Abstract
The long-term developmental and behavioral consequences of mammalian embryo culture are unknown. By altering the culture medium with the addition of FCS, we wanted to determine whether mouse embryos cultured under suboptimal conditions develop aberrant mRNA expression of imprinting genes at the blastocyst stage and whether fetal development, growth, and behavior of adult mice are affected. One-cell embryos obtained from superovulated female B6CBAF1 mice were cultured for 4 days in K+-modified simplex optimized medium in the presence of either 10% FCS or 1 g/liter BSA. After embryo transfer, born animals were submitted to several developmental and behavior tests. The mRNA expression of some imprinting genes was significantly affected in blastocysts cultured in the presence of FCS. Two of the eight measures of preweaning development and some specific measures of neuromotor development, such as the walking activity, were delayed in the group originated with FCS. After 34 weeks, the weight of female mice cultured in vitro in the presence of FCS was significantly higher than controls. In addition, the locomotion activity of mice was altered at 5 and 15 months. Anatomopathological and histological analysis of animals at 20 months of age showed some large organs and an increase in pathologies. We have found that mice derived from embryos cultured with FCS exhibited specific behavioral alterations in anxiety and displayed deficiencies in implicit memories. Our data indicate that long-term programming of postnatal development, growth, and physiology can be affected irreversibly during the preimplantation period of embryo development by suboptimal in vitro culture.
Keywords: embryo culture, organ weight, postnatal defects
In many of the new assisted reproductive technologies (ART), embryos are kept for a short time in a synthetic medium before transfer into their recipient mothers. Some evidences suggest that the culture process may alter normal development and may produce specific abnormalities during fetal and postnatal development (1, 2, 3). In sheep and cattle, large-offspring syndrome has been described, when the offspring is unusually large or presents aberrant phenotype. This phenomenon is found occasionally in calves and lambs that are born after embryo manipulations, such as in vitro fertilization (IVF) and nuclear transfer (4). Several culture systems have been associated with higher incidence of this condition, but all of them share a common feature: the use of serum (5, 6, 7). Serum contains active compounds (e.g., hormones and growth factors), providing a rich but undefined environment for embryo development (5). Serum reduces the early developmental potential of embryos (1), causes abnormal metabolic and ultrastructural embryo configurations (8, 9), reduces the inner-cell-mass:trophectoderm ratio by increasing the level of programmed cell death within the inner cell mass of embryos (10), affects the expression of developmentally important genes (11, 12, 13), and, at the same time, increases the embryo's sensitivity to cryopreservation and compromises its viability, as indicated by reduced embryo survival after thawing (14) and pregnancy rates (15). One hypothesis that may explain these abnormalities suggests that the embryo does not receive the right cues when cultured in vitro in the presence of FCS and that such abnormal signaling results in misregulation of genes (3) or aberrant epigenetic modifications in the genome (16). These alterations could be maintained somatically and might affect gene expression at later stages of development (17, 18). The subacute nature of at least some of the aberrant embryo changes induced by in vitro culture (IVC) with serum allows the changes to remain undetected in the short term. Blastocyst production, a hallmark for the efficiency of in vitro embryo culture systems, can often be achieved despite detrimental environmental effects. Embryo IVC with serum produces more blastocysts than culture without serum. However, implantation rates of embryos cultured in the presence of FCS remains very poor (19, 5), and the long-term effect of associated-early abnormalities is not known.
The use of ART to treat human infertility is gaining widespread use, and disconcerting to many researchers is that the clinical procedures used in ART are rapidly outpacing the underlying science. Moral, ethical, and legal issues complicate assessing the genetic quality of ART-derived human conception; therefore, appropriate animal models provide an important tool for studying potential effects of embryo culture on the health and development of mammalian embryos. It has been reported that culture conditions can perturb global patterns of gene expression in preimplantation mouse embryos (20). In particular, certain culture conditions result in biallelic expression of the imprinted H19 gene in the blastocyst, and this biallelic expression persists in extra-embryonic tissue after implantation (21). Genome imprinting includes a proportion of genes in mammals that are repressed on one of a pair of chromosomes, and this depends on the parental origin of the gene. Imprinting genes are specially implicated in the regulation of fetal growth, development and function of the placenta, brain development, and postnatal behavior (22). As imprinting is reestablished during preimplantation development, our aim was to determine the mRNA expression of four imprinted genes. Recent retrospective studies have unmasked an increased incidence of certain syndromes that are the result of loss-of-imprinting, highlighting the concern about the aggressive practice of ART. The long-term developmental and behavioral consequences of ART are unknown. To address this issue, we have developed a mouse model to study the effects of embryo culture in presence of FCS on development, mRNA expression of imprinting genes, and behavior of the offspring.
Materials and Methods
Embryo Production. All mice used in this experiment were maintained in an animal facility with controlled temperature conditions of 23 ± 1°C, with a 14-h light:10-h dark photoperiod and with free access to water and food. Experimental procedures followed ethical guidelines established by the Federation of European Laboratory Animal Science Associations. One-cell embryos obtained from superovulated female B6CBAF1 mice were cultured for 4 days in K+-modified simplex optimized medium (KSOM) in presence of 10% FCS (group A) or in presence of 1 g/liter BSA (control). Embryos that reached the blastocyst stage were transferred into CD1 pseudopregnant females or were frozen until mRNA purification and analysis.
