Abstract
Systemic administration of morphine typically produces greater tolerance than higher efficacy mu-opioid receptor (MOPr) agonists, such as fentanyl. The objective of the present study was to test this relationship by measuring antinociceptive efficacy and tolerance to morphine and fentanyl microinjected into the ventrolateral periaqueductal gray (vlPAG). MOPr agonist efficacy was evaluated by microinjecting the irreversible opioid receptor antagonist β-funaltrexamine hydrochloride (β-FNA) into the vlPAG prior to a dose-response analysis of morphine and fentanyl antinociception. In contrast to systemic administration of morphine and fentanyl, microinjection of these drugs into the vlPAG had similar efficacy as measured by similar reductions in maximal antinociception following β-FNA administration. Analysis of tolerance revealed a rightward shift in the dose-response curve to a single pretreatment with morphine, but not fentanyl. Moreover, the magnitude of tolerance to morphine was comparable following one, four, or eight pretreatments. Tolerance to fentanyl also was evident following four or eight microinjections. These data are surprising in that antinociceptive efficacy appears to vary depending on the site of administration. Moreover, the similar efficacy following microinjection of morphine and fentanyl into the vlPAG was associated with comparable tolerance, with the one exception of no tolerance to acute administration of fentanyl.
Perspective
These data reveal that antinociceptive tolerance following vlPAG administration of opioids develops rapidly, is evident with both morphine and fentanyl, and the magnitude is relatively consistent regardless of the number of pretreatments.
Keywords: Opioid, analgesia, efficacy, pain modulation, acute tolerance
Introduction
Mu opioid receptor (MOPr) agonists, such as morphine and fentanyl, are the most effective treatment for many types of pain. An important question is the degree to which tolerance develops to the antinociceptive effects of morphine and higher efficacy agonists used clinically. On one side of the debate, tolerance has been reported to occur following a single administration 4,5,6. On the other side, many pain patients can be maintained on the same opioid dose for months or even years 9,11. These seemingly disparate findings could both be true if tolerance develops rapidly and varies little with repeated administration.
The degree to which tolerance develops is complicated further by animal studies showing that the magnitude of tolerance varies inversely with agonist efficacy at the MOPr. Efficacy, defined as the maximal antinociceptive effect, is known to vary depending on the opioid. Previous research using systemic or spinal cord administration has shown that less antinociceptive tolerance develops to high efficacy MOPr agonists, such as fentanyl and DAMGO, compared to lower efficacy agonists, such as morphine 7,8,24,28,36,37. Given that the ventrolateral periaqueductal gray (vlPAG) is known to contribute to both morphine antinociception and tolerance, it is likely that this brain area contributes to these differences in tolerance between agonists. The vlPAG is part of a descending pain modulatory pathway that includes a relay in the rostral ventromedial medulla before terminating in the dorsal horn of the spinal cord. Microinjection of MOPr agonists into the vlPAG produces antinociception 2,3,13 and repeated opioid administration results in tolerance 14,23,25,39. Moreover, blocking opioid actions within the vlPAG attenuates tolerance to systemic morphine administration revealing the importance of this structure in the development of antinociceptive tolerance 18.
Although antinociceptive efficacy following vlPAG microinjection is expected to be consistent with differences in efficacy following systemic and intrathecal administration, the antinociceptive efficacy of MOPr agonists microinjected into the vlPAG has not been assessed. The present study will test this hypothesis by microinjecting low, medium, and high doses of the irreversible opioid receptor antagonist, β-FNA, into the vlPAG prior to microinjection of morphine or fentanyl. In addition, it is hypothesized that the agonist with the higher efficacy will produce less tolerance to repeated microinjections into the vlPAG. This hypothesis will be tested by measuring the magnitude of tolerance to one, four, or eight microinjections of morphine or fentanyl into the vlPAG.
Methods
Subjects
Subjects were 217 adult male Sprague-Dawley rats (mean weight = 274 ± 3.2 g) from Harlan Laboratories (Livermore, CA). Rats were anesthetized with pentobarbital (60 mg/kg; i.p.) and a 9 mm guide cannula (23 gauge) was implanted into the vlPAG using stereotaxic techniques (AP: +1.7 mm, ML: ±0.6 mm, DV: −4.6 mm from lambda). Dental cement anchored the guide cannula to two screws in the skull. A stylet was inserted into the guide cannula following surgery, and rats were allowed to recover under a heat lamp until awake.
