Abstract
Adiponectin and intracellular 5′adenosine monophosphate-activated protein kinase (AMPK) are important modulators of glucose and fat metabolism. Cinnamon exerts beneficial effects by improving insulin sensitivity and blood lipids, e.g., through increasing adiponectin concentrations and AMPK activation. The underlying mechanism is unknown. The Gi/Go-protein-coupled receptor (GPR) 109A stimulates adiponectin secretion after binding its ligand niacin. Trans-cinnamic acid (tCA), a compound of cinnamon is another ligand. We hypothesize whether AMPK activation and adiponectin secretion by tCA is transmitted by GPR signaling. Differentiated 3T3-L1 cells were incubated with pertussis toxin (PTX), an inhibitor of Gi/Go-protein-coupling, and treated with different tCA concentrations. Treatment with tCA increased adiponectin and the pAMPK/AMPK ratio (p ≤ 0.001). PTX incubation abolished the increased pAMPK/AMPK ratio and adiponectin secretion. The latter remained increased compared to controls (p ≤ 0.002). tCA treatment stimulated adiponectin secretion and AMPK activation; the inhibitory effect of PTX suggests GPR is involved in tCA stimulated signaling.
Keywords: trans-cinnamic acid, Adiponectin, 5′adenosine monophosphate-activated protein kinase, G-protein-coupled receptor 109A
1. Introduction
Cinnamon (CN) is known to exert several beneficial effects by improving insulin sensitivity and lipid profiles. Enhanced glucose uptake and glycogen synthesis were reported after stimulation of 3T3-L1 adipocytes with hydroxychalcone, a compound of cinnamon [1]. Khan et al. [2] demonstrated that supplementation with cinnamon reduces fasting serum glucose and improves blood lipid profiles in patients with type 2 diabetes. In mice treated with an extract of cinnamon bark, the concentration of the adipokine adiponectin (AdipoQ) was increased [3]. Adiponectin is mainly expressed in adipocytes [4] and is important for modulating glucose and fat metabolism in insulin-sensitive tissues like skeletal muscle and liver. Adiponectin exerts its effects via binding to its receptors AdipoR1/R2 and activation of peroxisome proliferator-activated receptor α (PPARα) and 5′adenosine monophosphate-activated protein kinase (AMPK) [5]. The AMPK is a heterotrimeric kinase complex, consisting of a catalytic α subunit and regulatory β and γ subunits [6]. Multiple isoforms of these subunits have been identified [7], and the α1-subunit represents the predominant isoform in adipose tissue [8] as well as in cultured 3T3-L1 cells [9]. Besides AdipoQ, metabolic active hormones like leptin or insulin, and an increased cellular AMP/ATP ratio activate AMPK through phosphorylation (pAMPK) of threonine 172 in the α1-subunit. Huang et al. [10] showed in 3T3-L1 cells in vitro, as well as in murine adipose tissue in vivo, an increased AMPK activation after supplementation with cinnamaldehyde, one compound of CN. Upon activation, AMPK switches on catabolic pathways (e.g., fatty-acid oxidation and glycolysis) and inhibits anabolic processes like cholesterol, glycogen, and protein synthesis in liver and muscle. The AMPK acts as an intra-cellular energy sensor and hence improves insulin sensitivity in insulin-sensitive tissues like adipose tissue, but here the data about AMPK and its effect remain poorly distinguished [11]. The effect of various ingredients of CN on AMPK and AdipoQ is reported, but the underlying mechanism is not characterized. Trans-cinnamic acid (tCA), another isolated compound of cinnamon, was recently identified as a ligand of the G-protein-coupled receptor (GPR) 109A [12]. The seven transmembrane GPR109A, a member of the recently deorphanized hydroxycarboxylic acid receptor family, which is also known as HCA2 [13], is expressed in activated macrophages and in adipocytes [14]. The binding of GPR109A agonists like niacin and its endogenous ligand β-hydroxybutyrate has been shown to activate this receptor and stimulate AdipoQ secretion in adipose tissue [15]. Therefore, we hypothesized that trans-cinnamic acid, as compound of CA, stimulates AdipoQ and AMPK also through G-protein-coupled receptor signaling.
