Abstract
Objective
Previous research has shown that biological (e.g., genetic, biochemical) accounts of depression—currently in ascendancy—are linked to the general public’s pessimism about the syndrome’s prognosis. This research examined for the first time whether people with depressive symptoms would associate biological accounts of depression with pessimism about their own prognoses and whether a psychoeducation intervention portraying the biology of depression as malleable could decrease prognostic pessimism among symptomatic individuals.
Method
In three studies, participants were recruited online and assessed for depression symptoms. Those with significant depressive symptomatology (a Beck Depression Inventory-II score of at least 16) rated their endorsement of biochemical and genetic causal attributions for their symptoms and indicated expected length of symptom duration. An audiovisual intervention emphasizing the malleability of gene effects and neurochemistry was developed, and its effects on symptomatic individuals’ prognostic pessimism, feelings of agency, guilt, and general hopelessness were measured.
Results
Biochemical and genetic causal attributions for depression were significantly associated with prognostic pessimism among symptomatic individuals. The malleability intervention significantly reduced prognostic pessimism, increased feelings of agency, and decreased general hopelessness.
Conclusions
Biochemical and genetic attributions for depression are related to prognostic pessimism among individuals with depressive symptoms, and not just among the general public. However, emphasizing the malleability of gene effects and brain chemistry in depression can foster more optimistic about depression-related beliefs.
Mental disorders are increasingly construed in terms of their biology, revolutionizing the conceptualization of psychopathology. Depression provides a compelling example of this conceptual shift: In 2006, 80% of Americans endorsed “a chemical imbalance in the brain,” as a cause of depression, while 64% endorsed “a genetic or inherited problem”—both figures representing double-digit increases from just ten years earlier (Pescosolido et al., 2010).
Unfortunately, studies have found that attributing mental disorders (e.g., depression) to such biological causes is associated with prognostic pessimism—a belief that mental health problems are relatively permanent and difficult to cure or treat effectively (Bennett, Thirlaway, & Murray, 2008; Deacon & Baird, 2009; Phelan, 2005; Phelan, Yang, & Cruz-Rojas, 2006). This association appears to stem from the belief that mental disorders have immutable essences (e.g., genes and neurobiology)—assumptions known as “genetic essentialism” and “neuroessentialism” (Dar-Nimrod & Heine, 2011; Haslam, 2011; Medin & Ortony, 1989). Popular media accounts of scientific advances often reinforce these essentialist biases by portraying genetic and neural influences as deterministic and immutable (Dar-Nimrod & Heine, 2011; Haslam, 2011).
Importantly, essentialist accounts contradict current understandings of genetics and neurochemistry. Neuroplasticity in the human brain persists throughout adulthood and is important for recovery from mental disorders, including depression (Brunoni, Lopes, & Fregni, 2008). Moreover, despite significant heritability, there is no “gene for” any psychiatric illness (Kendler, 2005), and epigenetics and gene-by-environment interactions show that experience can modulate genetic effects (Lau & Eley, 2010). The notion that biological explanations of mental disorders denote immutability is thus a misperception that should be corrected.
The present studies have two aims. First, they examine whether biochemical and genetic attributions for depression are associated with prognostic pessimism among individuals with depressive symptoms. Although considerable research has documented such an association in the general public (Haslam, 2011), the impact of biological attributions on people who actually have mental health problems has scarcely been examined. In one recent exception, genetic attributions for mental disorders were associated with implicit guilt among diagnosed individuals, but this research did not examine prognostic pessimism. Understanding how biological attributions relate to symptomatic individuals’ prognostic beliefs is clinically important, as depressed individuals’ prognostic expectancies can significantly affect their actual prognoses (Rutherford, Wager, & Roose, 2010).
The second goal is to examine whether psychoeducation about neuroplasticity and the malleability of gene effects can reduce prognostic pessimism among symptomatic individuals. Given the increasing prevalence of biological explanations for psychopathology, ways of presenting them without increasing prognostic pessimism are urgently needed, but research to date has not focused on this need. For this aim, we drew inspiration from previous research that has used educational interventions to dispel perceptions of immutability and promote the concept of malleability in domains like intelligence and social belonging (Aronson, Fried, & Good, 2002; Blackwell, Trzesniewski, & Dweck, 2007; Walton & Cohen, 2011).
Studies 1a and 1b
Methods
All study procedures were approved by the Institutional Review Board. U.S. Adults were recruited through Amazon.com’s Mechanical Turk (mTurk) website in exchange for small payments. Research has shown that mTurk participants tend to participate for their own enjoyment, provide data whose quality is independent of compensation rates, and are more demographically diverse than standard Internet and undergraduate samples (Buhrmester, Kwang, & Gosling, 2011). By recruiting from the general population rather than a clinical sample, we precluded possible selection biases (e.g. oversampling people with confidence in mental health treatment). Administering the studies online also eliminated potential experimenter effects.
After indicating informed consent via an online form, participants completed the Beck Depression Inventory-II, a measure of depression symptoms with high reliability and validity, on which higher scores indicate greater severity (Dozois, 2010). We omitted one BDI-II item, “Suicidal Thoughts or Wishes,” because our online procedures precluded appropriate responses to reports of suicidality. (See Table 2 for BDI-II score statistics.)
Table 2.
BDI-II data for all participants who completed the BDI-II in each study.
| Study Group | N | BDI-II Range |
BDI-II Mean (SD) |
|
|---|---|---|---|---|
| Study 1a | 108 | 16–50 | 24.41 (8.00) | |
| Study 1b | 40 | 16–56 | 25.42 (9.36) | |
| Study 2 (BDI-II High-scorers) |
Malleable Condition |
81 | 16–59 | 26.27 (9.70) |
| Control Condition |
65 | 16–46 | 24.69 (7.80) | |
| Bio. Illness Condition |
86 | 16–54 | 25.24 (8.96) | |
| Study 2 (BDI-II Low-scorers) |
Malleable Condition |
127 | 0–15 | 7.06 (4.35) |
| Control Condition |
131 | 0–15 | 6.65 (4.50) | |
| Bio. Illness Condition |
127 | 0– 15 | 6.95 (4.56) | |
To select individuals with depressive symptoms as participants, we used a somewhat conservative cutoff, including only those whose BDI-II scores were at least 16, which is higher than 14, the minimum for “mild depression” (Dozois, 2010). In Study 1a, these high-scoring individuals (N=108) ranged in age from 18 to 62 (M=34.34) and were 62.0% female, 36.1% male, and 1.9% unknown gender. Those in Study 1b (N=40) ranged in age from 18 to 57 (M=30.75) and were 72.5% female and 27.5% male.