RNA Extraction and Reverse Transcription. Poly(A)-RNA was prepared from four to five pools of 10 embryos by following the manufacturer's instructions and by using the Dynabeads mRNA Direct Extraction KIT (Dynal Biotech, Oslo). Poly(A)-mRNA was used in the RT-PCR in a total volume of 20 μlwith2.5 μM random hexamer primer/1× reverse transcription (RT) buffer/20 units of RNase inhibitor/50 units of Moloney murine leukemia virus reverse transcriptase enzyme (Promega)/5 mM MgCl2/1 mM of each dNTP. Tubes were heated to 70°C for 5 min to denature the secondary RNA structure before addition of RT enzyme. The RT reaction started with a 10-min incubation period at room temperature, followed by a 60-min period at 42°C to allow the RT of RNA, and ended after a 1-min enzyme denaturing step at 93°C.
Quantitative RT-PCR. The quantification of all gene transcripts was carried out by real-time quantitative RT-PCR. Four replicate PCR experiments were conducted for all genes of interest by using embryos collected from the experimental pools. Experiments were conducted to contrast relative levels of each transcript and mouse histone H2a in every sample. PCR was performed by adding 4 μl of each sample to the PCR mix containing the specific primers to amplify H2a and the imprinted genes [insulin-like growth factor 2 (Igf2), H19, mesoderm specific transcript homolog/epoxide hydrolase 1 (Mest/Peg1), and the growth factor receptor-bound protein 10/maternally expressed gene 1 (Grb10/Meg1)]. Primer sequences, annealing temperature, approximate sizes of amplified fragments, and GenBank accession numbers are shown in Table 4, which is published as supporting information on the PNAS web site. Murine histone H2a amplification was used as a standard control for the RT-PCR. PCR quantification was performed by using a Rotorgene 2000 Real Time Cycler (Corbett Research, Sydney) and SYBR Green (Molecular Probes) as a double-stranded DNA-specific fluorescent dye. The PCR reaction mixture (25 μl) contained 2.5 μl of 10× buffer, 3 mM MgCl2, 2 units of Taq Express (MWG Biotech, Ebersberg, Germany), 100 μM dNTPs, and 0.2 μM of each primer. In addition, the double-stranded DNA dye, SYBR Green I, (1:3,000 of 10,000× stock solution) was included in each reaction. The PCR protocol included an initial step of 94°C(2 min), followed by 40 cycles of 94°C (15 sec), 56–59°C (30 sec), and 72°C (30 sec). Fluorescent data were acquired at 85°C. The melting protocol consisted of a hold temperature at 40°C for 60 sec and then heating from 50°C to 94°C, holding at each temperature for 5 sec while monitoring fluorescence. Product identity was confirmed by ethidium-bromide-stained 2% agarose gel electrophoresis. As negative controls, RT reaction tubes were prepared without RNA or polymerase. In addition, amplified identities were confirmed by the appropriate restriction digestions of PCR products (data not shown).
The method used for quantification of expression was the relative standard curve method (13). Preweaning Developmental Studies. All of the pregnant dams were allowed to deliver spontaneously. The day of delivery was designated as day 1 of age of the neonates (error in estimations on time of birth was of ± 6 h). On delivery, the litter size of each dam was recorded, and each pup was checked for gross abnormalities. The pups were nursed by their natural dams until weaning. During the testing protocol, whole litters were separated from the dams and maintained for 30 min in a warmed environment. Males and females were pooled to perform the neurodevelopmental screening based on preliminary experiments, demonstrating no significant sex effect on the measures. The age range for each response observation was established from preliminary experiments so that the monitoring period could be defined minimizing handling. All the measures were performed between 9:30 a.m. and 11 a.m. Forty-three mice (24 males and 19 females) obtained from group A embryos (cultured in the presence of serum) and 35 control mice (13 males and 12 females cultured in serum-free medium) were submitted to preweaning developmental tests and postnatal behavioral tests. To assess with detail development throughout the neonatal period, our preweaning developmental study used several exams: body growth, fur appearance, incisor eruption, eye opening, surface righting, negative geotaxis, pivoting locomotion, walking test, and homing test (for a description, see Table 5, which is published as supporting information on the PNAS web site). Beginning at 2 days of age, newborn mice were examined daily for developmental milestones and complex development.
Postnatal Behavioral Studies. The tests performed were the Openfield test (23, 24), the elevated plus maze paradigm (24), and the free-choice exploration paradigm in Y-maze (25) (for a description, see Table 6, which is published as supporting information on the PNAS web site). All tests were performed by trained observers blind for experimental groups. Devices used for all behavioral studies were carefully cleaned with a diluted acetic acid solution between animals to prevent olfactory cues.
Postnatal Growth and Histological Analysis of Aged Animals. After weaning, mice were weighed weekly until 10 weeks of age and biweekly thereafter. Twenty months later, some viscera, including the liver, lung, heart, kidney, spleen, and testes were excised and weighed. In addition, histopathology analyses were done. Samples (liver, lung, heart, kidney, and spleen) were fixed in 10% buffered formalin and embedded in paraffin wax. Sections with 4 μm were stained with hematoxilyn-eosin and Congo red.
Data Analysis. Data on mRNA expression were analyzed with the sigmastat (Jandel, San Rafael, CA) software package. One-way repeated-measures ANOVA (followed by multiple pair-wise comparisons with the Student-Newman–Keul's method) were used for the analysis of differences in mRNA expression assayed by quantitative RT-PCR. Differences of P < 0.05 were considered significant. Significance of the effects of the preweaning exams was assessed by a one-way ANOVA or a multivariate ANOVA with a Bonferroni or Newman–Keul's test for post hoc analysis. Factor analysis included culture conditions, sex, novelty/familiarity, and time points. Student's t test was used for comparisons between two groups. Nonparametric data were analyzed by χ2. Analysis was processed by using the spss (Statistical Package for the Social Sciences) program.
