Abstract
Most female ixodid ticks, once mated, feed to repletion within 6–10 days. Previous studies indicate that an engorgement factor (EF), passed to the female during copulation, may be the stimulus for engorgement. Here, we show that extracts of the testis/vas deferens of fed (but not unfed) male Amblyomma hebraeum contain EF bioactivity when injected into the hemocoel of feeding virgins. We have produced recombinant proteins (recproteins) from 28 feeding-induced genes in the male gonad and have identified a recombinant A. hebraeum engorgement factor (recAhEF) among these recproteins. recAhEF is a combination of two peptides, recAhEFα (16.1 kDa) and recAhEFβ (11.6 kDa), neither of which has bioactivity on its own. recAhEF also stimulates salivary gland degeneration and partial development of the ovary, suggesting that it may be the same material as another male gonadal protein from this tick, male factor. We propose the name “voraxin” for the natural EF of ticks. When normal mated females were put on a rabbit immunized against recAhEF, 74% failed to feed beyond one-tenth the normal engorged weight within 14 days whereas all mated ticks put on a control rabbit engorged normally (mean duration of 8.8 ± 0.8 days). This result constitutes preliminary evidence that an anti-tick vaccine might be developed from voraxin.
Adult female ixodid ticks require 6–10 days to engorge fully. The feeding cycle consists of three phases: (i) a preparatory phase (1–2 days), during which the female establishes a feeding lesion and secretes a cement-like cone to securely attach herself to the skin, (ii) a slow phase (4–7 days), during which the female feeds to ≈10 times her unfed weight, and (iii) a 24- to 36-h rapid phase, during which the female increases her weight a further 10-fold (1).
In the African cattle tick, Amblyomma hebraeum Koch, the transition weight between the slow and rapid phases of feeding is called the critical weight (CW) (2). The CW, ≈10–14 times the unfed weight in A. hebraeum, depending on which parameter is used to measure it (3), is characterized by some marked behavioral and physiological changes (4). If a virgin or mated female is removed from a host below the CW, she (i) will reattach if given the opportunity, (ii) will not resorb her salivary glands (SGs), and (iii) will not lay a batch of eggs. In contrast, if a female is removed from the host above the CW, she (i) will not resume feeding even if given the opportunity, (ii) will resorb the SGs within 4 days (if mated) or 8 days (if virgin), and (iii) will lay a batch of eggs, and die. Most virgin A. hebraeum do not feed beyond the CW even after weeks on the host. The difference between mated and virgin females above the CW is due to a substance [male factor (MF)] passed to the female during copulation (2). MF is a protein produced in the testis/vas deferens (T/VD) during feeding but is not associated with the spermatozoa (5).
In ticks, three mating factors other than MF are known to induce specific physiological responses. A 12.5-kDa protein, sperm capacitation factor, is produced by the male accessory gland. It stimulates the final phase of tick sperm maturation after the spermatophore has been transferred to the female (6). Vitellogenesis-stimulating factor, from the argasid tick Ornithodorus moubata, seems to comprise two large proteins (≈100 and ≈200 kDa). This factor is released from recently capacitated sperm and stimulates vitellogenesis and subsequent oviposition (7). Finally, the stimulus for rapid engorgement is an engorgement factor (EF). EF, first described in Dermacentor variabilis, is produced by the male gonad and passes to the female during copulation (8, 9). We hypothesize that EF and MF are the same substance.
Weiss et al. (10) made a cDNA library from the T/VD of fed males and used a differential cross-screening approach to identify 35 feeding-induced genes. For this study, we successfully expressed 28 of the 35 up-regulated genes in Spodoptera frugiperda (Sf21) cells. The 28 recombinant proteins (recproteins) were injected into feeding virgins according to the protocol shown in Table 1. Two of the recproteins were sufficient and necessary for stimulating engorgement of the feeding virgins. Results presented here also support our hypothesis that MF and EF are the same substance.
