ABSTRACT
Niche-restricted pathogens are evolutionarily linked with the specific biological fluids that are encountered during infection. Neisseria gonorrhoeae causes the genital infection gonorrhea and is exposed to seminal fluid during sexual transmission. Treatment of N. gonorrhoeae with seminal plasma or purified semen proteins lactoferrin, serum albumin, and prostate-specific antigen each facilitated type IV pilus-mediated twitching motility of the bacterium. Motility in the presence of seminal plasma was characterized by high velocity and low directional persistence. In addition, infection of epithelial cells with N. gonorrhoeae in the presence of seminal plasma resulted in enhanced microcolony formation. Close association of multiple pili in the form of bundles was also disrupted after seminal plasma treatment leading to an increase in the number of single pilus filaments on the bacterial surface. Thus, exposure of N. gonorrhoeae to seminal plasma is proposed to alter bacterial motility and aggregation characteristics to influence the processes of transmission and colonization.
IMPORTANCE
There are greater than 100 million estimated new cases of gonorrhea annually worldwide. Research characterizing the mechanisms of pathogenesis and transmission of Neisseria gonorrhoeae is important for developing new prevention strategies, since antibiotic resistance of the organism is becoming increasingly prevalent. Our work identifies seminal plasma as a mediator of N. gonorrhoeae twitching motility and microcolony formation through functional modification of the type IV pilus. These findings provide insight into motility dynamics and epithelial cell colonization under conditions that are relevant to sexual transmission. Type IV pili are common virulence factors with diverse functions among bacterial pathogens, and this work identifies interactions between type IV pili and the host environment. Finally, this work illustrates the importance of the host environment and niche-specific fluids on microbial pathogenesis.
INTRODUCTION
Neisseria gonorrhoeae (the gonococcus) is the causative agent of gonorrhea and has been associated with humans for millennia (1). N. gonorrhoeae lacks a nonhuman reservoir, and the majority of infections are confined to the genital tract, indicating that this pathogen has evolved to thrive in a limited niche. Despite its narrow host range, N. gonorrhoeae must be well adapted to environmental changes that occur during infection and transmission. A dramatic modification to the local environment that occurs during sexual transmission is the introduction of seminal fluid, both to the male urethra and female vaginal mucosa. Semen is a complex biological fluid that includes abundant quantities of proteins, metal ions, and monosaccharides (2). The protein constituents of seminal plasma (SP) (lacking spermatozoa and nonsoluble material) are well defined, with serum albumin and lactoferrin being among the most abundant (2, 3). N. gonorrhoeae has previously been shown to bind spermatozoa through interactions with the bacterial type IV pili (T4P) (4) and lipo-oligosaccharide (5), and it was hypothesized that adherence to the highly motile sperm cells contributed to transmission of the disease from males to females (6). However, this “hitchhiker” hypothesis does not account for transmission from females to males, and it is difficult to model the establishment of an infectious dose in the male urethra without invoking bacterial motility. Despite the potential influence that SP may have on the pathogenesis of N. gonorrhoeae, little is known about the physiological response of gonococci to SP exposure.
T4P are well-conserved virulence factors among Gram-negative bacteria (7) and are also found in certain Gram-positive bacterial species (8). These surface structures play multiple roles in pathogenesis, including adhesion, motility, microcolony formation, and transformation. In Neisseria, the T4P consists of a polymer of the PilE major pilin subunit that assembles in the form of thin fiber of between 6 and 8 nm in diameter and several microns in length (9, 10). The pilE gene is subject to antigenic variation through gene conversion involving several silent pilS copies located throughout the gonococcal genome (11, 12). The T4P of N. gonorrhoeae has long been recognized as a primary mediator of adherence to and invasion of host epithelial cells (13–16), and gonococcal T4P are additionally required for high-frequency DNA transformation (17).
Twitching motility is the sole form of locomotion for N. gonorrhoeae. The basis for this motility is through cycles of T4P extension and retraction when pili are in contact with a surface. Retraction of T4P and motility are dependent on the ATPase motor protein PilT (18). When multiple filaments cooperate, pilus retraction is capable of generating remarkable force in the nanonewton range, approximately 10-fold greater than that of a single pilus fiber (19). A typical gonococcus (diplococcus or monococcus) has a peritrichous arrangement of T4P on its surface. Despite this, the motility of N. gonorrhoeae is directionally persistent through the cooperation of multiple pilus motors (20). This quality of persistence can be described as the movement of a bacterium in a certain direction during a time period that is longer than the time of a single pilus retraction, meaning that multiple pili pull the bacterium in the same direction. The mechanism by which gonococci coordinate pilus retraction is not yet known. However, it is unlikely to be controlled via a conventional chemosensory system, since the genes that control T4P motility in other organisms are lacking in the N. gonorrhoeae genome (21). In addition to facilitating the motility of individual bacteria, pilus retraction of clustered bacteria appears to have additional roles in host cell interactions. Bacterial aggregates called microcolonies form on the cell surface and are motile in a pilT-dependent manner (22). Furthermore, PilT is involved in the formation of cortical plaques at the microcolony-cell interface (23, 24) and cellular activation of the phosphatidylinositol (PI) 3-kinase (25) and NF-κB pathways (26).
Much progress has been made in defining the virulence factors and disease processes of N. gonorrhoeae, but the study of gonococcal transmission is limited by the lack of an appropriate model system. Since human semen is a prominent environmental component of the transmission process, we sought to characterize the response of N. gonorrhoeae to SP. In this study, we show that bacterial twitching motility and microcolony formation are facilitated by exposure to SP, leading to a phenotypic state in which N. gonorrhoeae are primed for transmission and colonization.
RESULTS
SP facilitates twitching motility.
To test the effect of SP on the twitching motility of a bacterial population, we assayed movement of N. gonorrhoeae through a porous membrane barrier using a Transwell system. For parental strain FA1090, a 1:20 dilution of SP caused an ~24-fold increase in the number of total bacteria (piliated and spontaneously nonpiliated) that crossed the barrier compared to medium alone (Fig. 1). When considering only piliated bacteria, a >200-fold increase in the presence of SP was observed. Conversely, a genetically engineered nonpiliated strain (∆pilE) exhibited minimal SP responsiveness, since approximately equivalent numbers of bacteria were observed to cross the Transwell barrier under both conditions. The T4P motility apparatus undergoes spontaneous high-frequency variation in the PilE coding sequence (11, 12) and phase variation of the pilus-associated protein gene pilC (27), both of which can result in a switch from a piliated state to a nonpiliated state. Consistent with the notion that pili are required for SP responsiveness, a strain designed to limit spontaneous pilus variation (nonvariable pilE gene [pilENV], phase-locked pilC1 gene [pilC1PL]) demonstrated an even greater SP response (~3.8 × 103-fold) than the variable parent strain (see Fig. S1A in the supplemental material). Furthermore, bacteria lacking the PilT ATPase failed to respond to SP, regardless of whether bacteria expressed pili (Fig. 1). Together, these data demonstrate that a functional pilus apparatus is required for N. gonorrhoeae to respond to SP and indicate that this response involves twitching motility. Other N. gonorrhoeae strains, including MS11, also exhibited enhanced barrier migration in the presence of SP (data not shown), demonstrating that this response is not strain specific.
FIG 1 .