Results
In Vitro Embryo Culture in the Presence of Serum. To analyze the impact of culture media supplementation with serum on embryo viability during preimplantation, one-cell embryos were cultured in control KSOM or KSOM supplemented with 10% FCS and transferred to recipient females at the blastocyst stage. Serum was found to reduce the percentage of blastocysts produced in vitro. Although 95% of one-cell embryos cultured in control KSOM reached the blastocyst stage after 72 h, only 43% of the embryos cultured in KSOM supplemented with FCS reached such a developmental stage (Table 1). Blastocyst development to term after transfer into recipient uteri was smaller when IVC was performed in the presence of FCS (23% vs. 44%). Newborn pups from both groups were healthy and rapidly gained active movement.
Table 1. Effect of FCS on in vitro culture of mouse embryos.
| No. of one-cell embryos set in culture | No. of embryos reaching the two-cell stage | No. of blastocysts (%) | No. of blastocysts transferred | No. of live offspring (%) | |
|---|---|---|---|---|---|
| +FCS | 441 | 435 | 190 (43)* | 187 | 43 (23)* |
| -FCS | 87 | 85 | 83 (95)* | 80 | 35 (44)* |
P < 0.05, values in the same column with significant differences (χ2 analysis).
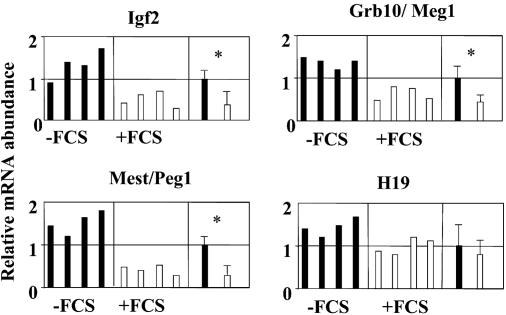
Alteration in the mRNA Expression of Imprinting Genes. We studied mRNA expression of 4 growth-related imprinting genes (Mest/Peg 1, Igf2, H19, and Grb10/Meg1) in mouse embryos at the blastocyst stage that were produced in KSOM and KSOM supplemented with 10% of FCS. The relative abundance of the gene transcripts studied is shown in Fig. 1. The level of mRNA expression of Igf2 mRNA was significantly higher in blastocysts that were cultured in absence of FCS. The relative abundance of Grb10/Meg1 and Mest/Peg1 transcripts was also significantly higher in embryos cultured in the absence of FCS.
Fig. 1.
Relative abundance of four growth-related imprinting genes (four replicates) in mice blastocysts cultured in the presence (white bars) or absence (black bars) of FCS. The mean of the four replicates was calculated for both groups, and the group with the higher value was assigned a value of 1. *, significant differences of P < 0.05.
There was no difference in H19 transcript abundance between blastocysts cultured in the presence or absence of FCS.
Preweaning Alteration of Development. Somatometric development was similar in mice with (group A) or without (control) preimplantation exposure to FCS, as demonstrated by the parallel growth increase. Regarding the appearance of developmental landmarks, only for incisor eruption and negative geotaxis, a significant difference was observed among groups.
At postnatal day 7 and day 10, neuromotor development was assessed by the pivoting and walking tests. At day 7 differences between groups A and control in the pivoting locomotion task were not seen. At day 10, the age-dependent increase in the pivoting activity, which is an index of adequate motor activity maturation and of the general maturation of the central nervous system, was smaller in mice with preimplantation exposure to FCS during IVC.
Regarding walking activity, there were no differences at day 7; however, at day 10, when the walking activity is usually already mature, a significant increase in latency to walk a distance exceeding its body length was observed in mice exposed during IVC to FCS (Table 2), suggesting again the presence of a neurodevelopmental impairment. Finally, the homing of group A pups to their nest was not significantly different from the homing of controls.
Table 2. Developmental behavior records of animals obtained from embryos cultured in vitro in the absence or presence of FCS.
| Conditions | Pivoting test day 7 | Walking test day 7, sec | Pivoting test day 10 | Walking test day 10, sec | Homing test, sec |
|---|---|---|---|---|---|
| +FCS | 9.4 ± 4.7 | 55.4 ± 3.0 | 17.3 ± 2.4* | 44.8 ± 3.5* | 30.4 ± 3.2 |
| -FCS | 15 ± 5.7 | 48 ± 3.3 | 23.9 ± 3.3* | 32.8 ± 5.5* | 25.1 ± 5.7 |
Forty-three mice derived from embryos cultured in the presence of FCS and 35 mice derived from embryos cultured in the absence of FCS were tested.
P < 0.05, values in the same column with significant differences (ANOVA).