Table 1. Bioassay of recproteins derived from feeding-induced mRNA transcripts expressed in the T/VD of male A. hebraeum.
| Exp. no. | Group no. (n) | Sf21 lysates injected*† | Mean weight of virgins (mg) at time of injection (± SEM) | Mean weight of virgins (mg) at detachment by day 14 (± SEM)‡§ | Fluid secretory competence (mg/gland/15 min) on day 4 postremoval§ | Ovary weight (mg) on day 10 postremoval§ |
|---|---|---|---|---|---|---|
| 1 | 1 (14) | 1-14 | 156 ± 8.9 | 182 ± 7.8 | — | — |
| 2 (14) | 15-28 | 191 ± 13.3 | 214 ± 6.6 | — | — | |
| 2 | 3 (14) | 1-7, 15-20 | 206 ± 5.1 | 211 ± 10.2 | 4.0 ± 0.6 (n = 4) | — |
| 4 (14) | 1-7, 21-28 | 219 ± 16.1 | 237 ± 10 | 3.9 ± 0.9 (n = 6) | — | |
| 5 (14) | 8-14, 15-20 | 183 ± 11.1 | 194 ± 11.1 | 3.6 ± 0.8 (n = 6) | — | |
| 6 (14) | 8-14, 21-28 | 169 ± 10.1 | 1070 ± 54.8 | 0.4 ± 0.1 (n = 13) | 15.91 ± 1.4 | |
| 7 (7) | Control 1 | 219 ± 14.3 | 214 ± 8.8 | 4.2 ± 0.3 (n = 8) | — | |
| 3 | 8 (7) | 8-14 | 221 ± 21.0 | 253 ± 8.5 | 4.1 ± 0.3 (n = 4) | 1.6 ± 0.43 |
| 9 (7) | 21-28 | 178 ± 18.2 | 199 ± 17.4 | 4.7 ± 0.7 (n = 6) | 1.7 ± 0.47 | |
| 10 (7) | 8-14, 21-24 | 236 ± 16.4 | 1651 ± 159 | 0.4 ± 0.1 (n = 10) | 18.12 ± 1.8 | |
| 11 (7) | 8-14, 25-28 | 200 ± 28.1 | 208 ± 18.2 | 3.7 ± 0.5 (n = 4) | 2.0 ± 0.47 | |
| 12 (7) | Control 2 | 207 ± 22.3 | 227 ± 12.9 | 4.5 ± 0.4 (n = 8) | 2.1 ± 0.17 | |
| 4 | 13 (7) | 8-10, 21, 22 | 185 ± 11.7 | 1979 ± 210 | 0.3 ± 0.1 (n = 8) | 12.5 ± 1.6 |
| 14 (7) | 11-14, 21, 22 | 202 ± 20.9 | 221 ± 17.2 | 4.7 ± 0.5 (n = 4) | 1.6 ± 0.44 | |
| 15 (7) | 8-10, 23, 24 | 245 ± 22.7 | 194 ± 16 | 4.5 ± 0.3 (n = 4) | 1.8 ± 1.3 | |
| 16 (7) | 11-14, 23, 24 | 192 ± 17.2 | 210 ± 15.7 | 4.0 ± 0.4 (n = 4) | 1.4 ± 0.22 | |
| 5 | 17 (7) | 8, 21 | 183 ± 14.8 | 234 ± 23.1 | ||
| 18 (7) | 8, 22 | 214 ± 15.1 | 206 ± 13.4 | |||
| 19 (7) | 9, 21 | 170 ± 26.4 | 206 ± 8.2 | |||
| 20 (7) | 9, 22 | 191 ± 22.9 | 1508 ± 81.0 | |||
| 21 (7) | 10, 21 | 241 ± 12.5 | 202 ± 9.3 | |||
| 22 (7) | 10, 22 | 139 ± 9.3 | 230 ± 12.2 |
Total protein injected per female ranged from 20 μg (groups 17-22) to 140 μg (groups 1 and 2; see Materials and Methods).
Control 1 = nontransfected cell lysates; control 2 = 7.5 μg of vector DNA.
The weight range for normal engorged ticks in our colony of A. hebraeum is roughly 1000-3,000 mg. In this study, a group of normal engorged ticks weighed 1,695 ± 150 mg (n = 8; Fig. 5, group C1), a mean that is well within the range observed for recAhEF in this table.
The means for all ticks receiving recproteins 9 plus 22 (recAhEF; in bold) were significantly different (P < 0.0001 in all cases; ANOVA) than the corresponding means for ticks receiving the other recproteins.
Materials and Methods
Ticks. Our colony of A. hebraeum was maintained at 26°C and >95% relative humidity (11). Unfed female A. hebraeum do not readily attach to a host in the absence of males. Thus, unfed virgin females were placed on rabbits along with a number of fed males that had their gonopores blocked with a small drop of cyanoacrylate glue to prevent spermatophore transfer. Virgin females were removed from the host after 7 days of feeding, at which point they were all below the CW. Individuals were allocated to the treatment groups indicated in Table 1.
