stage-infective larvae and then treated with gemcitabine (1.2 mg i.p. injection; 200 μl vol) or PBS on Days 1, 4, and 7 postinoculation before death on Day 9. Adult worms were counted in the intestine. (B) A second set of mice was treated as described in A, with gemcitabine injections continuing every 7 days, from Days 10 through 38 until T. spiralis larvae in muscle tissues were assessed on Day 41. Data shown represent the mean and sem from five mice. *P < 0.05; ***P < 0.0001.
MDSCs contribute to the chronic inflammatory environment of established asthma
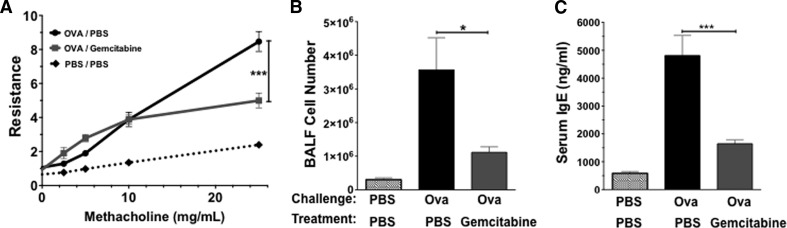
In addition to their protective role during parasite infection, mast cells are also known for eliciting destructive chronic inflammatory responses, such as atopic asthma. MDSCs have been identified recently in the lungs of mice during AHR. These studies identified suppressive or activating roles for MDSCs, depending on the MDSC subset [15–17]. Importantly, these models used an AHR assay that is not mast cell-dependent as a result of the inclusion of alum during immunization [30]. To test the importance of mast cell-MDSC interactions during mast cell-dependent chronic lung inflammation, mice were sensitized and challenged with OVA alone for 42 days to induce AHR and inflammation. Mice were then treated i.n. with PBS or gemcitabine to deplete airway MDSC prior to rechallenge with antigen. On Day 76, mice were killed, and airway resistance to methacholine challenge was measured. We found that gemcitabine treatment reduced airway resistance significantly compared with PBS (Fig. 6A). In addition, there was a significant decrease in the cellular infiltrate recovered in the BAL fluid of mice treated with gemcitabine compared with PBS (Fig. 6B). Mice treated with gemcitabine and therefore, depleted of MDSC, also exhibited significantly decreased serum IgE levels (Fig. 6C).
Figure 6. MDSC depletion reduces AHR.
BALB/c mice were sensitized and challenged with OVA, as described in Materials and Methods. After establishing inflammation with OVA challenge, i.n. gemcitabine treatment was given every 3 days from Days 47 through 75. OVA restimulation was performed on Days 66 through 75, with AHR and inflammation assessed on Day 76. (A) Airway resistance to methacholine challenge, as measured by Flexivent. (B) Trypan blue cell counts of cellular infiltrate recovered by BAL fluid (BALF). (C) Quantification of IgE levels in the serum by ELISA. Data shown represent the mean and sem from four to six mice/group. *P < 0.05; ***P < 0.0001.
To confirm the importance of mast cell-MDSC interactions in the AHR model, we increased MDSC numbers by adoptive cell transfer during the allergen challenge phase of the assay, using mice that possess or lack mast cells. As shown in Fig. 7, adoptively transferring MDSCs, starting 4 days prior to i.n. challenge and continuing every 3 days throughout the challenge period, exacerbated lung resistance in WT C57BL/6 mice. By comparison, Wsh mice did not exhibit AHR when compared with WT mice, as expected from this mast cell-dependent model, and MDSC transfer had no impact on AHR. Therefore, the inflammatory effects of MDSCs in the AHR model require the presence of mast cells.
Figure 7. MDSC transfer exacerbates AHR and requires mast cells.
C57BL/6 or Wsh/Wsh mice were sensitized and challenged with OVA, as described in Materials and Methods. MDSCs (1×107/mouse) were injected i.p. every 3 days from Days 18 through 27. Mice were challenged with OVA on Days 22–27. AHR was assessed on Day 28. Data shown represent the mean and sem from three to five mice/group. ΔRL, change in lung resistance.
