Abstract
Bothrops mattogrossensis snake is widely distributed throughout eastern South America and is responsible for snakebites in this region. This paper reports the purification and biochemical characterization of three new phospholipases A2 (PLA2s), one of which is presumably an enzymatically active Asp49 and two are very likely enzymatically inactive Lys49 PLA2 homologues. The purification was obtained after two chromatographic steps on ion exchange and reverse phase column. The 2D SDS-PAGE analysis revealed that the proteins have pI values around 10, are each made of a single chain, and have molecular masses near 13 kDa, which was confirmed by MALDI-TOF mass spectrometry. The N-terminal similarity analysis of the sequences showed that the proteins are highly homologous with other Lys49 and Asp49 PLA2s from Bothrops species. The PLA2s isolated were named BmatTX-I (Lys49 PLA2-like), BmatTX-II (Lys49 PLA2-like), and BmatTX-III (Asp49 PLA2). The PLA2s induced cytokine release from mouse neutrophils and showed cytotoxicity towards JURKAT (leukemia T) and SK-BR-3 (breast adenocarcinoma) cell lines and promastigote forms of Leishmania amazonensis. The structural and functional elucidation of snake venoms components may contribute to a better understanding of the mechanism of action of these proteins during envenomation and their potential pharmacological and therapeutic applications.
1. Introduction
Snake venoms contain a complex mixture of components with a wide range of biological and pharmacological activities. More than 90% of their dry weight is composed of proteins, including a variety of enzymes, such as phospholipases A2, proteases (metallo and serine), L-amino acid oxidases, esterases, as well as many other nonenzymatic proteins and peptides [1–3]. These proteins and peptides can be grouped into a small number of superfamilies based on remarkable similarities in their primary, secondary, and tertiary structures, while showing distinct pharmacologic effects [3].
One important protein superfamily present in all snake venoms is phospholipase A2 (PLA2, E.C. 3.1.1.4). PLA2s are a class of heat-stable and highly homologous enzymes, which catalyze the hydrolysis of the 2-acyl bond of cell membrane phospholipids releasing free fatty acids such as arachidonic acid and lysophospholipids. PLA2s have been characterized as the major component of snake venoms, being responsible for several pathophysiological effects caused by snake envenomation, such as neurotoxic, cardiotoxic, myotoxic, cytotoxic, hypotensive, and anticoagulant activities triggering an intense inflammatory reaction with the release of cytotoxins and eicosanoids [4–6]. PLA2's involvement in a variety of inflammatory diseases and accidents caused by venomous animals has raised medical and scientific interest in this enzyme [7, 8].
Myotoxic PLA2s from the Bothrops species are composed of approximately 110 to 135 amino acid residues and can be divided into two groups: “classical”, which contain an aspartate residue at position 49 (Asp49) and catalyze ester bond hydrolysis at the glycerophospholipid sn-2 position in a Ca2+-dependent manner; and “variant”, which contain a lysine residue at the same position (Lys49). This substitution affects the ability of these proteins to bind to Ca2+, which is an essential cofactor for catalysis, leading to decreased or no catalytic activity [1–5, 7, 9, 10].
The Bothrops mattogrossensis snake belongs to the Bothrops neuwiedi complex [11]. This snake is found in the eastern region of South America including Bolivia, Brazil, Southeast Peru, Paraguay, Uruguay, and Argentina [12]. The present study describes for the first time the isolation, identification, and functional characterization of three myotoxic phospholipases A2, named: BmatTX-I (Lys49 PLA2-like), BmatTX-II (Lys49 PLA2-like), and BmatTX-III (Asp49 PLA2) and evaluates their activity against Leishmania and tumor cells.
2. Materials and Methods
2.1. Venom
The venom from the Bothrops mattogrossensis snake was acquired from Serpentário de Proteínas Bioativas, Batatais-SP, Brazil. This study was authorized by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis—IBAMA, Instituto Chico Mendes de Conservação da Biodiversidade—ICMBio (number: 27131-1) and Conselho de Gestão do Patrimônio Genético—CGEN/Brazil (number 010627/2011-1).
2.2. Animals
Swiss male mice (18–20 g) were used. These animals were housed in temperature-controlled rooms and received water and food ad libitum until used. Animal care was in accordance with the guidelines of the Brazilian College for Animal Experimentation (COBEA) and was approved by the Committee of Ethics on Animal Utilization in Research from the Institute of Research of Tropical Pathologies (IPEPATRO/FIOCRUZ-Rondônia) (protocol number 2012/1).
2.3. Bothrops mattogrossensis Venom Fractioning
Around 250 mg of B. mattogrossensis venom was eluted in 1.2 mL of sterile deionized water and centrifuged at 1,530 ×g for 10 minutes and then submitted to cation exchange chromatography in an Akta purifier 10 (GE-Healthcare) using a CM-Sepharose column (27 × 300 mm, GE Healthcare), equilibrated with 50 mM ammonium bicarbonate buffer, pH 8.0, and eluted with a linear gradient of 0–100% 500 mM ammonium bicarbonate, pH 8.0 at a flow rate of 5.0 mL/minute. The fractions were monitored at an absorbance of 280 nm, collected manually, identified, lyophilized, and stored at −20°C. All fractions were submitted to SDS-PAGE and enzymatic activity analyses. The isolation of the three PLA2s was obtained by liquid chromatography using a Discovery C18 column (25 × 4.6 mm, Supelco) equilibrated with deionized water with 0.1% trifluoroacetic acid (v/v) and eluted with ten volumes of a linear gradient of 0–100% acetonitrile with 0.1% trifluoroacetic acid (v/v) at a flow rate of 1.0 mL/minute. The elution was monitored at an absorbance of 280 nm, manually collected, lyophilized, and stored at −20°C. In order to determine protein concentration, the Bradford method (Bio-Rad) was used with bovine serum albumin (BSA) as a standard [14].
2.4. Monodimensional Electrophoresis (SDS-PAGE)
Polyacrylamide gel electrophoresis (PAGE) in the presence of sodium dodecyl sulfate (SDS) was conducted using the method described by Laemmli [15]. The electrophoresis was carried out at 15 mA and 5 W using a conventional molecular weight standard (BioLabs) and a Lys49-PLA2 homologue isolated from B. jararacussu snake venom (BthTX-I) as markers. The gel was stained with Coomassie Brilliant Blue (CBB) G-250 and images of the gel were obtained using Image Scanner (GE Healthcare).
2.5. Bidimensional Electrophoresis (2D-SDS-PAGE) and pI Determination
The protein fractions were submitted to electrofocusing in 13 cm strips with pHs ranging from 3 to 10 in a nonlinear form, using an Ettan IPGphor 3 IEF System (GE Healthcare). At the end of the electrofocusing, the strips containing the PLA2s were equilibrated and transferred to a 12.5% polyacrylamide gel where they were separated according to molecular mass. The electrophoretic run was performed at 25 mA and 100 W during 5.5 hours and at the end of the protein separation the gel was stained with Coomassie Brilliant Blue (CBB) G-250 and images of the gel were captured using Image Scanner (Amershan Bioscience).
2.6. Mass Spectrometry (MALDI-TOF)
The samples (1 μL) were mixed with a matrix solution composed of sinapinic acid and acetonitrile with 0.1% trifluoroacetic solubilized at a 1 : 1 ratio. The average mass of the protein was obtained in a MALDI-TOF system (Shimatzu Biotech), operated in linear mode using insulin (5,734.5 Da), cytochrome C (12,361.9 Da), apomyoglobin (16,952.2 Da), aldolase (39,212.2 Da), and albumin (66,430.0 Da) as calibrators. The mass spectra obtained were submitted to automatic baseline subtraction.
2.7. N-Terminal Sequencing and Similarity Search
The amino terminal sequence of each isolated PLA2 previously determined by automated Edman degradation was used to search for sequence similarity. Approximately 50 μg of each isolated PLA2 corresponding to approximately 2 nmols/mL sample was submitted for N-terminal amino acid sequencing using the automated Edman degradation method [13] on a PPSQ-33A (Shimadzu) automatic sequencer. A sequence similarity search and multiple sequence alignment were performed in the SWISS-PROT/TREMBL database using the programs FASTA, BLAST, and CLUSTALW2.
2.8. Functional Characterization
2.8.1. Phospholipase Activity with 4-Nitro-3(octanoyloxy)benzoic (4N3OBA)
Phospholipase activity of the three PLA2s was assayed following the protocols described by Holzer and Mackessy [16], modified for 96-well plates. The standard assay mixture contained 200 μL of buffer (10 mM Tris-HCl plus 10 mM CaCl2, and 100 mM NaCl, pH 8.0), 20 μL of 4-nitro-3(octanoyloxy)benzoic (4N3OBA – Biomol, EUA), 20 μL of deionized water, and 20 μL of PLA2 (5 μg). After the addition of the PLA2s, the mixture was incubated for 30 minutes at 37°C and the absorbance was determined at 425 nm using an Eon (Biotek) microplate spectrophotometer, for 3-minute intervals. The enzymatic activity was expressed as the reaction's initial velocity (V o) calculated based on the increase in absorbance.