Construct Design and Preparation. The 28 feeding-induced genes expressed in this study were identified previously by differentially cross-screening a cDNA library; their differential status was confirmed by Northern blot analysis (10).
All constructs used in this study were drafted by using Gene Construction Kit 2 (SciQuest, Research Park, NC). All PCR primers, designed using Genetool software (Biotools, Edmonton, Canada), were engineered with 5′-EcoRI and 3′-XhoI restriction endonuclease cleavage sites (Invitrogen). The PCR primers used to amplify the clone inserts subsequently found to have EF bioactivity had the following sequences: AhT/VD 9-F, 5′-GGG AAT TCG GGA TGT TGA TCA CCA AGG ACC TGA-3′; AhT/VD 9-R, 5′-GGC TCG AGG GTC GAC CAG TGT CAA GCT CGG-3′; AhT/VD 22-F, 5′-GGG AAT TCG GGA TGG CGA AAC AGG GAC TT-3′; and AhT/VD 22-R, 5′-GGC TCG AGG GCC GCA GGC TCC CCA-3′. PCR was performed as described by Weiss et al. (10).
All PCR products were digested with EcoRI and XhoI restriction endonucleases (New England Biolabs) and ligated into an insect-based plasmid expression vector having either a C-terminal (pIB/V5-His) or N-terminal (pIB/His A, B, or C) 6x-polyhistidine tag. Constructs were propagated in DH5α competent cells (GIBCO/BRL), purified by using a Qiagen plasmid miniprep kit and subjected to EcoRI and XhoI restriction endonuclease digestion followed by electrophoresis on 1% agarose gels to verify the presence of insert and vector DNA. Finally, we sequenced all constructs using a DYEnamic ET terminator cycle sequencing premix kit (Amersham Pharmacia) and a PE Applied Biosystems 377 automated sequencer.
Expression and Detection. Sf21 cells were transfected with construct DNA by using Cellfectin liposome reagent (Invitrogen). recProteins were produced over 48 h at 27°C. Cell lysis buffer (100 μl) [50 mM Tris, pH 7.8/150 mM NaCl/1% (vol/vol) Igepal CA-630] was streamed repeatedly over the dishes to dislodge cells. Complete lysis was assured by vortexing the cells rapidly for 15 s and pelleting the cellular debris at 10,000 × g for 15 min at 4°C.
Protein concentration of cell lysis supernatant was determined by a Bradford assay (12). Proteins (≈ 20 μg) were electrophoresed on 3% stacked, 12% continuous separating polyacrylamide gels, and transferred to 0.2-μm nitrocellulose membranes (BioRad). Membranes were incubated in blocking buffer [50 mM Tris·HCl, pH 8.0/150 mM NaCl/3% (wt/vol) ovalbumin/0.1% (vol/vol) Triton X-100/0.1% (wt/vol) NaN3] for 30 min at room temperature, covered with anti-6x histidine antibody (diluted at 1:3,000 in fresh blocking buffer), and incubated on a rocking platform overnight at 4°C. Protein bands were visualized by using a goat anti-mouse secondary antibody conjugated to an IRDye 800 and an LI-COR (Lincoln, NE) Odyssey infrared imaging system.
EF Bioassay. T/VDs of fed males were homogenized in chilled saline [1.2% NaCl; 7.5 μl per T/VD pair (1 gonad equivalent)] and centrifuged at 8,000 × g for 5 min at 4°C. The pellet was discarded, and the supernatant was stored at –80°C. Fed virgin experimentals (all below the CW) were injected with a volume of this supernatant equivalent to 0.5, 1.0, or 1.5 gonad equivalents. Controls were injected with nothing, or 1.2% NaCl, or a similar extract of accessory gland from fed males (1 gonad equivalent), or a similar extract of T/VD from unfed males (1 gonad equivalent). Injected females were applied to a fresh rabbit and monitored for engorgement over 7 days.
All injections were made into the hemocoel by means of a coxal leg segment, by using a 30-gauge needle attached to a Hamilton (Reno, NV) microliter syringe. After injection, ticks were allowed up to 14 days to feed on fresh rabbits [except for the initial experiment (Fig. 2) in which only 7 days were allowed]. All engorged females were weighed and stored in the colony incubator, as were all ticks still attached at 14 days. Once all ticks were off the host, some were dissected at 4 days to measure SG degeneration, others at 10 days to measure ovary weight, and some left to lay eggs. SG degeneration was determined by measuring rate of fluid secretion in vitro (2).