DISCUSSION
Although mast cells and MDSCs have been shown to accumulate in tumor microenvironments or during parasitic infections, interactions between these cells are poorly understood [6, 22–24]. Yang and coworkers [14] showed that mast cells can recruit MDSCs to the tumor microenvironment and enhance MDSC IL-17 secretion. This finding encouraged our study of these interactions. We found recently that mast cells are required for a MDSC-mediated increase in tumor growth and metastasis and that increased MDSC numbers enhance resistance to Nb. In the current report, we show that mast cell-MDSC interactions are bidirectional, as MDSCs enhance mast cell-mediated inflammatory responses.
The diverse roles of MDSCs are becoming more evident, as exhibited by the effects of MDSC depletion in the disease models presented here. In two parasitic nematode models, using infection with Nb and T. spiralis, MDSC loss exacerbated infection, suggesting an evolutionary function of MDSC for controlling helminths. These findings are complemented by our previous study showing that MDSC transfer increased resistance to Nb in WT but not Wsh mice [18]. Importantly, we found that gemcitabine therapy did not alter mast cell numbers in multiple tissues and did not block the mast cell response in a systemic anaphylactic shock model. Thus, the effects of gemcitabine are unlikely a result of mast cell depletion or suppression. Our in vitro data showed that mast cell-MDSC interactions enhanced IL-13 secretion, which is critical for Nb clearance [31]. These data suggest that some of the antiparasitic effects mediated by mast cells are a result of interactions with MDSCs. This represents an unexplored area of immune regulation and confirms that MDSCs are not solely suppressive.
Unlike acute Nb infection, our established AHR model represents a role for mast cell-MDSC interactions in chronic inflammation. This model elicits a mast cell-dependent pulmonary, inflammatory response that was fully engaged prior to depleting MDSCs with gemcitabine, and gemcitabine therapy clearly reduced inflammation and improved pulmonary function. The in vitro data showed that MDSCs enhance mast cell-mediated secretion of several inflammatory cytokines, including TNF, IL-6, IL-13, MIP-1α, and MCP-1. Each of these can contribute to immunopathology. For example, TNF has been shown to be a critical player in the pathology of asthma, and anti-TNF therapy has been beneficial in some severely asthmatic patients [32]. TNF may contribute to MDSC recruitment, given that granulocytic MDSCs share characteristics with neutrophils, which migrate toward a TNF gradient [33]. Thus, the sustained contact between MDSCs and mast cells could result in exacerbated inflammation and AHR.
Distinct MDSC populations and our use of a mast cell-dependent AHR model may explain differences between our work and a recent report. Deshane and coworkers found that intratracheal transfer of a Ly6C+ MDSC-like myeloid population improved AHR measurements [17]. Their data are in keeping with the known suppressive capacity of MDSCs. However, this paper also found that Ly6C−/G+ MDSCs exacerbated AHR [17], which agrees with our observation that MDSC depletion improved AHR. Our model also differed in that we did not use the adjuvant alum, which enhances the Th2 response and allows for mast cell-independent AHR and eosinophil recruitment [30]. The effects of alum on MDSCs are not known, although these cells are activated through TLRs [15–17].
Further support for the importance of mast cells in interpreting our model is the demonstration that MDSCs did not elicit AHR when injected into the c-kit mutant, mast cell-deficient Wsh mice. Therefore, the effect of MDSCs in asthma may vary with the requirement for mast cells and with the antigens presented. These data are particularly interesting in light of the recent demonstration that MDSCs are increased in Wsh mice, owing to extramedullary hematopoiesis [34]. It appears that MDSCs are able to enhance the mast cell response but are not sufficient to elicit AHR, even when present in increased numbers. The effect of MDSCs on mast cell responses needs to be taken into account when interpreting mast cell reconstitution of Wsh mice, a common means of determining a role for mast cells in vivo. Our data suggest that a simple means of determining the impact of MDSCs in these assays is gemcitabine therapy. Mast cells and MDSCs appear to be linked by c-Kit signaling. Many tumors secrete SCF, and blocking SCF/c-Kit interactions has been shown to reduce MDSC recruitment to tumors [35, 36]. As MDSCs have not been found to express c-Kit, one explanation for the effect of c-Kit blockade is mast cell suppression. These data indicate a dynamic interaction between these two cell types that contributes to inflammatory responses.
