Abstract
Alzheimer’s disease (AD) is the most common form of dementia, characterized by a decline in memory and cognitive function. Clinical manifestations of AD are closely associated with the formation of senile plaques and neurofibrillary tangles, neuronal loss and cognitive decline. Apoptosis signal regulating kinase 1 (ASK1) is a mediator of the MAPK pathway, which regulates various cellular responses such as apoptosis, cell survival, and differentiation. Accumulating evidence indicates that ASK1 plays a key role in the pathogenesis of neurodegenerative disorders such as Huntington’s disease and AD. Of particular interest, ASK1 is associated with many signaling pathways, which include endoplasmic reticulum (ER) stress-mediated apoptosis, Aβ-induced neurotoxicity, tau protein phosphorylation, and insulin signal transduction. Here, we review experimental evidence that links ASK1 signaling and AD pathogenesis and propose that ASK1 might be a new point of therapeutic intervention to prevent or treat AD.
Keywords: apoptosis signal regulating kinase 1 (ASK1), Alzheimer’s disease (AD), oxidative stress, endoplasmic reticulum (ER) stress, Aβ neurotoxicity, tau protein phosphorylation, insulin signal transduction
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by neuronal loss, aggregation of senile plaques derived from amyloid beta (Aβ) peptides, abnormal phosphorylation of tau protein and cognitive decline in the hippocampus or cortex [1,2]. Reactive oxygen species (ROS) production and activation of c-Jun N-terminal kinases (JNKs) are involved in many pathological mechanisms in AD [3]. Apoptosis signal-regulating kinase 1 (ASK1) is a protein kinase of the mitogen-activated protein kinase kinase kinase (MAPKKK) family that activates the JNK and p38 MAPK signaling cascades [4,5]. ASK1 is related to various cellular responses including apoptosis, cell survival, and differentiation [6,7]. ASK1 is activated in response to various stresses including tumor necrosis factor (TNF), endoplasmic reticulum (ER) stress, and H2O2 [4,5,8–10]. In addition, Aβ leading to AD pathology [11] can activate ASK1 that is required for ROS and ER stress-induced JNK activation [12–15]. Insulin like growth factor-1 receptor (IGF-IR) signaling suppresses the ASK1 mediated activation of JNK/p38 pathway. Insulin-like growth factor-1 (IGF-I) can suppress apoptosis, interfere downstream of tumor necrosis factor receptor (TNF-R) activation [16] and block the ASK1 mediated JNK activation by Aβ [17]. The activation of ASK1 also leads to tau phosphorylation that aggravates AD pathology [18]. Therefore, the deterioration of central nervous system (CNS) insulin receptor functions is related to the pathogenesis of sporadic Alzheimer’s disease [19–23]. The cognitive decline is involved in brain insulin dysfunction [24]. ASK1 is involved in insulin signal transduction through TNF-α-induced JNK signaling [25]. In conclusion, ASK1 is associated with various mechanisms, which include cell death, Aβ neurotoxicity, abnormal phosphorylation of tau protein and impaired insulin signal transduction. Hence, ASK1 is involved in mechanisms related to AD pathology.
2. ASK1 and Oxidative Stress
The various pathologies in AD are associated with neuronal cell death by oxidative stress. ROS are produced as part of normal cellular metabolic activity. However, excessive production of ROS under oxidative stress causes cell death via apoptosis. MAP kinase signaling involves pathways linking ROS. ASK1 is a MAPKKK and activates both the mitogen-activated protein kinase kinase 4 (MKK4)/MKK7-JNK pathway and MKK3/MKK6-p38 pathway [26]. ASK1 is activated in response to various stresses including TNF, ER stress, and H2O2 [4,5,8–10,27]. Tobiume et al. demonstrated that the activation of the JNK/p38 pathway is attenuated in fibroblasts from ASK1-knockout mice after H2O2 treatment [5]. The ASK1 activation is regulated by multiple steps including dimerization, phosphorylation, and protein-protein interactions [8,28–31]. Thioredoxin (TRX), which regulates the cellular reduction and oxidation (redox) status, is bound directly to the N-terminal region of ASK1 [15]. In the oxidative stress state, ROS induce dissociation of Trx from ASK1. ASK1 is subsequently activated by inducing the oligomerization and the phosphorylation of a critical threonine residue [15,32]. A recent study using gel filtration column chromatography demonstrated that ASK1 constitutively forms the ASK1 signalosome as a high molecular mass complex [33]. The ASK1 signalosome forms a molecular mass complex by recruiting at least two TNF receptor-associated factor (TRAF) family proteins, TRAF2 and TRAF6, which appear to stabilize the complex and promote the activation of ASK1 phosphorylation [33]. Also, in the oxidative stress state, the attenuation of TNF-α expression in the cells isolated from ASK1-knockout mice suggests that ASK1 may act as a regulator of cytokine [5]. ASK1 is associated with TNF-α-induced apoptosis cascades [4,5,26]. To sum up, ASK1 is activated by forming “ASK1 signalosome” with TRAF family proteins in response to oxidative stress and ASK1 is involved in the TNF-α-induced apoptosis pathway (Figure 1).