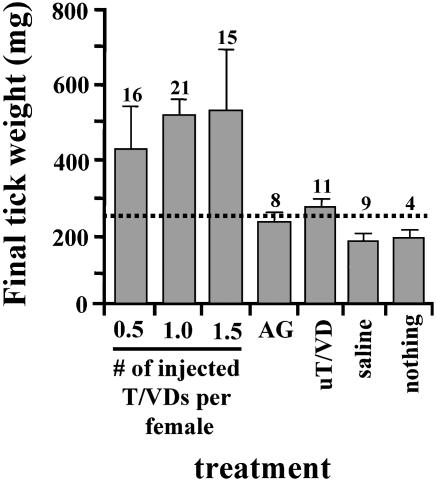
Fig. 2.
EF bioassay using a crude extract of T/VD from fed males. Shown are results for virgin females injected with all three doses (0.5, 1.0, and 1.5 whole gonad equivalents) of T/VD homogenate fed to significantly above the CW (≈ 250 mg for this population of ticks; indicated by dashed line) after being allowed to feed on fresh hosts for 7 days. However, those females injected with an extract of accessory gland (AG; one gonad equivalent) from fed males or an extract of T/VD from unfed males (uT/VD; one gonad equivalent) remained at or below the CW. Controls injected with 1.2% NaCl (saline) or nothing also remained below the CW. Numbers above each error bar indicate sample size.
Bioassay of the 28 recproteins and Purified Recombinant A. hebraeum Engorgement Factor (recAhEF). Lysates of Sf21 cells, corresponding to each of the feeding-induced recproteins, were grouped together and bioassayed in partially fed virgin females (Table 1). Each lysate in a group contributed 10 μg of protein. Thus, the total protein injected ranged from 20 μg (groups 17–22) to 140 μg (groups 1 and 2). The 28 recproteins shown in Table 1 were initially randomly allocated to two groups, each containing 14 recproteins. We did not attempt to include a group receiving a mixture of all 28 lysates, primarily because the injected volume would have been excessive. Neither group of 14 recproteins had EF bioactivity, suggesting that at least two proteins were necessary (one or more together from each of the original two groups). The subsequent groupings of recproteins in Table 1 were designed to progressively eliminate those without EF bioactivity. Controls received (i) nontransfected cell lysates, or (ii) 7.5 μg of vector DNA (both pIB/V5-His and pIB/His C).
From cell lysates, we purified the two recproteins eventually found to be necessary for EF bioactivity (group 20, Table 1) using a HisTrap 6x histidine-binding column according to the manufacturer's protocol (Amersham Pharmacia). Purified protein was stored at –20°C in elution buffer containing 500 mM imidazole.
Immunizations with recAhEF. A Flemish lop-eared rabbit was inoculated with 150 μg of recAhEF in Freund's complete adjuvant, followed by two booster inoculations in Freund's incomplete adjuvant, 4 weeks apart. Thirty-one unfed females and 31 males were fed on the inoculated rabbit for up to 14 days, and engorgement was compared with a similar group of ticks feeding on a normal rabbit.
Statistics. All data are expressed as mean ± SEM. Statistical significance was determined by Student's t test by using Microsoft excel software, or one-way ANOVA by using statview software (Abacus Concepts, Berkeley, CA), on a Macintosh computer.
Results
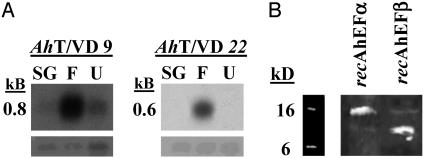
Differential Expression of EF RNA Transcripts. Fig. 1A shows Northern blots demonstrating that the mRNA encoding recAhEFα and recAhEFβ (clones AhT/VD9 and AhT/VD22, respectively) are up-regulated specifically in the gonads of males fed for 4 days.
Fig. 1.
(A) Northern blot analysis of differentially expressed clones. Radio-labeled clone AhT/VD 9 or AhT/VD 22 PCR product was used to probe 3 μg per lane of total RNA from the following tissues: fed salivary gland (SG), fed T/VD (F), and unfed T/VD (U). RNA was electrophoresed on 1.0% agaroseformaldehyde gels and subsequently transferred to nylon membranes. 18S ribosomal RNA (Lower) was used as a loading standard. (B) Western blots of crude cell lysates containing recAhEFα and recAhEFβ. Sf21 cells used for expression were lysed and centrifuged, and the resulting supernatants were subjected to electrophoresis on 12% polyacrylamide gels (see Materials and Methods). Proteins were transferred to nylon membranes, and blots were probed with an anti-6x histidine antibody (Invitrogen).