To verify this hypothesis, we investigated the changes in AdipoQ secretion and the prevalence of the phosphorylated form of AMPK in differentiated 3T3-L1 adipocytes stimulated with different concentrations of the recent characterized GPR109A ligand tCA. To prove signaling by G-protein-coupled receptors, the adipocytes were additionally pre-incubated with pertussis toxin (PTX), an inhibitor of Gi/Go protein coupling.
2. Results
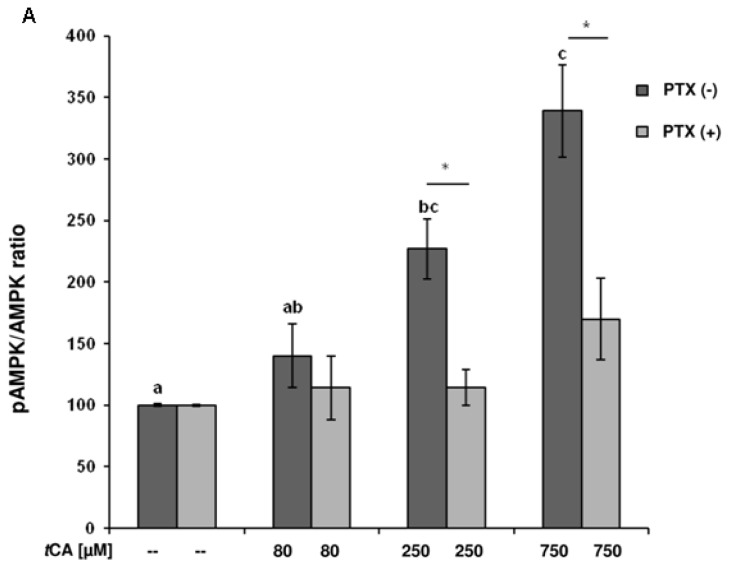
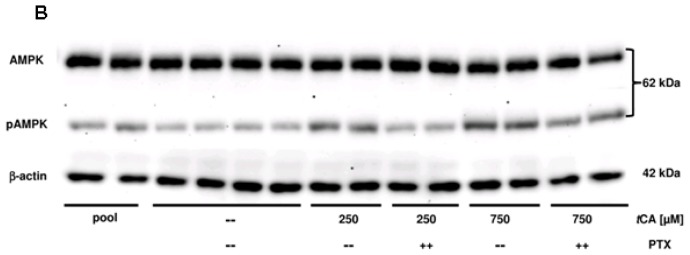
To test whether tCA has an effect on the phosphorylation of AMPK, the differentiated 3T3-L1 cells were stimulated with three different concentrations of tCA (80, 250, 750 μM) for 5 h. tCA acid increased (p ≤ 0.001) the extent of phosphorylation of Thr 172 of AMPK in a dose dependent manner (Figure 1a). When compared with the controls, activation of the AMPK was 2 and 3 times higher in cells treated with 250 or 750 μM tCA (p = 0.009 and p ≤ 0.001, respectively), whereas the pAMPK/AMPK ratio in 80 μM tCA treatment was similar to controls. To assess whether the effects of tCA were mediated by Gi/Go-protein-coupled receptor signaling, the experiments were conducted following pre-incubation with PTX (100 ng/mL) for 16 h. Treatment with PTX dampened the increase of pAMPK/AMPK ratios after tCA treatment, no differences were observed neither among the treatment groups, nor in comparison to controls pre-incubated with PTX. Cells treated with 250 and 750 μM tCA, respectively, but without PTX pre-incubation showed two times higher pAMPK/AMPK ratios (p = 0.011 and p = 0.026) when compared to the same treatment groups with PTX incubation (Figure 1A). A representative picture of Western blot analyses is shown in Figure 1B.
Figure 1.
Trans-cinnamic acid (tCA) affects the intracellular 5′adenosine monophosphate-activated protein kinase (AMPK) activation by phosphorylation (pAMPK) in differentiated 3T3-L1 cells. (A) tCA effects on pAMPK/AMPK ratios in differentiated 3T3-L1 cells. After 4 h of starvation, the adipocytes were pre-incubated with (PTX (+)) or without pertussis toxin (PTX (−)) (100 ng/mL) for 16 h and then treated for 5 h with 80, 250 or 750 μM tCA, or with buffered saline (PBS) as controls respectively. Different lower case letters designate significant differences (p ≤ 0.01) between tCA treatments and controls. Significant differences (p ≤ 0.05) due to (+) or (−) PTX pre-incubation are designated with asterisks for each tCA treatment group. Data are expressed as means ± SEM (n = 6); (B) Representative Western blot analyses. After gel electrophoreses, membranes were incubated with specific antibodies against AMPK, pAMPK or with β-actin as loading control.