Participants scoring at least 16 on the BDI-II were told, “Based on your answers to the preceding questions, it seems that you are feeling sad, blue, or depressed.” They then rated, on 7-point scales (1=”very unlikely,” 7=”very likely”), the extent to which they believed each of several factors “might be causing the sad, blue, or depressed feelings [in your case].” The bracketed phrase was used only in Study 1b (omitting the brackets in the actual administration) to emphasize that participants’ ratings referred to their own symptoms. Ten causal factors were presented in a randomized order, two of which—“Genetics” and “Brain chemistry or other biochemical imbalance”—were of primary interest. Endorsement of these two causes were significantly correlated, r=.58, p<.01 for Study 1a and r=.74, p<.01 for Study 1b, so we averaged them to form a “biochemical/genetic attribution” score for each participant. The other eight items, which served as fillers, were: “Day-to-day problems and/or stress,” “Beliefs of style of thinking (cognitive factors),” “Abnormal brain structure/development,” “Brain Injury,” “Substance Abuse,” “Weakness of Character,” “Problems from childhood or the way you were raised,” and “Recent traumatic events.” Biochemical and genetic attributions had the strongest correlation of any two causal factors in both studies, and factor analyses with maximum-likelihood extraction and varimax rotation revealed that genetic and biochemical attributions loaded onto the same factor (with respective loadings of .85 and .68 in Study 1a and 1.0 and .74 in Study 1b). No other attribution loaded onto this biochemical/genetic factor with a loading above .42 in either study.
After rating causal attributions, participants answered the question, “How long do you think that you will continue to feel sad, blue, or depressed?” Study 1a used a 7-point scale comprising“Less than 1 week” (coded as 1), “1 to 2 weeks,” “2 to 4 weeks,” “1 month to 6 months,” “6 months to 1 year,” “More than 1 year, but not indefinitely,” and “Indefinitely” (coded as 7). Study 1b used a 9-point scale for greater granularity. The first 5 and final scale points were the same as in Study 1a, and the 6th-8th were “1 to 2 years,” “2 to 5 years,” and “More than 5 years, but not indefinitely.”
Results
We conducted linear regressions with biochemical/genetic attributions and BDI-II scores as independent predictors and symptom-duration ratings as the dependent variable. Biochemical/genetic attributions significantly predicted higher scores on the symptom-duration scale in both Study 1a, β=.23, p=.02, and Study 1b, β=.42, p<.01. (Because of the symptom-duration measure’s ordinal nature, we also confirmed these results using ordinal regressions, p=.01 in Study 1a, p=.02 in Study 1b.) BDI-II scores were a significant predictor in Study 1a only, β=.28, p<.01. (See Table 1 for R-square values). To our knowledge, these results are the first empirical demonstration that the more people with depressive symptoms attribute those symptoms to genetic and biochemical causes, the longer they tend to expect their symptoms to last.
Table 1.
Linear regressions modeling the effects of biochemical/genetic attributions.
| Dependent Variable | n | Model R-Square |
Biochemical/Genetic Attributions |
BDI-II Score | ||
|---|---|---|---|---|---|---|
| β | p | β | p | |||
| Predicted Duration of Symptoms (study 1a) |
108 | .14 | .227* | .015 | .277* | .003 |
| Predicted Duration of Symptoms (study 1b) |
40 | .23 | .421* | .006 | .185 | .210 |
| Predicted Duration of Symptoms (study 2) |
193 (BDI-II ≥ 16) |
.16 | .171* | .015 | .311** | <.001 |
| 328 (BDI-II < 16) |
.02 | .124* | .024 | (not included) | ||
| Predicted Duration of Symptoms, With Treatment (study 2) |
193 | .09 | .112 | .099 | .243** | .001 |
| Perceived Odds of Symptom Desistance (study 2) |
191 (BDI-II ≥ 16) |
.19 | −.136* | .048 | −.378** | <.001 |
| 324 (BDI-II < 16) |
.01 | −.113* | .042 | (not included) | ||
| Perceived Agency Regarding Depressive Symptoms (study 2) |
193 | .12 | −.089 | .213 | −.310** | <.001 |
| Guilt Concerning Depressive Symptoms (study 2) |
192 | .07 | .041 | .576 | .253** | .001 |
| BHS Score (study 2) | 179 | .34 | .103 | .106 | .548** | <.001 |
Results are for individuals with BDI-II scores of at least 16, except where noted. Regression models used endorsement of biochemical/genetic attributions and/or BDI-II score as independent predictors. The number of subjects varies slightly in each of the study 2 regressions reported above because some participants did not respond to all measures.
p<.05
p<.01
Study 2
Study 2 examined an approach to counteracting the prognostic pessimism associated with biochemical and genetic attributions for depression.
Methods
All study procedures were approved by the Institutional Review Board. The procedures for participant recruitment, administering the BDI-II, notifying high-scorers that they seemed to be feeling “sad, blue, or depressed,” and measuring their causal attributions for their symptoms were identical to those used in study 1b. In study 2, we also collected data from participants who scored under 16 on the BDI-II. (Table 2 shows BDI-II descriptive statistics for all participants.) BDI-II low-scorers were told, “Based on your answers to the preceding questions, it seems that you are NOT feeling particularly sad, blue, or depressed.” For causal attributions, the low-scores rated the likelihood that each factor “might be causing the average depressed person's sad, blue, or depressed feelings.”
After rating their causal attributions, all participants were randomly assigned to one of three conditions. In the “Malleable” condition, participants watched an audiovisual psychoeducation intervention emphasizing the malleability of gene expression and brain chemistry associated with depression. The video provided a basic primer on epigenetics, explaining that genes can be “turned on or off” by environmental factors, and described how experience affects brain chemistry and activity. Previous research has found that providing environmental explanations for depression (e.g. stressful experiences) along with biological ones can reduce negative effects of biological explanations (Deacon & Baird, 2009). We avoided describing such environmental factors so that any effect of this video could not be attributed to mentioning environmental causes of depression. Instead, the malleability video discussed environmental factors only in terms of their ability to moderate the influence of biology on mood (e.g. “Aerobic exercise and exposure to sunlight have also been shown to change brain chemistry and activity in a way that helps with feelings of depression”). In the “Biological-Illness” condition, participants watched a video focusing on the concept of depression as a biomedical condition. Similar to arguments promoted in scientific literature and popular media, the video explained that depression tends to run in families and that studies have documented differences between the brains of depressed and non-depressed individuals. Both videos were approximately 6 minutes long and narrated by the same person. Every effort was made to ensure that the two videos were similar in comprehensibility and in the amount of scientific information and treatment options described, such that the explanatory emphasis was the only dimension on which they differed. The audio narrations for both videos are transcribed in the Appendix. In the Control condition, participants received no intervention.