Postnatal Studies: Hyperactivity, Anxiety, and Deficiencies in Implicit Memories. In a preliminary study (Tables 7 and 8, which are published as supporting information on the PNAS web site) we characterized the normal pattern of behavior of normal mice of the same genotype in the open-field test and the elevated plus maze. Female mice were more active than males under novelty conditions [F(1, 40) = 17.5, P < 0.0005]. They all exhibited a profound decrease of exploration 24 h after the exposure to the test conditions [F(1, 40) = 10.5, P < 0.01]. Both sexes showed similar rates of exploration of the exposed arms of the elevated plus maze, and they both reduced dramatically the exploratory activity 24 h after a single exposure. This attenuated exploration has been previously described in rats and mice (23, 24). By using this paradigm, we characterized the behavioral response of animals produced with or without FCS. At 5 months of age (see Fig. 4, which is published as supporting information on the PNAS web site), males produced by IVC + FCS displayed higher locomotor activity than controls in both novelty and familiarity [sex × culture condition × day of study; F(9,360) = 2.54, P < 0.01]. Males from the FCS group were more active than females [F(1,40) = 17.5, P < 0.0005]. There was a clear sex × culture condition interaction [F(1,40) = 15.6, P < 0.001], reflecting that hyperactivity appears in males exposed to FCS [simple effect of FCS on males; (F(1,40) = 3.2, P < 0.0001]. At 15 months of age (see Fig. 5, which is published as supporting information on the PNAS web site) they displayed lower locomotor activity than control mice [F(1,40) = 5.9, P < 0.02]. The age-dependent changes in locomotor activity were not observed in females produced with FCS. Control mice showed a significant habituation (decreased number of squares crossed) under familiarity. Lastly, the attenuation in locomotor responses exhibited 24 h after the first test was not observed in FCS-produced mice of both sexes at any age studied. Although young male animals explored more the center of the field, suggesting a lower anxiety state, they did the opposite at 15 months of age, indicating increased anxiety levels associated with aging.
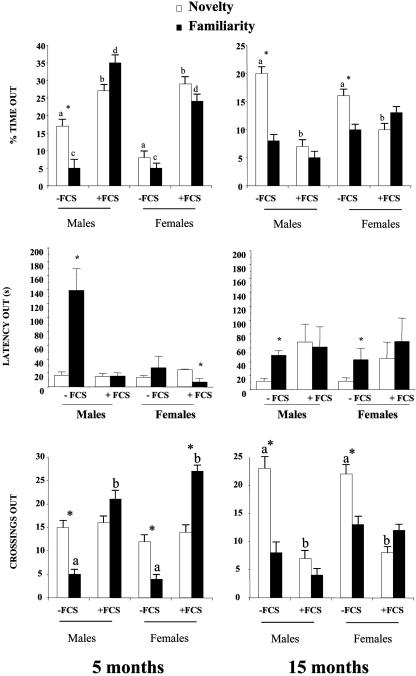
This age-dependent anxiolysis/anxiety was confirmed by using the elevated plus maze. In this test, animals are exposed to an approach/avoidance conflict between exploratory behavior and a natural aversion to heights and open spaces. Reduced exploration of exposed arms is considered to reflect increased anxiety. The number of crossing out (number of visits) provides a measure for general activity and anxiolysis. At 5 months of age (Fig. 2), males and females produced with FCS exhibited a marked anxiolysis, exploring the open arms of the maze more than animals not produced with FCS. Retesting after 24 h of IVC + FCS mice showed a lack of the normal attenuation in exploration, suggesting a sustained anxiolytic state and decreased fear responses. These effects were more clear in males on which the marked increase in the latency to explore the exposed arms 24 h after the first exposure did not occur. Both male and female IVC + FCS mice showed higher crossing activity than control after retesting (familiarity). Again, as found in the open field, these responses were reversed with aging. At 15 months of age, male and female IVC + FCS mice always showed a lower exploratory rate than control mice in the open arms. As happened in the open-field studies, mice did not keep a memory trace of the first exposure, and they did not show the attenuation of exploration after 24 h. Altogether, these observations indicate that IVC + FCS mice have a lower anxiety tone at young ages and a higher anxiety state when aged.
Fig. 2.
Elevated plus maze paradigm. At 5 months of age, mice produced with FCS exhibited a greater anxiolysis than controls, as reflected in the percentage of time spent in the exposed arms of the elevated plus maze under novelty conditions (Top Left). Retesting 24 h after (familiarity) induced a fear response in controls, characterized by enhanced latency to enter into the exposed arms (Middle Left). Young mice of both sexes produced with FCS did not exhibit this conditioned fear, spending more time, exhibiting lower latencies, and displaying greater activity (Bottom) than controls. This pattern was completely reversed with aging. Animals at 15 months of age produced with FCS displayed higher anxiety states and lower activity in the exposed arms of the maze (Right). Again, animals exposed to FCS did not exhibit fear responses during retesting. Data are means ± SEM of at least 10 determinations per group. Within each color bar, different letters denote significant differences (a ≯ b; c ≯ d, P < 0.05). *, P < 0.05 versus –FCS group, Newman–Keul's test.
To assess whether the young animals were constitutively hyperactive and that the lack of habituation to the open field was not derived of impaired recognition of novelty/familiarity we used the free-choice Y-maze test (ref. 25 and its supplementary materials). Results indicate that mice produced with FCS do recognize novelty, retaining a good memory trace for very short intertrials (2 min; see Figs. 6 and 7, which are published as supporting information on the PNAS web site). Moreover, males produced with FCS spent more time on the novel arm than control animals in the longest intertrial interval tested (30 min).
Postnatal Growth and Histology of Aged Mice. Body weight data for male and female mice produced in vitro in media with or without FCS are presented in Fig. 3. Weights were not significantly different between males; whereas, from the 31st week on, weights of females produced with FCS were higher than those of controls. However, this difference disappeared at week 70. Although the body weight did not show significant differences, liver and heart weights were significantly greater (P < 0.01) in 20-month-old mice derived from in vitro culture with FCS treatment than in control animals (Table 3).