Bioassay of T/VD Homogenates for EF Bioactivity. Extracts of T/VD from fed males induced virgins to feed beyond the CW (250 mg for this population of A. hebraeum; Fig. 2). The differences in fed weight, 431 ± 129, 538 ± 43, and 545 ± 141 mg, among the three doses (0.5, 1.0, and 1.5 gonad equivalents) were not statistically significant (P > 0.05; ANOVA); neither were the differences in weight among the four controls (231 ± 11, 284 ± 17, 181 ± 6, and 199 ± 9 mg). However, all of the ticks receiving 0.5–1.5 gonad equivalents fed significantly more (505 ± 42 mg; n = 52) than the controls (224 ± 11 mg; n = 32; P < 0.0001; Student's t test).
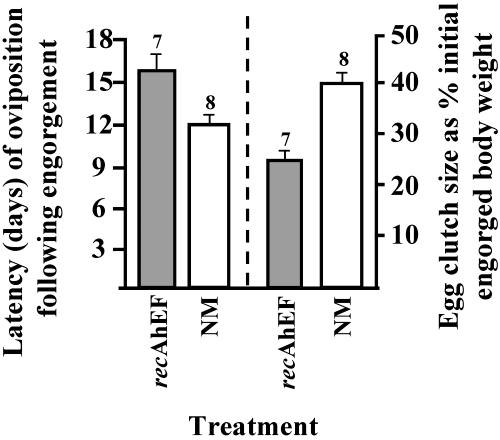
Bioassay of the 28 recproteins. Table 1 presents the sequence of bioassays performed to identify the two recproteins that together are sufficient and necessary for EF bioactivity. The mean weight of virgins at the time of injection was always below the CW. The only treatments resulting in engorgement and spontaneous detachment (groups 6, 10, 13, and 20 in Table 1) were those groups receiving recproteins 9 plus 22. Henceforth, rec9 is designated as recAhEFα and rec22 as recAhEFβ, and the two together as recAhEF. Table 1 also demonstrates that recAhEF possesses MF activity because it induced a marked reduction of fluid secretory competence (a physiological index of SG degeneration) in 4 days and stimulated a significant increase in ovary weight after 10 days. Finally, latency to oviposition in virgins injected with recAhEF was significantly higher (15.3 ± 1.4 days, n = 7, P < 0.001), and egg clutch size significantly lower (25 ± 2.1%, n = 7, P < 0.0001), than occurred in normal mated females (12.3 ± 0.5 days, n = 8, and 39.4 ± 7%, n = 8, respectively; Fig. 3).
Fig. 3.
Effects of recAhEF on egg production in A. hebraeum. Virgins injected with recAhEF (shaded bars) showed a significant increase in latency to oviposition (15.3 ± 1.4 days) compared with normally mated (NM) controls (12.3 ± 0.5 days) and a significant reduction in egg clutch size (25 ± 2.1%) compared with NM controls (39.4 ± 7%). Numbers above each error bar indicate sample size. Data from NM controls are from ref. 14.
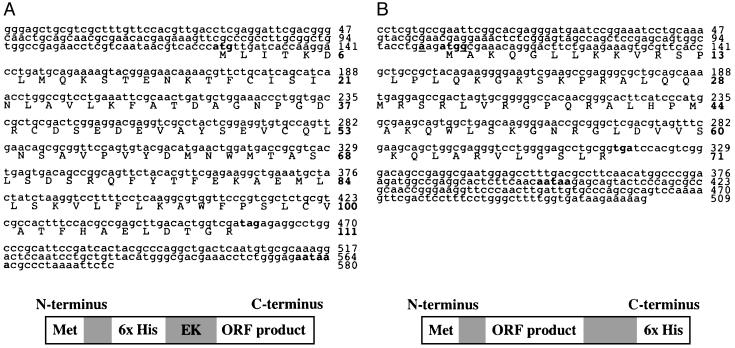
recAhEFα (16.1 kDa; Fig. 1B) is a protein encoded by a 333-nt ORF (GenBank accession no. AY442319) and contains 111 aa. The ORF, with a start codon (atg) at position 125 and a stop codon (tag) beginning at position 458, is followed by a polyadenylation [poly(A)+] signal at position 560. The ORF constitutes 57% of a 580-nt insert from clone AhT/VD 9 (Fig. 4A). recAhEFβ (11.6 kDa; Fig. 1B) is a protein encoded by a 213-nt ORF (GenBank accession no. AY442320) and contains 71 aa. The ORF, with a start codon (atg) at position 104 and a stop codon (tga) at position 317, is followed by a poly(A)+ signal at position 400. The ORF constitutes 42% of a 509-nt insert from clone AhT/VD 22 (Fig. 4B). The faint band visible in each lane in Fig. 1B may be the result of spillover while loading the samples, or perhaps nonspecific binding between the anti-6x histidine antibody and another protein in the lysate. A blast search (www.ncbi.nlm.nih.gov/blast/) revealed no significant homology between the cDNA and putative amino acid sequences of recAhEFα and recAhEFβ and any catalogued genes or proteins.