Rats were housed individually on a reverse light cycle (lights off at 7:00) so that behavioral testing could be conducted during the active dark phase. Food and water were available at all times except during testing. Rats were handled daily for a week prior to testing. All procedures were approved by the Washington State University Animal Care and Use Committee and conducted in accordance with the guidelines for animal use described by the International Association for the Study of Pain.
Microinjections and Behavioral Testing
Morphine sulfate (a gift from the National Institute on Drug Abuse), fentanyl citrate (Sigma-Aldrich; St. Louis, MO), β-funaltrexamine hydrochloride (Tocris Bioscience; Ellisville, MO), were administered through a 31-gauge injection cannula that extended 2 mm beyond the guide cannula. Drugs were dissolved in 50-100% methanol (β-FNA) or normal saline (morphine and fentanyl). To prevent confounds from mechanical stimulation of neurons during the experiment, the injection cannula was inserted into the guide cannula without drug administration one day prior to testing. Microinjections were administered in a volume of 0.4 μL (for opioids) or 0.5 μL (for β-FNA and vehicle) at a rate of 0.1 μL/10 s. To minimize backflow up the cannula tract, the injector remained in place an additional 20 s. A stylet was inserted into the guide cannula and the rat was returned to its home cage immediately following the injection.
Test times and doses for MOPr agonists were chosen based on a previous vlPAG microinjection study 2 which determined that the peak effects for fentanyl and morphine antinociception are 3 and 15 – 30 min, respectively. Doses for fentanyl (3 μg/0.4 L) and morphine (5 μg/0.4 μL) were chosen based on D50 values from that study2. Microinjections and testing for MOPr agonist pretreatment were conducted at approximately 10:00 and 16:00.
Nociception was assessed by placing rats on a 52.5°C hotplate and measuring the latency to lick a hind paw 17,19. If there was no response by 50 s, the rat was removed from the apparatus. Any rat with a baseline hotplate latency greater than 25 s (n = 18 of 235 rats; 0-3/group) was not included in further testing.
Experiment 1- MOPr agonist efficacy in vlPAG
The purpose of this experiment was to determine the relative antinociceptive efficacy of morphine and fentanyl in the vlPAG. Efficacy was assessed by irreversibly blocking a subset of opioid receptors by microinjecting β-FNA (0.1, 1, or 10 μg/0.5μL; n = 8 – 14/group) into the vlPAG. The vehicle for β-FNA was 50% methanol/saline except rats receiving the highest β-FNA dose (10 μg/0.5μL) requiring a 100% methanol vehicle. A subset of each control group received 50% (n = 8) or 100% methanol (n = 6). Six hours after β-FNA administration, cumulative doses of fentanyl or morphine were microinjected into the vlPAG. Immediately prior to agonist administration, baseline hotplate latency was measured to assure no alteration in antinociception as a result of β-FNA or vehicle pretreatment.
Dose-response curves were generated on the test day by repeated microinjections of fentanyl at 4 min intervals resulting in cumulative third-log doses of 0.46, 1, 2.2, 4.6, 10 μg/0.4 μL. A hotplate test was conducted 2 min after each injection. Cumulative doses of morphine were administered at 20 min intervals resulting in third–log doses of 1, 2.2, 4.6, 10, and 22 μg/0.4 μL. The hotplate test was conducted 15 min following each injection. These test times were as close to the peak effect of the agonist (3 min for fentanyl and 15 min for morphine) to assure completion of the cumulative procedure before the effects of the first agonist microinjection wore off 2,25. Previous studies from our lab and others have shown the value of the cumulative dosing procedure to reduce the number of animals despite slightly different effective doses than standard single-dose experiments, but allowing for the important comparisons between experimental and control animals to be made 7,20,25,26,34. Repeated vlPAG microinjections of MOPr agonist or a single MOPr antagonist administration has been shown to cause a rightward shift in the dose-response curve 25.
Experiment 2- Tolerance to a single vlPAG microinjection
The purpose of this experiment was to evaluate the development of acute tolerance in the vlPAG to fentanyl and morphine at two different time points. A single microinjection of fentanyl (3 μg/0.4 μL), morphine (5 μg/0.4 μL), or saline (0.4 μL) was administered into the vlPAG. Antinociception was assessed on the hotplate test 3 min after fentanyl microinjection and 30 min following microinjection of morphine. A subset of saline pretreated rats were tested at each time point (3 and 30 min) as controls for fentanyl and morphine pretreated rats, respectively.