Participants who watched either video were instructed, before proceeding, to write a short letter to a depressed individual, using information from the video they watched to persuade the person to see depression “in a new light.” This approach took advantage of the “saying-is-believing” effect, a tendency for people to internalize viewpoints they have advocated (Aronson et al., 2002; Higgins, 1999; Walton & Cohen, 2011).
The subsequent procedure differed for high-scorers and low-scorers. High-scoring participants in all three conditions rated their expectations regarding the prognosis of their depression symptoms, perceptions of personal agency regarding their mood, feelings of guilt concerning their depression symptoms, and outlook for the future. Prognostic expectations were measured using the same symptom-duration scale from Study 1b, plus an identical scale asking how long they expected their symptoms would last if they received treatment, and a 0–100% scale asking the odds that their depressed mood would “go away”. Agency perceptions were gauged using ratings of agreement with the statements “There are things I can do to eliminate my sad, blue, or depressed mood” and “I am able to improve my sad, blue, or depressed mood” (1, “Completely Disagree” to 7, “Completely Agree”). These two agency items were significantly correlated (r=.68, p<.01), so they were averaged to create an agency score for each participant. As a measure of guilt, the same agreement scale was used for the statement “I feel guilty about my sad, blue, or depressed mood.” We gauged participants’ outlook for the future using the Beck Hopelessness Scale (BHS) (Beck, Weissman, Lester, & Trexler, 1974), but modified each of the 20 true/false judgments to a 6-point scale (“very false,” “false,” “somewhat false,” “somewhat true,” “true,” “very true”); scores thus ranged from 20 to 120, with higher scores indicating more hopelessness.
BDI-II low-scorers responded to the same prognosis and agency items as the high-scorers, but with respect to “the average depressed person” ( e.g. “What do you think are the odds that the average depressed person's sad, blue, or depressed mood will go away?”).
Finally, all participants were debriefed that depression “likely results from a combination of genetic, biochemical, environmental, and psychological factors” and received resources to find treatment for depression.
Some participants did not complete the study; most attrition appeared to result from difficulties viewing the videos using their own devices. The number of participants completing each dependent measure, by condition, is displayed in Figures 1–4. We computed demographics for participants who completed at least one dependent measure, regardless of BDI-II score. Those in the Malleable condition ranged in age from 18 to 60 (M=26.44) and were 43.5% female, 51.3% male, and 5.2% unknown gender. Those in the Biological-Illness condition ranged in age from 18 to 62 (M=30.26) and were 47.7% female, 48.9% male, and 3.4% unknown gender. Those in the Control condition ranged in age from 18 to 64 (M=29.04) and were 46.6% female, 50.3% male, and 3.1% unknown gender.
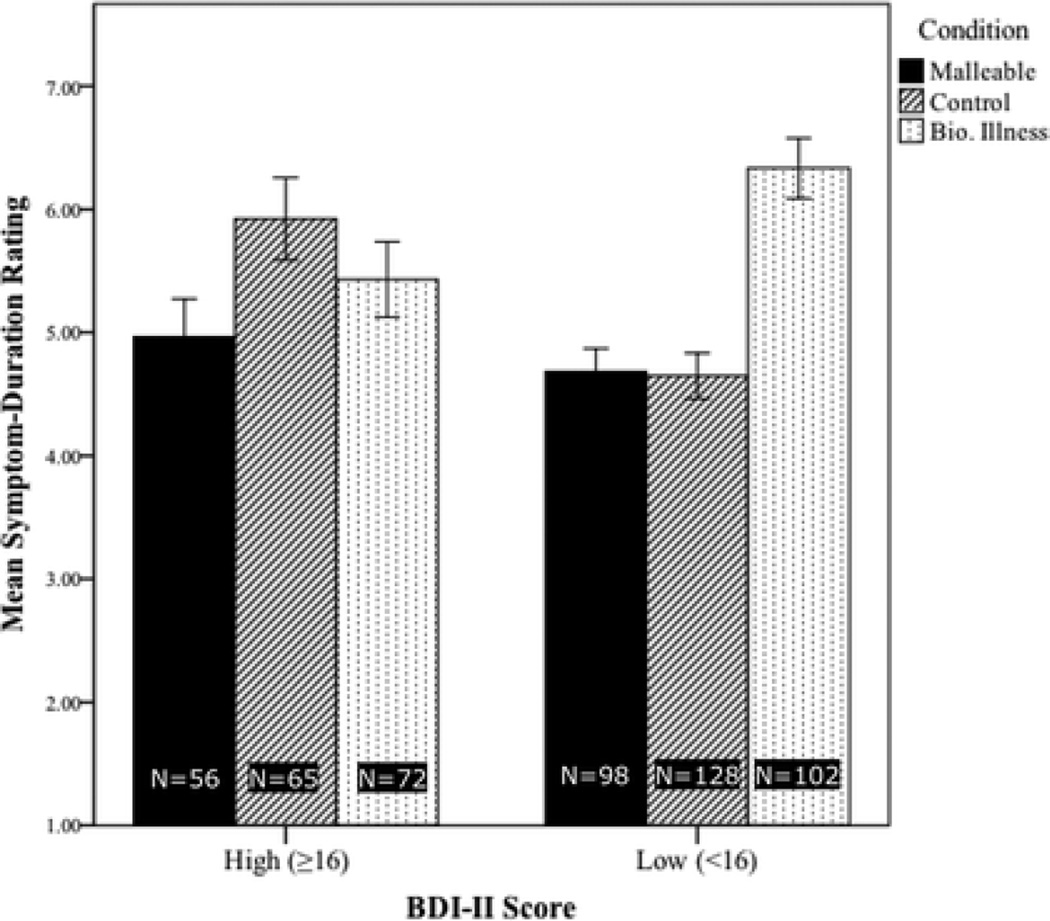
Figure 1.