There remains much to uncover in understanding mast cell-MDSC interactions. Our previous study showed that Ly6C/G+ MDSCs selectively enhanced resistance to Nb, whereas we find that Ly6C+ and Ly6C/G+ MDSCs enhance mast cell cytokine production. The reason for these differences is not clear but could be related to selective homing of these subsets to an inflammatory environment. We also find that much of the mast cell-enhancing activity provided by MDSCs is from a soluble factor, as physically separating the cells by a semipermeable membrane did not reduce production of most cytokines tested. We have yet to identify the factor(s) involved. IL-6, TNF, MIP-1α, or platelet-activating factor did not replicate MDSC effects on IgE-mediated cytokine production (data not shown). Collectively, our data support the hypothesis that mast cell-MDSC interactions are bidirectional, enhancing the activities of each cell type in ways that can be protective or pathological, depending on the eliciting stimulus. We demonstrate the efficacy of the FDA-approved chemotherapeutic gemcitabine in suppressing mast cell-dependent inflammation, apparently by diminishing MDSCs. These clinically relevant findings support further studies of how this cellular interaction regulates immune homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
Support for this work was provided by grants from the U.S. National Institutes of Health (1R01AI59638 to J.J.R. and U19AI077435 to J.J.R. and D.H.C.).
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ADAM10
- a disintegrin and metalloproteinase 10
- AHR
- airway hyper-responsiveness
- cRPMI
- complete RPMI
- FDA
- U.S. Food and Drug Administration
- HSA
- human serum albumin
- i.n.
- intranasal
- L
- larval stage
- Ly6C+
- Ly6C+/Ly6G−
- Ly6C/G+
- Ly6C+/Ly6G+
- MDSC
- myeloid-derived suppressor cell
- Nb
- Nippostrongylus brasiliensis
- RBC
- red blood cell
- SCF
- stem cell factor
AUTHORSHIP
J.K.M., S.J.S., R.K.M., B.L.S., B.O.B., T.W.F., N.A.P.,E.M.K., K.B.B., S.K.N., J.S., L.G., J.F.U., C.S.L., and J.J.R. conducted experiments. J.K.M., H.D.B., J.F.U., C.S.L., D.H.C., and J.J.R. participated in experimental design. J.K.M. and J.J.R. wrote the manuscript.
REFERENCES
- 1. Galli S. J., Tsai M. (2012) IgE and mast cells in allergic disease. Nat. Med. 18, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalesnikoff J., Galli S. J. (2010) Anaphylaxis: mechanisms of mast cell activation. Chem. Immunol. Allergy 95, 45–66 [DOI] [PubMed] [Google Scholar]
- 3. Gilfillan A. M., Rivera J. (2009) The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 228, 149–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor M. D., van der Werf N., Maizels R. M. (2012) T cells in helminth infection: the regulators and the regulated. Trends Immunol. 33, 181–189 [DOI] [PubMed] [Google Scholar]
- 5. Cuervo H., Guerrero N. A., Carbajosa S., Beschin A., De Baetselier P., Girones N., Fresno M. (2011) Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J. Immunol. 187, 2656–2665 [DOI] [PubMed] [Google Scholar]
- 6. Van Ginderachter J. A., Beschin A., De Baetselier P., Raes G. (2010) Myeloid-derived suppressor cells in parasitic infections. Eur. J. Immunol. 40, 2976–2985 [DOI] [PubMed] [Google Scholar]
- 7. Solito S., Pinton L., Damuzzo V., Mandruzzato S. (2012) Highlights on molecular mechanisms of MDSC-mediated immune suppression: paving the way for new working hypotheses. Immunol. Invest. 41, 722–737 [DOI] [PubMed] [Google Scholar]
- 8. Lindau D., Gielen P., Kroesen M., Wesseling P., Adema G. J. (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138, 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raber P., Ochoa A. C., Rodriguez P. C. (2012) Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol. Invest. 41, 614–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ostrand-Rosenberg S. (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 59, 1593–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waldron T. J., Quatromoni J. G., Karakasheva T. A., Singhal S., Rustgi A. K. (2013) Myeloid derived suppressor cells: targets for therapy. Oncoimmunology 2, e24117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hensley M. L. (2010) Update on gemcitabine and docetaxel combination therapy for primary and metastatic sarcomas. Curr. Opin. Oncol. 22, 356–361 [DOI] [PubMed] [Google Scholar]