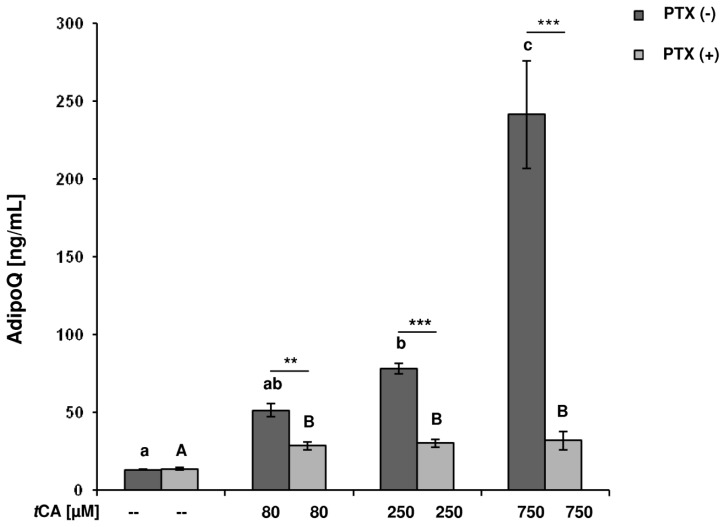
Treatment with tCA increased (p ≤ 0.001) AdipoQ concentrations in the cell culture supernatant dose dependently (Figure 2). When compared to controls, AdipoQ concentrations were increased five-fold (78 ± 3.4 ng/mL) after stimulation with 250 μM tCA (p = 0.005) and were about 18 times higher (241 ± 34.4 ng/mL) after treatment with 750 μM tCA (p ≤ 0.001). For all tCA treatment groups, pre-incubation with PTX lowered the AdipoQ concentrations to values between 28.5 ± 2.6 and 32 ± 5.9 ng/mL, but consistently higher concentrations than in the related controls (p ≤ 0.002) were retained.
Figure 2.
Trans-cinnamic acid (tCA) effects on AdipoQ concentrations in cell culture supernatant of differentiated 3T3-L1 cells. After 4 h of starvation, the adipocytes were pre-incubated with (PTX (+)) or without pertussis toxin (PTX (−)) (100 ng/mL) for 16 h and then treated for 5 h with 80, 250 or 750 μM tCA, with PBS as controls, respectively. Different lower case letters designate significant differences (p ≤ 0.01) between tCA treatments vs. controls for PTX (−) cells, different capital letters designate significant differences (p ≤ 0.01) between tCA treatments vs. controls for PTX (+) cells. Significant differences (**: p ≤ 0.01; ***: p ≤ 0.001) due to PTX (+) or PTX (−) pre-incubation for each tCA treatment group are indicated with asterisks. Data are expressed as means ± SEM (n = 6).
Comparing PTX pre-incubation groups, AdipoQ concentrations decreased by 1.8 and 2.5 times after PTX pre-incubation in the 80 μM tCA (p = 0.002) and 250 tCA (p ≤ 0.001) treatment groups, respectively, compared to the corresponding PTX (−) group. In addition, the AdipoQ concentrations in the supernatant of the 750 μM treated cells decreased seven-fold (p ≤ 0.001) with PTX pre-incubation.
Correlation analysis across all samples confirmed a linear relationship between the AdipoQ concentrations and the pAMPK/AMPK ratio (p ≤ 0.001, r = 0.534).