Mean ratings of expected symptom duration, by condition, in study 2. Ratings were made on a scale from 1 (“Less than 1 week”) to 9 (“Indefinitely”). As such, higher bars indicate greater prognostic pessimism.
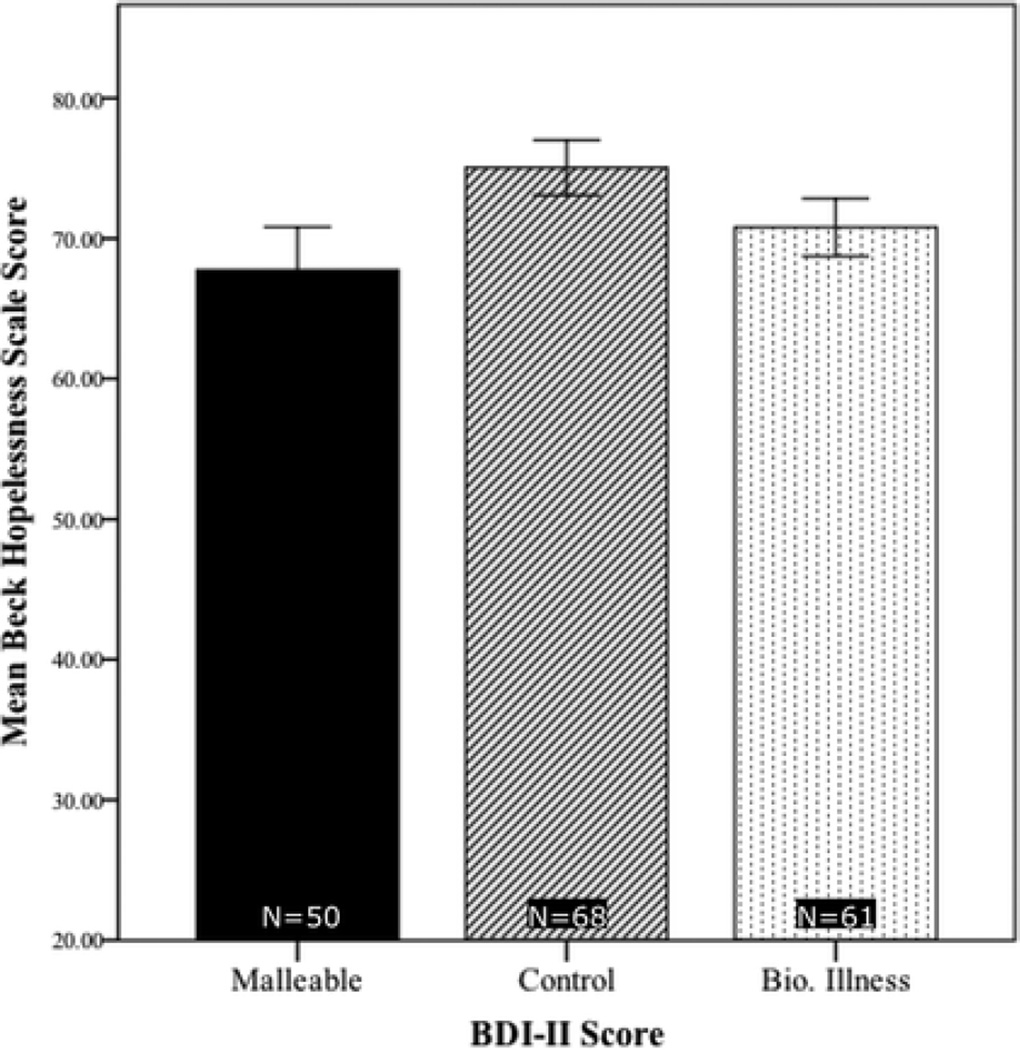
Figure 4.
Mean scores among individuals scoring at least 16 on the BDI-II, by condition, on the modified Beck Hopelessness Scale (BHS). The range of possible scores was 20–120, with higher scores denoting a greater degree of hopelessness.
Results
a. Associations between Biochemical/Genetic Attributions and Prognostic Pessimism
We first examined relationships between BDI-II high-scorers’ biochemical/genetic attributions and their ratings on our dependent measures. A moderated multiple regression approach (O'Connor, 1998), conducted to determine whether condition moderated the effects of pre-manipulation biochemical/genetic attributions, controlling for BDI-II score, revealed no significant attributions × condition interaction for any of our dependent variables. As such, we collapsed participants across conditions and conducted regression analyses for each of our dependent variables using the same predictor variables as in studies 1a and 1b. Table 1 shows details of the results for all measures. In particular, among BDI-II high-scorers, biochemical/genetic attribution scores were a significant predictor of longer expected symptom duration (replicating studies 1a and 1b), β=.18, p=.01, and lower perceived odds of recovery, β=.15, p=.02.
Regression models with low BDI-II scorers showed that their biochemical/genetic attributions were also a significant independent predictor of longer expected symptom duration (β=.12, p=.012) and lower perceived odds of recovery (β =−.11, p=.04). Ordinal regressions also showed biochemical/genetic attributions to significantly predict expected symptom duration (p=.01 among BDI-II high-scorers, p=.03 among low-scorers).
b. Effects of Experimental Manipulations
To examine the experimental manipulations’ effects, we first conducted 2 (symptomatology: high vs. low BDI-II score) × 3 (condition) ANOVAs for symptom-duration ratings (with and without treatment), predicted odds of recovery, and agency scores. These revealed significant interactions (p<.05) for all variables. Thus, subsequent analyses considered high- and low-scoring participants separately.
To examine the effects of our manipulations on individuals with high BDI-II scores, we first conducted a series of ANOVAs with BDI-II scores as a covariate to control for symptom severity. These omnibus ANOVAs revealed significant effects of condition on expected symptom duration, F(2,189)=3.10, p<.05†; perceived odds of recovery, F(2,187)=5.31, p<.01; agency perceptions, F(2,189)=15.15, p<.01; and BHS scores, F(2,175)=5.67, p<.01. Figures 1–4 display each condition’s mean for these variables. There were no significant effects of condition on the measures of guilt or expected symptom duration with treatment.