2.8.2. Phospholipase Activity with Fluorescent Substrate
Phospholipase A2's (PLA2) enzymatic activity was evaluated through the hydrolysis of synthetic fluorescent phospholipid, using the fluorescent substrate Acyl 6 : 0 NBD phospholipid, NBD-phosphatidylcholine (NBD-PC) (Avanti Polar Lipids Inc., Alabaster, AL, USA). The assay was performed in a spectrofluorometer (Shimadzu, RF-5301PC, software RFPC) with excitation and emission wavelengths of 460 and 534, respectively. The enzymatic activity of each B. mattogrossensis chromatographic fraction (7, 12, 15, 16, 17, and 20) was evaluated over 250 seconds, after the addition of substrate (3.3 μg/mL, final concentration) in a reaction media containing 50 mM Tris-HCl and 8 mM CaCl2 at pH 7.5 at room temperature.
2.8.3. Platelet Aggregation
A platelet aggregation assay was carried out according to the process described by Fuly et al. [17], with modifications. Platelet aggregation was monitored in an Aggregometer (Chrono Log model 490 2D, Havertown, USA) using Platelet-Rich-Plasma (PRP). PRP was obtained from human whole blood of health volunteers (CAAE: 14204413.5.0000.0011). BmatTX-III (rechromatography fraction 15) was incubated with PRP for five minutes at 37°C being stirred constantly, and then, platelet aggregation was triggered by the addition of ADP (15 μM) or collagen (16 μg/mL). Assays were performed at 37°C in siliconized glass cuvettes in a final volume of 300 µL. Control experiments were performed by adding agonists in the absence of peak 15. One hundred percent (100%) platelet aggregation was obtained with a supramaximal concentration of each agonist and determined 6 minutes after the addition of each, while PRP's light transmittance showed 0% aggregation.
2.8.4. Hemorrhage Activity
Mice were injected intradermally in the dorsal region with 50 μg of crude venom dissolved in 50 μL of physiological solution [18]. Controls received 50 μL of physiological solution in identical conditions. After 3 hours, the animals were euthanized through cervical dislocation and the skin was removed. The hemorrhagicactivitywas expressed as size of thehemorrhagic area on the inner surface measured in mm2.
2.8.5. Coagulation Activity and Minimal Coagulation Dose (MCD) Determination
Coagulation activity was tested using a methodology previously described by Gené et al. [19]. In brief, 200 μL of plasma from mice was distributed in a 96-well plate, and 10 μL of crude venom containing different concentrations (0.312, 0.625, 1.25, 2.5, 5, and 10 μg) of proteins was added. The plate was placed in a thermostatically controlled environment and the optical density was measured every 3 seconds at 600 nm using an Eon microplate spectrophotometer (Biotek) in order to evaluate the smallest concentration of venom able to coagulate 200 μL of plasma/minute.
2.8.6. Proteolytic Activity
Proteolytic activitywas ascertained using azocasein (Sigma) as a substrate according to the procedure described by Charney and Tomarelli [20]. Azocasein solubilized in distilled water (150 μL) was added to 7 μL of the crude venom in different protein concentrations (0.312, 0.625, 1.25, 2.5, 5, and 10 μg) and then the mixture was incubated in a water bath at 37°C for 1 hour. The reaction stopped when 150 μL of 20% (m/v) trichloroacetic acid was added, which was followed by incubation for 30 min and centrifugation at 10,000 ×g for 10 more minutes. Then, 100 μL of the supernatant was transferred to a multiple-well plate and the absorbance was measured at 440 nm using an Eon microplate spectrometer (Biotek) and one enzymatic unit (U) was defined as the amount of enzyme necessary to increase the absorbance by 0.05.
2.8.7. Myotoxic Activity
Myotoxic activity was determined by measuring the creatine kinase (CK) activity in the plasma [21]. Groups of mice were injected in the gastrocnemius muscle with 25 μg of isolated myotoxins diluted in 50 μL of phosphate buffered saline (PBS). Negative controls received an injection of 50 μL of PBS. Three hours after the injections, aliquots of mice blood were collected from the caudal vein, in heparinized capillaries and centrifuged at 1,530 ×g for 20 minutes. Creatine kinase's enzymatic activity was determined using the CK-NAC kinetic kit (Bioclin, Brazil) according to the manufacturer's protocol. Absorbance was measured for 3 minutes at 37°C, in a spectrophotometer at 340 nm. Enzymatic activity was expressed in units/liter (U/L) and each unit consists of the result of the phosphorylation of one nanomol of creatine per minute.
2.8.8. Neutrophil Viability
Neutrophils were collected 6 hours after the intraperitoneal (IP) injection of 1.5 mL of 3% thioglycollate sterile solution according to the method previously described by Call et al. [22]. The animals were euthanized in order to collect the cells and the peritoneal cavity was washed with 3 mL of PBS. The predominance of neutrophils in the liquid obtained was confirmed by microscopic analysis with glass slides stained with a panoptic dye. The peritoneal neutrophils obtained were suspended in an RPMI culture medium (Gibco-BRL) supplemented with gentamicin (100 μg/mL), L-glutamine (2 mM), and 10% bovine fetal serum (SFB) in order to obtain 2 × 105 cells/100 μL. Next, cellular viability was assayed over 1, 12, and 24 h at 37°C, and 5% CO2 in which the cells were incubated in a 96-well plate with the previously isolated PLA2s at a concentration of 12 μg/mL using RPMI as a negative control. After this, the samples were centrifuged and the supernatant removed. Cellular viability was determined using the MTT method [23].
2.8.9. Quantification of Cytokine
EIA was used to evaluate IL-1β cytokine as described by Schumacher et al. [24]. Briefly, the neutrophils (2 × 105 cells/200 μL) were either incubated with the isolated proteins at 3, 6, and 12 μg/mL concentrations (experimental group) or with PMA (positive control group) or with RPMI (negative control) and kept for 12 and 24 hours at 37°C in a humid atmosphere with 5% CO2. After that, 96-well plates were coated with 100 μL of the capture monoclonal antibody anti-IL-1β and incubated for 18 hours at 37°C. The plate was then washed with washer buffer (PBS/Tween20). After that, 200 μL of blocking buffer, containing 5% bovine serum albumin (BSA) in PBS/Tween20, was added to the wells and the plates were incubated for 1 hour at 37°C. Following this, the wells were washed and 50 μL of either samples or standard were dispensed into each well and the plates were incubated for 2 hours at 37°C. After this, the plate was washed and 100 μL of detection antibody anti-IL-1β was added for 2 hours at 37°C. After incubation and washing, 100 μL of streptavidin-peroxidase was added, followed by incubation and addition of the substrate (100 μL/mL 3,3′,5,5′-tetramethylbenzidine). Finally, sulfuric acid (50 μL) was added to stop the reaction. Absorbances were recorded at 540 and 450 nm and concentration of IL-1β was estimated from standard curves prepared with recombinant cytokine. The results were expressed as pg/mL of IL-1β.
2.8.10. Antitumor Activity
Cytotoxic activity of isolated PLA2s on human T-cell leukemia (JURKAT) and breast adenocarcinoma (SK-BR-3) lines obtained from the American Culture Collection of Cells (ATCC, American Type Culture Collection, Rockville, MD, USA) were investigated (Figure 6). This activity was assayed by MTT staining as described by Mosmann [25] and adapted by Stábeli et al. [21]. Cells were dispensed in 96-well plates at a density of 5 × 105 cells/mL. After 24 h of incubation, the medium was removed and fresh medium, with or without different concentrations of PLA2s (BmatTX-I, BmatTX-II, BmatTX-III, or methotrexate), was added to the wells and incubated for another 24 hours. The evaluation of the cytotoxic activity was measured in a spectrophotometer using an interference filter of 570 nm and expressed as a percentage.
Figure 6.
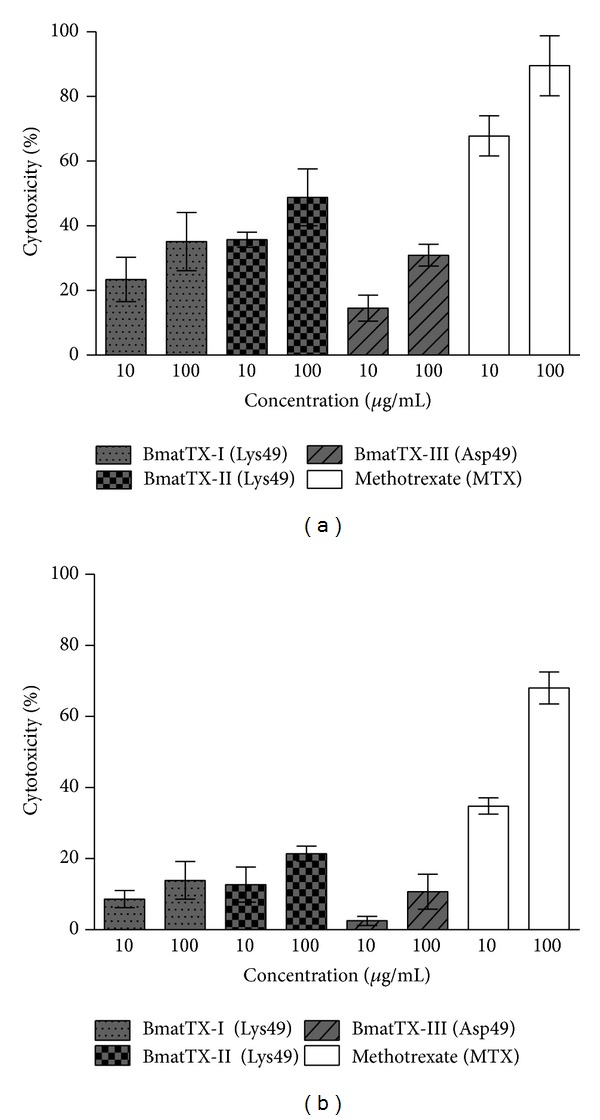
Antitumoral activity of PLA2s from B. mattogrossensis. (a) Antitumoral activity on human acute T-cell leukemia (JURKAT) lines. (b) Antitumoral activity on human breast adenocarcinoma (SK-BR-3). Different concentrations of the PLA2s were incubated with cell lines. Methotrexate was used as the positive control. Results are presented as mean +/− SD (n = 3).