3. Discussion
We investigated the changes in AdipoQ secretion and the prevalence of the phosphorylated form of AMPK in differentiated 3T3-L1 adipocytes stimulated with different concentrations of the recently identified GPR109A ligand tCA. In addition, it was to be characterized if these changes were mediated through Gi/Go-protein-coupled receptor signaling. The major findings were as follows: (1) tCA increased secretion of AdipoQ and phosphorylation of AMPK; (2) Inhibition of GPR signaling by PTX abrogated the activating effect of tCA on secretion of AdipoQ and phosphorylation of AMPK but not completely. Several studies characterized CN to improve glucose and lipid profiles [1,2,16]. Various components and sources of CN were tested but we introduced tCA, another isolated compound, for the first time as the influencing variable on the AdipoQ system and, thereby, on glucose and fat metabolism. Corresponding to the study of Kim et al. [3] in which liquid Cinnamon bark extract was administered to mice, we showed that AdipoQ secretion increased in a dose dependent manner by tCA treatment. Adiponectin, one of the most important adipokines, improves insulin resistance and lipid metabolism [17]. The effects are mediated through its receptors AdipoR1/R2 and can at least partially be explained by their direct activation of AMPK in skeletal muscle, liver and adipose tissue [18]. Here, we showed a correlation between AdipoQ and the pAMPK/AMPK ratio, presuming an activation of AMPK subsequent to the increased secretion of AdipoQ after tCA treatment, supporting the study of Yamauchi et al. [19]. In our study, treatment with tCA induced phosphorylation of AMPK up to three times more than in non-treated cells. That concurs with findings of Huang et al. [10] where activation of AMPK after treatment with cinnamaldehyde in 3T3-L1 adipocytes was observed and confirmed by dampened effects after adding compound C, a specific inhibitor of AMPK. In addition, Huang et al. [10] showed in consequence of cinnamaldehyde treatment an increase in phosphorylation and, thereby, inactivation of acetyl-CoA carboxylase (ACC), which is associated with a decreased lipogenic rate and reduced lipolysis [8]. Furthermore, phosphorylation of both proteins is said to be related to increased mitochondrial fatty acid oxidation in adipose tissue [8,10]. Although the beneficial effects of CN and its compounds were proven in several studies, little is known about its signaling pathway. Ren et al. [12] recently identified tCA as a ligand of GPR109A (PUMA-G in mice), mainly expressed in immune cells and adipocytes. Niacin as another ligand of GPR109A is known to increase AdipoQ secretion [20]. Plaisance et al. [15] demonstrated that the AdipoQ modulating effect of niacin is mediated through the GPR109A. Mice deficient in PUMA-G (GPR109A) showed no increase in serum AdipoQ concentration after treatment with niacin. To test if the modulating effect of tCA on AdipoQ and AMPK is mediated by Gi/Go-protein-coupled receptors, we pre-incubated the adipocytes with PTX, an inhibitor of G-protein coupling. The tCA-induced activation of AMPK was abolished after blocking of Gi/Go signaling, indicating this pathway is involved in the signal transmission of tCA. The AdipoQ secretion was, as expected, significantly decreased after receptor blocking irrespective of the tCA treatment group, but still significantly increased according to controls. This is in contrast to the findings of Plaisance et al. [15], who showed an abrogated increase in AdipoQ secretion after stimulation with niacin and PTX in rat adipocytes. Due to diminished but still higher AdipoQ concentration after PTX incubation, another possible stimulator for AdipoQ after tCA treatment should be discussed. Kim and Choung [3] showed an up-regulated mRNA expression of peroxisome proliferation-activated receptor (PPARγ) in adipose tissue after treatment with an extract of cinnamon bark. The transcription factor PPARγ is a known stimulator of AdipoQ expression [21] and a regulator of several genes involved in controlling insulin sensitivity [22]. Besides increased PPARγ expression, an increase of AdipoQ secretion was observed after administration of cinnamon extract, supporting this regulation as possible stimulus of AdipoQ secretion after tCA treatment, besides the signaling through the GPR109A [3]. The data about the expression of GPR109A and its signaling capability in 3T3-L1 adipocytes is controversial. Zhang et al. [23] were unable to detect gene expression of GPR109A; Jeninga et al. [24] showed clearly an increasing mRNA as well as protein expression of GPR109A in 3T3-L1 cells throughout differentiation, which was increased by the PPARγ agonist rosiglitazone. Also, Digby et al. [25] observed the expression of GPR109A mRNA, which was upregulated by TNFα. Plaisance et al. [15] showed protein expression of GPR109A in 3T3-L1 cells but observed no effect on AdipoQ secretion after niacin treatment, whereas Ge et al. [26] detected increased glycerol release after stimulation with niacin. The findings of Plaisance et al. [15] were annihilated when the cells were transfected with the human GPR109A orthologon HM74A. In our study, the presence of GPR109A mRNA in the differentiated 3T3-L1 in vitro model was proven (data not shown). The GPR109A ligand tCA increased the AdipoQ secretion via GPR signaling in 3T3-L1 adipocytes. Due to our experimental design, it was not possible to specify the Gi/Go-protein-coupled receptors mediating the effects of tCA, but we assume that further studies using e.g., a specific GPR109A agonist, or primary adipocytes from GPR109A knockout mice, will define the GPR109A being involved in tCA signaling pathways. Furthermore, our findings after PTX pre-incubation indicate a potential, but still unknown tCA mediated signaling pathway besides the one through GPR signaling that might be related to the activation of PPARγ, and which should be verified in the future.