Figure 1.
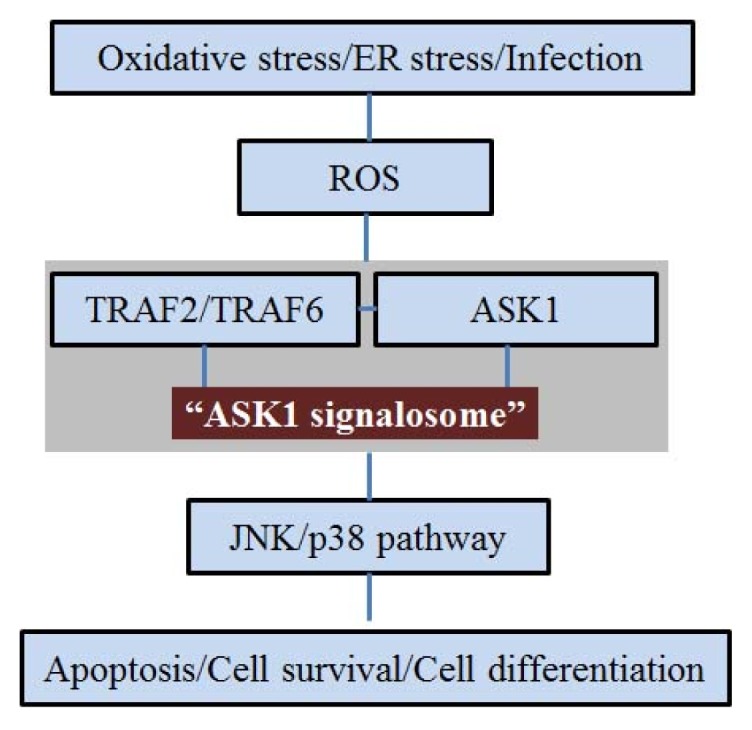
Schematic representation of the relationship between apoptosis signal regulating kinase 1 (ASK1) and c-Jun N-terminal kinases (JNK)/p38 pathway activated by various stresses. Various stresses including oxidative stress, ER stress, and bacterial infection, generate reactive oxygen species (ROS). ASK1 activates by forming ASK1 signalosome with TNF receptor-associated factor (TRAF)2/TRAF6. The ASK1 signalosome induces the JNK/p38 pathway and regulates a variety of cellular signal pathways including cell death, cell survival, and the cell differentiation pathway.
3. ASK1 and ER Stress
The ER stress is caused by the accumulation of unfolded and misfolded proteins in the ER lumen and triggers multiple signals leading to translational and transcriptional apoptosis [34,35]. The ER stress is related to neuronal death occurring in AD [14,36–38]. Mutations of presenilin-1 (PS1) located in the ER are the most common finding in patients with AD. Cells that express PS1 mutants have been reported to be more sensitive to ER stress compared to normal cells [36,38–40]. Based on this relationship, ER stress is an important point to study AD pathology. ASK1 is activated in response to ER stress [4,41] and is required for ER stress-mediated apoptosis [15]. Inositol-requiring enzyme 1 (IRE1) is associated with neuronal death related to ER stress [42] and specifically combines with ASK1. ER stress induces formation of an IRE1-TRAF2-ASK1 complex and activates the ASK1-JNK pathway [15]. The TRAF2-ASK1-JNK pathway plays a central role in ER stress-induced apoptosis [15]. Kadowaki et al. demonstrated that primary neurons derived from ASK1-knockout mice brain were resistant to ER stress-induced cell death [12]. In conclusion, ASK1 ultimately activates the JNK pathway in ER stress-induced apoptosis as a component of the IRE1-TRAF2-ASK1 cascade.