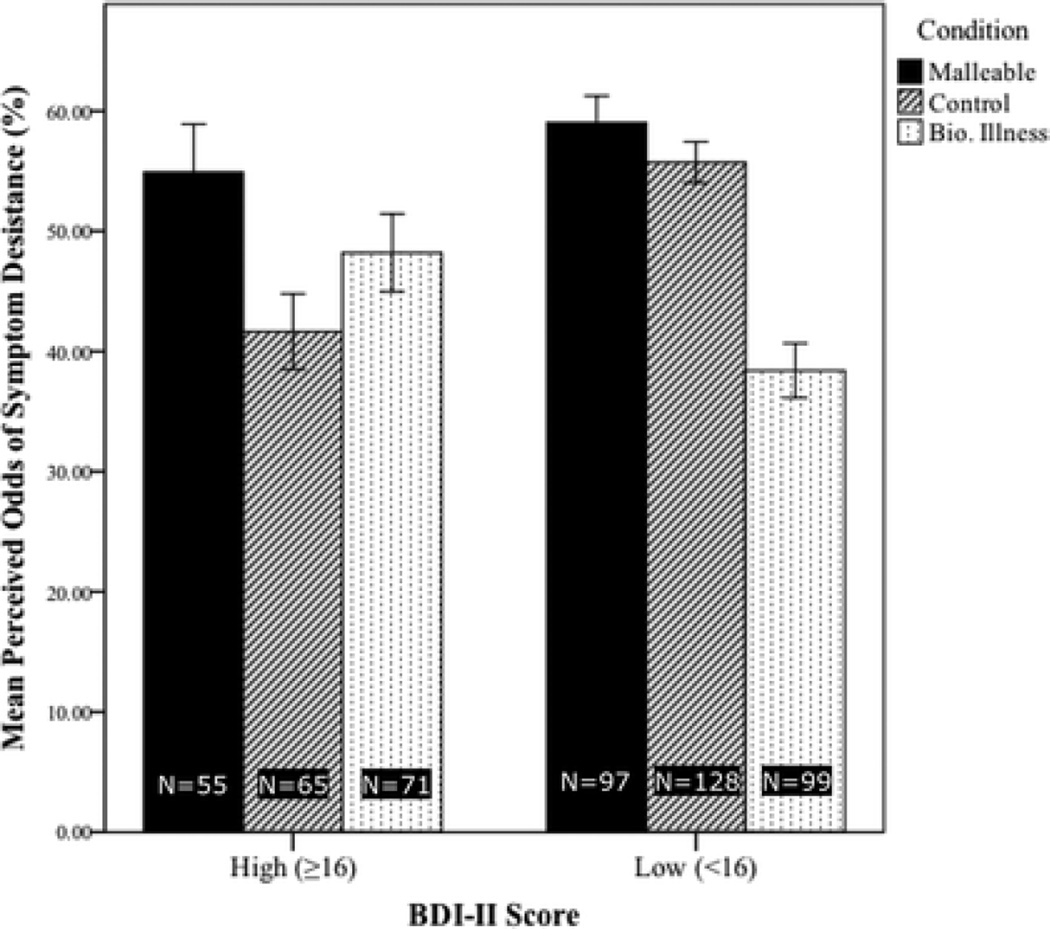
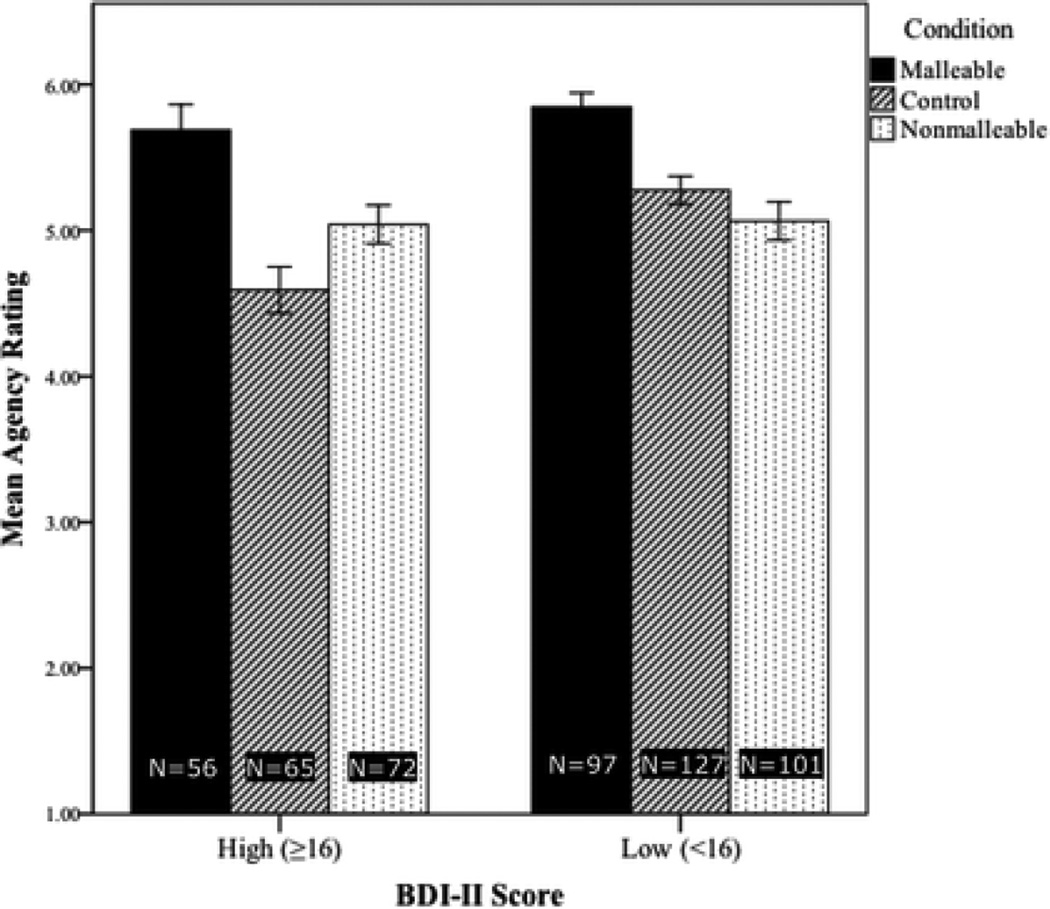
Then, on the four variables for which omnibus ANOVAs yielded significant effects of condition, we compared the responses of BDI high-scorers in the Malleable and Control conditions using simple weighted contrasts. Overall, the malleability intervention yielded more optimistic views among BDI-II high-scorers about their depressive symptoms. Specifically, those in the Malleable condition expected shorter symptom durations (p=0.1, Figure 1, confirmed with rank-transformation analysis) and better odds of recovery (p<.01, Figure 2), perceived more agency (p<.01, Figure 3), and gave more optimistic BHS scores (p<.01, Figure 4) than those in the Control condition.