2.8.11. Anti-Leishmania Activity
Promastigote forms of Leishmania amazonensis (IFLA/BR/67/PH8) were dispensed in a 96-well plate with 1 × 105 cells/well. Different concentrations (3.12, 6.25, 12.5, 25, 50, and 100 μg/mL) of the crude venom of B. mattogrossensis and the isolated PLA2s (BmatTX-I and BmatTX-II) were added to each well. 100 mg/mL of pentamidine was used as a positive control. After an incubation period of 48 h, 10 μL of a 5 mg/mL MTT solution was added. Then, the plates were placed in the oven at 33°C with 5% CO2 for 4 hours of incubation after which 50 μL of SDS (20%, w/v) was added. Absorbance was monitored at 570 nm. Results were expressed in toxicity percentage following the equation: 1 − (D.O. sample/D.O. control) × 100.
2.9. Statistical Analysis
Results were expressed as mean +/− standard deviation. An ANOVA test was used to evaluate the significance of the differences observed with P value ≤ 0.05 considered to be significant.
3. Results and Discussion
3.1. Crude Venom Biological Activities
B. mattogrossensis snake venom induced hemorrhage, coagulation, proteolytic, and phospholipase activities in vitro (Table 1). The hemorrhagic activity of B. mattogrossensis crude venom was evaluated based on the dimensions of the average hemorrhagic halo which was 3.33 cm2. This result is in agreement with the results recently obtained with B. atrox [26].
Table 1.
Activities induced by B. mattogrossensis snake venom.
| Effecta | Activity |
|---|---|
| Phospholipase activityb (U/mg) | 1,864.05 |
| Proteolytic activityc (U/10 μg) | 3.0 ± 0.1 |
| Hemorrhagic halod (cm2) | 3.33 ± 0.05 |
| Coagulation activitye (MCD, μg) | 0.325 |
aAll experiments were carried out in triplicate. bActivity using NOB stained substrate. cOne enzymatic unit (U) was defined as the quantity of enzyme needed to increase the absorbance by 0.05 UA/440 nm. dValues 3 h after incubation with crude venom of B. mattogrossensis (50 μg). eMCD: the minimum coagulation dose was the dose capable of coagulating 200 μL of citrated plasma in less than a minute.
Coagulation activity was confirmed after incubation of different concentrations of B. mattogrossensis crude venom with plasma in which the minimum coagulation dose capable of promoting coagulation in less than 1 min was 0.325 μg of protein. Analysis of the proteolytic activity of B. mattogrossensis crude venom demonstrated a concentration-dependent response (data not shown). Regarding phospholipase, proteolytic, coagulating, and hemorrhagic activities of B. mattogrossensis crude venom, assays confirmed the presence and activity of proteases and phospholipases. The presence of metalloproteases was evidenced by the important formation of an extensive hemorrhagic halo in vivo. The presence of serine proteases was evidenced by coagulating activity present even in low concentrations of the venom (325 μg/mL).
Phospholipase activity of B. mattogrossensis crude venom assayed with 4N3OBA synthetic substrate was 1,864.05 U/mg measured by the number of moles of chromophores released per minute (n°mols/min or U) for a given quantity of protein in milligrams (mg).
The properties of snake venom components observed in this study are characteristic of accidents caused by snakes of the Bothrops sp genus; symptoms such as pain, edema, hemorrhage, and necrosis and, additionally, systemic disturbances are characteristic and corroborate the literature that describes that proteases of snake venom proteins are closely related to interference in the hemostatic system promoting blood coagulation, fibrinolysis, and platelet aggregation [27–30].
3.2. Isolation and Biochemical Characterization of Phospholipases
The present study showed, for the first time, the isolation of the three phospholipases A2 from B. mattogrossensis snake venom, obtained by two chromatographic steps. First, ionic exchange chromatography was performed on a CM-Sepharose column with an ammonium bicarbonate gradient (50 to 500 mM, pH 8.0). The elution of absorbed proteins with a linear gradient of concentrated buffer resulted in thirteen fractions (Figure 1(a)), of which fraction nine (9) was related to PLA2s because it showed phospholipase activity of 221.09 U/mg on artificial substratum. All fractions were lyophilized and submitted to unidimensional electrophoresis revealing many protein bands.
Figure 1.
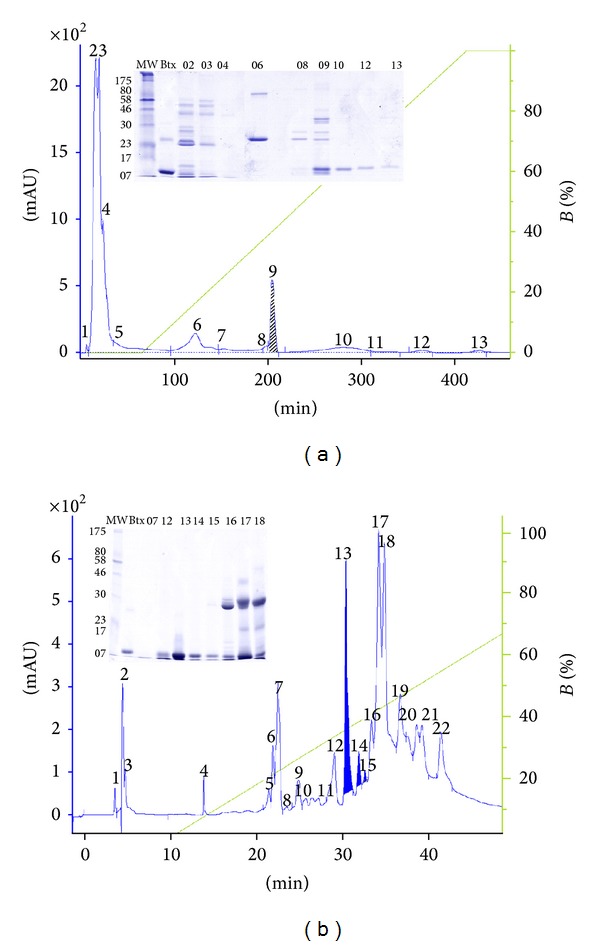
Chromatographic and electrophoretic profile of Bothrops mattogrossensis venom fractioning. (a) CM-Sepharose columns equilibrated with solvent A (50 mM ammonium bicarbonate, pH 8.0) and eluted with a 0–100% concentration gradient of solvent B (500 mM ammonium bicarbonate, pH 8.0) at a 5.0 mL/minute flow. Emphasis on peak 9, rechromatographed. (b) Rechromatography of fraction 9 on Discovery C18 column equilibrated with solvent A (0.1% TFA) and eluted with a concentration gradient of 0–100% of solvent B (99.9% acetonitrile and 0.1% trifluoroacetic acid) and a 1.0 mL/min flow. Emphasized in blue are fractions 13, 14, and 15 characterized as phospholipases BmatTX-I, BmatTX-II, and BmatTX-III, respectively. Controls: MW: molecular weight standard; BTx: BthTX-I a basic enzymatically inactive PLA2 (Lys49) (10 μg) isolated from Bothrops jararacussu venom. Absorbances read at 280 nm. Electrophoresis gel made with 12.5% (w/w) acrylamide/bis-acrylamide.
Fraction 9 was submitted to the second chromatographic step in a reverse column phase on a Discovery C18 column, using 0.1% Trifluoroacetic (TFA) and 99.9% Acetonitrile (ACN) as solvents for the separation of other venom components. The elution of absorbed proteins with a linear gradient of concentrated buffer resulted in twenty-two (22) fractions (Figure 1(b)). The PLA2s were highly purified with approximately 40% ACN. Similar results were observed in the isolation of other PLA2s from snake venoms in high performance liquid chromatography on reverse phase columns where the elution profile of these proteins occurs between 30 and 40% ACN [5].
The association of chromatographic techniques such as ionic exchange and reverse phase has commonly been used, and many snake venoms have been fractioned this way, highlighting the phospholipase purification of a species belonging to the old complex “Bothrops neuwiedi”, as well as the target species studied in this research. Two PLA2 basic isoforms from B. (neuwiedi) pauloensis venom were purified by Rodrigues et al. [31], using biochemical techniques similar to the ones used in this study, with the combination of ionic exchange (cationic) and reverse phase chromatographies.
Other PLA2s have also been purified using simplified procedures based in CM-Sepharose and/or reverse phase, as for example, venom PLA2s from Bothrops moojeni [21, 32, 33], B. pirajai [34, 35], B. jararacussu [36, 37], B. alternatus [8, 38], Cerastes cerastes [39], and Elaphe climacophora [40].