4. ASK1 and Aβ
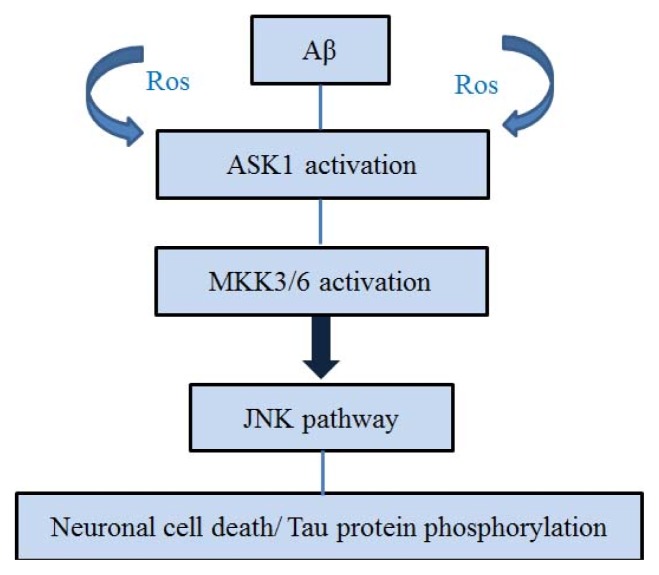
The extracellular deposition of senile plaques composed of Aβ and the formation of intracellular neurofibrillary tangles (NFT) caused by abnormal phosphorylation of tau proteins related to regulation of microtubule stability aggravate the progression of AD [43–45]. Aβ is a product of the cleaved amyloid precursor protein (APP) and accumulates as extracellular plaque in the AD brain [46–48]. Kadowaki et al. suggested the possibility that Aβ neurotoxicity might be mediated by activation of the ASK1 [12]. Hsu et al. demonstrated that the activation of the ASK1-MKK3/6-p38MAPK signaling cascade triggered Aβ-induced cell death in cerebral endothelial cell [49]. Also, tau proteins undergo abnormal phosphorylation and dissociate from microtubules to aggregate into NFTs [50,51]. Hyperphosphorylated tau proteins accumulate to form insoluble paired helical filaments (PHF) within neuronal cell bodies. Aβ induces activation of JNK and phosphorylation of c-Jun [52,53]. Previous studies have demonstrated that Aβ-induced neuronal cell death is inhibited by the expression of a dominant-negative mutant of c-Jun, by the treatment with a JNK inhibitor, or by the disruption of c-Jun or JNK3 [52–54]. Also, Reynolds et al. demonstrated that, in the Tg2576/ PS1P264L brain, JNK activation was localized in reactive neurites containing phosphorylated tau proteins. Previous studies have demonstrated that JNK can regulate hyperphosphorylated tau proteins in AD [55]. MKK6 and p38 are recruited by tau, leading to tau phosphorylation at specific and distinct p38-dependent sites [18]. MKK3, MKK4 and MKK6 are JNK-activating MAPK kinases that can be activated by a number of MAPKK kinases including ASK1 [56]. Hashimoto et al. demonstrated that the dimerization of the cytoplasmic domains of APP induces ASK1- and JNK-dependent apoptosis in neuronal cells [57,58]. Aβ-induced ASK1 activation is mediated by ROS. Aβ can activate ASK1 that is required for ER-stress-induced JNK activation and apoptosis by ROS [12]. ASK1 is activated by APP dimerization, and both ASK1 and MKK6 are activated by Aβ dimerization of APP [58–60]. Aβ causes an early, strong and transient oxidation of both glutaredoxin-1 (GRX1) and thioredoxin-1 (TRX1). Also, Aβ induces apoptosis by activation of the ASK1 cascade in SH-SY5Y cells [61]. Accordingly, Aβ neurotoxicity is related to the activation of ASK1. Considering the association between Aβ and ASK1, ASK1 might be a potential target for enhancing AD pathology (Figure 2).
Figure 2.
Schematic representation of the relationship between ASK1 and Aβ. Aβ activates ASK1 and MKK3/6 by ROS. Aβ induces the JNK signal pathway, which induces neuronal cell death and phosphorylation of tau protein. Finally, activation of ASK1 by Aβ is associated with neuronal cell death and phosphorylation of tau protein.