Fig. 3.
Body weights of male and female mice produced in vitro in the presence or absence of FCS (0–76 weeks old). *, P < 0.05, versus–FCS group, Student's t test.
Table 3. Organ weights of 2-year-old mice produced in vitro with or without FCS.
| Males
|
Females
|
|||
|---|---|---|---|---|
| Organs | +FCS | -FCS | +FCS | -FCS |
| Liver | 1.90 ± 0.09* | 1.38 ± 0.10* | 1.59 ± 0.05* | 1.00 ± 0.07* |
| Heart | 0.29 ± 0.02* | 0.20 ± 0.01* | 0.19 ± 0.01* | 0.13 ± 0.01* |
| Spleen | 0.12 ± 0.02 | 0.11 ± 0.06 | 0.09 ± 0.02 | 0.07 ± 0.01 |
| Kidneys | 0.63 ± 0.03 | 0.57 ± 0.04 | 0.36 ± 0.01 | 0.36 ± 0.02 |
| Testicles | 0.19 ± 0.01 | 0.17 ± 0.02 | ||
Data are mean ± SD. Asterisk indicates significant difference (P < 0.05). Statistics used Student's t test for independent samples.
The postmortem histology examination of selected organs (liver, heart, spleen, kidney, lung, and testicles) showed some abnormalities of liver, kidney, and lung. We found presence of pneumonia (47% of FCS cases vs. 16% of control). Females produce with FCS showed steatosis in the liver with the presence of vacuoles of fat and glycogen (Periodic acid-Schiff-positive) in the cytoplasm. Steatosis was mild in males produced with FCS and was mild or absent in control mice. These vacuoles displaced the nucleus to a peripheral position in the cell. The presence of glycogen vacuoles are probably related to a disorder on glycogen metabolism, and the abundant fatty infiltration in the liver may be caused by mobilization of fat as an energy source. Furthermore, mice produced with FCS showed perivascular kidney inflammatory infiltration that only were occasionally found in the control mice. In addition, two males produced with FCS presented testicular atrophy.
Discussion
Many scientific, medical, and commercial interventions today use the culture of mammalian preimplantation embryos, including the generation of transgenic animals, gene targeting, assisted reproduction technologies in human and domestic animals, cloning from embryonic and adult cells, and cloning for the generation of human stem cells. Recently, adverse effects of such procedures on postimplantation growth and development have been documented. For example, the manipulation of bovine embryos during preimplantation stages often produce unpredictable results in the delivery of unusually large offspring, an event commonly associated with the IVC of in vitro-produced embryos. These perturbations occurring during the first days after fertilization may profoundly interfere with the postimplantation embryonic, fetal, and placental growths. However, the physiological or molecular bases of these perturbations still remain unknown. Changes in the controlled program of activation/inactivation of parental alleles of imprinted genes during IVC and, subsequently, early development could lead to a differential fetal growth regulation by either the fetal or placental system or even both.
We have found here that serum inhibited the in vitro growth of one-cell embryos. More embryos cultured in KSOM supplemented with FCS died before they reached the blastocyst stage. In agreement with our results, serum has been found to reduce the postimplantation viability of embryos (20, 26, 27). Blastocysts that were cultured in the presence of FCS showed a normal appearance; however, a higher proportion of them did not survive after transfer into recipient females compared with blastocysts obtained in the control group. Serum appears to have adverse effects on gene expression (12) and may also affect mRNA expression of imprinting genes. Studies on the mRNA expression of imprinted genes may provide some clues to the way in which the manipulation of the embryo or its environment during early development might influence the phenotype of the offspring.
To date, ≈74 imprinted genes have been found in mice and humans, and many more are expected to become identified in the future (www.mgu.har.mrc.ac.uk). Major changes in the DNA methylation of imprinted genes occur in the mouse preimplantation embryo, leading to the silencing or expression of particular genes. It was suggested that epigenetic modification of imprinted genes may result from culture environments during early embryogenesis and that these modifications may affect gene expression during later fetal development and result in conditions such as large-offspring syndrome. During preimplantation, extensive changes in genomewide methylation take place, and any perturbation caused in this process may result in deregulation of development at later stages. Imprinting genes are particularly susceptible to methylation changes during early development (28). The fetal abnormalities observed as a consequence of IVC have been proposed to result from aberrant changes in the methylation status of imprinting genes (17), and this deregulation has effects on growth and development (5, 29). In this work, we have analyzed the mRNA expression of Igf2 and three other growth-related imprinting genes: H19, Mest/Peg1, and Grb10/Meg1. H19 and Igf2 are reciprocally imprinted; meanwhile, H19 is expressed from the maternal allele during all stages of embryonic development, including blastocysts, and Igf2 is expressed only from the paternal chromosome (21). Alterations in the expression of the Igf2 have been shown to severely affect growth in mice (29, 30) and humans (16). Also in humans, deregulation of Igf2 has been implicated in cases of the congenital overgrowth syndrome, Beckwith–Weidemann syndrome (31, 32). Grb10 is another imprinting gene whose product affects the Igf1/insulin axis and it is expressed from the maternal chromosome exclusively (33). Mest is another growth-related imprinted gene that is paternally expressed. Doherty et al. (21) have reported that culture conditions of preimplantation mice embryos altered the expression and imprinting status of H19. Under our experimental conditions, we have found in all four replicates a lower expression of mRNA H19, but differences were nonsignificant. However, the three other mRNAs analyzed showed significantly smaller expression in the IVC + FCS group, indicating that, in agreement with Doherty et al. (21), culture conditions can selectively affect the expression of imprinted genes at the blastocyst stage. The aberrant expression of these growth-related genes could be a stochastic mechanism induced by higher levels of methylation produced by the FCS. A significantly higher level of methylation in fetuses produced from the culture of embryos in the presence of serum has been reported (20). Serum increases the developmental speed of preimplantation embryos (34), and these alterations in the pace of cell cycles can result in the improper maintenance of methylation and chromatin imprints. In addition, some components of FCS may be responsible of the aberrant expression of these mRNAs.