Fractions 13 and 14 were related to enzymatically inactive PLA2s, whereas fraction 15 was related to enzymatically active PLA2s. The phospholipase activity of the collected fractions were analyzed with synthetic NOB and NBD-PC substrates (Figures 2(a) and 2(b)). The amount of activity was compared to BthTX-II, a basic enzymatically active PLA2 (Asp49), and BthTX-I, a basic enzymatically inactive PLA2 (Lys49), both previously isolated from Bothrops jararacussu venom [36, 41]. The BmatTX-III PLA2s (Asp49) were not able to induce platelet aggregation and did not inhibit collagen or ADP induced platelet aggregation (data not shown).
Figure 2.
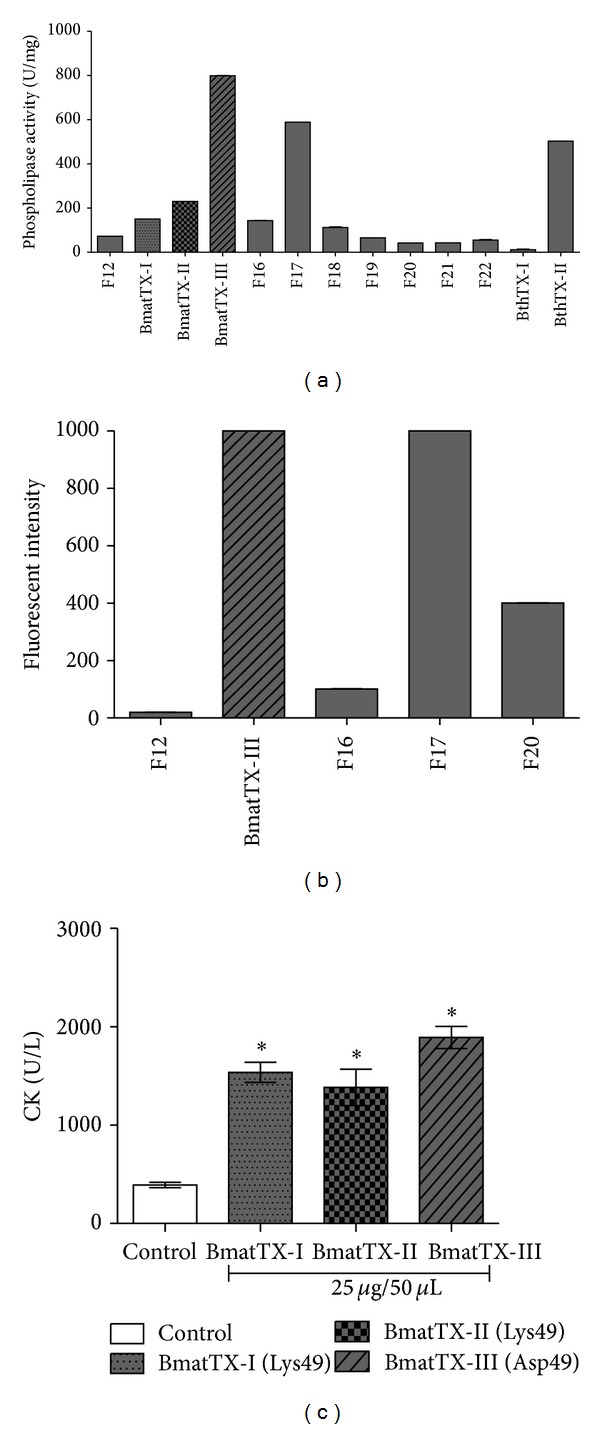
Enzymatic activity and myotoxic activity of PLA2s isolated from the venom of B. mattogrossensis. (a) Phospholipase activity of the fractions collected from the rechromatography of fraction 9 done in C18 column assayed using an NOB stained substrate. This activity was assessed through the measurement of the number of moles of the released chromophore per minute (n°mols/min or U) per milligram of protein. (b) Phospholipase activity of the fractions collected from the rechromatography of fraction 9 done in C18 column assayed using a fluorescent substrate. (c) Myotoxic activity evaluated for inoculation of PLA2s (25 μg/50 µL) or PBS (control) done intramuscularly, in the gastrocnemius muscle of mice. After 3 hours, the creatine kinase (CK) level, an important marker of muscular lesion, was assayed in the animal's plasma. Each bar represents the average +/− SD of three independent groups. *P < 0.05 compared to the control. F13: BmatTX-I (Lys49); F14: BmatTX-II (Lys49); F15: BmatTX-III (Asp49); Controls: BthTX-I (Lys49) and BthTX-II (Asp49).
The degree of purity of the isolated proteins was further demonstrated by SDS-PAGE, mass spectrometry (Figure 3), and N-terminal sequencing (Figures 4(a) and 4(b)). The purified PLA2s were named BmatTX-I, BmatTX-II, and BmatTX-III. They were characterized as single polypeptide chains, with isoelectric points around 10 (data not shown). This result agrees with data from published literature, where most basic PLA2s, with or without catalytic activity on artificial substrates [32, 42], show an isoelectric point between 8 and 10.
Figure 3.
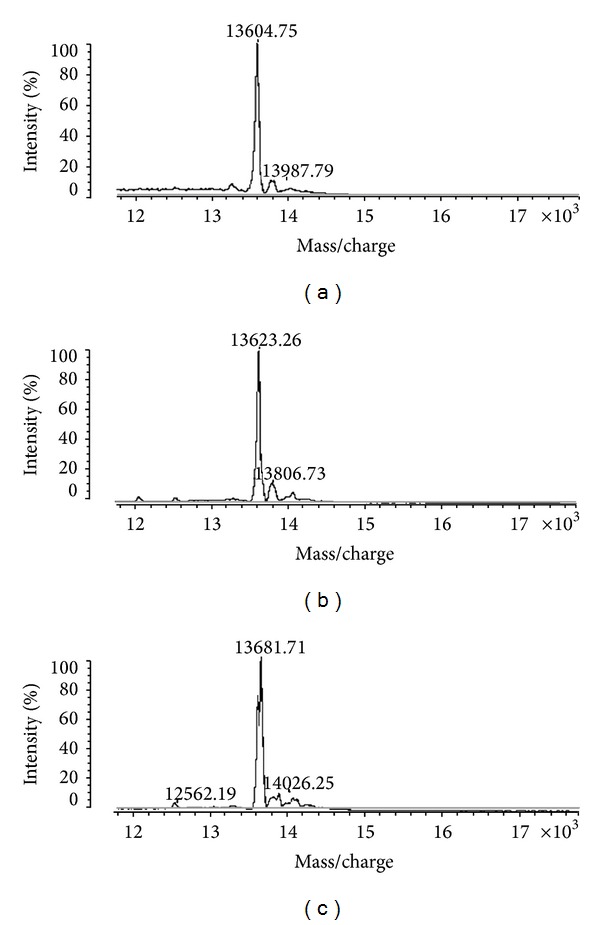
Mass determination by spectrometry. The average protein masses of BmatTX-I (a), BmatTX-II (b), and BmatTX-III (c) were obtained in a MALDI-TOF system, operated in linear mode using external standards for calibration. The resulting mass spectra were submitted to automatic baseline subtraction.
Figure 4.
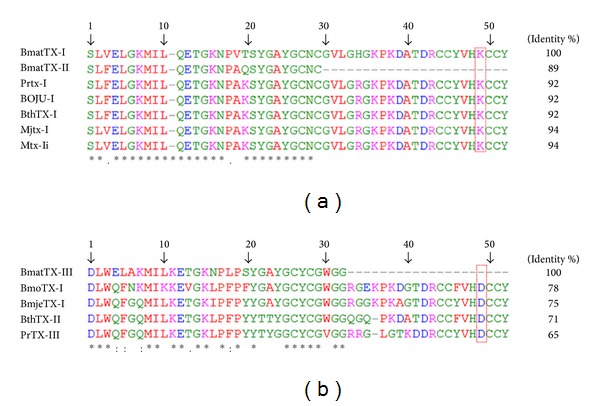
Comparison of the N-terminal sequence of the snake's PLA2s with the PLA2s isolated from B. mattogrossensis venom. N-terminal sequencing of the PLA2s obtained by Edman [13] degradation and alignment done with CLUSTALW2 expressed as % of similarity. (a) BmatTX-I, BmatTX-II with Lys49 residues in marked sequences. (b) BmatTX-III with Asp49 residues in marked sequences. Sequences used for the alignment and their respective access numbers: BthTX-I (gi:51890398); MjTX-I (gi:17368325); Mtx-II (gi:390981003); PrTX-I (gi:190016174); BOJU-I (gi:209572966); BthTX-II (gi:1171971); PrTX-III (gi:90016174); BmjeTX-I (gi:313471399); BmoTX-I (gi:221272396). BthTX-I (Lys49), BthTX-II (Asp49), and BOJU-I (Lys49): isolated from the venom of B. jararacussu; MjTX-I (Lys49), BmoTX-I (Asp49), and BmjeTX-I (Asp49): isolated from the venom of B. moojeni; Mtx-II (Lys49): isolated from the venom of B. brazili; PrTX-I (Lys49) and PrTX-III (Asp49): isolated from the venom of B. pirajai; the “∗” was used to indicate the amino acid residues that are the same between the sequences.