5. ASK1 and Insulin Signal Transduction
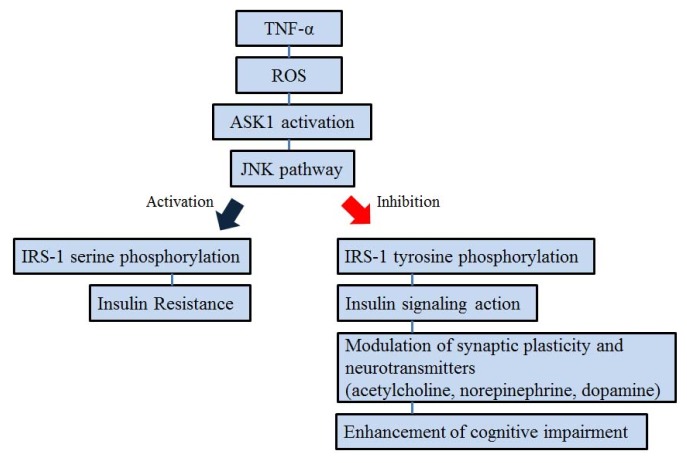
Insulin signaling plays an important role in AD pathology such as cognitive impairment [62,63]. Insulin facilitates glucose uptake in peripheral tissue by binding to the insulin receptor (IR), which belongs to the family of tyrosine kinase receptors. Binding of insulin leads to a rapid auto-phosphorylation on several tyrosine residues that provide docking sites for the insulin receptor substrate (IRS) proteins [64,65]. In the brain, insulin signal transduction is associated with acognitive function, irrespective of changes in peripheral glucose [62,63,66–68]. Several studies have demonstrated that the binding between insulin and IRs regulates the learning and memory functions in brain [62,69–72]. Insulin also modulates the concentration of neurotransmitters associated with cognitive function such as acetylcholine, norepinephrine, and dopamine in the central nervous system (CNS) [73,74]. Additionally, the insulin signaling pathway modulates synaptic plasticity by promoting the recruitment of gamma amino butyric acid (GABA) receptors on post-synaptic membranes, regulating N-methyl-d-aspartate receptor (NMDA) receptor conductance and 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propionic acid (AMPA) receptor cycling [75–81]. Tyrosine phosphorylation of the insulin receptor substrates including IRS-1 and IRS-2 is an early and important process of the insulin signal transduction [82,83]. Impaired tyrosine phosphorylation of IRS-1 is correlated with insulin resistance in vivo [64,84]. In addition, TNF-α causes insulin resistance through attenuation of IR signaling [64]. TNF-α triggers the activation of ASK1-mediated JNK signaling. The TNF-α-induced JNK signaling increases in Ser307 phosphorylation of IRS-1 and decreases in tyrosine phosphorylation of IRS-1. Finally, the Ser307 phosphorylation of IRS-1 through TNF-α induced JNK signaling results in insulin resistance [25,65,84]. In conclusion, the increased tyrosine phosphorylation of IRS-1 enhances insulin signal transduction in modulating of neurotransmitters associated with cognitive function and alleviating cognitive decline in brain. ASK1 is involved in insulin signal transduction through TNF-α-induced JNK signaling. Hence, ASK1 serves as a key factor in modulating insulin signal transduction, and its regulation might enhance cognitive decline in AD (Figure 3).
Figure 3.
Schematic representation of the relationship between ASK1 and insulin signal transduction. TNF-α triggers the activation of ASK1 mediated JNK signaling. The activation of TNF-α-induced JNK signaling induces serine phosphorylation of insulin receptor substrate (IRS)-1 and insulin resistance whereas the inhibition of TNF-α-induced JNK signaling induces tyrosine phosphorylation of IRS-1 and enhancement of cognitive decline. Finally, ASK1 is related to insulin signal transduction through TNF-α-induced JNK signaling. The inhibition of ASK1 enhances the cognitive decline in AD.
6. Conclusions
AD is characterized by neuronal loss, Aβ accumulation, abnormal phosphorylation of tau protein, and cognitive decline in the hippocampus or cortex. ASK1 activates by forming an “ASK1 signalosome” with TRAF family proteins and activates the JNK signaling pathway in response to oxidative stress and ER stress. In addition, Aβ neurotoxicity is associated with the activation of ASK1, and ASK1 is involved in the phosphorylation of tau protein via JNK signaling. Moreover, ASK1 is associated with insulin signal transduction, an important signaling component in cognitive decline. The inhibition of ASK1 induces tyrosine phosphorylation of IRS-1 and prevents the cognitive decline in the brain. Even though activation of ASK1 has not been reported in the AD brain until now, previous studies have indirectly demonstrated that GRX1 and TRX1 modulating ASK1 [41] decreased in the AD brain [61]. Thus, the apparent association between ASK1 and AD pathology related mechanisms advocates the potential of ASK1 to modify the progression of AD. In conclusion, we suggest that ASK1 in the AD brain be more thoroughly investigated in relation with AD pathology.