Figure 2.
Mean responses by condition, to the question “What do you think are the odds that your sad, blue, or depressed mood will go away?” Ratings could range from 0% to 100%; higher bars indicate greater optimism.
Figure 3.
Mean agency scores by condition. Agency scores represent the mean of participants’ agreement with the statements “There are things I can do to eliminate my sad, blue, or depressed mood” and “I am able to improve my sad, blue, or depressed mood,” rated on a scale from 1 (Completely Disagree) to 7 (Completely Agree). Higher bars indicate greater perceptions of personal agency.
Next, we compared the Biological-Illness condition with the Malleable condition among the high BDI-II scorers. Both conditions presented videos describing treatment options for depression; perhaps as a result, there were no significant differences between the two conditions in prognostic pessimism or hopelessness. Nonetheless, those in the Malleable condition had significantly higher agency ratings than those in the Biological-Illness condition, t(126)=2.99, p<.01.
We also used simple weighted contrasts to compare the Biological-Illness and Control conditions among BDI-II high-scorers. Participants in the Biological-Illness condition showed more optimism than those in the Control condition only on two measures—lower BHS scores (p<.05) and higher agency ratings (p=.02). This suggests that the Biological-Illness video was less powerful in changing the outlooks of symptomatic individuals from their baseline than the malleability intervention, which showed more differences from the Control condition.
Next, we examined the effects of our manipulations on BDI-II low-scorers. A series of ANOVAs revealed significant omnibus effects of condition on symptom-duration ratings (with and without rank transformation), perceived odds of recovery, and agency ratings (all Fs>12, all ps<.01). Replicating and extending previous findings with members of the general public (Deacon & Baird, 2009), BDI-II low-scorers in the Biological-Illness condition were significantly more pessimistic about symptom duration (with and without rank transformation) and odds of recovery than those in the Malleable and Control conditions (all ps<.01, from weighted contrasts). Yet, those in the Malleable and Control conditions did not differ significantly on these measures, suggesting that the negative effects of biological explanations were absent when such information included psychoeducation about malleability. Furthermore, BDI-II low-scorers in the Malleable condition perceived depressed individuals to have more agency than did those in either of the other two conditions (all ps<.01 from weighted contrasts).
Finally, we compared BDI-II high- and low-scoring participants in each condition. At baseline (i.e. the Control condition), prognostic pessimism was unsurprisingly stronger among BDI-II high-scorers, as measured by symptom-duration ratings, F(1,191)=13.02, p<.01, and perceived odds of recovery, F(1,191)=18.46, p<.01. However, in the Malleable condition, BDI-II high- and low-scorers did not differ significantly on either of these measures (see Figures 1 and 2). Agency scores showed an analogous pattern: in the Control condition BDI-II low-scorers’ agency ratings were higher than those of high-scorers, F(1,190)=15.15, p<.01, but there was no such difference in the Malleable condition (Figure 3). These patterns suggest that the malleability manipulation successfully elevated optimism among individuals with depressive symptoms to the level of non-symptomatic individuals.
In the Biological-Illness condition, BDI-II high- and low-scorers did not differ significantly in their agency ratings. Interestingly, the Biological-Illness video actually resulted in more prognostic pessimism among BDI-II low-scorers than among high-scorers, as measured by expected symptom duration, F(1,172)=5.38, p=.02, and predicted odds of recovery, F(1,168)=6.52, p=.01.
Discussion
The current studies examined how beliefs about the biology of depression might be related to prognostic pessimism among symptomatic individuals. Among people with elevated depressive symptomatology, endorsement of biochemical and genetic causal attributions for depressive symptoms was significantly associated with more pessimistic symptom-duration predictions in all three studies and lower ratings of the likelihood of symptom desistance in study 2. To our knowledge, this is the first time such effects have been documented in a sample of people who report significant depressive symptomatology. Given the increasing prevalence of biomedical conceptualizations of depression, the notion that depressed individuals who hold such beliefs might be more vulnerable to pessimism about the course of their disorder is alarming, particularly as positive outcome expectancies are an important determinant of actual prognosis (Rutherford et al., 2010).
While this association was correlational, there are reasons to believe that it did not arise merely because people who are already pessimistic are more likely to endorse biochemical and genetic attributions. First, we controlled for BDI-II scores in all regression models, meaning that the effect of biochemical/genetic attributions in predicting pessimism was independent from severity of depressive symptomatology, which includes general pessimism. Moreover, in study 2, there was no significant independent association between biochemical/genetic attributions and hopelessness scores, suggesting that such attributions are not a placeholder for general hopelessness, but rather are associated specifically with pessimism in prognostic beliefs about depression itself.
Study 2 investigated whether such pessimism could be reduced by emphasizing the malleability of gene effects and brain chemistry in education about the biology of depression. Considering the ample evidence of the power of biological explanations in shaping views of psychopathology (Bennett et al., 2008; Dar-Nimrod & Heine, 2011; Deacon & Baird, 2009; Haslam, 2011; Pescosolido et al., 2010; Phelan, 2005; Phelan et al., 2006), we sought to harness this power to promote optimism and feelings of agency by teaching that biology is not deterministic or fixed. Indeed, our malleability intervention successfully reduced symptomatic individuals’ prognostic pessimism and increased their feelings of agency concerning their moods, relative to those in the Control condition. In fact, BDI-II high-scorers who received this intervention were the only ones who rated the odds of recovery as greater than 50% on average. This intervention also yielded reduced feelings of general hopelessness.
Furthermore, the malleability intervention did not significantly increase feelings of guilt among BDI-II high-scorers. While caution is required in interpreting nonsignificant differences, this finding may suggest that the malleability intervention had specific, targeted effects rather than simply functioning as a positive mood induction. Also, the absence of increased guilt indicates that our study found no evidence of a potential negative side effect of emphasizing malleability—namely, that it could make symptomatic individuals feel accountable for their symptoms.