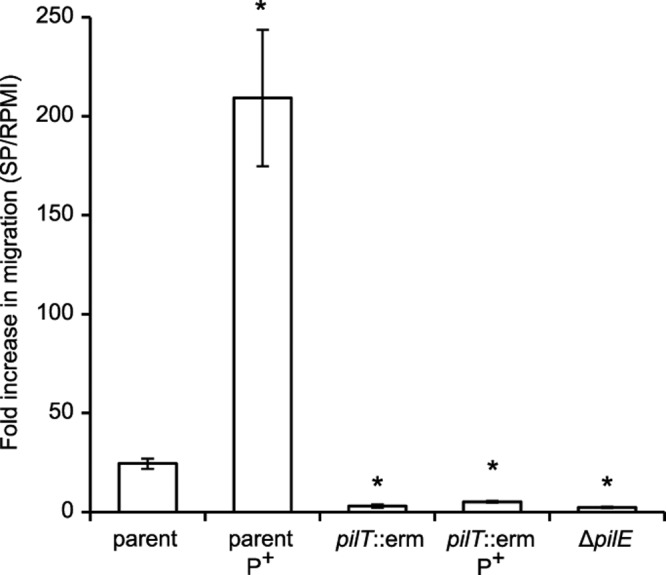
SP stimulates Transwell migration of piliated N. gonorrhoeae. Transwells were seeded with parental (FA1090), T4P mutant (∆pilE), or pilus retraction mutant (pilT::erm) strains. The mean number of bacteria ± standard deviation (SD) that cross the Transwell barrier after exposure to SP (1:20) relative to the number of bacteria in RPMI 1640 that cross the barrier is reported for the entire population or just piliated bacteria (P+). Values that are significantly different (P < 0.01) from the value for the parent strain by the two-sample t test are indicated by an asterisk.
To determine the effect of SP concentration on twitching motility, 2-fold serial dilutions of SP starting from 1:20 were assayed for Transwell migration-stimulating activity. A significant decrease in motility-enhancing activity was not observed until the 1:320 dilution (see Fig. S1B in the supplemental material), indicating that the stimulatory effect of SP is possible even at low concentrations and beyond an initial influx of seminal fluid into the genital environment. In addition to high concentrations of total protein, SP contains large amounts of small molecules such as metal ions, citrate, and monosaccharides (2). However, these small molecules were discounted as a source of motility-stimulating activity, since SP passed through a 10-kDa-nominal-molecular-size-limit filter did not stimulate Transwell barrier migration beyond that of the negative control, while the activity of the retentate (>10 kDa) was similar to that of whole SP (Fig. S1C).
Dynamics of SP-mediated motility.
Twitching motility was assessed microscopically to quantitate the effect of SP on individual bacteria. In this assay, bacteria were treated in suspension with the tested compounds, then spotted onto glass coverslips, and tracked over a 30-s interval at room temperature to allow for rapid assessment of several treatments on a single bacterial culture. Bacteria treated with SP were highly motile with a median velocity of 0.78 µm/s and few observed stationary cells (Fig. 2A; see Fig. S2 in the supplemental material). In contrast, the majority of untreated bacteria were nonmotile and exhibited background-level velocities similar to that of the nonmotile pilT::erm strain, which was unaffected by SP exposure (Fig. 2B). Proteins found in high abundance in SP include lactoferrin, serum albumin, and prostate-specific antigen, all of which were tested for the ability to stimulate gonococcal motility at levels approximating those found in SP from healthy males (28, 29). All three protein components resulted in high levels of surface motility similar to whole SP (Fig. 2C), indicating that the activity of SP is not specific to one component and consistent with the observation that activity is generated by large compounds.
FIG 2 .
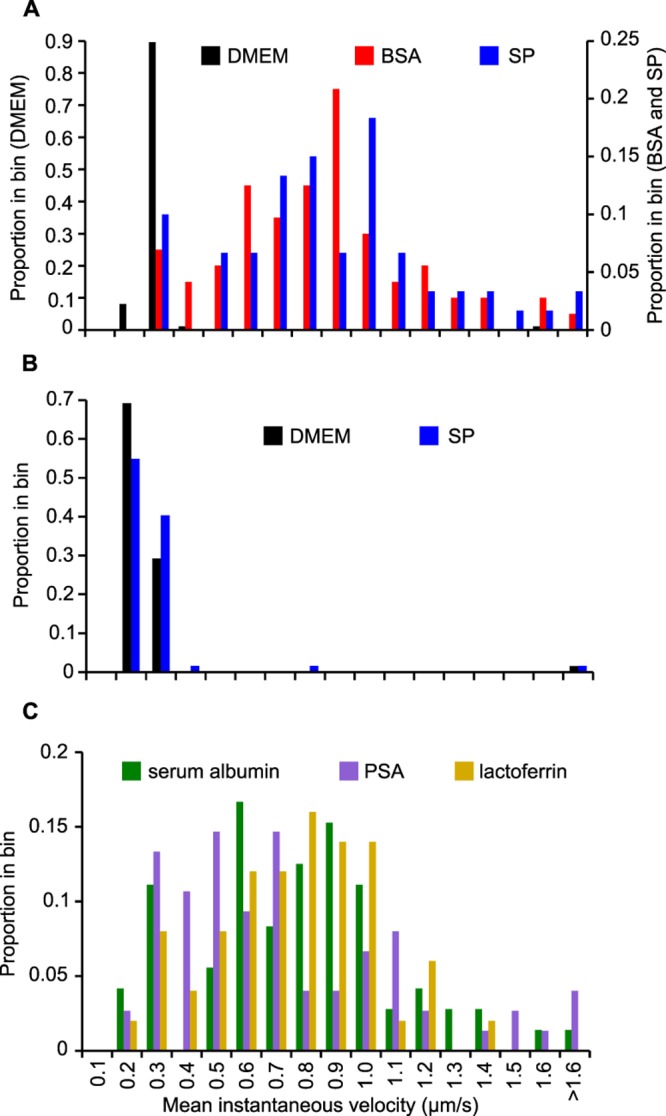
SP and SP proteins facilitate N. gonorrhoeae twitching motility. The pilus-locked FA1090 pilENV pilC1PL strain (A and C) and the nonmotile FA1090 pilENV pilT::erm strain (B) were used to quantitate the motility of N. gonorrhoeae on glass coverslips. The distributions of mean instantaneous velocity for individual bacteria treated with the indicated test components are reported (temperature of 23°C). The median velocities of the bacteria with the different test components are as follows: with DMEM, 0.22 µm/s (n = 87) (A) and 0.20 µm/s (n = 65) (B); with BSA, 0.78 µm/s (n = 72); with SP, 0.78 µm/s (n = 60) (A) and 0.20 µm/s (n = 62) (B); with human serum albumin, 0.74 µm/s (n = 72); with lactoferrin, 0.60 µm/s (n = 75); and with prostate-specific antigen (PSA), 0.74 µm/s (n = 50).
Previous studies have utilized bovine serum albumin (BSA)-coated glass as a substrate for N. gonorrhoeae motility, since little motility is observed on pure glass (18, 20, 30). Substituting BSA in the microscopic motility assay at room temperature resulted in a motility velocity (median = 0.78 µm/s) matching that of SP (Fig. 2A). Similarly, BSA facilitated approximately equivalent Transwell migration levels compared to SP at similar total protein concentrations (see Fig. S1B in the supplemental material). Although gonococci are clearly motile when BSA is provided as a substrate, BSA is not relevant to the human genital environment. In contrast, the presence of SP in vivo exposes the bacteria to numerous proteins, including human serum albumin, during sexual transmission.