Fig. 4.
Nucleotide and putative amino acid sequences of AhT/VD 9 (580 bases) (A) and AhT/VD 22 (509 bases) (B). The start codon (atg), stop codons (tag and tga), and polyadenylation signals are bolded, and the Kozak consensus sequence (in B) is bolded and underlined (13). Upper numbers adjacent to each sequence indicate nucleotide position, and bolded numbers indicate amino acid position. Below each nucleotide sequence is a diagrammatic representation of the corresponding recprotein after expression. recAhEFα has an N-terminal 6x histidine detection tag. recAhEFβ has an C-terminal 6x histidine detection tag. Shaded boxes represent binding sites for other commercially available antibodies (anti-Xpress and anti-V5 monoclonals; Invitrogen), spacer regions, and an enterokinase cleavage site (EK).
Purification and Bioassay of recAhEF. recAhEFα and recAhEFβ, after elution from a 6x histidine-binding column, constituted ≈89% and 96% (respectively) of the total protein, as determined by NIH image software analysis (http://rsb.info.nih.gov/nihimage) of SDS/PAGE gels (data not shown).
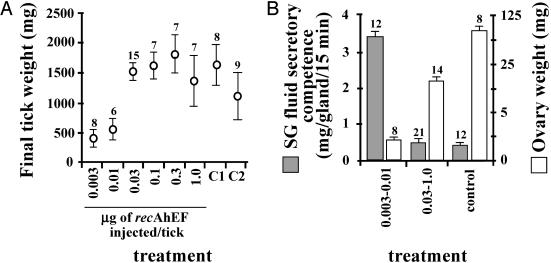
Purified recAhEF also stimulated engorgement, SG degeneration, and a significant increase in ovary weight in virgin females below the CW. Injection of as little as 0.03 μg of recAhEFα plus 0.03 μg recAhEFβ into virgins stimulated complete engorgement and detachment (Fig. 5A). Based on the combined MW of the two peptides (27.7 kDa), and an average preinjection tick weight of ≈200 mg (Table 1), the lowest concentration for a complete response was ≈11 nmol per kg of tick. The lowest doses (0.003 and 0.01 μg per tick) stimulated feeding to significantly beyond the CW, but not engorgement or detachment. Purified recAhEF also triggered SG degeneration and ovarian development in virgins at the same concentrations as were effective in stimulating engorgement (≥0.03 μg per tick of each peptide), but not at lower concentrations (Fig. 5B).
Fig. 5.
Effect of purified recAhEF on engorgement (A) and SG degeneration and ovarian development (B). Doses indicated are for each peptide (recAhEFα and recAhEFβ). Controls (C1 and C2 respectively, in A) are normally mated females and normally mated females injected with 500 mM imidazole (elution buffer). In B, controls have been pooled. Sample size is shown for each treatment.
Engorgement Success of Normal Ticks Fed on a Rabbit Immunized with recAhEF. Thirty-one normal mated females were placed on a rabbit immunized with recAhEF. The mean weight after 14 days was 521 ± 140 mg, a 72% reduction compared with the 28 normal females that fed to engorgement on a control rabbit (1,899 ± 74 mg). Whereas all of the females feeding on the control rabbit reached full engorgement within 14 days, only 8 engorged normally on the immunized rabbit (mean weight of 1,783 ± 140 mg; not significantly different from the control); the remaining 23 did not even achieve the CW (83 ± 10 mg). The mean engorgement period for the 8 engorged ticks from the immunized rabbit (11.8 ± 0.5 days) was significantly longer (P < 0.0001) than that for ticks feeding on the control rabbit (8.8 ± 0.8 days).