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (2011-0017276). This work was supported by the Brain Korea 21 Plus Project for Medical Science, Yonsei University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hyman B.T., Damasio H., Damasio A.R., van Hoesen G.W. Alzheimer’s disease. Annu. Rev. Public Health. 1989;10:115–140. doi: 10.1146/annurev.pu.10.050189.000555. [DOI] [PubMed] [Google Scholar]
- 2.Van Hoesen G.W., Augustinack J.C., Dierking J., Redman S.J., Thangavel R. The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. Ann. N. Y. Acad. Sci. 2000;911:254–274. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- 3.Ebenezer P.J., Weidner A.M., LeVine H., 3rd, Markesbery W.R., Murphy M.P., Zhang L., Dasuri K., Fernandez-Kim S.O., Bruce-Keller A.J., Gavilan E., et al. Neuron specific toxicity of oligomeric amyloid-beta: Role for JUN-kinase and oxidative stress. J. Alzheimer’s Dis. 2010;22:839–848. doi: 10.3233/JAD-2010-101161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobiume K., Matsuzawa A., Takahashi T., Nishitoh H., Morita K., Takeda K., Minowa O., Miyazono K., Noda T., Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda K., Hatai T., Hamazaki T.S., Nishitoh H., Saitoh M., Ichijo H. Apoptosis signal-regulating kinase 1 (ASK1) induces neuronal differentiation and survival of PC12 cells. J. Biol. Chem. 2000;275:9805–9813. doi: 10.1074/jbc.275.13.9805. [DOI] [PubMed] [Google Scholar]
- 7.Sayama K., Hanakawa Y., Shirakata Y., Yamasaki K., Sawada Y., Sun L., Yamanishi K., Ichijo H., Hashimoto K. Apoptosis signal-regulating kinase 1 (ASK1) is an intracellular inducer of keratinocyte differentiation. J. Biol. Chem. 2001;276:999–1004. doi: 10.1074/jbc.M003425200. [DOI] [PubMed] [Google Scholar]
- 8.Hwang J.R., Zhang C., Patterson C. C-terminus of heat shock protein 70-interacting protein facilitates degradation of apoptosis signal-regulating kinase 1 and inhibits apoptosis signal-regulating kinase 1-dependent apoptosis. Cell Stress Chaperones. 2005;10:147–156. doi: 10.1379/CSC-90R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman E.H., Chen L., Fu H. Activation of apoptosis signal-regulating kinase 1 by reactive oxygen species through dephosphorylation at serine 967 and 14-3-3 dissociation. J. Biol. Chem. 2004;279:10442–10449. doi: 10.1074/jbc.M311129200. [DOI] [PubMed] [Google Scholar]
- 10.Ouyang M., Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J. Neurochem. 2006;97:234–244. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- 11.Jucker M., Walker L.C. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann. Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadowaki H., Nishitoh H., Urano F., Sadamitsu C., Matsuzawa A., Takeda K., Masutani H., Yodoi J., Urano Y., Nagano T., et al. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 14.Imaizumi K., Miyoshi K., Katayama T., Yoneda T., Taniguchi M., Kudo T., Tohyama M. The unfolded protein response and Alzheimer’s disease. Biochim. Biophys. Acta. 2001;1536:85–96. doi: 10.1016/s0925-4439(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 15.Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002;16:1345–1355. doi: 10.1101/gad.992302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hueber A.O., Zornig M., Lyon D., Suda T., Nagata S., Evan G.I. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- 17.Wei W., Norton D.D., Wang X., Kusiak J.W. Abeta 17–42 in Alzheimer’s disease activates JNK and caspase-8 leading to neuronal apoptosis. Brain: J. Neurol. 2002;125:2036–2043. doi: 10.1093/brain/awf205. [DOI] [PubMed] [Google Scholar]
- 18.Peel A.L., Sorscher N., Kim J.Y., Galvan V., Chen S., Bredesen D.E. Tau phosphorylation in Alzheimer’s disease: Potential involvement of an APP-MAP kinase complex. Neuromol. Med. 2004;5:205–218. doi: 10.1385/NMM:5:3:205. [DOI] [PubMed] [Google Scholar]
- 19.Hoyer S. Is sporadic Alzheimer disease the brain type of non-insulin dependent diabetes mellitus? A challenging hypothesis. J. Neural Transm. 1998;105:415–422. doi: 10.1007/s007020050067. [DOI] [PubMed] [Google Scholar]
- 20.Hoyer S. The aging brain. Changes in the neuronal insulin/insulin receptor signal transduction cascade trigger late-onset sporadic Alzheimer disease (SAD). A mini-review. J. Neural Transm. 2002;109:991–1002. doi: 10.1007/s007020200082. [DOI] [PubMed] [Google Scholar]