Although both BSA and SP are capable of facilitating N. gonorrhoeae twitching motility, the two substrates were differentiated by analyzing the directional persistence of motility in the presence of each substrate. The persistence of motile cells is measured by determining their mean-squared displacement (MSD) as a function of time. The use of BSA as a substrate was previously shown to result in persistent bacterial movement (20). In this assay, the correlation time is derived from the fit to a correlated random walk and is a measure of directional persistence (20). Strikingly, bacteria treated with SP had a lower correlation time (0.12 ± 0.01 s) than those treated with BSA (0.50 ± 0.05 s), indicating that movement is less coordinated in the presence of SP (Fig. 3A). The decrease in persistence of SP-treated bacteria compared to BSA-treated bacteria could be observed qualitatively when comparing the motility tracks generated in the presence of both substrates, with SP-treated bacteria often displaying movement that does not carry the cell far from the point of origin (see Fig. S2 in the supplemental material). The persistence assay tracks the movement of bacteria at 37°C. Under these conditions, the temperature of the host environment is more accurately represented, and SP stimulated more rapid motility of N. gonorrhoeae than BSA did (1.80 ± 0.10 µm/s for SP and 1.31 ± 0.06 µm/s for BSA). A potential explanation for the observed decrease in persistence is that SP results in weaker interactions between pili and the surface compared to BSA. To test this hypothesis, optical tweezers were used to trap individual bacteria and measure the force of cooperative pilus retraction (Fig. S3). Interestingly, the force applied to the cell body by pilus retraction did not differ between SP-treated and BSA-treated bacteria (Fig. 3B). This result is inconsistent with an SP-mediated reduction in pilus-surface binding strength and suggests that SP modifies pilus function in another manner.
FIG 3 .
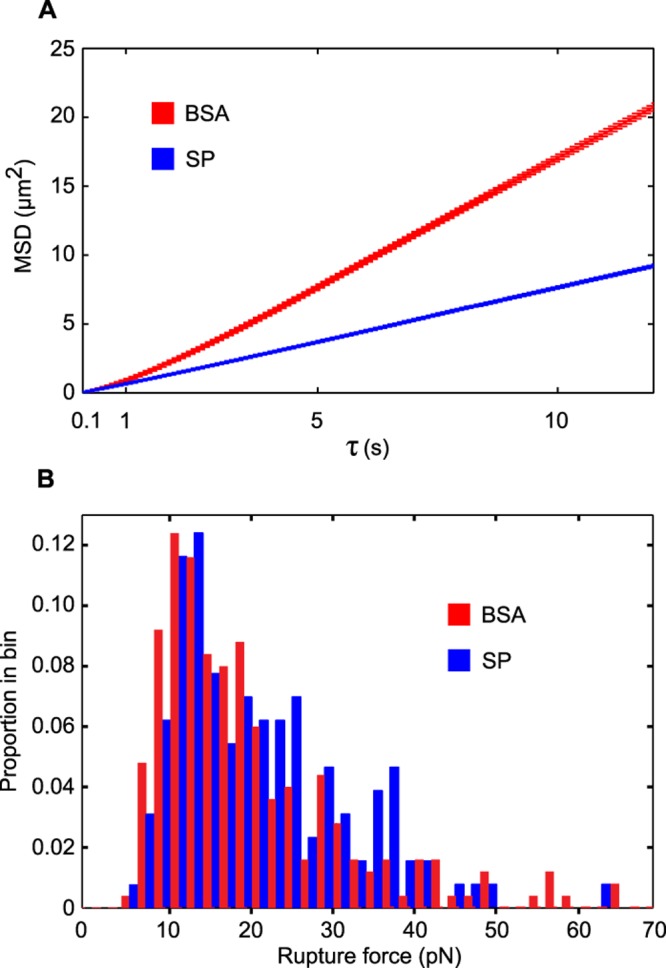
SP-treated bacteria are less persistent but generate equivalent force compared to BSA-treated bacteria. (A) The trajectories of individual bacteria were recorded after exposure to BSA or SP. SP-treated bacteria exhibit shorter average displacement lengths (MSD) over the given time interval. The curves represent the mean trajectories ± SDs from >1,000 bacteria in three independent experiments. (B) Single bacteria treated with BSA or SP were held in an optical trap, and displacement from the trap was used to quantitate the force generated by retraction of surface-adhered T4P. The force distributions were not significantly different by the Wilcoxon-Mann-Whitney rank sum test (U = 8,560; P > 0.05). The temperature was 37°C.
SP modulates pilus morphology and function.
Individual Neisseria pilus fibers have a width of 6 to 8 nm (9, 10) but can interact to form bundles on the bacterial surface (31). Pilus filaments imaged by transmission electron microscopy (TEM) from untreated gonococci had a width of >8 nm at a frequency of 82% compared to only 28% from the SP-treated bacterial population (Fig. 4). Therefore, exposure to SP disrupts pilus bundles into single filaments or small bundles.
FIG 4 .
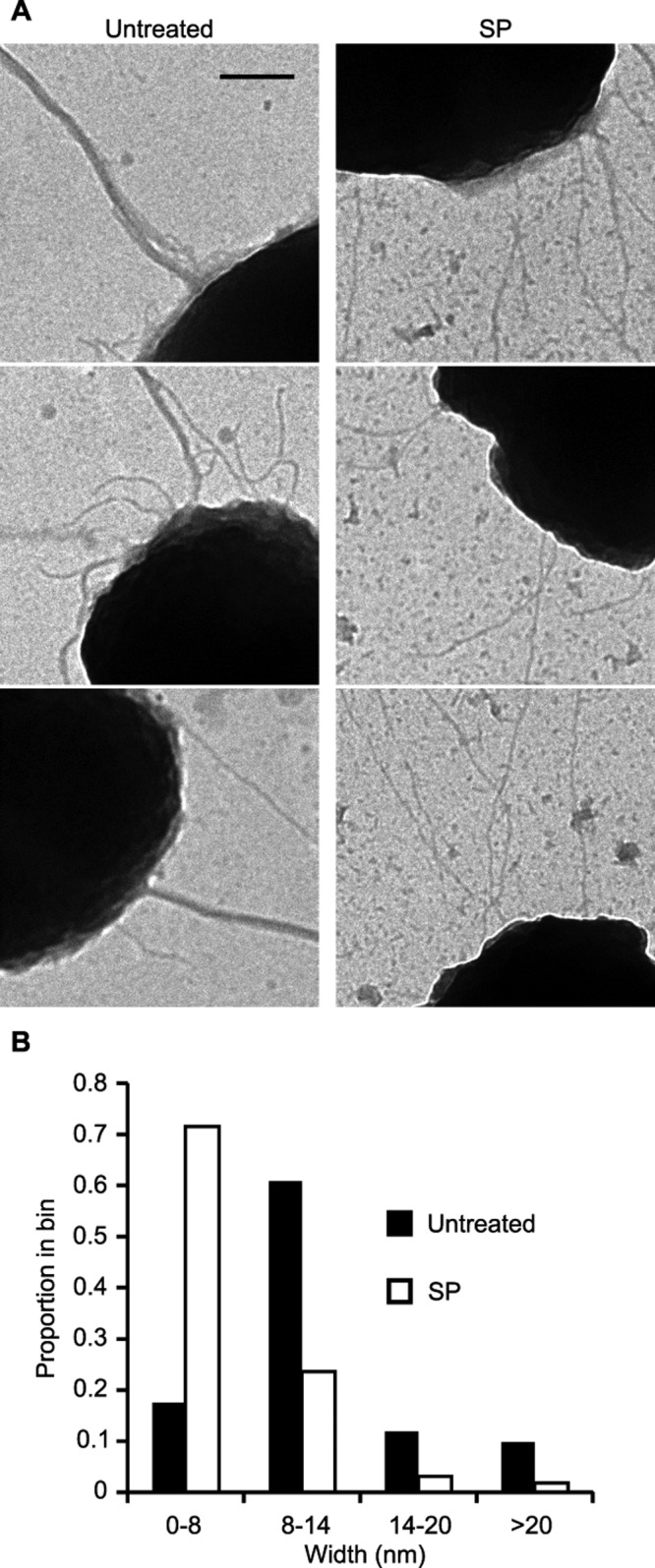
Exposure to SP reduces T4P bundling. (A) Representative TEM images of N. gonorrhoeae T4P in the presence or absence of SP. Bar = 200 nm. (B) The widths of the pili and pilus bundles were determined from TEM micrographs and plotted as a distribution. The median pilus widths for untreated and SP-treated bacteria were 0.011 µm (n = 195) and 0.007 µm (n = 225), respectively. The distributions are significantly different by the Wilcoxon-Mann-Whitney rank sum test (U = 2,100; P < 0.01).