The abnormal appearance of some developmental landmarks, such as incisor eruption and negative geotaxis, could be indications of some abnormalities in the general maturation of the nervous system. Regarding the neurobehavioral facts, measures reflecting crania-caudal maturation, such as the latency to initiate walking or other measures, also depend on colliculi maturation, such as the pivoting activity, and were significantly different in pups produced in culture with FCS. The age-dependent increase in pivoting activity at day 10, a measure that reflects the adequate maturation of motor activity and that is also an index of general maturation of the central nervous system (35), was lower in pups from group A compared with controls, indicating either a hypoactive behavior or retardation in the acquisition of motor skills. The higher walking latency at this age supports the possible hypoactivity observed in the pivoting test. It has been argued that retardation in body growth can affect general developmental parameters and neuromotor behavior; however, because no significant differences were attained in the growth curve at these ages, this effect might be excluded.
The behavioral data obtained in the open-field test and elevated plus maze suggests that mice produced with FCS develop a sexdimorphic hyperactivity and low anxiety state at youth. They showed poor habituation to the field of study, exhibiting a nearly constant hyperactivity during the 10 min of test. This phenotype, found to be more marked in males than in females, is reversed with aging, being substituted by hypoactivity and generalized anxiety. Independently of age, animals exposed to FCS did not exhibit attenuated exploratory activity associated with preexposure to aversive environments, such as the elevated plus maze, suggesting a profound disturbance of the subcortical limbic circuits that process this type of implicit memory. It is worth noting that decreased response habituation has been implicated in impaired attention in human studies (36, 37). Habituation diminishes unwanted responses as a result of repeated exposure to neutral stimuli and enables the organism to attend selectively to relevant stimuli. The impact of these alterations in more complex behavioral tasks remains to be elucidated. Preliminary studies with the Y-maze showed that young animals exposed to FCS behave not only normally but better than controls at short intertrial intervals. These data indicate a normal functioning of working and short-term memory circuits, on which the cortico-hippocampal loops are involved. Whether aging deterioration is accelerated in FCS-exposed animals remains to be studied. However, the lack of memory trace of the aversive properties of the elevated plus maze suggests that implicit aversive memories, on which the extended amygdalar circuits are involved, are affected. Whether FCS exposure produces either an impaired memory consolidation, or the induction of a potent endogenous anxiolytic tone counteracting the aversive nature of the fear response, remains to be conclusively elucidated. Anyway, the overall picture shows a profound modification of emotional and motivational responses derived of FCS exposure.
Malformation in some viscera has been found in many mammalian animals produced by nuclear transfer (38) and in some in vitro-produced animals (39). These authors observed that oversized calves derived from in vitro-produced embryos had abnormally large hearts compared with their control counterparts when slaughtered at the same weight at 1 year of age, suggesting that, at least for the heart, some effects of aberrant prenatal development can persist into adulthood. These findings agree with our results for the hearts and livers of the mice produced with FCS. In addition, the postmortem histology indicates that when IVC + FCS mice were produced, careful postmortem examination may reveal that the welfare of these animals is compromised more severely that control.
The higher body weight of females produced with FCS could be caused by epigenetic alterations. These female mice showed an increased adipose mass. We did not study other characteristics of obesity, but obesity is a state of sufficient magnitude to produce adverse health consequences, such as type 2 diabetes and hypertension. We do not know why only females have a higher body weight; however, there are many differences between the sexes that can affect obesity; it has been reported that sex hormones influence obesity (40), that the association between body mass index and lipids/lipoproteins is stronger in females than in males (41), and that leptin-deficient obesity is affected by female sex (42). The finding that mice generated by culture in FCS or by micromanipulation (nuclear transfer) are susceptible to obesity (43, 44) is consistent with a supposed phenotypic influence by preimplantation in vitro procedures. Tamashiro et al. (44) reported that as the obese phenotype was not transmitted to offspring of the cloned mice produced by natural mating; it probably resulted from epigenetic errors in donor cells or errors that arose as a result of inadequate nuclear remodeling. Moreover, some of the changes described in cloned mice were also present in a second control group of mice, in which zygotes were recovered and cultured in the same system as the cloned embryos, before transfer of a few embryos and ultimately delivery by cesarean section. We confirm here that the well documented perturbations in development of sheep and cattle that result from manipulating embryos are evident also in a different order of eutherian mammals (mice). Long-term postnatal survivors might have subtle epigenetic defects that are below the threshold, threaten viability, which can produce longterm deleterious effects.