The development of tolerance to this initial pretreatment was assessed using a cumulative dosing procedure either 1, 3, or 18 hrs following the initial injection. Just prior to tolerance assessment, baseline hotplate latency was measured. Given the short duration (< 30 min) of antinociception following microinjection of fentanyl 2, acute tolerance was assessed at 1 hour. Morphine has a longer duration (approximately 90 min) so acute tolerance was tested at 3 hours post pretreatment injection 2. These time points were chosen as double the duration of antinociception for the particular agonist to limit the impact of residual drug influencing hotplate latency. These test times are similar to the times used for systemic studies on acute tolerance to these opioids 22. Cumulative doses of fentanyl or morphine were microinjected into the vlPAG as described in Experiment 1.
It is possible that the molecular changes underlying tolerance take time to develop. Therefore, acute tolerance also was assessed 18 hours following pretreatment with fentanyl, morphine, or saline. Tolerance to fentanyl and morphine was assessed using the same cumulative dosing procedure described above.
Experiment 3- Tolerance to repeated opioids in the vlPAG
The purpose of this experiment was to compare the magnitude of tolerance that develops to repeated microinjections of fentanyl or morphine into the vlPAG. Tolerance was induced by microinjecting fentanyl (3 μg/0.4 μL), morphine (5 μg/0.4 μL) or saline(0.4 μL) into the vlPAG twice a day for 2 or 4 days. Antinociception was assessed using the hotplate test 3 min following microinjection of fentanyl and 30 min following microinjection of morphine on Trial 1. Tolerance was assessed by comparing shifts in the dose-response curve for opioid and saline pretreated controls 18 hours after the last opioid injection. That is, tolerance was assessed on Day 3 for rats pretreated with four injections and on Day 5 for rats pretreated with eight injections. Following a baseline hotplate test, cumulative doses of fentanyl or morphine were microinjected into the vlPAG as described in Experiment 1.
Histology
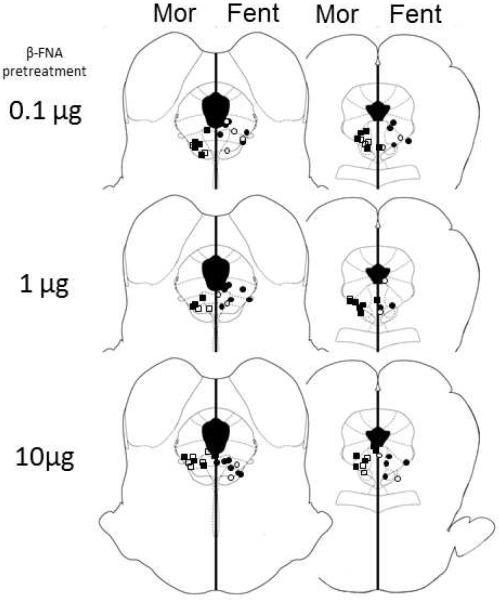
Following testing, rats were given a lethal dose of Halothane. The brain was removed and placed in formalin (10%). At least two days later, the brain was sectioned coronally (100 μm) to determine the location of the microinjection cannula. Only rats with placements in or immediately adjacent to the vlPAG were included in data analysis (Figure 1) 30. Placements are shown for Experiment 1, but placements were similar for all experiments.
Figure 1. Location of injection sites within the vlPAG for Experiment 1.
Cannula placements for animals pretreated with 0, 0.1, 1, or 10 μg/0.5 μL β-FNA. Open symbols represent placements for vehicle groups, closed squares for morphine, and closed circles for fentanyl pretreated groups. Injection sites were similar for rats tested with morphine (left side) and fentanyl (right side). All cannula were implanted on the right side and are separated here for clarity. Placements for Experiments 2 and 3 were similar to those shown here.