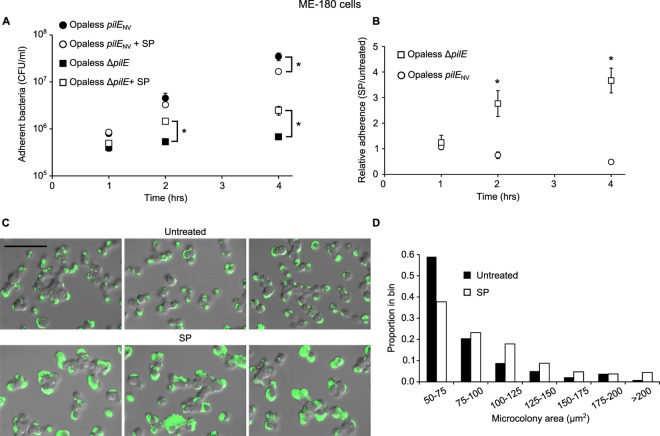
To determine whether SP-mediated disruption of pilus bundles altered interactions with host cells, adherence of N. gonorrhoeae to human epithelial cells was measured. Analysis of pilus-mediated adherence was carried out in strains lacking Opa protein adhesins (Opaless) (32) to avoid introducing variability in adherence originating from the high-frequency phase variation of Opa paralogs (33). The number of bacteria expressing the nonvariable pilE gene that were adherent to ME-180 cells (Fig. 5A) and HEC-1B cells (see Fig. S4 in the supplemental material) was largely unaffected by the presence of SP. A small SP-mediated decrease in the adherence of piliated bacteria at 4 h postinfection was observed using ME-180 cells, but this was not reproducible between cell lines. Interestingly, the adherence of nonpiliated bacteria increased significantly at 2 and 4 h postinfection when SP was present. Furthermore, the relative levels of adherent bacteria in SP-treated versus untreated bacterial infections clearly demonstrate that SP facilitates adherence of gonococci in the absence of pili (Fig. 5B and Fig. S4B). These results indicate that SP mediates pilus-independent bacterial interactions with epithelial cells and suggest that these interactions may be masked by the much higher levels of adherence observed when pili are present. Even though SP did not consistently modulate the total number of piliated bacteria that adhere to epithelial cells, the nature of the bacterium-cell interaction was affected. SP exposure caused a significant increase in the size of bacterial microcolonies on ME-180 cells (Fig. 5C and D) and HEC-1B cells (Fig. S4C and S4D) compared to untreated controls. Bacteria harboring the ∆pilE allele were unable to form large microcolonies regardless of the presence of SP (data not shown). Together, these data support a model in which SP exposure enhances microcolony formation through bacterium-bacterium interactions between unbundled pili and additionally facilitates adherence of nonpiliated gonococci to host cells.
FIG 5 .
SP exposure stimulates gonococcal microcolony formation and adherence of nonpiliated bacteria. (A) Total number of piliated (Opaless pilENV) (MOI of 180) or nonpiliated (Opaless ∆pilE) (MOI of 210) bacteria adherent to ME-180 epithelial cells. Values are mean numbers (± SDs) of viable adherent bacteria from triplicate determinations. (B) Relative adherence of bacteria from SP-treated bacterial infections compared to control infections from panel A. (C) Formation of bacterial microcolonies on ME-180 cells infected with the Opaless pilENV strain (MOI of 113). Bacteria were labeled using an anti-gonococcal antibody and a FITC-conjugated secondary antibody. Representative merged fluorescent and DIC images are shown. Bar = 100 µm. (D) Distribution of fluorescent microcolony sizes (≥50 µm2). The median microcolony area from untreated infections and SP-treated infections was 68 µm2 (n = 239) and 86 µm2 (n = 297), respectively. The distributions are significantly different by the Wilcoxon-Mann-Whitney rank sum test (U = 25,297; P < 0.01). Values that are significantly different (P < 0.01) as determined by the two-sample t test are indicated. Values that are significantly different between treatments in panel A are shown by a bar and an asterisk. Values that are significantly different between strains in panel B are shown by an asterisk.
DISCUSSION
Transmission of N. gonorrhoeae occurs through human sexual contact. Thus, gonococci must be adapted to thrive under these conditions in order to successfully replicate and cause infection. We have shown that whole SP and SP proteins are able to facilitate motility of N. gonorrhoeae. Given that multiple SP proteins and BSA are able to facilitate twitching motility, it is likely that there is little specificity in the interactions between these proteins and the bacteria or T4P. Consistent with this notion is the fact that multiple cellular pilus receptor proteins have been proposed in the literature (34–37). Bacteria undoubtedly encounter numerous proteins during the course of infection, both in contact with the male and female epithelium and in female vaginal secretions (38). However, bacterial exposure to SP represents a massive influx of soluble protein that is specific to the transmission process. The amount of free protein present in SP varies considerably depending on the sample and measurement technique used, but it ranges from ~10 to 70 mg/ml (2). The total protein content of the SP used in this study was approximated at 25 mg/ml (data not shown). Although other environmental factors differ markedly between SP and the genital mucosa, notably pH (2, 39), our data indicate that it is the interaction between T4P and SP proteins that facilitates the twitching motility of N. gonorrhoeae.
The nature of the pilus-surface interactions in part determines the characteristics of N. gonorrhoeae twitching motility. Previous studies have determined that gonococci have limited motility on fluid phospholipid surfaces, but when pili establish an anchor point with BSA-coated or nonfluid membranes, bacteria are able to move at higher velocities (30). In this study, the velocity of motility of SP-treated bacteria was similar to that of BSA-treated bacteria at room temperature, but it was elevated at 37°C. However, the combined retraction force generated by all pili on the surface during SP- and BSA-mediated motility was not significantly different, suggesting that pili were capable of similarly strong surface interactions under both conditions. In support of the idea that SP proteins bind pili, we have shown that SP-treated bacteria have altered morphology in the form of fewer bundled pili (Fig. 4). A similar observation was previously made for bacteria treated with BSA (19), and it is likely that in both cases, nonspecific interactions between soluble proteins and the pilus filaments causes bundle dissolution. However, in contrast to the report of Biais et al. (19), we observed ample pilus bundle formation in the absence of PilT, since the bundle width quantitation experiments reported here were performed with the FA1090 pilENV pilT::erm strain.
The mechanism by which SP exposure decreases motility persistence remains unresolved, but the result is that SP-treated bacteria display strongly unbiased movement. The availability of oxygen has previously been shown to influence the velocity of N. gonorrhoeae motility (40). Although motility and T4P retraction switch to a low-speed state when oxygen becomes depleted, bacterial movement is still persistent under these conditions. Since persistent movement requires multiple cooperative pilus retraction events (20), the decreased persistence observed upon exposure to SP suggests that motor complexes are more disorganized along the perimeter of the bacterial cell and that retraction events occur in competing directions. In a previous study, we observed that BSA treatment reduces directional persistence (20). Since BSA also disrupts bundle formation (19), it is reasonable to propose that T4P bundles enhance persistence. Here we found that SP reduced persistence of movement even more strongly than BSA, demonstrating that T4P coordination is considerably affected by SP.