The average molecular mass defined by mass spectrometry was 13,304 Da for BmatTX-I, 13,623 Da for BmatTX-II, and 13,681 Da for BmatTX-III (Figure 3). This molecular mass is consistent with most isolated PLA2s from snake venoms which are around 13 to 16 kDa [27, 36, 43].
BmatTX-I sequencing showed a lysine (Lys) at position 49 and although only the first 28 amino acid residues of BmatTX-II have been sequenced, both are highly similar to the PLA2 Lys49 homologue subgroup. The N-terminal sequence alignment of BmatTX-I and BmatTX-II has revealed that these basic proteins are PLA2s similar to homologous Lys49 and other Lys49 myotoxins from snake venoms (Figure 4(a)). BmatTX-I showed 94% similarity with MjTX-I present in B. moojeni venom, 92% with BthTX-I and BOJU-I present in B. jararacussu venom, and 94% similarity with MTX-II present in B. brazili venom.
Additionally, multiple sequence alignment of BmatTX-III showed a PLA2-Asp49 basic myotoxin with another Asp49 of the Bothrops genera (Figure 4(b)). It can be observed that BmatTX-III presented 78% similarity with BmjeTX-I and 75% with BmoTX-I, both from B. moojeni venom, 71% similarity with BthTX-II isolated from B. jararacussu venom, and 65% similarity with PrTX-III from B. pirajai venom.
In the analysis of the sequences of the PLA2s isolated from B. mattogrossensis in this study, highly preserved constituent residues of the α-helix structure, characteristic of phospholipases were identified, as well as the presence of many cysteine residues, which suggests the existence of many disulfide bridges, important for the stabilization of PLA2's molecular structure [21, 44].
Some studies regarding the amino acid composition of PLA2s demonstrate that these proteins are rich in basic and hydrophobic amino acids containing three long α-helixes, two beta sheets, and a Ca2+ binding site [45–47]. Calcium is absolutely necessary for hydrolysis; therefore, almost all PLA2s have a highly preserved region for a Ca2+ bond (XCGXGG) and a catalytic site (DXCCXXHD) [48]. It is observed in BmatTX-III that the calcium binding site (27YCGWGG 32) is preserved, indicating that it is a catalytically active PLA2 belonging to the PLA2 Asp49 subgroup.
3.3. Biological Activities of Phospholipases A2
The main objective for the isolation and characterization of components of snake venom is to better understand the participation of each component in different pathophysiological processes resulting from envenomation. Local lesions can be attributed to proteases, phospholipase activity, and hemorrhagic factors of these venoms, followed by the release of vasoactive agents causing hemorrhaging in various organs and tissues [5, 44, 49–52].
The PLA2s isolated from B. mattogrossensis venom, BmatTX-I, BmatTX-II, and BmatTX-III, showed high myotoxic activity (Figure 2(c)). At a concentration of 25 µg/50 µL, the PLA2s induced a significant release of CK, an important muscular lesion marker, when compared to the control. Myotoxicity is the characteristic presented by most basic phospholipases A2 from snake venoms. Several studies demonstrate that the myotoxic effect begins quickly by direct action of the myotoxic PLA2s on the plasma membrane of muscle cells or it is mediated by metalloproteases, due to consequent degeneration and ischemia [27, 28, 44].
Regarding the Lys49-PLA2 myotoxins, it is evident that they lyse the plasma membrane of the muscle cell infected in vivo; however the exact mechanism has not been described yet. Furthermore, it is not known if the toxin is internalized before, during, or after the initial lysis or if it is not internalized. Although myotoxicity can be induced by the production of fatty acids, there is a second mechanism that seems to be independent of the enzymatic activity and is mediated by the C-terminal region at sites 115–129 of the Lys49 molecules [43, 53, 54].
In an attempt to better understand the development of the inflammatory process unleashed by the protein complex present in snake venoms, many studies have been done, such as edema induction [55], leukocyte participation [56, 57], mast cells degranulation [55], participation of various cytotoxins in the inflammatory system [58, 59], participation of cyclooxygenases [60, 61], and the participation of venom PLA2s in the inflammatory process [6, 51, 56, 62].
Many studies about PLA2 activity in macrophages have already been done [8, 63, 64]. Little is known about PLA2's effect on neutrophils however. Escocard et al. [65] described an influx of inflammatory cells including many neutrophils into the peritoneal cavity of mice after the injection of Bothrops atrox venom. The induction of reactive oxygen species (ROS), cytokines like IL-6 and IL-1β, was seen in these neutrophils. These data were also observed in a study carried out by Souza et al. [26] where besides the influx of neutrophils into the peritoneal cavity of mice after injection of the venom of B. atrox, there was also induction of superoxide by these cells, mast cell degranulation, and phagocytosis by macrophages. Regarding the activity of PLA2 Gambero et al. [66] have observed the ability of some myotoxins (bothropstoxin-I,-II and piratoxin-I) to induce neutrophil chemotaxis in a concentration-dependent manner.
In order to evaluate the activation of leukocytes, the toxicity of B. mattogrossensis myotoxins on neutrophil cells was investigated. The cells were incubated with different concentrations (3, 6, and 12 μg/mL) of BmatTX-I (Lys49), BmatTX-II (Lys49), and BmatTX-III (Asp49) myotoxins during 1, 12, and 24 hours (data not shown). These myotoxins did not affect the neutrophils' viability, which agrees with Zuliani et al. [64], showing low toxicity on thioglycollate elicited mice macrophages.
Nonetheless, our data showed that neutrophils incubated with BmatTX-I and BmatTX-II myotoxins induced the release of IL-1β (Figures 5(a) and 5(b)). Moreover, these results suggest that phospholipid hydrolysis is not essential for the activity observed and argue with the hyphotesis that other molecular regions distinct from the active site may be involved in this effect.
Figure 5.
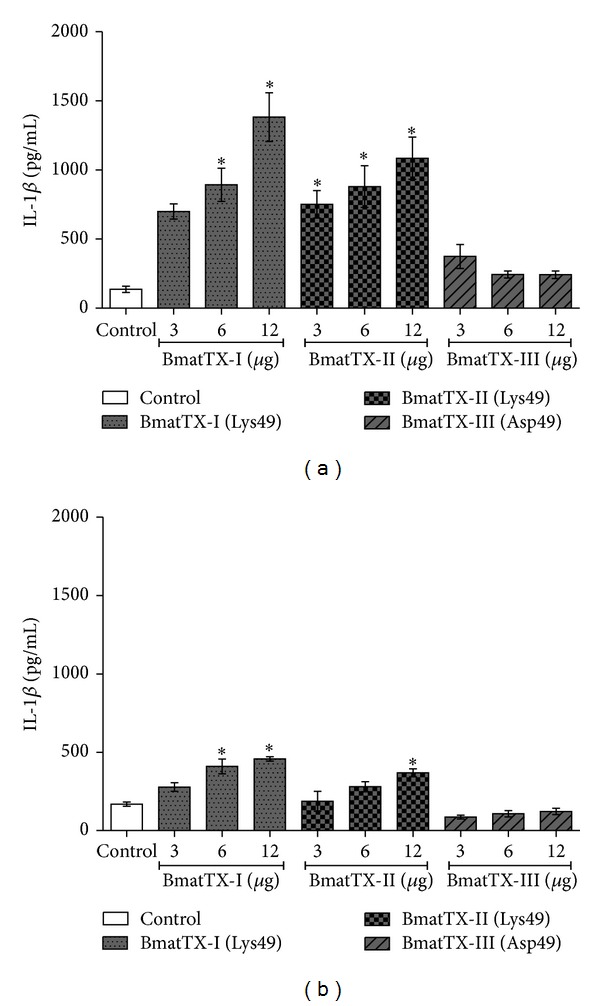
Production of IL-1β by mice neutrophils. These cells were incubatedwith PLA2 (3, 6, and 12 μg/mL) or RPMI (control) for 12 hours (a) and 24 hours (b) at 37°C in a humid atmosphere with 5% CO2. Quantification was done by ELISA as described in 2.9. Each bar represents the average +/− SD of three independent groups. *P < 0.05 compared to the control.
PLA2s are multifunctional proteins that can be used as mediators in several areas of medicine, such as in the treatment of rheumatoid arthritis, as a new class of HIV inhibitors by blocking the host cell invasion, as a potential treatment against malaria, and as an antibiotic by inducing cytotoxicity via the disruption of bacterial membranes [21, 23, 30, 67, 68]. A study by Costa et al. [67] with PLA2s isolated from B. brazili, MTX-I and II, demonstrated cytotoxic activity against Jurkat tumor cells as well as antimicrobial activity against E. coli and C. albicans and antiparasitic activity against Leishmania sp.
In the present study we evaluated the cytotoxic activity of PLA2s isolated from B. mattogrossensis on JURKAT (T leukemia) and SK-BR-3 (breast adenocarcinoma), both of which are human tumor cell lines. Like MTX-II of B. brazili [67] the cytotoxic activity of PLA2s BmatTX-I and BmatTX-II on JURKAT cells was independent of their catalytic activity, since these are characterized as Lys49 PLA2s and are therefore catalytically inactive. BmatTX-III, characterized as Asp49, despite being enzymatically active also showed a lower level of toxicity. Some authors propose that the cytotoxic activity on tumor cell lines is associated with the induction of apoptosis, considering the fact that PLA2 promotes alterations in the cell membrane. And some studies involving Lys49 PLA2s isolated from B. asper demonstrated that the C-terminal region comprised of amino acids 115–129 is concerned with the cytotoxic and bactericidal activities of this protein [69–71]. The same observation was made by Costa et al. [67] with synthetic peptides derived from the C-terminal portion of MTX-I and II PLA2s.