Comparisons of prognostic pessimism among high- and low-scorers also highlighted the benefits of the malleability intervention. While all comparisons of BDI-II low- and high-scorers in the Control condition predictably revealed that high-scorers were more pessimistic, the same comparisons in the Malleable condition showed no such differences. That is, after symptomatic individuals viewed the malleability intervention, their perceptions of their own agency and prognoses were as positive as non-symptomatic individuals’ views of depression.
In contrast to those in the Malleability condition, BDI-II high-scorers in the Biological-Illness condition, who also received information about the biology of depression but without an emphasis on malleability, were no less pessimistic about their prognoses than those who received no intervention. This may suggest that this video’s content did not differ greatly from BDI-II high-scorers’ pre-existing beliefs about depression or that it was not powerful enough to change their pre-existing beliefs. BDI-II high-scorers in the Biological-Illness condition did report less hopelessness and more agency than those in the Control condition, in a departure from previous research (Dar-Nimrod & Heine, 2011). This may have occurred because the Biological-Illness video referred to several effective treatments (Lebowitz & Ahn, 2012), which may have introduced the notion of malleability in some small way. However, they were significantly less confident in their own agency than participants in the Malleable condition. The less generalizable and less powerful benefits of the Biological-Illness intervention for BDI-II high-scorers suggest that the advantageous effects of the malleability intervention were not due merely to its inclusion of biological information, but rather to its specific emphasis on malleability.
Our manipulations did not affect high-scorers’ expectations regarding symptom duration with treatment. One possible explanation is that mentioning treatment in the question strongly suggested the existence of effective treatment, which could have overpowered the differences in prognostic pessimism among the conditions. Additionally, research has shown that treatments are seen as more effective when they are congruent with causal accounts (e.g. medication is seen as more effective given biomedical explanations) (Iselin & Addis, 2003). Thus, medication may have been seen as equally effective in all conditions, since none questioned the importance of biological causes for depression. However, only the malleability intervention mentioned that psychotherapy can affect the brain, so prognostic expectations given psychotherapy (as opposed to treatment generally) could have differed among the conditions. Additionally, the absence of significant effects on our measure of symptom duration with treatment might be attributable to the fact that the measure did not specify a type of treatment, which could make this result somewhat difficult to interpret. Among participants who defined “treatment” as pharmacotherapy, the Biological-Illness video might have had yielded more optimistic expectations of prognosis with treatment, with the opposite occurring among participants who defined “treatment” as psychotherapy. Such a pattern of responses could have “canceled out” any significant effects.
Interestingly, we did not find pre-existing endorsement of biochemical/genetic causes for depression to moderate the effects of our manipulations. In previous research people with strong genetic essentialist beliefs were affected more powerfully by the suggestion that biological differences underlie social categories (Keller, 2005), but this research used methodology quite different from ours, measuring nationalist prejudices. Future studies could clarify conditions in which pre-existing biological essentialism affects sensitivity to biological explanations for psychopathology.
Study 2 also replicated previous findings that members of the general public, a majority of whom are not depressed, often associate biological explanations for psychopathology with prognostic pessimism (Haslam, 2011). Specifically, the Biological-Illness condition increased prognostic pessimism among low-scorers so much that their responses on the symptom-duration scale and the rating of perceived odds of recovery were actually more pessimistic than those of BDI-II high-scorers (who were unaffected by the Biological-Illness video, vis-à-vis the Control condition). It remains unclear why our Biological-Illness video did not significantly increase prognostic pessimism among BDI-II high-scorers, as it did among low-scorers. One possibility is that some of its content (e.g. information about genetic heritability) may have seemed irrelevant to some high-scoring participants (e.g. those with no family history of depression). Another possibility is that before any experimental manipulation, many BDI-II high-scorers already held essentialist views of their depression symptoms as immutable consequences of largely unchangeable genetic and neurochemical factors. Indeed, this would be consistent with existing research suggesting that individuals with depression are more likely to view the disorder as resulting from stable biological causes (Prins, Verhaak, Bsing, & van der Meer, 2008). If BDI-II high-scorers’ pre-existing default beliefs already accorded with the content of the Biological-Illness video in such a manner, this could explain why the prognostic expectations of those in the Biological-Illness condition did not differ significantly from those of BDI-II high-scorers in the control condition. By contrast, individuals without depression (i.e. a majority of the general public) may be more likely to view the disorder as resulting from psychosocial factors by default (Prins et al., 2008). If such causes are seen as more malleable, this could explain why BDI-II low-scorers in the Biological-Illness condition—but not those in the Malleability condition—differed in prognostic expectations from those in the control condition.
The benefits of our malleability intervention have clear clinical implications concerning psychoeducation. In particular, the intervention was delivered online in the form of a short video viewable on most Web browsers, making it highly scalable. Although Internet access, a computer with relatively up-to-date technical specifications, and some minimal familiarity with technology are necessary for consumers to access the video, it has the benefit of requiring no special expertise to administer. The development of psychological interventions that can be effectively administered remotely on a large scale—of which our malleability video is a prime example—has been identified as a pressing need in the field of mental healthcare (Kazdin & Blase, 2011).
The present studies also suggest important directions for further research. In particular, the long-term effects of our malleability intervention await future study. Since the intervention appears to promote a sense of agency and decrease feelings of pessimism among those with symptoms of depression, future studies could examine whether it might increase help-seeking behavior or enhance responsiveness to treatment.
Furthermore, future research could investigate how a history of treatment for depression—which we did not assess in our samples—might relate to our findings or moderate the effects of our manipulations. For example, symptomatic individuals’ previous experience with using antidepressant medications or with healthcare providers who promoted a particular causal theory regarding depression might influence their beliefs about the disorder’s causes or its malleability. While our results speak to the ways in which individuals’ causal attributions for depression relate to their beliefs about its prognosis, our data do not allow us to examine how these beliefs formed, whether they were influenced by treatment history, or how such factors might relate to prognostic pessimism. Moreover, a history of successful treatment might bolster the notion that depression can be overcome, which could render the malleability intervention more plausible and efficacious. By contrast, a history of unsuccessful treatment (which might be more common among people with high BDI-II scores, since successfully treated individuals would likely not score as high) could make the malleability intervention seem less plausible. Nonetheless, our finding that the malleability intervention benefitted people with current depression symptoms suggests that its effects are not limited to those who have been successfully treated for depression. More definite answers require future research.