4. Experimental Section
4.1. Cell Culture
Murine 3T3-L1 fibroblast cells were seeded in 25 cm2 flasks at a density of 4000 cells per cm2 and cultured with Dulbecco’s modified eagle’s medium high glucose (DMEM) containing 10% fetal calf serum (FCS) and 10 mg/mL penicillin/streptomycin (pen/strep) (basic medium) (all from PAA, Pasching, Austria) in a humidified atmosphere of 95% air and 5% CO2 at 37 °C for 24 h. To induce differentiation of the 3T3-L1 fibroblasts into adipocytes, 0.5 mM 3-isobutyl-methylxanthine (IBMX) (Applichem, Darmstadt, Germany), 0.25 μM dexamethasone and 5 μg/mL bovine insulin (both from Sigma-Aldrich, St. Louis, MO, USA) were added to the basic medium for 48 h. Cells were then maintained in basic medium supplemented with 5 μg/mL bovine insulin. Media were replaced every 2 days until 85%–95% of the cells were differentiated (day 12 after initiation of differentiation), which was documented by the accumulation of lipid droplets (Oil Red O staining, 0.2%).
4.2. Treatment of Cells
Prior to the treatments, cells were cultured in basic medium for 24 h, then serum starved in DMEM supplemented only with 0.1% fatty acid-free bovine serum albumin (BSA) (Carl Roth, Karlsruhe, Germany) for 4 h. The adipocytes were subsequently incubated for 16 h with 100 ng/mL pertussis toxin (PTX) (Sigma-Aldrich, St. Louis, MO, USA), which selectively affects Gi/Go signaling, to characterize possible effects of tCA by GPR signaling. Cells were then treated with 80 μM, 240 μM or 750 μM tCA (Sigma-Aldrich, St. Louis, MO, USA) for 5 h (n = 6). Equal volumes of the solvent (phosphate buffered saline (PBS)) were applied to controls instead of PTX and tCA, respectively. At the end of the incubation time, supernatant was collected and stored at −20 °C until analysis. The adherent adipocytes were washed twice with ice cold PBS and lysed with pre-chilled lysis buffer as described previously [27]. The cell lysates were harvested by scraping, transferred into pre-chilled 1.5 mL tubes and centrifuged at 16,000 g for 20 min at 4 °C. Protein concentrations were measured according to Bradford [28].
4.3. Western Blot
For the detection of AMPK and pAMPK, respectively, 18 μg total protein were treated with Laemmli buffer and reduced with 4% Dithiothreitol (DTT) (Applichem, Darmstadt, Germany), boiled for 5 min at 95 °C, centrifuged for 5 min at 10,000 g at 4 °C, and subsequently loaded in duplicates on a 10% Mini-PROTEAN TGX Precast Gel (Bio Rad Laboratories, Munich, Germany). After electrophoresis, the fractionated proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (GE Healthcare, Buckinghamshire, UK) by Trans Turbo Blot (Bio Rad Laboratories, Munich, Germany). To avoid unspecific antibody binding, the membranes were incubated in tris-buffered saline containing 0.05% Tween 20 (TBST) and 10% Rotiblock (Carl Roth, Karlsruhe, Germany) for 60 min at RT. The membranes were cut horizontally at 50–55 kDa. The upper parts of the membranes were exposed to the primary rabbit antibodies against AMPK in a dilution of 1:1000 or its phosphorylated form (pAMPK) (both 62 kDa), respectively (AMPKα, pAMPKα, Cell Signaling, Danvers, MA, USA) in a dilution of 1:500, each diluted in TBST with 5% BSA overnight at 4 °C. The lower parts of the membranes, with proteins ≤50 kDa, were incubated with a primary mouse antibody against β-actin (42 kDa) (Biovision, Milpitas, CA, USA) diluted 1:6000 in blocking solution under the same conditions. After rinsing, a horseradish peroxidase-labeled secondary anti-rabbit antibody (1:50,000; Cell Signaling, Danvers, MA, USA) or a horseradish peroxidase-labeled secondary anti-mouse antibody (1:20,000) (SouthernBiotech, Birmingham, AL, USA) were applied for 60 min at RT. Antigen-antibody immunocomplexes were revealed using enhanced chemiluminescence detection system (GE Healthcare) and densitometry analysis was performed using a Versa Doc 1000 and Image Lab software (both Bio Rad Laboratories Munich, Germany). Specific band intensities were normalized to β-actin values as an internal standard. To be able to compare the band intensities from different membranes, a 3T3-L1 pool sample was electrophoresed and blotted in duplicates on each membrane and used as reference standard. The mean intensity of the duplicate bands of the samples in relation to the mean of the standard was estimated and the ratio of pAMPK to AMPK was calculated.