Discussion
Here, we report the isolation of a recombinant A. hebraeum EF from a group of 28 feeding-induced recproteins produced in vitro. Table 1 and Fig. 5A indicate that recAhEF induces a normal degree of SG degeneration but incomplete ovary development. Ovary weight of normal, mated A. hebraeum is ≈5–7% of engorged weight, 10 days postfeeding (14, 15). Hence, the mean ovary weight of virgins in groups 6, 10, and 13 (Table 1) was at most 30%, 22%, and 12.6%, respectively, of what would be calculated for weight-matched controls. The factor(s) responsible for this difference between recAhEF-injected virgins and normal mated females is not known. Moreover, the latency to oviposition was longer in the engorged virgins displayed in Table 1 compared with normal, mated engorged females, and the total egg mass was significantly less than that laid by normal engorged females (Fig. 3).
Previous work from our lab demonstrated that extracts of unfed gonad had little, if any, MF activity (2, 5). The results from our bioassay of crude T/VD homogenates (Fig. 2) also indicate that EF bioactivity is markedly enhanced as the result of feeding, and this finding was confirmed by Northern blot analysis (Fig. 1 A).
The molecular mass of native MF, as determined by gel filtration, was reported to be in the range of 20–100 kDa (4). Western blots (Fig. 1B) and computer analysis using peptool software (Biotools) both indicate that the combined molecular masses of recAhEFα and recAhEFβ (≈ 27.7 kDa) fall within this range. This molecular mass is different from tick sperm-capacitation factor (12.5 kDa) and vitellogenesis-stimulating factor (100 and 200 kDa; refs. 6 and 16), the only two other known mating factors from male ticks. Native EF may conceivably be larger than 27.7 kDa.
The site and mode of action of native EF and MF have not been determined. Both factors are introduced to the female during copulation, and both factors are effective when injected into the hemocoel. Moreover, the hemolymph of mated (but not virgin) females contains MF bioactivity (2, 17). This finding suggests one of the following: (i) EF and MF may be transported to the hemolymph from the female genital tract, as occurs with some insect sex peptides (18–20), or (ii) EF and MF may stimulate release into the hemocoel of a substance from the wall of the seminal receptacle. We should be better able to determine which of the latter mechanisms applies to EF once an ELISA is developed.
MF is characterized by its ability to hasten the onset of SG degeneration by 4 days, a process initiated by an early release of 20E (17). Lomas et al. (21) demonstrated that a peptide produced by the synganglion stimulates integumental ecdysteroidogenesis by means of a cAMP-dependent second messenger system. That the aforementioned synganglial peptide is necessary for this ecdysteroid production suggests that the synganglion may be the target tissue for MF.
We propose the generic name “voraxin” (Latin, vorax: voracious, gluttonous) for native AhEF and homologous proteins in other ticks.
Ticks serve as vectors for a diverse variety of pathogens, and the worldwide incidence of tick-borne diseases has increased significantly in the past 25 years (22, 23). We believe that this study has important implications for controlling ticks and the transmission of tick-borne diseases. If an effective vaccine can be derived from voraxin, the anticipated results from reduced feeding would include less salivation (hence reduced pathogen transmission to the host) and a reduction in oocyte development. Current methods for inhibiting the rapid growth of tick populations in areas where they transmit pathogens to humans and domestic animals include acaricides (24), biological control agents (25, 26), and recombinant anti-tick vaccines (27, 28). The two currently available commercial anti-tick vaccines are both based on the Bm86 antigen, which resulted in up to a 37% reduction in mean weight of the surviving ticks (29). The reduction we observed here (72%) suggests that a vaccine based on voraxin has the potential to provide significant protection to immunized hosts.
Acknowledgments
We thank Dr. Paul Wong (Department of Biological Sciences, University of Alberta) for much advice and encouragement offered throughout this study, Dr. Michael Pickard (Department of Biological Sciences, University of Alberta) for supplying Taq and Pfu DNA polymerases, and Ms. Pat Murray and Ms. Lisa Ostifichuk (Molecular Biology Research facility, University of Alberta) for performing all sequencing electrophoresis. This work was generously supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada (to W.R.K.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CW, critical weight; EF, engorgement factor; MF, male factor; rec, recombinant; recAhEF, recombinant Amblyomma hebraeum engorgement factor; SG, salivary gland; T/VD, testis/vas deferens.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY442319 and AY442320).