We also evaluated the antiparasitic activity of the crude venom and isolated PLA2 of B. mattogrossensis on promastigote forms of L. amazonensis. After the analysis, it was observed that the crude venom of B. mattogrossensis presents increasing toxic activity (approximately 50 to 80%) against promastigote forms of L. amazonensis after 48 h of incubation (Figure 7(a)). The PLA2s isolated from B. mattogrossensis, at 100 μg/mL, characterized as BmatTX-I (Lys49) and BmatTX-III (Asp49) presented toxic activity between 25% and 30%, respectively, even with values close to those presented after incubation of the protozoan with Pentamidine, a drug used as a positive control (Figures 7(b) and 7(c)).
Figure 7.
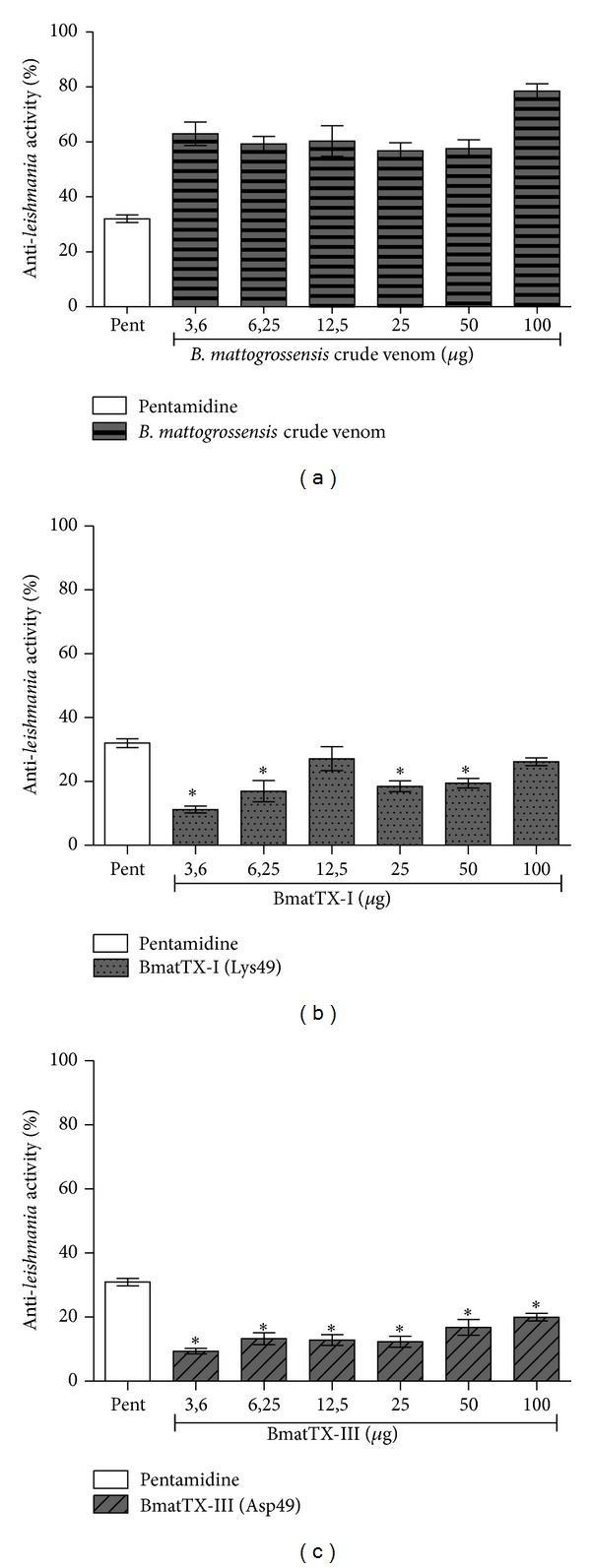
Antileishmanial activity of the crude venom of and PLA2s from B. mattogrossensis. The promastigote forms of L. amazonensis were plated with 1 × 105 cells/well. Then, different concentrations of crude venom and isolated PLA2s were added to each well. The experiment was done in a 48 h period, with the (a) crude venom and the PLA2 enzymes, (b) BmatTX-I, and (c) BmatTX-III. MTT was added and after the incubation period at 33°C, the formazan crystal formed was dissolved in SDS. Readings were done in a spectrophotometer at 570 nm. Each bar represents the average +/− SD of the three independent experimental groups, sixfold total. *P < 0.05 compared to the control.
When compared, the cytotoxicity values of PLA2s against promastigote forms of L. amazonensis show similar activity between the Lys49-PLA2 and the Asp49-PLA2. Comparison of this activity with the crude venom (70%) showed that PLA2s are responsible for almost half of the observed effect. Nonetheless, notably, the results suggest that other toxins present in the venom contribute to the parasite's death.
Similar to the results obtained in the present study, Stábeli et al. [21] demonstrated that MjTX-II, a Lys49-PLA2 homologue isolated from B. moojeni venom, in different concentrations (5, 25 and 75 μg) was effective as a parasiticide agent against Schistosoma mansoni and promastigote forms of Leishmania (L. amazonensis, L. braziliensis, L. donovani, and L. major).
The action of the PLA2s, BmatTX-I (Lys49), and BmatTX-III (Asp49), on promastigote forms of L. amazonensis, was independent of its catalytic activity, since catalytically inactive Lys49 myotoxin also demonstrated toxicity against Leishmania. It is believed that the observed cytotoxic activity might be related to the C-terminal regions of these phospholipase-homologues that are able to promote a disturbance in the cellular membranes independent of their catalytic activity [67, 72]. However, more studies are necessary to define the exact mechanism of action of these enzymes on parasites.
Growing interest in the comprehension of the structure and function of snake venom components, especially PLA2, contributes to a better understanding of the mechanism of action of their enzymatic and toxic activities. It opens the path to better understand the intoxication caused by envenomation and the physiopathology behind its side effects. Future studies will potentially improve serum therapy and help develop the pharmaceutical potential that molecules isolated from animal venoms can have, such as the PLA2s isolated from the B. mattogrossensis venom which show anti-Leishmania and antitumor activities.
4. Conclusion
In conclusion, the venom of Bothrops mattogrossensis has a qualitatively similar toxicological profile to previously studied snake venoms of the Bothrops sp. genera despite the observation of quantitative variations. Of the three basic PLA2s from B. mattogrossensis venom, now isolated for the first time, two are characterized as Lys49-PLA2 homologues, BmatTX-I and -II, and the other as an Asp49-PLA2, named BmatTX-III. This showed high phospholipase activity. The PLA2s isolated induced myotoxic effects as well as the release of proinflammatory cytokines by neutrophils. BmatTX-I and -III PLA2s were cytotoxic to human tumor cell lines JURKAT and SK-BR-3 and showed activity against promastigote forms of L. amazonensis.
Acknowledgments
The authors are grateful to the Ministry of Science and Technology (MCTI), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Fundação de Tecnologia do Acre (FUNTAC/FDCT), Coordenação de Aperfeiçoamento de Nível Superior (CAPES)—Projeto NanoBiotec, Rede de Biodiversidade e Biotecnologia da Amazônia Legal (BIONORTE/CNPq/MCTI), Instituto Nacional para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica (INCT-INPeTAm/CNPq/MCTI) and Instituto Nacional para Pesquisa em Toxinas (INCT-Tox), and Secretary of Development of Rondonia State (SEPLAN/PRONEX/CNPq), for financial support. We are grateful to Sr. Guilherme G. Galassi (Mantenedouro da Fauna Silvestre, autorização IBAMA 02027.008991/2001-95, Americana-SP) for their helpful technical collaboration in provide the snake venom. Amy Grabner provided the English editing of the paper.
Conflict of Interests
The authors state that there is no conflict of interests.