4.4. Measurement of AdipoQ Secreted from 3T3-L1 Adipocytes
The AdipoQ content in the cell supernatant was quantified by a recently developed in-house ELISA [29] for which parallelism of mouse AdipoQ was approved. The intra- and interassay coefficients of variation were 7% and 11%, respectively.
4.5. Statistical Analyses
Data were analysed using IBM SPSS 20 (IBM, Ehningen, Germany) and are presented as means ± SEM. The results of the controls were not different and thus merged for further analyses, within the PTX (+) and PTX (−) treatment. For comparisons within treatment groups and between treatment and controls, ANOVA with either Bonferroni or Dunnett-T3 post-hoc analysis, depending on homogeneity of variances, was performed. To compare the PTX treated versus non PTX treated samples, data were examined using the Student’s t-test. Spearman-Rho correlation coefficients were calculated between the results of AdipoQ and pAMPK/AMPK. Statistical significance was declared at p ≤ 0.05.
5. Conclusions
In conclusion, treatment with tCA stimulated the secretion of AdipoQ and the phosphorylation of AMPK in 3T3-L1 adipocytes and therefore improves insulin sensitivity; the inhibitory effect of PTX points to a tCA stimulated Gi/Go-protein-coupled receptor signaling pathway.
Acknowledgments
The authors thank Barbara Heitkönig and Birgit Mielenz for their excellent technical assistance. The donation of a graduate scholarship by the University of Bonn to Christina Kopp is gratefully acknowledged. Shiva Pratap Singh is the recipient of an International Fellowship by Indian Council of Agricultural Research (ICAR) at the University of Bonn, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jarvill-Taylor K.J., Anderson R.A., Graves D.J. A Hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J. Am. Coll. Nutr. 2001;20:27–36. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- 2.Khan A., Safdar M., Khan M., Khattak K.N., Anderson R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.H., Choung S.Y. Antihyperglycemic and antihyperlipidemic action of Cinnamomi cassiae (cinnamon bark) extract in C57BL/Ks db/db mice. Arch. Pharm. Res. 2010;33:325–333. doi: 10.1007/s12272-010-0219-0. [DOI] [PubMed] [Google Scholar]
- 4.Scherer P.E., Williams S., Fogliano M., Baldini G., Lodish H.F. A novel serum protein similar to C1q exclusively in adipocytes. J. Biol. Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 5.Kadowaki T., Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 6.Thornton C., Snowden M.A., Carling D. Identification of a novel AMP-activated protein kinase b subunit isoform that is highly expressed in skeletal muscle. J. Biol. Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- 7.Hardie D.G., Sakamoto K. AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology. 2006;21:48–60. doi: 10.1152/physiol.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Daval M., Foufelle F., Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J. Physiol. 2006;574:55–62. doi: 10.1113/jphysiol.2006.111484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salt I.P., Connell J.M., Gould G.W. 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes. Diabetes. 2000;49:1649–1656. doi: 10.2337/diabetes.49.10.1649. [DOI] [PubMed] [Google Scholar]
- 10.Huang B., Yuan H.D., Kim D.Y., Quan H.Y., Chung S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-γ (PPARγ) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 2011;59:3666–3673. doi: 10.1021/jf104814t. [DOI] [PubMed] [Google Scholar]
- 11.Bijland S., Mancini S.J., Salt I.P. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin. Sci. 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- 12.Ren N., Kaplan R., Hernandez M., Cheng K., Jin L., Taggart A.K., Zhu A.Y., Gan X., Wright S.D., Cai T.Q. Phenolic acids suppress adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G) J. Lipid Res. 2009;50:908–914. doi: 10.1194/jlr.M800625-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Offermanns S., Colletti S.L., Lovenberg T.W., Semple G., Wise A., Ijzerman A.P. International union of basic and clinical pharmacology. LXXXII: Nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B) Pharmacol. Rev. 2011;63:269–290. doi: 10.1124/pr.110.003301. [DOI] [PubMed] [Google Scholar]