Our data demonstrate that exposure to SP enhances the formation of bacterial microcolonies on epithelial cells. The model for attachment of gonococci to epithelial cells consists of a continuum of adhesion processes that includes pilus-mediated interactions with the cell surface (13–15). Bacterial aggregates in the form of microcolonies interact with host microvilli and cause rearrangement of the host cytoskeleton and cortical plaque formation (14, 23, 24). PilT function and pilus retraction in microcolonies have also been associated with induction of host cell signaling and even prevention of infection-associated cell death (22, 25, 26). While microcolonies form on epithelial cells that are not treated with SP, the presence of SP significantly increases the size of microcolony aggregates. This suggests that SP exposure creates conditions that favor bacterium-bacterium interactions rather than bacterium-cell interactions. Consistent with this hypothesis is our observation that aggregation of N. gonorrhoeae in the absence of epithelial cells is also increased in the presence of SP (data not shown). The enhanced microcolony formation in the presence of SP therefore promotes the establishment of infection during transmission by allowing bacteria to form robust cell surface aggregates. As has been observed for the related bacterium Neisseria meningitidis, formation of gonococcal aggregates may also be important for resisting shear forces on the cell surface (41). Since microcolony aggregates exist in three dimensions on the cell surface and require intimate bacterium-bacterium contact, these structures may represent a small biofilm that increases in size when exposed to SP.
The presence of seminal fluid in the male and female genital tracts modifies the genital environment via introduction of high concentrations of soluble protein and other SP constituents. This work demonstrates that exposure of the genital pathogen N. gonorrhoeae to SP results in dramatic changes in bacterial motility, pilus morphology, microcolony formation, and adherence. The gonococcal response to SP has the potential to facilitate transmission and colonization of the organism and cumulatively results in a physiological state that we have termed transmission competent. We propose a model in which transmission competence is achieved as a result of SP-mediated physical changes in pilus-surface and pilus-pilus interactions that allows N. gonorrhoeae to achieve favorable motility dynamics and enhanced bacterial aggregation in the host.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
N. gonorrhoeae strain FA1090 expressing the 1-81-S2 pilE variant sequence (42) and strain N400 carrying the recA6 allele (43) were used as the wild-type strains for this study. All strains, with the exception of pilE mutants, expressed type IV pili (T4P) as determined by colony morphology at the outset of each experiment. Derivatives of FA1090 were constructed by spot transformation (44) using genomic DNA from previously constructed N. gonorrhoeae strains and confirmed genotypically. The FA1090 pilENV pilC1PL strain was used to minimize spontaneous loss of pilus expression through the processes of antigenic variation and phase variation. This strain harbors a mutation in the guanine nucleotide repeat region adjacent to pilE that reduces antigenic variation (45). The pilC1PL allele was previously constructed in strain FA7458-1A (54) and maintains the pilC1 gene in a “phase-on” sequence conformation through mutation of the phase-variable poly(G) tract of pilC1 as confirmed by sequencing. The pilE mutant allele consists of a 924-bp deletion that includes the promoter and ribosome binding site of pilE (46). The pilT mutant allele contains an erythromycin resistance cassette within the pilT open reading frame (ORF) and was described previously (47). The pilT mutant strain used here also harbored the pilENV allele. The pilENV strain lacking Opa protein adhesins (Opaless) harboring loss-of-function mutations in all 11 opa genes has been described previously (32) and was transformed with the ∆pilE allele to generate the Opaless ∆pilE derivative. All strains used in this study contained phase-variable opa genes unless indicated as Opaless. N. gonorrhoeae strains were routinely grown in 5% CO2 at 37°C on gonococcal medium base (GCB) (Difco) or in GCB liquid broth (GCBL) modified by the addition of Kellogg’s supplements (48).
Reagents.
Pooled human semen samples from donors who gave consent were obtained commercially from Lee Biosolutions and Innovative Research Biologicals. Seminal plasma (SP) was prepared by separation of spermatozoa and nonsoluble material through centrifugation. Prostate-specific antigen purified from human seminal fluid and recombinant hololactoferrin were also obtained from Lee Biosolutions. Human serum albumin was purchased from Sigma-Aldrich.
Transwell assays.
Growth from GCB agar plates incubated ~18 h was used to inoculate GCBL cultures at a low initial density. Liquid cultures were incubated for 3 h, and bacteria were harvested by centrifugation. Bacteria were then washed and resuspended in RPMI 1640 with l-glutamine without phenol red (Cellgro). Transwells with 3.0-µm-pore polycarbonate membranes (Costar) were seeded with 2 × 106 CFU in a 0.2-ml volume, and 0.6-ml volumes of test solutions diluted in RPMI 1640 were added to the lower chambers. Transwells were incubated at 37°C for 1 h, and the number of bacteria that migrated through the membrane barrier was determined by serial dilution and colony counts. Piliated and nonpiliated N. gonorrhoeae bacteria were differentiated by characteristic colony morphology on GCB plates and enumerated separately. Transwell assays involving the FA1090 pilENV pilC1PL strain did not distinguish between piliated and nonpiliated bacteria, since this strain undergoes little spontaneous loss of T4P expression.
Size filtration of SP was accomplished using Centriprep 10,000-Da-nominal-molecular-size-limit filters (Millipore) according to the manufacturer’s recommendations. Filtrate and retentate volumes were normalized to account for concentration of the retentate during filtration.
Quantitation of motility.
The velocity of motility was determined using the FA1090 pilENV pilC1PL strain or the FA1090 pilENV pilT::erm strain as indicated. The bacteria were grown on GCB agar for ~18 h and then equilibrated to room temperature along with all other reagents prior to analysis. The bacteria were suspended in Dulbecco modified Eagle medium (DMEM) (with 4 mM glutamine, 4.5 g/liter glucose, and 8 mM sodium pyruvate and without phenol red) (HyClone) to an optical density of 0.3 at a 550-nm wavelength. The bacteria were treated in suspension with the following test solutions at the indicated concentrations: SP, 1:20; bovine serum albumin (BSA), 1.25 mg/ml; human serum albumin, 1 mg/ml; lactoferrin, 1 mg/ml; and prostate-specific antigen, 0.75 mg/ml. All SP proteins were solubilized in phosphate-buffered saline (PBS), and an appropriate volume of PBS was added to DMEM as the negative control. Samples of bacterial suspensions were immediately spotted onto slides and imaged via differential interference contrast (DIC) microscopy using a Nikon 90i microscope and a 100× lens objective at room temperature. Ten fields were captured for a duration of 30 s at a rate of 20 frames/s for each condition. All bacteria in a given field were subjected to quantitation using the NIS Elements v.4 tracking module. Bacteria that yielded tracks of less than 10 s were eliminated from the analysis, as this often corresponded with detachment from the motility surface.
Transmission electron microscopy.
The FA1090 pilENV pilT::erm strain was used to visualize bacterium-associated pili via transmission electron microscopy. Bacteria were gently suspended in PBS after growth on GCB agar and treated with either a 1:100 dilution of SP or left untreated for 20 min at room temperature. Bacterial suspensions were placed on 300-mesh nickel grids with carbon support films (Ted Pella) and allowed to adhere for 20 min. Bacteria were then fixed in 1% glutaraldehyde, washed, and stained with 1% phosphotungstic acid. Images were captured on a FEI Tecnai Spirit G2 120-kV transmission electron microscope at a magnification of ×18,500. Pilus widths were quantitated from captured images of well-isolated bacteria using the ImageJ software (49). Only bacterium-associated pili were considered, and measurements were taken proximal to the bacterium.
Quantitation of motility persistence.
The persistence of motility in the presence of BSA or SP was determined as described previously (20) by calculating the mean-squared displacement (MSD)
of a motile bacterium as a function of time as follows:
Here, the data were fitted for 0.1 s < τ < 15 s, a characteristic speed of v = (1.6 ± 0.1) µm/s, a correlation time τc = (2.3 ± 0.5) s, and an offset A = (0.02 ± 0.04) µm2. Strain N400 was treated in DMEM suspension with either 1 mg/ml BSA or a 1:20 dilution of SP. Images were recorded at 10 frames/s and the temperature was set at 37°C. The speed of pilus retraction and consequently surface motility is temperature dependent. Therefore, the velocities determined for persistence measurements are greater than those reported in Fig. 1. The conditions for measuring persistence time were different than in previous studies (20) in agreement with lower observed values in the presence of BSA here.