- 21.Frolich L., Blum-Degen D., Riederer P., Hoyer S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer’s disease. Ann. N. Y. Acad. Sci. 1999;893:290–293. doi: 10.1111/j.1749-6632.1999.tb07839.x. [DOI] [PubMed] [Google Scholar]
- 22.Hoyer S., Lannert H. Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann. N. Y. Acad. Sci. 1999;893:301–303. doi: 10.1111/j.1749-6632.1999.tb07842.x. [DOI] [PubMed] [Google Scholar]
- 23.Watson G.S., Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: Implications for treatment. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 24.Cholerton B., Baker L.D., Craft S. Insulin, cognition, and dementia. Eur. J. Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishikawa T., Kukidome D., Sonoda K., Fujisawa K., Matsuhisa T., Motoshima H., Matsumura T., Araki E. Impact of mitochondrial ROS production in the pathogenesis of insulin resistance. Diabetes Res. Clin. Pract. 2007;77:S161–S164. doi: 10.1016/j.diabres.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 26.Ichijo H., Nishida E., Irie K., ten Dijke P., Saitoh M., Moriguchi T., Takagi M., Matsumoto K., Miyazono K., Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh C.C., Papaconstantinou J. Thioredoxin-ASK1 complex levels regulate ROS-mediated p38 MAPK pathway activity in livers of aged and long-lived Snell dwarf mice. FASEB J. 2006;20:259–268. doi: 10.1096/fj.05-4376com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzawa A., Nishitoh H., Tobiume K., Takeda K., Ichijo H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: Advanced findings from ASK1 knockout mice. Antioxid. Redox Signal. 2002;4:415–425. doi: 10.1089/15230860260196218. [DOI] [PubMed] [Google Scholar]
- 29.Chen J.T., Fong Y.C., Li T.M., Liu J.F., Hsu C.W., Chang C.S., Tang C.H. DDTD, an isoflavone derivative, induces cell apoptosis through the reactive oxygen species/apoptosis signal-regulating kinase 1 pathway in human osteosarcoma cells. Eur. J. Pharmacol. 2008;597:19–26. doi: 10.1016/j.ejphar.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 30.Zhang L., Chen J., Fu H. Suppression of apoptosis signal-regulating kinase 1-induced cell death by 14-3-3 proteins. Proc. Natl. Acad. Sci. USA. 1999;96:8511–8515. doi: 10.1073/pnas.96.15.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lau J.M., Jin X., Ren J., Avery J., DeBosch B.J., Treskov I., Lupu T.S., Kovacs A., Weinheimer C., Muslin A.J. The 14-3-3 tau phosphoserine-binding protein is required for cardiomyocyte survival. Mol. Cell. Biol. 2007;27:1455–1466. doi: 10.1128/MCB.01369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotoh Y., Cooper J.A. Reactive oxygen species- and dimerization-induced activation of apoptosis signal-regulating kinase 1 in tumor necrosis factor-alpha signal transduction. J. Biol. Chem. 1998;273:17477–17482. doi: 10.1074/jbc.273.28.17477. [DOI] [PubMed] [Google Scholar]
- 33.Matsuzawa A., Saegusa K., Noguchi T., Sadamitsu C., Nishitoh H., Nagai S., Koyasu S., Matsumoto K., Takeda K., Ichijo H. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat. Immunol. 2005;6:587–592. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- 34.Harding H.P., Ron D. Endoplasmic reticulum stress and the development of diabetes: A review. Diabetes. 2002;51:S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 35.Rutkowski D.T., Kaufman R.J. A trip to the ER: Coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Katayama T., Imaizumi K., Sato N., Miyoshi K., Kudo T., Hitomi J., Morihara T., Yoneda T., Gomi F., Mori Y., et al. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 37.Sato N., Imaizumi K., Manabe T., Taniguchi M., Hitomi J., Katayama T., Yoneda T., Morihara T., Yasuda Y., Takagi T., et al. Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. J. Biol. Chem. 2001;276:2108–2114. doi: 10.1074/jbc.M006886200. [DOI] [PubMed] [Google Scholar]
- 38.Katayama T., Imaizumi K., Honda A., Yoneda T., Kudo T., Takeda M., Mori K., Rozmahel R., Fraser P., George-Hyslop P.S., et al. Disturbed activation of endoplasmic reticulum stress transducers by familial Alzheimer’s disease-linked presenilin-1 mutations. J. Biol. Chem. 2001;276:43446–43454. doi: 10.1074/jbc.M104096200. [DOI] [PubMed] [Google Scholar]