References
- 1.Balashov, Y. S. (1972) Misc. Publ. Entomol. Soc. Am. 8, 161–376. [Google Scholar]
- 2.Harris, R. A. & Kaufman, W. R. (1984) J. Exp. Biol. 109, 281–290. [Google Scholar]
- 3.Weiss, B. L. & Kaufman, W. R. (2001) J. Insect Physiol. 47, 1261–1267. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman, W. R. & Lomas, L. O. (1996) Invertebr. Reprod. Dev. 30, 191–198. [Google Scholar]
- 5.Lomas, L. O. & Kaufman, W. R. (1992a) J. Insect Physiol. 38, 595–601. [Google Scholar]
- 6.Shephard, J., Oliver, J. H. & Hall, J. D. (1982) Int. J. Invertebr. Reprod. 5, 129–137. [Google Scholar]
- 7.Sahli, R, Germond, J. E., Diehl & P. E. (1985) Exp. Parasitol. 60, 383–395. [DOI] [PubMed] [Google Scholar]
- 8.Pappas, P. J. & Oliver, J. H. (1971) J. Ga. Entomol. Soc. 6, 122–124. [Google Scholar]
- 9.Pappas, P. J. & Oliver, J. H. (1972) J. Med. Entomol. 9, 47–50. [DOI] [PubMed] [Google Scholar]
- 10.Weiss, B. L., Stepczynski, J. M., Wong, P. & Kaufman, W. R. (2002) Insect Biochem. Mol. Biol. 32, 785–793. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman, W. R. & Phillips, J. E. (1973) J. Exp. Biol. 58, 523–536. [Google Scholar]
- 12.Bradford, M. M. (1976) Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 13.Kozak, M. (1990) Proc. Natl. Acad. Sci. USA 87, 8301–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lunke, M. D. & Kaufman, W. R. (1992) Exp. Appl. Acarol. 13, 249–259. [DOI] [PubMed] [Google Scholar]
- 15.Lunke, M. D. & Kaufman, W. R. (1993) Invertebr. Reprod. Dev. 23, 25–38. [Google Scholar]
- 16.Connat, J. L., Ducommun, J., Diehl, P. A. & Aeschlimann, A. A. (1986) in Morphology, Physiology and Behavioral Biology of Ticks, eds. Sauer, J. R & Hair, J. A. (Ellis Horwood, Chichester, U.K.), pp. 194–216.
- 17.Lomas, L. O. & Kaufman, W. R. (1992) Arch. Insect Biochem. Physiol. 21, 169–178. [DOI] [PubMed] [Google Scholar]
- 18.Yi, S. X. & Gillot, C. (1999) J. Insect Physiol. 45, 143–150. [DOI] [PubMed] [Google Scholar]
- 19.Yamaoka, K. & Hirao, T. (1977) J. Insect Physiol. 23, 57–63. [DOI] [PubMed] [Google Scholar]
- 20.Smid, H. M. (1998) Invertebr. Reprod. Dev. 34, 47–53. [Google Scholar]
- 21.Lomas, L. O., Turner, P. C. & Rees, H. H. (1997) Proc. R. Soc. London B 264, 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gayle, A. & Ringdahl, E. (2001) Am. Fam. Physician 64, 461–466. [PubMed] [Google Scholar]
- 23.Young, J. D. (1998) N. Engl. J. Med. 338, 1629 (lett.). [DOI] [PubMed] [Google Scholar]
- 24.Frisch, J. E. (1999) Int. J. Parasitol. 29, 57–71. [DOI] [PubMed] [Google Scholar]
- 25.Samish, M. & Rehacek, J. (1999) Annu. Rev. Entomol. 44, 159–182. [DOI] [PubMed] [Google Scholar]
- 26.Samish, M. (2000) Ann. N.Y. Acad. Sci. 916, 172–178. [DOI] [PubMed] [Google Scholar]
- 27.Willadsen, P., Bird, P., Cobon, G. S. & Hungerford, J. (1995) Parasitology 110, S43–S50. [DOI] [PubMed] [Google Scholar]
- 28.Trimnell, A. R., Hails, R. S. & Nuttall, P. A. (2002) Vaccine 20, 3560–3568. [DOI] [PubMed] [Google Scholar]
- 29.Tellam, R. L., Smith, D., Kemp, D. H. & Willadsen, P. (1992) in Animal Parasite Control Utilizing Biotechnology, ed. Yong, W. K. (CRC, Boca Raton, FL), pp. 303–331.