Retraction force measurement.
The optical tweezers were assembled on a Zeiss Axiovert 200 microscope as described previously (50). The retraction force measurement assay was modified here to measure the force generated by a single surface-attached bacterium as a function of time (see Fig. S3 in the supplemental material). The trap was calibrated by spectrum analysis of Brownian motion of individual monococci and was found to have a stiffness of 0.065 pN/nm ± 10%. The position of trapped bacteria was detected at 20 kHz using a quadrant photodiode, and the sample stage was movable via a combined piezo and electric motor.
The retraction experiments were performed using a low-density suspension of strain N400, allowing for undisturbed measurement of single bacteria over a period of minutes. During the experiment, a single bacterium was trapped near the surface using optical tweezers. Retraction of surface-bound pili resulted in deflection of the bacterium from the center of the optical trap as measured by the four-quadrant photodiode. The force acting on a bacterium is proportional to this deflection and was calculated using the calibration described above. After several minutes, a detrimental effect of the optical trap on the bacterium became apparent, and the bacterium was abandoned.
Adherence to epithelial cells.
The influence of SP on N. gonorrhoeae adherence was tested using the FA1090 Opaless and Opaless ∆pilE strains (32), since Opa proteins are known mediators of gonococcal adherence (51, 52) and spontaneously undergo “on-off” phase variation at high frequency (33). ME-180 or HEC-1B cells were seeded at a density of 1 × 105 cells per well in 24-well plates and allowed to incubate overnight in McCoy’s 5A medium or minimal essential medium both containing 10% fetal bovine serum (FBS) (Gibco). Bacteria were grown in GW medium (53) for 3 h prior to infection. Subconfluent monolayers were washed with PBS to remove the media and FBS and then infected with normalized bacterial suspensions at a multiplicity of infection (MOI) of ~100 in GW medium either containing a 1:20 dilution of SP or lacking SP. Cells incubated at 37°C were washed extensively with PBS to remove nonadherent bacteria at the indicated time points, after which cells were permeabilized by treatment with 1% saponin, and the number of adherent bacteria was determined by serial dilution and colony counts.
Microcolony formation.
For analysis of microcolony formation, ME-180 and HEC-1B cells were seeded at a density of 2 × 105 on poly-l-lysine-treated glass coverslips and allowed to adhere overnight. After the coverslips were washed to remove media and FBS, cells were infected with the Opaless pilENV strain as described above for adherence assays. Following incubation at 37°C for 2 h, the coverslips were washed extensively to remove nonadherent bacteria, then fixed in 4% paraformaldehyde, and blocked with 10% normal goat serum. Bacteria were detected using a rabbit anti-gonococcal primary antibody (Meridian Life Sciences) and a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit secondary antibody (Jackson Immunoresearch). Images were obtained using a Nikon 90i microscope and a 40× objective lens. The area of bacterial aggregates was determined from 10 fields for each condition using the Nikon NIS Elements software, and areas of fluorescence of ≥50 µm2 were considered microcolonies.
SUPPLEMENTAL MATERIAL
Stimulatory activity of dilute SP and retention of active components after size filtration. (A) Comparison between the Transwell migration of the parent strain (FA1090) and a pilus-locked strain (pilENV pilC1PL). The number of bacteria that cross the membrane barrier in response to RPMI 1640 (open bars) or RPMI 1640 containing a 1:20 dilution of SP (closed bars) is reported. Asterisks indicate a significant increase in migration compared to the RPMI 1640 condition. (B) Two-fold serial dilutions from initial concentrations of 1:20 SP and 1.25 mg/ml BSA (shaded bars) were tested for motility-stimulating activity in comparison to RPMI 1640 (open bars) using the FA1090 pilENV pilC1PL strain. The dilution series contain approximately equivalent total protein concentrations. Asterisks indicate a significant decrease in activity compared to the highest concentration of test solution. (C) Ability of SP to stimulate FA1090 barrier migration after filtration with a 10,000-Da-nominal-molecular-size limit filter. Unfiltered SP was tested at a 1:20 dilution. Asterisks indicate a significant increase in activity compared to the RPMI 1640 condition. All values are means (± SD) from triplicate determinations. Statistical significance was determined by the two-sample t test (P < 0.01). Download
Tracking motility of N. gonorrhoeae. Representative images from motility quantitation experiments showing an overlay of tracks after 30 s of time-lapse capture. Untreated bacteria (DMEM) were nonmotile. Greater persistence of motility can be observed for bacteria treated with BSA than for bacteria treated with SP. Images were captured by DIC microscopy, and bacteria and tracks were colored (false colors) during the analysis. Bar = 10 µm. Download
Schematic of pilus retraction force measurements. (A) A single bacterium was trapped and maintained near the glass coverslip surface using optical tweezers. Attachment of the T4P to the glass coverslip surface (x) provided anchor points for retraction. (B) Movement of the bacterium was strongly inhibited by the optical trap, but deflection of the bacterium from the center of the trap was achieved by cooperative retraction of multiple surface-bound pili and was measured as displacement (d). The displacement was used to calculate the force (F) generated by pilus retraction as a function of time. (C) Representative force measurement for a single bacterium treated with SP. In the absence of pilus retraction, no displacement occurs, and the force is zero (left of the thick black line). Pilus retraction events result in displacement of the bacterium from the optical trap (right of the thick black line), allowing for calculation of pilus retraction force. Download
SP exposure stimulates gonococcal microcolony formation and adherence of nonpiliated bacteria. (A) Total number of piliated (Opaless pilENV) (MOI of 165) or nonpiliated (Opaless ∆pilE) (MOI of 160) bacteria adherent to HEC-1B epithelial cells. Values are mean numbers (± SDs) of viable adherent bacteria from triplicate determinations. (B) Relative adherence of bacteria from SP-treated bacterial infections compared to control bacterial infections from panel A. (C) Formation of bacterial microcolonies on HEC-1B cells infected with the Opaless pilENV strain (MOI of 113). Bacteria were labeled using an anti-gonococcal antibody and a FITC-conjugated secondary antibody. Representative merged fluorescent and DIC images are shown. Bar = 100 µm. (D) Distribution of fluorescent microcolony sizes (≥50 µm2). The median microcolony areas from untreated bacterial infections and SP-treated bacterial infections were 70 µm2 (n = 225) and 94 µm2 (n = 270), respectively. The distributions are significantly different by the Wilcoxon-Mann-Whitney rank sum test (U = 21,108; P < 0.01). Asterisks indicate values that are significantly different (P < 0.01) between treatments (A) or between strains (B) as determined by the two-sample t test. Download
ACKNOWLEDGMENTS
This work was supported by the NIH (R37 AI033493 and R01 AI044239 to H.S.S.) and DFG (MA3898 to B.M.). Electron microscopy and NIS Elements analysis were performed at the Northwestern University Cell Imaging Facility that is supported by CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center.
We thank C. Thomas for providing strain FA7458-1A, C. Mauer and G. Smith for assistance with image analysis, and K. Satchell, N. Cianciotto, and A. Chen for critical reading of the manuscript.
Footnotes
Citation Anderson MT, Dewenter L, Maier B, Seifert HS. 2014. Seminal plasma initiates a Neisseria gonorrhoeae transmission state. mBio 5(2):e01004-13. doi:10.1128/mBio.01004-13.