- 13. Sun C., Ansari D., Andersson R., Wu D. Q. (2012) Does gemcitabine-based combination therapy improve the prognosis of unresectable pancreatic cancer? World J. Gastroenterol. 18, 4944–4958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Z., Zhang B., Li D., Lv M., Huang C., Shen G. X., Huang B. (2010) Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS One 5, e8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arora M., Poe S. L., Oriss T. B., Krishnamoorthy N., Yarlagadda M., Wenzel S. E., Billiar T. R., Ray A., Ray P. (2010) TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol. 3, 578–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arora M., Poe S. L., Ray A., Ray P. (2011) LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation. Int. Immunopharmacol. 11, 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deshane J., Zmijewski J. W., Luther R., Gaggar A., Deshane R., Lai J. F., Xu X., Spell M., Estell K., Weaver C. T., Abraham E., Schwiebert L. M., Chaplin D. D. (2011) Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol. 4, 503–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saleem S. J., Martin R. K., Morales J. K., Sturgill J. L., Gibb D. R., Graham L., Bear H. D., Manjili M. H., Ryan J. J., Conrad D. H. (2012) Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J. Immunol. 189, 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gibb D. R., Saleem S. J., Kang D. J., Subler M. A., Conrad D. H. (2011) ADAM10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of Notch. J. Immunol. 186, 4244–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford J. W., Sturgill J. L., Conrad D. H. (2009) 129/SvJ Mice have mutated CD23 and hyper IgE. Cell. Immunol. 254, 124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Urban J. F., Jr., Schopf L., Morris S. C., Orekhova T., Madden K. B., Betts C. J., Gamble H. R., Byrd C., Donaldson D., Else K., Finkelman F. D. (2000) Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J. Immunol. 164, 2046–2052 [DOI] [PubMed] [Google Scholar]
- 22. Abraham S. N., St John A. L. (2010) Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 10, 440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribatti D. (2013) Mast cells and macrophages exert beneficial and detrimental effects on tumor progression and angiogenesis. Immunol. Lett. 152, 83–88 [DOI] [PubMed] [Google Scholar]
- 24. Ostrand-Rosenberg S., Sinha P. (2009) Myeloid-derived suppressor cells: linking inflammation and cancer J. Immunol. 182, 4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Youn J. I., Nagaraj S., Collazo M., Gabrilovich D. I. (2008) Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crowle P. K., Reed N. D. (1981) Rejection of the intestinal parasite Nippostrongylus brasiliensis by mast cell-deficient W/Wv anemic mice. Infect. Immun. 33, 54–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Le H. K., Graham L., Cha E., Morales J. K., Manjili M. H., Bear H. D. (2009) Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int. Immunopharmacol. 9, 900–909 [DOI] [PubMed] [Google Scholar]
- 28. Sinha P., Clements V. K., Bunt S. K., Albelda S. M., Ostrand-Rosenberg S. (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 [DOI] [PubMed] [Google Scholar]
- 29. Ha T. Y., Reed N. D., Crowle P. K. (1983) Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect. Immun. 41, 445–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams C. M., Galli S. J. (2000) Mast cells can amplify airway reactivity and features of chronic inflammation in an asthma model in mice. J. Exp. Med. 192, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Urban J. F., Jr., Noben-Trauth N., Donaldson D. D., Madden K. B., Morris S. C., Collins M., Finkelman F. D. (1998) IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8, 255–264 [DOI] [PubMed] [Google Scholar]
- 32. Desai D., Brightling C. (2010) TNF-α antagonism in severe asthma? Recent Pat. Inflamm. Allergy Drug Discov. 4, 193–200 [DOI] [PubMed] [Google Scholar]
- 33. Smart S. J., Casale T. B. (1994) TNF-α-induced transendothelial neutrophil migration is IL-8 dependent. Am. J. Physiol. 266, L238–L245 [DOI] [PubMed] [Google Scholar]
- 34. Michel A., Schuler A., Friedrich P., Doner F., Bopp T., Radsak M., Hoffmann M., Relle M., Distler U., Kuharev J., Tenzer S., Feyerabend T. B., Rodewald H. R., Schild H., Schmitt E., Becker M., Stassen M. (2013) Mast cell-deficient Kit(W-sh) “Sash” mutant mice display aberrant myelopoiesis leading to the accumulation of splenocytes that act as myeloid-derived suppressor cells. J. Immunol. 190, 5534–5544 [DOI] [PubMed] [Google Scholar]
- 35. Pan P. Y., Wang G. X., Yin B., Ozao J., Ku T., Divino C. M., Chen S. H. (2008) Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111, 219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kao J., Ko E. C., Eisenstein S., Sikora A. G., Fu S., Chen S. H. (2011) Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit. Rev. Oncol. Hematol. 77, 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.