References
- 1.Angulo Y, Lomonte B. Biochemistry and toxicology of toxins purified from the venom of the snake Bothrops asper . Toxicon. 2009;54(7):949–957. doi: 10.1016/j.toxicon.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Calvete JJ, Juárez P, Sanz L. Snake venomics. Strategy and applications. Journal of Mass Spectrometry. 2007;42(11):1405–1414. doi: 10.1002/jms.1242. [DOI] [PubMed] [Google Scholar]
- 3.Kang TS, Georgieva D, Genov N, et al. Enzymatic toxins from snake venom: structural characterization and mechanism of catalysis. The FEBS Journal. 2011;278(23):4544–4576. doi: 10.1111/j.1742-4658.2011.08115.x. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira CZ. Caracterização funcional e estrutural de inibidores de fosfolipases A2 isolados do plasma de serpente Bothrops jararacussu [Ph.D. thesis] Ribeirão Preto, Brazil: Faculdade de Ciências Farmacêuticas de Ribeirão Preto; 2009. [Google Scholar]
- 5.Stábeli RG, Simões-Silva R, Kayano AM, et al. Chromatography—The Most Versatile Method of Chemical Analysis. chapter 1. Vienna, Austria: InTech; 2012. Purification of phospholipases A2 from American snake venoms; pp. 1–34. [Google Scholar]
- 6.Zuliani JP, Fernandes CM, Zamuner SR, Gutiérrez JM, Teixeira CFP. Inflammatory events induced by Lys-49 and Asp-49 phospholipases A2 isolated from Bothrops asper snake venom: role of catalytic activity. Toxicon. 2005;45(3):335–346. doi: 10.1016/j.toxicon.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Marcussi S, Stabeli RG, Santos-Filho NA, et al. Genotoxic effect of Bothrops snake venoms and isolated toxins on human lymphocyte DNA. Toxicon. 2013;65:9–14. doi: 10.1016/j.toxicon.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Setúbal SS, Pontes AS, Furtado JL, et al. Action of two phospholipases A2 purified from Bothrops alternatus snake venom on macrophages. Biochemistry. 2013;78(2):194–203. doi: 10.1134/S0006297913020089. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez JM, Ponce-Soto LA, Marangoni S, Lomonte B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon. 2008;51(1):80–92. doi: 10.1016/j.toxicon.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Rueda AQ, Rodríguez IG, Ara EC, et al. Biochemical characterization, action on macrophages, and superoxide anion production of four basic phospholipases A2 from Panamanian Bothrops asper snake venom. BioMed Research International. 2013;2013:9 pages. doi: 10.1155/2013/789689.789689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva VX, Rodrigues MT. Taxonomic revision of the Bothrops neuwiedi complex (Serpentes, Viperidae) with description of a new species. Phyllomedusa. 2008;7(1):45–90. [Google Scholar]
- 12.Campbell JA, Lamar WW. The Venomous Reptiles of the Western Hemisphere. Ithaca, NY, USA: Comstock; 2004. [Google Scholar]
- 13.Edman P. Method for determination of the amino acid sequence in peptides. Acta Chemica Scandinavica. 1950;4:283–293. [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Holzer M, Mackessy SP. An aqueous endpoint assay of snake venom phospholipase A2 . Toxicon. 1996;34(10):1149–1155. doi: 10.1016/0041-0101(96)00057-8. [DOI] [PubMed] [Google Scholar]
- 17.Fuly AL, de Miranda ALP, Zingali RB, Guimarães JA. Purification and characterization of a phospholipase A2 isoenzyme isolated from Lachesis muta snake venom. Biochemical Pharmacology. 2002;63(9):1589–1597. doi: 10.1016/s0006-2952(02)00873-0. [DOI] [PubMed] [Google Scholar]
- 18.Nikai T, Mori N, Kishida M, Sugihara H, Tu AT. Isolation and biochemical characterization of hemorrhagic toxin f from the venom of Crotalus atrox (Western Diamondback Rattlesnake) Archives of Biochemistry and Biophysics. 1984;231(2):309–319. doi: 10.1016/0003-9861(84)90393-x. [DOI] [PubMed] [Google Scholar]
- 19.Gené JA, Roy A, Rojas G, Gutierrez JM, Cerdas L. Comparative study on coagulant, defibrinating, fibrinolytic and fibrinogenolytic activities of Costa Rican crotaline snake venoms and their neutralization by a polyvalent antivenom. Toxicon. 1989;27(8):841–848. doi: 10.1016/0041-0101(89)90096-2. [DOI] [PubMed] [Google Scholar]
- 20.Charney MS, Tomarelli RM. A colorimetric method for the determination of the proteolytic activity of duodenal juice. The Journal of Biological Chemistry. 1947;171:501–505. [PubMed] [Google Scholar]
- 21.Stábeli RG, Amui SF, Sant'Ana CD, et al. Bothrops moojeni myotoxin-II, a Lys49-phospholipase A2 homologue: an example of function versatility of snake venom proteins. Comparative Biochemistry and Physiology C. 2006;142(3-4):371–381. doi: 10.1016/j.cbpc.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Call DR, Nemzek JA, Ebong SJ, Bolgos GL, Newcomb DE, Remick DG. Ratio of local to systemic chemokine concentrations regulates neutrophil recruitment. The American Journal of Pathology. 2001;158(2):715–721. doi: 10.1016/S0002-9440(10)64014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberto PG, Kashima S, Marcussi S, et al. Cloning and identification of a complete cDNA coding for a bactericidal and antitumoral acidic phospholipase A2 from Bothrops jararacussu venom. The Protein Journal. 2004;23(4):273–285. doi: 10.1023/b:jopc.0000027852.92208.60. [DOI] [PubMed] [Google Scholar]
- 24.Schumacher JH, O'Garra A, Shrader B, et al. The characterization of four monoclonal antibodies specific for mouse IL-5 and development of mouse and human IL-5 enzyme-linked immunosorbent. The Journal of Immunology. 1988;141(5):1576–1581. [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Souza CAT, Kayano AM, Setúbal SS, et al. Local and systemic biochemical alterations induced by Bothrops atrox snake venom in mice. Journal of Venom Research. 2012;3:28–34. [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez JM, Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33(11):1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 28.Gutiérrez JM, Rucavado A. Snake venom metalloproteinases: their role in the pathogenesis of local tissue damage. Biochimie. 2000;82(9-10):841–850. doi: 10.1016/s0300-9084(00)01163-9. [DOI] [PubMed] [Google Scholar]
- 29.Matsui T, Fujimura Y, Titani K. Snake venom proteases affecting hemostasis and thrombosis. Biochimica et Biophysica Acta. 2000;1477(1-2):146–156. doi: 10.1016/s0167-4838(99)00268-x. [DOI] [PubMed] [Google Scholar]
- 30.Soares AM, Fontes MRM, Giglio JR. Phospholipase A2 myotoxins from Bothrops snake venoms: structure-function relationship. Current Organic Chemistry. 2004;8(17):1677–1690. [Google Scholar]
- 31.Rodrigues VM, Marcussi S, Cambraia RS, et al. Bactericidal and neurotoxic activities of two myotoxic phospholipases A2 from Bothrops neuwiedi pauloensis snake venom. Toxicon. 2004;44(3):305–314. doi: 10.1016/j.toxicon.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Santos-Filho NA, Silveira LB, Oliveira CZ, et al. A new acidic myotoxic, anti-platelet and prostaglandin I2 inductor phospholipase A2 isolated from Bothrops moojeni snake venom. Toxicon. 2008;52(8):908–917. doi: 10.1016/j.toxicon.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Soares AM, Andrião-Escarso SH, Ângulo Y, et al. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from Bothrops moojeni (Caissaca) snake venom. Archives of Biochemistry and Biophysics. 2000;373(1):7–15. doi: 10.1006/abbi.1999.1492. [DOI] [PubMed] [Google Scholar]
- 34.Soares AM, Andrião-Escarso SH, Bortoleto RK, et al. Dissociation of enzymatic and pharmacological properties of piratoxins-I and -III, two myotoxic phospholipases A2 from Bothrops pirajai snake venom. Archives of Biochemistry and Biophysics. 2001;387(2):188–196. doi: 10.1006/abbi.2000.2244. [DOI] [PubMed] [Google Scholar]
- 35.Teixeira SS, Silveira LB, da Silva FMN, et al. Molecular characterization of an acidic phospholipase A2 from Bothrops pirajai snake venom: synthetic C-terminal peptide identifies its antiplatelet region. Archives of Toxicology. 2011;85(10):1219–1233. doi: 10.1007/s00204-011-0665-6. [DOI] [PubMed] [Google Scholar]
- 36.Andrião-Escarso SH, Soares AM, Rodrigues VM, et al. Myotoxic phospholipases A2 in Bothrops snake venoms: effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu . Biochimie. 2000;82(8):755–763. doi: 10.1016/s0300-9084(00)01150-0. [DOI] [PubMed] [Google Scholar]
- 37.Andrião-Escarso SH, Soares AM, Fontes MRM, et al. Structural and functional characterization of an acidic platelet aggregation inhibitor and hypotensive phospholipase A2 from Bothrops jararacussu snake venom. Biochemical Pharmacology. 2002;64(4):723–732. doi: 10.1016/s0006-2952(02)01210-8. [DOI] [PubMed] [Google Scholar]