Despite the fact that human IVF has been practiced for >20 years, we still do not know the potential long-term effects of the technique on the health of the individuals conceived. Although several national and international registers on the outcome of IVF have been in use for many years, they do not allow detailed studies on postnatal health on the children and adults. In human IVF, no large-organ syndrome has been reported; on the contrary, several studies have shown lower birth weights of babies conceived after IVF (45), and a clear relationship between low birth-weight and cardiovascular disease (hypertension, cardiac disease, and stroke), noninsulin-dependent diabetes mellitus, and osteoporosis in middle age has been reported (46). One of the most widely used supplements for in vitro culture of bovine embryos is serum. Furthermore, until the early 1990s, most human embryos produced by IVF were cultured in media supplemented with 10–20% maternal serum (47); however, implantation rates remained poor (19). We know that the use of serum albumin as the protein source yields very good embryo development and quality; however, even today most of the in vitro-produced bovine embryos are cultured with serum, and some of the most prominent laboratories in the world use maternal of fetal cord serum in their human culture system. We now know that preimplantation culture in the presence of serum can influence the mRNA expression of multiple growth-related imprinted genes, thus leading to aberrant fetal growth and development. In addition, the use of serum produces profound consequences on the behavior of adult mice. It is likely also that with the use of serum in the culture of other mammalian embryos there is a possibility of producing abnormal growth, diseases, and behavioral problems.
Supplementary Material
Acknowledgments
This work was supported by Ministerio de Ciencia y Tecnología of Spain Grant AGL2003-05783 and Fondo de Investigación Sanitaria Redes GO3/028 and CO3/06.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ART, assisted reproductive technologies; IVF, in vitro fertilization; IVC, in vitro culture; KSOM, K+-modified simplex optimized medium; Igf2, insulin-like growth factor 2; Mest/Peg1, mesoderm specific transcript homolog/epoxide hydrolase 1; Grb10/Meg1, growth factor receptor-bound protein 10/maternally expressed gene 1.
References
- 1.Bavister, B. D. (1995) Hum. Reprod. Update 1, 91–148. [DOI] [PubMed] [Google Scholar]
- 2.McEvoy, T. G. (2003) Reprod. Dom. Anim. 38, 268–275. [DOI] [PubMed] [Google Scholar]
- 3.Walker, S. K., Hartwick, K. M. & Seamark, R. F. (1996) Theriogenology 45, 111–120. [Google Scholar]
- 4.Young, L. E., Sinclair, K. D. & Wilmut, I. (1998) Rev. Reprod. 3, 155–163. [DOI] [PubMed] [Google Scholar]
- 5.Gardner, D. K. (1994) Cell Biol. Int. 18, 1163–1179. [DOI] [PubMed] [Google Scholar]
- 6.Gardner, D. K. & Lane, M. (2003) Reprod. Biomed. Online 6, 470–481. [DOI] [PubMed] [Google Scholar]
- 7.Thompson, J. G., Gardner, D. K., Pugh, P. A., McMillan, W. H. & Tervit, H. R. (1995) Biol. Reprod. 53, 1385–1391. [DOI] [PubMed] [Google Scholar]
- 8.Abe, H., Yamashita, S., Itoh, T., Satoh, T. & Hoshi, H. (1999) Mol. Reprod. Dev. 53, 325–335. [DOI] [PubMed] [Google Scholar]
- 9.Stojkovic, M., Kölle, S., Zakhartchenko, V., Stojkovic, P., Sinowatz, F., Wolf, E. (2000) Adv. Reprod. 5, 35–44. [Google Scholar]
- 10.Byrne, A. T., Southgate, J., Brison, D. R. & Leese, H. J. (1999) J. Reprod. Fertil. 117, 97–105. [DOI] [PubMed] [Google Scholar]
- 11.Lonergan, P., Rizos, D., Gutierrez-Adan, A., Moreira, P. M., Pintado, B., de la Fuente, J. & Boland, M. P. (2003) Biol. Reprod. 69, 1424–1431. [DOI] [PubMed] [Google Scholar]
- 12.Rizos, D., Lonergan, P., Boland, M. P., Arroyo-Garcia, R., Pintado, B., De la Fuente, J. & Gutiérrez-Adán A. (2002a) Biol. Reprod. 66, 589–595. [DOI] [PubMed] [Google Scholar]
- 13.Rizos, D., Gutiérrez-Adán, A., Perez-Garnalo, S., De la Fuente, J., Boland, M. P. & Lonergan, P. (2003) Biol. Reprod. 68, 236–243. [DOI] [PubMed] [Google Scholar]
- 14.Rizos, D., Fair, T., Papadopoulos, S., Boland, M. P. & Lonergan, P. (2002b) Mol. Reprod. Dev. 62, 320–327. [DOI] [PubMed] [Google Scholar]