REFERENCES
- 1. Lee KC, Ladizinski B. 2012. The clap heard round the world. Arch. Dermatol. 148:223. 10.1001/archdermatol.2011.2716 [DOI] [PubMed] [Google Scholar]
- 2. Owen DH, Katz DF. 2005. A review of the physical and chemical properties of human semen and the formulation of a semen simulant. J. Androl. 26:459–469. 10.2164/jandrol.04104 [DOI] [PubMed] [Google Scholar]
- 3. Pilch B, Mann M. 2006. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 7:R40. 10.1186/gb-2006-7-5-r40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James-Holmquest AN, Swanson J, Buchanan TM, Wende RD, Williams RP. 1974. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect. Immun. 9:897–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harvey HA, Porat N, Campbell CA, Jennings M, Gibson BW, Phillips NJ, Apicella MA, Blake MS. 2000. Gonococcal lipooligosaccharide is a ligand for the asialoglycoprotein receptor on human sperm. Mol. Microbiol. 36:1059–1070. 10.1046/j.1365-2958.2000.01938.x [DOI] [PubMed] [Google Scholar]
- 6. Howard TL. 1971. Bacterial hitch-hikers. J. Urol. 106:94. [DOI] [PubMed] [Google Scholar]
- 7. Craig L, Pique ME, Tainer JA. 2004. Type IV pilus structure and bacterial pathogenicity. Nat. Rev. Microbiol. 2:363–378 [DOI] [PubMed] [Google Scholar]
- 8. Varga JJ, Nguyen V, O’Brien DK, Rodgers K, Walker RA, Melville SB. 2006. Type IV pili-dependent gliding motility in the Gram-positive pathogen Clostridium perfringens and other clostridia. Mol. Microbiol. 62:680–694. 10.1111/j.1365-2958.2006.05414.x [DOI] [PubMed] [Google Scholar]
- 9. Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. 2006. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol. Cell 23:651–662. 10.1016/j.molcel.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 10. Chamot-Rooke J, Mikaty G, Malosse C, Soyer M, Dumont A, Gault J, Imhaus AF, Martin P, Trellet M, Clary G, Chafey P, Camoin L, Nilges M, Nassif X, Duménil G. 2011. Posttranslational modification of pili upon cell contact triggers N. meningitidis dissemination. Science 331:778–782. 10.1126/science.1200729 [DOI] [PubMed] [Google Scholar]
- 11. Hagblom P, Segal E, Billyard E, So M. 1985. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature 315:156–158. 10.1038/315156a0 [DOI] [PubMed] [Google Scholar]
- 12. Cahoon LA, Seifert HS. 2011. Focusing homologous recombination: pilin antigenic variation in the pathogenic Neisseria. Mol. Microbiol. 81:1136–1143. 10.1111/j.1365-2958.2011.07773.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swanson J. 1973. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137:571–589. 10.1084/jem.137.3.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McGee ZA, Johnson AP, Taylor-Robinson D. 1981. Pathogenic mechanisms of Neisseria gonorrhoeae: observations on damage to human fallopian tubes in organ culture by gonococci of colony type 1 or type 4. J. Infect. Dis. 143:413–422. 10.1093/infdis/143.3.413 [DOI] [PubMed] [Google Scholar]
- 15. Merz AJ, Rifenbery DB, Arvidson CG, So M. 1996. Traversal of a polarized epithelium by pathogenic Neisseriae: facilitation by type IV pili and maintenance of epithelial barrier function. Mol. Med. 2:745–754 [PMC free article] [PubMed] [Google Scholar]
- 16. Mosleh IM, Boxberger HJ, Sessler MJ, Meyer TF. 1997. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect. Immun. 65:3391–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sparling PF. 1966. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 92:1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merz AJ, So M, Sheetz MP. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98–102. 10.1038/35024105 [DOI] [PubMed] [Google Scholar]
- 19. Biais N, Ladoux B, Higashi D, So M, Sheetz M. 2008. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 6:e87. 10.1371/journal.pbio.0060087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holz C, Opitz D, Greune L, Kurre R, Koomey M, Schmidt MA, Maier B. 2010. Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys. Rev. Lett. 104:178104. 10.1103/PhysRevLett.104.178104 [DOI] [PubMed] [Google Scholar]
- 21. Whitchurch CB, Leech AJ, Young MD, Kennedy D, Sargent JL, Bertrand JJ, Semmler AB, Mellick AS, Martin PR, Alm RA, Hobbs M, Beatson SA, Huang B, Nguyen L, Commolli JC, Engel JN, Darzins A, Mattick JS. 2004. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Mol. Microbiol. 52:873–893. 10.1111/j.1365-2958.2004.04026.x [DOI] [PubMed] [Google Scholar]
- 22. Higashi DL, Lee SW, Snyder A, Weyand NJ, Bakke A, So M. 2007. Dynamics of Neisseria gonorrhoeae attachment: microcolony development, cortical plaque formation, and cytoprotection. Infect. Immun. 75:4743–4753. 10.1128/IAI.00687-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Merz AJ, So M. 1997. Attachment of piliated, Opa− and Opc− gonococci and meningococci to epithelial cells elicits cortical actin rearrangements and clustering of tyrosine-phosphorylated proteins. Infect. Immun. 65:4341–4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merz AJ, Enns CA, So M. 1999. Type IV pili of pathogenic Neisseriae elicit cortical plaque formation in epithelial cells. Mol. Microbiol. 32:1316–1332. 10.1046/j.1365-2958.1999.01459.x [DOI] [PubMed] [Google Scholar]
- 25. Lee SW, Higashi DL, Snyder A, Merz AJ, Potter L, So M. 2005. PilT is required for PI(3,4,5)P3-mediated crosstalk between Neisseria gonorrhoeae and epithelial cells. Cell. Microbiol. 7:1271–1284. 10.1111/j.1462-5822.2005.00551.x [DOI] [PubMed] [Google Scholar]
- 26. Dietrich M, Bartfeld S, Munke R, Lange C, Ogilvie LA, Friedrich A, Meyer TF. 2011. Activation of NF-κB by Neisseria gonorrhoeae is associated with microcolony formation and type IV pilus retraction. Cell. Microbiol. 13:1168–1182. 10.1111/j.1462-5822.2011.01607.x [DOI] [PubMed] [Google Scholar]
- 27. Jonsson AB, Nyberg G, Normark S. 1991. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J. 10:477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elzanaty S, Erenpreiss J, Becker C. 2007. Seminal plasma albumin: origin and relation to the male reproductive parameters. Andrologia 39:60–65. 10.1111/j.1439-0272.2007.00764.x [DOI] [PubMed] [Google Scholar]
- 29. Lilja H, Oldbring J, Rannevik G, Laurell CB. 1987. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J. Clin. Invest. 80:281–285. 10.1172/JCI113070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holz C, Opitz D, Mehlich J, Ravoo BJ, Maier B. 2009. Bacterial motility and clustering guided by microcontact printing. Nano Lett. 9:4553–4557. 10.1021/nl903153c [DOI] [PubMed] [Google Scholar]
- 31. Todd WJ, Wray GP, Hitchcock PJ. 1984. Arrangement of pili in colonies of Neisseria gonorrhoeae. J. Bacteriol. 159:312–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ball LM, Criss AK. 2013. Constitutively Opa-expressing and Opa-deficient Neisseria gonorrhoeae strains differentially stimulate and survive exposure to human neutrophils. J. Bacteriol. 195:2982–2990. 10.1128/JB.00171-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mayer LW. 1982. Rates of in vitro changes of gonococcal colony opacity phenotypes. Infect. Immun. 37:481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blom AM, Rytkönen A, Vasquez P, Lindahl G, Dahlbäck B, Jonsson AB. 2001. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4B-binding protein. J. Immunol. 166:6764–6770 [DOI] [PubMed] [Google Scholar]
- 35. Coureuil M, Lécuyer H, Scott MG, Boularan C, Enslen H, Soyer M, Mikaty G, Bourdoulous S, Nassif X, Marullo S. 2010. Meningococcus hijacks a β2-adrenoceptor/β-arrestin pathway to cross brain microvasculature endothelium. Cell 143:1149–1160. 10.1016/j.cell.2010.11.035 [DOI] [PubMed] [Google Scholar]