- 38.Denegri MEG, Acosta OC, Huancahuire-Vega S, et al. Isolation and functional characterization of a new acidic PLA2 Ba SpII RP4 of the Bothrops alternatus snake venom from Argentina. Toxicon. 2010;56(1):64–74. doi: 10.1016/j.toxicon.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 39.Zouari-Kessentini R, Luis J, Karray A, et al. Two purified and characterized phospholipases A2 from Cerastes cerastes venom, that inhibit cancerous cell adhesion and migration. Toxicon. 2009;53(4):444–453. doi: 10.1016/j.toxicon.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Shirai R, Toriba M, Hayashi K, Ikeda K, Inoue S. Identification and characterization of phospholipase A2 inhibitors from the serum of the Japanese rat snake, Elaphe climacophora . Toxicon. 2009;53(6):685–692. doi: 10.1016/j.toxicon.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Homsi-Brandenburgo MI, Queiroz LS, Santo-Neto H, Rodrigues-Simoni L, Giglio JR. Fractionation of Bothrops jararacussu snake venom: partial chemical characterization and biological activity of bothropstoxin. Toxicon. 1988;26(7):615–627. doi: 10.1016/0041-0101(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 42.Fernández GP, Segura A, Herrera M, et al. Neutralization of Bothrops mattogrossensis snake venom from Bolivia: experimental evaluation of llama and donkey antivenoms produced by caprylic acid precipitation. Toxicon. 2010;55(2-3):642–645. doi: 10.1016/j.toxicon.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 43.Ownby CL, de Araujo HSS, White SP, Fletcher JE. Lysine 49 phospholipase A2 proteins. Toxicon. 1999;37(3):411–445. doi: 10.1016/s0041-0101(98)00188-3. [DOI] [PubMed] [Google Scholar]
- 44.Soares AM, Sestito WP, Marcussi S, et al. Alkylation of myotoxic phospholipases A2 in Bothrops moojeni venom: a promising approach to an enhanced antivenom production. International Journal of Biochemistry & Cell Biology. 2004;36(2):258–270. doi: 10.1016/s1357-2725(03)00237-1. [DOI] [PubMed] [Google Scholar]
- 45.Lomonte B, Gutiérrez JM, Furtado MF, et al. Isolation of basic myotoxins from Bothrops moojeni and Bothrops atrox snake venoms. Toxicon. 1990;28(10):1137–1146. doi: 10.1016/0041-0101(90)90114-m. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso LC, Correa MM, Vieira CA, et al. Fractionation of Bothrops pirajai snake venom: isolation and characterization of piratoxin-I, a new myotoxic protein. Toxicon. 1995;33(5):615–626. doi: 10.1016/0041-0101(95)00012-b. [DOI] [PubMed] [Google Scholar]
- 47.Selistre HS, Queiroz LS, Cunha OAB, de Souza GEP, Giglio JR. Isolation and characterization of hemorrhagic, myonecrotic and edema-inducing toxins from Bothrops insularis (jararaca ilhoa) snake venom. Toxicon. 1990;28(3):261–273. doi: 10.1016/0041-0101(90)90062-c. [DOI] [PubMed] [Google Scholar]
- 48.Dennis EA, Cao J, Hsu Y-H, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical Reviews. 2011;111(10):6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardoso AGT. Ação do veneno de Bothrops moojeni e sua fração L-aminoácido oxidase, submetida ao tratamento com raios gama de 60CO, em Leishmania spp [M.S. thesis] São Paulo, Brazil: IPEN-CNEN/SP; 1999. [Google Scholar]
- 50.Gallacci M, Cavalcante WLG. Understanding the in vitro neuromuscular activity of snake venom Lys49 phospholipase A2 homologues. Toxicon. 2010;55(1):1–11. doi: 10.1016/j.toxicon.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira SS. Caracterização funcional e estrutural de uma fosfolipase A2 ácida isolada do veneno de Bothrops pirajai [M.S. thesis] Ribeirão Preto, Brazil: Faculdade de Ciências Farmacêuticas de Ribeirão Preto/USP; 2009. [Google Scholar]
- 52.Valentin E, Lambeau G. Increasing molecular diversity of secreted phospholipases A2 and their receptors and binding proteins. Biochimica et Biophysica Acta. 2000;1488(1-2):59–70. doi: 10.1016/s1388-1981(00)00110-4. [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez JM, Lomonte B. Phospholipases A2 from Viperidae snake venoms: how do they induce skeletal muscle damage? Acta Chimica Slovenica. 2011;58(4):647–659. [PubMed] [Google Scholar]
- 54.Gutiérrez JM, Ownby CL, Odell GV. Isolation of a myotoxin from Bothrops asper venom: partial characterization and action on skeletal muscle. Toxicon. 1984;22(1):115–128. doi: 10.1016/0041-0101(84)90144-2. [DOI] [PubMed] [Google Scholar]
- 55.Nascimento NG, Sampaio MC, Olivo RA, Teixeira C. Contribution of mast cells to the oedema induced by Bothrops moojeni snake venom and a pharmacological assessment of the inflammatory mediators involved. Toxicon. 2010;55(2-3):343–352. doi: 10.1016/j.toxicon.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Teixeira CFP, Landucci ECT, Antunes E, Chacur M, Cury Y. Inflammatory effects of snake venom myotoxic phospholipases A2 . Toxicon. 2003;42(8):947–962. doi: 10.1016/j.toxicon.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Zamuner SR, Teixeira CFP. Cell adhesion molecules involved in the leukocyte recruitment induced by venom of the snake Bothrops jararaca . Mediators of Inflammation. 2002;11(6):351–357. doi: 10.1080/0962935021000051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chaves F, Teixeira CFP, Gutiérrez JM. Role of TNF-α, IL-1β and IL-6 in the local tissue damage induced by Bothrops asper snake venom: an experimental assessment in mice. Toxicon. 2005;45(2):171–178. doi: 10.1016/j.toxicon.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Teixeira CDFP, Fernandes CM, Zuliani JP, Zamuner SF. Inflammatory effects of snake venom metalloproteinases. Memórias do Instituto Oswaldo Cruz. 2005;100(supplement 1):181–184. doi: 10.1590/s0074-02762005000900031. [DOI] [PubMed] [Google Scholar]
- 60.Olivo RDA, Teixeira CFP, Wallace JL, Gutierrez JM, Zamuner SR. Role of cyclooxygenases in oedema-forming activity of bothropic venoms. Toxicon. 2007;49(5):670–677. doi: 10.1016/j.toxicon.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 61.Silveira LB, Marchi-Salvador DP, Santos-Filho NA, et al. Isolation and expression of a hypotensive and anti-platelet acidic phospholipase A2 from Bothrops moojeni snake venom. Journal of Pharmaceutical and Biomedical Analysis. 2013;73:35–43. doi: 10.1016/j.jpba.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Rucavado A, Escalante T, Teixeira CFP, Fernándes CM, Díaz C, Gutiérrez JM. Increments in cytokines and matrix metalloproteinases in skeletal muscle after injection of tissue-damaging toxins from the venom of the snake Bothrops asper . Mediators of Inflammation. 2002;11(2):121–128. doi: 10.1080/09629350220131980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leiguez E, Zuliani JP, Cianciarullo AM, Fernandes CM, Gutiérrez JM, Teixeira C. A group IIA-secreted phospholipase A2 from snake venom induces lipid body formation in macrophages: the roles of intracellular phospholipases A2 and distinct signaling pathways. Journal of Leukocyte Biology. 2011;90(1):155–166. doi: 10.1189/jlb.0510263. [DOI] [PubMed] [Google Scholar]
- 64.Zuliani JP, Gutiérrez JM, Casais e Silva LL, Sampaio SC, Lomonte B, Teixeira CDFP. Activation of cellular functions in macrophages by venom secretory Asp-49 and Lys-49 phospholipases A2 . Toxicon. 2005;46(5):523–532. doi: 10.1016/j.toxicon.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 65.Escocard RDCM, Kanashiro MM, Petretski JH, et al. Neutrophils regulate the expression of cytokines, chemokines and nitric oxide synthase/nitric oxide in mice injected with Bothrops atrox venom. Immunobiology. 2006;211(1-2):37–46. doi: 10.1016/j.imbio.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Gambero A, Landucci ECT, Toyama MH, et al. Human neutrophil migration in vitro induced by secretory phospholipases A2: a role for cell surface glycosaminoglycans. Biochemical Pharmacology. 2002;63(1):65–72. doi: 10.1016/s0006-2952(01)00841-3. [DOI] [PubMed] [Google Scholar]
- 67.Costa FLS, Rodrigues RS, Izidoro LFM, et al. Biochemical and functional properties of a thrombin-like enzyme isolated from Bothrops pauloensis snake venom. Toxicon. 2009;54(6):725–735. doi: 10.1016/j.toxicon.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 68.Villalobos JC, Mora R, Lomonte B, Gutiérrez JM, Angulo Y. Cytotoxicity induced in myotubes by a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops asper: evidence of rapid plasma membrane damage and a dual role for extracellular calcium. Toxicology in Vitro. 2007;21(8):1382–1389. doi: 10.1016/j.tiv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 69.Cummings BS, McHowat J, Schnellmann RG. Phospholipase A2s in cell injury and death. The Journal of Pharmacology and Experimental Therapeutics. 2000;294(3):793–799. [PubMed] [Google Scholar]
- 70.Panini SR, Yang L, Rusinol AE, Sinensky MS, Bonventre JV, Leslie CC. Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. Journal of Lipid Research. 2001;42(10):1678–1686. [PubMed] [Google Scholar]
- 71.Páramo L, Lomonte B, Pizarro-Cerdá J, Bengoechea J-A, Gorvel J-P, Moreno E. Bactericidal activity of Lys49 and Asp49 myotoxic phospholipases A2 from Bothrops asper snake venom: synthetic Lys49 myotoxin II-(115-129)-peptide identifies its bactericidal region. European Journal of Biochemistry. 1998;253(2):452–461. doi: 10.1046/j.1432-1327.1998.2530452.x. [DOI] [PubMed] [Google Scholar]
- 72.Lomonte B, Angulo Y, Moreno E. Synthetic peptides derived from the C-terminal region of Lys49 phospholipase A2 homologues from viperidae snake venoms: biomimetic activities and potential applications. Current Pharmaceutical Design. 2010;16(28):3224–3230. doi: 10.2174/138161210793292456. [DOI] [PubMed] [Google Scholar]