- 15.Massip, A., Mermillod, P. & Dinnyes, A. (1995) Hum. Reprod. 10, 3004–3011. [DOI] [PubMed] [Google Scholar]
- 16.Reik, W., Romer, I., Barton, S. C., Surani, M. A., Howlett, S. K. & Klose, J. (1993) Development (Cambridge, U.K.) 119, 933–942. [DOI] [PubMed] [Google Scholar]
- 17.Dean, W., Bowden, L., Aitchison, A., Klose, J., Moore, T., Meneses, J. J., Reik, W. & Feil, R. (1998) Development (Cambridge, U.K.) 125, 2273–2282. [DOI] [PubMed] [Google Scholar]
- 18.Moore, T. & Reik, W. (1996) Rev. Reprod. 1, 73–77. [DOI] [PubMed] [Google Scholar]
- 19.Bolton, V. N., Wren, M. E. & Parsons, J. H. (1991) Fertil. Steril. 55, 830–832. [DOI] [PubMed] [Google Scholar]
- 20.Khosla, S., Dean, W., Reik, W. & Feil, R. (2001) Hum. Reprod. Update 7, 419–427. [DOI] [PubMed] [Google Scholar]
- 21.Doherty, A. S., Mann, M. R. W., Tremblay, K. D., Bartolomei, M. S. & Schultz, R. M. (2000) Biol. Reprod. 62, 1526–1535. [DOI] [PubMed] [Google Scholar]
- 22.Isles, A. & Wilkinson, L. (2000) Trends Cogn. Sci. 4, 309–318. [DOI] [PubMed] [Google Scholar]
- 23.File, S. E. (1993) Behav. Brain Res. 58, 199–202. [DOI] [PubMed] [Google Scholar]
- 24.File, S. E., Zangrossi, H., Jr., Viana, M. & Graefff, F. G. (1996) Psychopharmacologia 111, 491–494. [DOI] [PubMed] [Google Scholar]
- 25.Dellu, F., Mayo, W., Cherkaoui, J., Le Moal, M. & Simon, H. (1992) Brain Res. 588, 132–139. [DOI] [PubMed] [Google Scholar]
- 26.Arny, M., Nachtigall, L. & Quagliarello, J. (1987) Fertil. Steril. 48, 861–865. [DOI] [PubMed] [Google Scholar]
- 27.Caro, C. M. & Trounson, A. (1984) J. In Vitro Fert. Embryo Transf. 1, 183–187. [DOI] [PubMed] [Google Scholar]
- 28.Constancia, M., Pickard, B., Kelsey, G. & Reik, W. (1998) Genome Res. 8, 881–900. [DOI] [PubMed] [Google Scholar]
- 29.Sun, F. L., Dean, W. L., Kelsey, G., Allen, N. D. & Reik, W. (1997) Nature 389, 809–815. [DOI] [PubMed] [Google Scholar]
- 30.DeChiara, T. M., Efstratiadis, A. & Robertson, E. J. (1990) Nature 345, 78–80. [DOI] [PubMed] [Google Scholar]
- 31.Maher, E. R. & Reik, W. (2000) J. Clin. Invest. 105, 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reik, W. & Maher, E. R. (1997) Trends Genet. 13, 330–334. [DOI] [PubMed] [Google Scholar]
- 33.Miyoshi, et al. (1998) Proc. Natl. Acad. Sci. USA 95, 1102–1107.9448292 [Google Scholar]
- 34.Gutiérrez-Adán, A., Lonergan, P., Rizos, D., Ward, F. A., Boland, M. P., Pintado, B. & De la Fuente, J. (2001) Theriogenology 55, 1117–1126. [DOI] [PubMed] [Google Scholar]
- 35.Cripps, M. M. & Nash, D. J. (1983) Behav. Neural. Biol. 38, 127–132. [DOI] [PubMed] [Google Scholar]
- 36.Cohen, R. A. & Sparling-Cohen, Y. A. (1993) in The Neuropsychology of Attention, ed. Cohen, R. A. (Plenum, New York), pp. 75–93.
- 37.Tipper, S. P., Bourque, T. A., Anderson, S. H. & Brehaut, J. C. (1989) J. Exp. Child Psychol. 48, 353–378. [DOI] [PubMed] [Google Scholar]
- 38.Pennisi, E. & Vogel, G. (2000) Science 288, 1722–1727. [DOI] [PubMed] [Google Scholar]
- 39.McEvoy, T. G., Robinson, J. J. & Sinclair, K. D. (2001) Reproduction 122, 507–518. [PubMed] [Google Scholar]
- 40.Lovejoy, J. C. (1998) J. Women's Health 7, 1247–1256. [DOI] [PubMed] [Google Scholar]
- 41.Margolis, C. F., Sprecher, D. L., Simbartl, L. A. & Campaigne, B. N. (1996) Int. J. Obes. Relat Metab Disord 20, 784–790. [PubMed] [Google Scholar]
- 42.Polotsky, V. Y., Wilson, J. A., Smaldone, M. C., Haines, A. S., Hurn, P. D., Tankersley, C. G., Smith, P. L., Schwartz, A. R. & O'Donnell, C. P. (2001) Am. J. Respir. Crit. Care Med. 164, 1470–1475. [DOI] [PubMed] [Google Scholar]
- 43.Inui, A. (2003) Trends Pharmacol. Sci. 24, 77–80. [DOI] [PubMed] [Google Scholar]
- 44.Tamashiro, K. L., Wakayama, T., Akutsu, H., Yamazaki, Y., Lachey, J. L., Wortman, M. D., Seeley, R. J., D'Alessio, D. A., Woods, S. C., Yanagimachi, R. & Sakai, R. R. (2002) Nat. Med. 8, 262–267. [DOI] [PubMed] [Google Scholar]
- 45.Schieve, L. A., Meikle, S. F., Ferre, C., Peterson, H. B., Jeng, G. & Wilcox, L. S. (2002) N. Engl. J. Med. 346, 731–737. [DOI] [PubMed] [Google Scholar]
- 46.Barker, D. J. (1994) Horm. Res. 42, 223–230. [DOI] [PubMed] [Google Scholar]
- 47.FitzGerald, L. & DiMattina, M. (1992) Fertil. Steril. 57, 641–647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.