Overall, our findings suggest reason for concern as well as reason for hopefulness. They establish that biological conceptualizations of depression—which are ever more popular both among scientists and the public—are associated with prognostic pessimism among symptomatic people. However, information about the biology of depression can be presented so that it actually reduces such pessimism.
The current results represent a call to arms for scientists studying the biology of mental disorders and those responsible for disseminating their findings. The association between biological attributions and prognostic pessimism may itself be malleable, and discussions of biology’s role in mental health need not suggest that psychopathology is permanent and immutable.
Supplementary Material
Acknowledgments
This research was conducted and written up prior to Susan Nolen-Hoeksema's untimely death. Mr. Lebowitz and Dr. Ahn gratefully and affectionately acknowledge her invaluable contributions to this article, the discipline of psychology, and the lives of all who knew her.
References
- Aronson J, Fried CB, Good C. Reducing the effects of stereotype threat on African American college students by shaping theories of intelligence. Journal of Experimental Social Psychology. 2002;38(2):113–125. [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: The hopelessness scale. Journal of Consulting and Clinical Psychology. 1974;42(6):861–865. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Bennett L, Thirlaway K, Murray AJ. The stigmatising implications of presenting schizophrenia as a genetic disease. Journal of Genetic Counseling. 2008;17(6):550–559. doi: 10.1007/s10897-008-9178-8. [DOI] [PubMed] [Google Scholar]
- Blackwell LS, Trzesniewski KH, Dweck CS. Implicit theories of intelligence predict achievement across an adolescent transition: A longitudinal study and an intervention. Child Development. 2007;78(1):246–263. doi: 10.1111/j.1467-8624.2007.00995.x. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. The International Journal of Neuropsychopharmacology. 2008;11:1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Buhrmester M, Kwang T, Gosling SD. Amazon's mechanical Turk. Perspectives on Psychological Science. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- Dar-Nimrod I, Heine SJ. Genetic essentialism: On the deceptive determinism of DNA. Psychological Bulletin. 2011;137:800–818. doi: 10.1037/a0021860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon BJ, Baird GL. The chemical imbalance explanation of depression: Reducing blame at what cost? Journal of Social and Clinical Psychology. 2009;28(4):415–435. [Google Scholar]
- Dozois DJA. Beck Depression Inventory-III. In: Weiner IB, Craighead WE, editors. The Corsini Encyclopedia of Psychology. 4 ed. New York: Wiley; 2010. pp. 210–211. [Google Scholar]
- Haslam N. Genetic essentialism, neuroessentialism, and stigma: Commentary on Dar-Nimrod and Heine (2011) Psychological Bulletin. 2011;137:819–824. doi: 10.1037/a0022386. [DOI] [PubMed] [Google Scholar]
- Higgins ET. “Saying is believing” effects: When sharing reality about something biases knowledge and evaluations. In: Thompson LL, Levine JM, Messick DM, editors. Shared Cognition in Organizations: The Management of Knowledge. Hillsdale, NJ: Lawrence Erlbaum Associates; 1999. pp. 33–49. [Google Scholar]
- Iselin MG, Addis ME. Effects of etiology on perceived helpfulness of treatments for depression. Cognitive Therapy and Research. 2003;27(2):205–222. [Google Scholar]
- Kazdin AE, Blase SL. Rebooting psychotherapy research and practice to reduce the burden of mental illness. Perspectives on Psychological Science. 2011;6(1):21–37. doi: 10.1177/1745691610393527. [DOI] [PubMed] [Google Scholar]
- Keller J. In genes we trust: The biological component of psychological essentialism and its relationship to mechanisms of motivated social cognition. Journal of Personality and Social Psychology. 2005;88(4):686–702. doi: 10.1037/0022-3514.88.4.686. [DOI] [PubMed] [Google Scholar]
- Kendler KS. “A gene for…”: The nature of gene action in psychiatric disorders. American Journal of Psychiatry. 2005;162(7):1243–1252. doi: 10.1176/appi.ajp.162.7.1243. [DOI] [PubMed] [Google Scholar]
- Lau JY, Eley TC. The genetics of mood disorders. Annual Review of Clinical Psychology. 2010;6:313–337. doi: 10.1146/annurev.clinpsy.121208.131308. [DOI] [PubMed] [Google Scholar]
- Lebowitz MS, Ahn W. Combining biomedical accounts of mental disorders with treatability information to reduce mental illness stigma. Psychiatric Services. 2012;63(5):496–499. doi: 10.1176/appi.ps.201100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medin DL, Ortony A. Psychological essentialism. In: Vosniadou S, Ortony A, editors. Similarity and Analogical Reasoning. New York: Cambridge University Press; 1989. pp. 179–195. [Google Scholar]
- O'Connor BP. SIMPLE: All-in-one programs for exploring interactions in moderated multiple regression. Educational and Psychological Measurement. 1998;58(5):836–840. [Google Scholar]
- Pescosolido BA, Martin JK, Long JS, Medina TR, Phelan JC, Link BG. "A disease like any other"? A decade of change in public reactions to schizophrenia, depression, and alcohol dependence. The American Journal of Psychiatry. 2010;167(11):1321–1330. doi: 10.1176/appi.ajp.2010.09121743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan JC. Geneticization of deviant behavior and consequences for stigma: The case of mental illness. Journal of Health and Social Behavior. 2005;46(4):307–322. doi: 10.1177/002214650504600401. [DOI] [PubMed] [Google Scholar]
- Phelan JC, Yang LH, Cruz-Rojas R. Effects of attributing serious mental illnesses to genetic causes on orientations to treatment. Psychiatric Services. 2006;57(3):382–387. doi: 10.1176/appi.ps.57.3.382. [DOI] [PubMed] [Google Scholar]
- Prins MA, Verhaak PFM, Bensing JM, van der Meer K. Health beliefs and perceived need for mental health care of anxiety and depression: The patients' perspective explored. Clinical Psychology Review. 2008;28(6):1038–1058. doi: 10.1016/j.cpr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Rutherford BR, Wager TD, Roose SP. Expectancy and the treatment of depression: A review of experimental methodology and effects on patient outcome. Current Psychiatry Reviews. 2010;6(1):1–10. doi: 10.2174/157340010790596571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton GM, Cohen GL. A brief social-belonging intervention improves academic and health outcomes of minority students. Science. 2011;331(6023):1447–1451. doi: 10.1126/science.1198364. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.