- 14.Taggart A.K., Kero J., Gan X., Cai T.-Q., Cheng K., Ippolito M., Ren N., Kaplan R., Wu K., Wu T.-J., et al. D-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- 15.Plaisance E.P., Lukasova M., Offermanns S., Zhang Y., Cao G., Judd R.L. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am. J. Physiol. Endocrinol. Metab. 2009;296:549–558. doi: 10.1152/ajpendo.91004.2008. [DOI] [PubMed] [Google Scholar]
- 16.Imparl-Radosevich J., Deas S., Polansky M.M., Baedke D.A., Ingebrutsen T.S., Anderson R.A., Graves D.J. Regulation of phosphotyrosine phosphatase (PTP-1) and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signaling. Horm. Res. 1998;50:177–182. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]
- 17.Ohara K., Uchida A., Nagasaka R., Ushio H., Ohshima T. The effects of hydroxycinnamic acid derivatives on adiponectin secretion. Phytomedicine. 2009;16:130–137. doi: 10.1016/j.phymed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Lafontan M., Viguerie N. Role of adipokines in the control of energy metabolism: Focus on adiponectin. Curr. Opin. Pharmacol. 2006;6:580–585. doi: 10.1016/j.coph.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T., Kamon J., Minokoshi Y., Ito Y., Waki H., Uchida S. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 20.Westphal S., Borucki K., Taneva E., Makarova R., Luley C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis. 2007;193:361–365. doi: 10.1016/j.atherosclerosis.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Iwaki M., Matsuda M., Maeda N., Funahashi T., Matsuzawa Y., Makishima M., Shimomura I. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 22.Chiarelli F., Di M.D. Peroxisome proliferator-activated receptor-gamma agonists and diabetes: current evidence and future perspectives. Vasc. Health Risk Manag. 2008;4:297–304. doi: 10.2147/vhrm.s993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y., Schmidt R.J., Foxworthy P., Emkey R., Oler J.K., Large T.H., Wang H., Su E.W., Mosior M.K., Eacho P.I., et al. Niacin mediates lipolysis in adipose tissue through its G-protein coupled receptor HM74A. Biochem. Biophys. Res. Commun. 2005;334:729–732. doi: 10.1016/j.bbrc.2005.06.141. [DOI] [PubMed] [Google Scholar]
- 24.Jeninga E.H., Bugge A., Nielsen R., Kersten S., Hamers N., Dani C., Wabitsch M., Berger R., Stunnenberg H.G., Mandrup S., et al. Peroxisome proliferator-activated receptor regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81) J. Biol. Chem. 2009;25:26385–26393. doi: 10.1074/jbc.M109.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Digby J.E., McNeill E., Dyar O.J., Lam V., Greaves D.R., Choudhury R.P. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis. 2010;209:89–95. doi: 10.1016/j.atherosclerosis.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge H., Li X., Weiszmann J., Wang P., Baribault H., Chen J., Tian H., Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008;149:4519–4526. doi: 10.1210/en.2008-0059. [DOI] [PubMed] [Google Scholar]
- 27.Locher L.F., Meyer N., Weber E.-M., Rehage J., Meyer U., Dänicke S., Huber K. Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow. J. Dairy Sci. 2011;94:4514–4523. doi: 10.3168/jds.2011-4145. [DOI] [PubMed] [Google Scholar]
- 28.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Mielenz M., Mielenz B., Singh S.P., Kopp C., Heinz J., Häussler S., Sauerwein H. Development, validation, and pilot application of a semiquantitative Western blot analysis and an ELISA for bovine adiponectin. Domest. Anim. Endocrinol. 2013;44:121–130. doi: 10.1016/j.domaniend.2012.10.004. [DOI] [PubMed] [Google Scholar]