- 39.Guo Q., Sebastian L., Sopher B.L., Miller M.W., Ware C.B., Martin G.M., Mattson M.P. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: Central roles of superoxide production and caspase activation. J. Neurochem. 1999;72:1019–1029. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- 40.Guo Q., Fu W., Sopher B.L., Miller M.W., Ware C.B., Martin G.M., Mattson M.P. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat. Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 41.Nishitoh H., Saitoh M., Mochida Y., Takeda K., Nakano H., Rothe M., Miyazono K., Ichijo H. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell. 1998;2:389–395. doi: 10.1016/s1097-2765(00)80283-x. [DOI] [PubMed] [Google Scholar]
- 42.Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H.P., Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 43.Johnson G.A., Spencer T.E., Hansen T.R., Austin K.J., Burghardt R.C., Bazer F.W. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol. Reprod. 1999;61:312–318. doi: 10.1095/biolreprod61.1.312. [DOI] [PubMed] [Google Scholar]
- 44.Selkoe D.J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- 45.Selkoe D.J. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimer’s Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- 46.Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Muller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 47.Selkoe D.J. Translating cell biology into therapeutic advances in Alzheimer’s disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 48.Yankner B.A. Mechanisms of neuronal degeneration in Alzheimer’s disease. Neuron. 1996;16:921–932. doi: 10.1016/s0896-6273(00)80115-4. [DOI] [PubMed] [Google Scholar]
- 49.Hsu M.J., Hsu C.Y., Chen B.C., Chen M.C., Ou G., Lin C.H. Apoptosis signal-regulating kinase 1 in amyloid beta peptide-induced cerebral endothelial cell apoptosis. J. Neurosci. 2007;27:5719–5729. doi: 10.1523/JNEUROSCI.1874-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farias G., Cornejo A., Jimenez J., Guzman L., Maccioni R.B. Mechanisms of tau self-aggregation and neurotoxicity. Curr. Alzheimer Res. 2011;8:608–614. doi: 10.2174/156720511796717258. [DOI] [PubMed] [Google Scholar]
- 51.Johnson G.V., Stoothoff W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 2004;117:5721–5729. doi: 10.1242/jcs.01558. [DOI] [PubMed] [Google Scholar]
- 52.Morishima Y., Gotoh Y., Zieg J., Barrett T., Takano H., Flavell R., Davis R.J., Shirasaki Y., Greenberg M.E. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song S., Kim S.Y., Hong Y.M., Jo D.G., Lee J.Y., Shim S.M., Chung C.W., Seo S.J., Yoo Y.J., Koh J.Y., et al. Essential role of E2-25K/Hip-2 in mediating amyloid-beta neurotoxicity. Mol. Cell. 2003;12:553–563. doi: 10.1016/j.molcel.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Kihiko M.E., Tucker H.M., Rydel R.E., Estus S. c-Jun contributes to amyloid beta-induced neuronal apoptosis but is not necessary for amyloid beta-induced c-jun induction. J. Neurochem. 1999;73:2609–2612. doi: 10.1046/j.1471-4159.1999.0732609.x. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds C.H., Betts J.C., Blackstock W.P., Nebreda A.R., Anderton B.H. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: Differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J. Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- 56.Kyriakis J.M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto Y., Niikura T., Chiba T., Tsukamoto E., Kadowaki H., Nishitoh H., Yamagishi Y., Ishizaka M., Yamada M., Nawa M., et al. The cytoplasmic domain of Alzheimer’s amyloid-beta protein precursor causes sustained apoptosis signal-regulating kinase 1/c-Jun NH2-terminal kinase-mediated neurotoxic signal via dimerization. J. Pharmacol. Exp. Ther. 2003;306:889–902. doi: 10.1124/jpet.103.051383. [DOI] [PubMed] [Google Scholar]
- 58.Maruoka S., Hashimoto S., Gon Y., Nishitoh H., Takeshita I., Asai Y., Mizumura K., Shimizu K., Ichijo H., Horie T. ASK1 regulates influenza virus infection-induced apoptotic cell death. Biochem. Biophys. Res. Commun. 2003;307:870–876. doi: 10.1016/s0006-291x(03)01283-x. [DOI] [PubMed] [Google Scholar]
- 59.Lu D.C., Shaked G.M., Masliah E., Bredesen D.E., Koo E.H. Amyloid beta protein toxicity mediated by the formation of amyloid-beta protein precursor complexes. Ann. Neurol. 2003;54:781–789. doi: 10.1002/ana.10761. [DOI] [PubMed] [Google Scholar]