- 36. Edwards JL, Brown EJ, Uk-Nham S, Cannon JG, Blake MS, Apicella MA. 2002. A co-operative interaction between Neisseria gonorrhoeae and complement receptor 3 mediates infection of primary cervical epithelial cells. Cell. Microbiol. 4:571–584. 10.1046/j.1462-5822.2002.t01-1-00215.x [DOI] [PubMed] [Google Scholar]
- 37. Källström H, Liszewski MK, Atkinson JP, Jonsson AB. 1997. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol. Microbiol. 25:639–647. 10.1046/j.1365-2958.1997.4841857.x [DOI] [PubMed] [Google Scholar]
- 38. Huggins GR, Preti G. 1981. Vaginal odors and secretions. Clin. Obstet. Gynecol. 24:355–377. 10.1097/00003081-198106000-00005 [DOI] [PubMed] [Google Scholar]
- 39. Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. 10.1016/S0010-7824(99)00010-4 [DOI] [PubMed] [Google Scholar]
- 40. Kurre R, Maier B. 2012. Oxygen depletion triggers switching between discrete speed modes of gonococcal type IV pili. Biophys. J. 102:2556–2563. 10.1016/j.bpj.2012.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mikaty G, Soyer M, Mairey E, Henry N, Dyer D, Forest KT, Morand P, Guadagnini S, Prévost MC, Nassif X, Duménil G. 2009. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 5:e1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seifert HS, Wright CJ, Jerse AE, Cohen MS, Cannon JG. 1994. Multiple gonococcal pilin antigenic variants are produced during experimental human infections. J. Clin. Invest. 93:2744–2749. 10.1172/JCI117290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seifert HS. 1997. Insertionally inactivated and inducible recA alleles for use in Neisseria. Gene 188:215–220. 10.1016/S0378-1119(96)00810-4 [DOI] [PubMed] [Google Scholar]
- 44. Howell-Adams B, Wainwright LA, Seifert HS. 1996. The size and position of heterologous insertions in a silent locus differentially affect pilin recombination in Neisseria gonorrhoeae. Mol. Microbiol. 22:509–522. 10.1046/j.1365-2958.1996.00128.x [DOI] [PubMed] [Google Scholar]
- 45. Cahoon LA, Seifert HS. 2009. An alternative DNA structure is necessary for pilin antigenic variation in Neisseria gonorrhoeae. Science 325:764–767. 10.1126/science.1175653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen CJ, Tobiason DM, Thomas CE, Shafer WM, Seifert HS, Sparling PF. 2004. A mutant form of the Neisseria gonorrhoeae pilus secretin protein PilQ allows increased entry of heme and antimicrobial compounds. J. Bacteriol. 186:730–739. 10.1128/JB.186.3.730-739.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Long CD, Tobiason DM, Lazio MP, Kline KA, Seifert HS. 2003. Low-level pilin expression allows for substantial DNA transformation competence in Neisseria gonorrhoeae. Infect. Immun. 71:6279–6291. 10.1128/IAI.71.11.6279-6291.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9:671–675. 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Clausen M, Koomey M, Maier B. 2009. Dynamics of type IV pili is controlled by switching between multiple states. Biophys. J. 96:1169–1177. 10.1016/j.bpj.2008.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Swanson J, Sparks E, Young D, King G. 1975. Studies on gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect. Immun. 11:1352–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sadarangani M, Pollard AJ, Gray-Owen SD. 2011. Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria. FEMS Microbiol. Rev. 35:498–514. 10.1111/j.1574-6976.2010.00260.x [DOI] [PubMed] [Google Scholar]
- 53. Wade JJ, Graver MA. 2007. A fully defined, clear and protein-free liquid medium permitting dense growth of Neisseria gonorrhoeae from very low inocula. FEMS Microbiol. Lett. 273:35–37. 10.1111/j.1574-6968.2007.00776.x [DOI] [PubMed] [Google Scholar]
- 54. Cheng Y, Johnson MD, Burillo-Kirch C, Mocny JC, Anderson JE, Garrett CK, Redinbo MR, Thomas CE. 2013. Mutation of the conserved calcium-binding motif in Neisseria gonorrhoeae PilC1 impacts adhesion but not piliation. Infect. Immun. 81:4280–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stimulatory activity of dilute SP and retention of active components after size filtration. (A) Comparison between the Transwell migration of the parent strain (FA1090) and a pilus-locked strain (pilENV pilC1PL). The number of bacteria that cross the membrane barrier in response to RPMI 1640 (open bars) or RPMI 1640 containing a 1:20 dilution of SP (closed bars) is reported. Asterisks indicate a significant increase in migration compared to the RPMI 1640 condition. (B) Two-fold serial dilutions from initial concentrations of 1:20 SP and 1.25 mg/ml BSA (shaded bars) were tested for motility-stimulating activity in comparison to RPMI 1640 (open bars) using the FA1090 pilENV pilC1PL strain. The dilution series contain approximately equivalent total protein concentrations. Asterisks indicate a significant decrease in activity compared to the highest concentration of test solution. (C) Ability of SP to stimulate FA1090 barrier migration after filtration with a 10,000-Da-nominal-molecular-size limit filter. Unfiltered SP was tested at a 1:20 dilution. Asterisks indicate a significant increase in activity compared to the RPMI 1640 condition. All values are means (± SD) from triplicate determinations. Statistical significance was determined by the two-sample t test (P < 0.01). Download
Tracking motility of N. gonorrhoeae. Representative images from motility quantitation experiments showing an overlay of tracks after 30 s of time-lapse capture. Untreated bacteria (DMEM) were nonmotile. Greater persistence of motility can be observed for bacteria treated with BSA than for bacteria treated with SP. Images were captured by DIC microscopy, and bacteria and tracks were colored (false colors) during the analysis. Bar = 10 µm. Download
Schematic of pilus retraction force measurements. (A) A single bacterium was trapped and maintained near the glass coverslip surface using optical tweezers. Attachment of the T4P to the glass coverslip surface (x) provided anchor points for retraction. (B) Movement of the bacterium was strongly inhibited by the optical trap, but deflection of the bacterium from the center of the trap was achieved by cooperative retraction of multiple surface-bound pili and was measured as displacement (d). The displacement was used to calculate the force (F) generated by pilus retraction as a function of time. (C) Representative force measurement for a single bacterium treated with SP. In the absence of pilus retraction, no displacement occurs, and the force is zero (left of the thick black line). Pilus retraction events result in displacement of the bacterium from the optical trap (right of the thick black line), allowing for calculation of pilus retraction force. Download
SP exposure stimulates gonococcal microcolony formation and adherence of nonpiliated bacteria. (A) Total number of piliated (Opaless pilENV) (MOI of 165) or nonpiliated (Opaless ∆pilE) (MOI of 160) bacteria adherent to HEC-1B epithelial cells. Values are mean numbers (± SDs) of viable adherent bacteria from triplicate determinations. (B) Relative adherence of bacteria from SP-treated bacterial infections compared to control bacterial infections from panel A. (C) Formation of bacterial microcolonies on HEC-1B cells infected with the Opaless pilENV strain (MOI of 113). Bacteria were labeled using an anti-gonococcal antibody and a FITC-conjugated secondary antibody. Representative merged fluorescent and DIC images are shown. Bar = 100 µm. (D) Distribution of fluorescent microcolony sizes (≥50 µm2). The median microcolony areas from untreated bacterial infections and SP-treated bacterial infections were 70 µm2 (n = 225) and 94 µm2 (n = 270), respectively. The distributions are significantly different by the Wilcoxon-Mann-Whitney rank sum test (U = 21,108; P < 0.01). Asterisks indicate values that are significantly different (P < 0.01) between treatments (A) or between strains (B) as determined by the two-sample t test. Download