- 60.Galvan V., Banwait S., Spilman P., Gorostiza O.F., Peel A., Ataie M., Crippen D., Huang W., Sidhu G., Ichijo H., et al. Interaction of ASK1 and the beta-amyloid precursor protein in a stress-signaling complex. Neurobiol. Dis. 2007;28:65–75. doi: 10.1016/j.nbd.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akterin S., Cowburn R.F., Miranda-Vizuete A., Jimenez A., Bogdanovic N., Winblad B., Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006;13:1454–1465. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- 62.Craft S., Asthana S., Newcomer J.W., Wilkinson C.W., Matos I.T., Baker L.D., Cherrier M., Lofgreen C., Latendresse S., Petrova A., et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 63.Kern W., Peters A., Fruehwald-Schultes B., Deininger E., Born J., Fehm H.L. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- 64.Hotamisligil G.S., Peraldi P., Budavari A., Ellis R., White M.F., Spiegelman B.M. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 65.Aguirre V., Werner E.D., Giraud J., Lee Y.H., Shoelson S.E., White M.F. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J. Biol. Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 66.Kern W., Born J., Schreiber H., Fehm H.L. Central nervous system effects of intranasally administered insulin during euglycemia in men. Diabetes. 1999;48:557–563. doi: 10.2337/diabetes.48.3.557. [DOI] [PubMed] [Google Scholar]
- 67.Park C.R., Seeley R.J., Craft S., Woods S.C. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol. Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 68.Craft S., Asthana S., Schellenberg G., Cherrier M., Baker L.D., Newcomer J., Plymate S., Latendresse S., Petrova A., Raskind M., et al. Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology. 1999;70:146–152. doi: 10.1159/000054469. [DOI] [PubMed] [Google Scholar]
- 69.Van der Heide L.P., Ramakers G.M., Smidt M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 70.Plum L., Schubert M., Bruning J.C. The role of insulin receptor signaling in the brain. Trends Endocrinol. Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 71.Cole A.R., Astell A., Green C., Sutherland C. Molecular connexions between dementia and diabetes. Neurosci. Biobehav. Rev. 2007;31:1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 72.Dou J.T., Chen M., Dufour F., Alkon D.L., Zhao W.Q. Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn. Mem. 2005;12:646–655. doi: 10.1101/lm.88005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Figlewicz D.P., Szot P., Israel P.A., Payne C., Dorsa D.M. Insulin reduces norepinephrine transporter mRNA in vivo in rat locus coeruleus. Brain Res. 1993;602:161–164. doi: 10.1016/0006-8993(93)90258-o. [DOI] [PubMed] [Google Scholar]
- 74.Kopf S.R., Baratti C.M. Effects of posttraining administration of insulin on retention of a habituation response in mice: Participation of a central cholinergic mechanism. Neurobiol. Learn. Mem. 1999;71:50–61. doi: 10.1006/nlme.1998.3831. [DOI] [PubMed] [Google Scholar]
- 75.Wang Y.T., Salter M.W. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 76.Wan Q., Xiong Z.G., Man H.Y., Ackerley C.A., Braunton J., Lu W.Y., Becker L.E., MacDonald J.F., Wang Y.T. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 77.Van der Heide L.P., Kamal A., Artola A., Gispen W.H., Ramakers G.M. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J. Neurochem. 2005;94:1158–1166. doi: 10.1111/j.1471-4159.2005.03269.x. [DOI] [PubMed] [Google Scholar]
- 78.Man H.Y., Lin J.W., Ju W.H., Ahmadian G., Liu L., Becker L.E., Sheng M., Wang Y.T. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 79.Kneussel M. Dynamic regulation of GABA(A) receptors at synaptic sites. Brain Res. Brain Res. Rev. 2002;39:74–83. doi: 10.1016/s0165-0173(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 80.Malenka R.C. Synaptic plasticity and AMPA receptor trafficking. Ann. N. Y. Acad. Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- 81.Huang C.C., Lee C.C., Hsu K.S. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J. Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- 82.Araki E., Lipes M.A., Patti M.E., Bruning J.C., Haag B., 3rd, Johnson R.S., Kahn C.R. Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature. 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 83.Saltiel A.R., Kahn C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 84.Aguirre V., Uchida T., Yenush L., Davis R., White M.F. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J. Biol. Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]