Data Analysis
Differences in baseline and opioid-induced antinociception were compared using analysis of variance (ANOVA) followed by a Bonferroni post-hoc test when necessary. Dose-response data were converted to Percent Maximum Possible Effect (%MPE) to allow for comparison between pretreatment trials and drugs: (hotplate latency – baseline hotplate latency)/(cutoff hotplate latency – baseline hotplate latency) X 100%. Dose-response curves were plotted using GraphPad (Prism; La Jolla, CA). The half maximal antinociceptive dose (D50) was calculated for each pretreatment group using nonlinear regression. Given that our data produces a graded response and not quantal responses, the term D50 was used instead of ED50 38. Differences in D50s between treatment groups were assessed with ANOVA. Significance was assigned at an alpha level of 0.05. All data are presented as the mean (± S.E.M.) unless otherwise stated.
Results
Experiment 1- MOPr agonist efficacy in vlPAG
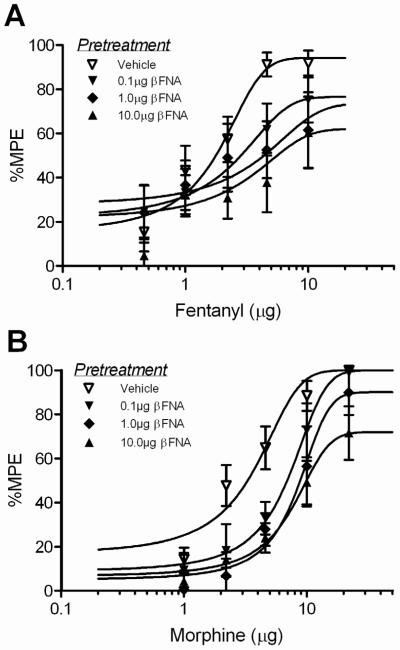
There was no significant difference in mean baseline hotplate latencies (13.5 ±0.9 – 16.1 ± 1.7 s) for the different groups pretreated with β-FNA or vehicle (F(3, 81) = 0.228; n.s.) or between morphine and fentanyl conditions (F(1, 81) = 2.69; n.s.). Microinjection of fentanyl or morphine into the vlPAG produced a dose-dependent increase in hot plate latency. Pretreatment with β-FNA 6 hours prior to opioid administration caused a dose-dependent decrease in fentanyl and morphine induced antinociception (Figure 2). Comparison of equipotent sub-maximal doses of fentanyl(4.6 μg/0.4 μL) and morphine (10 μg/0.4 μL) revealed that pretreatment with β-FNA (0.1, 1, 10 μg/0.5 μL) caused a dose-dependent decrease in antinociception (Figure 3; F(3,80) = 9.32; p < 0.05) evident by a decrease in efficacy (Emax) and potency (D50) for each agonist (Table 1). This decrease in antinociception caused by pretreatment with β-FNA was not statistically significant between fentanyl and morphine (Figure 3; F(1, 80) = 2.16; n.s.).
Figure 2. Microinjection of β-FNA into the vlPAG produces a dose dependent decrease in fentanyl and morphine efficacy.
Microinjection of β-FNA (0, 0.1, 1, or 10 μg/0.5 μL) into the vlPAG caused a dose-dependent decrease in the maximal antinociceptive effect of a subsequent microinjection of A) fentanyl or B) morphine.
Figure 3. Equal antinociceptive efficacy for fentanyl and morphine.
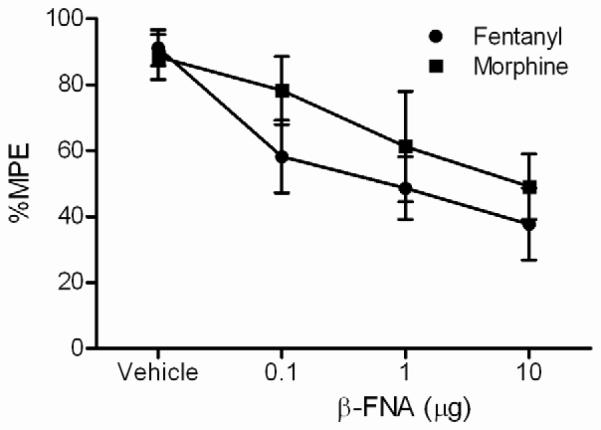
β-FNA (0, 0.1, 1, or 10 μg/0.5 μL) causes an equal decrease in antinociception for equipotent maximal doses of fentanyl (4.6 μg/0.4 μL) and morphine (10 μg/0.4 μL) p > 0.05. Data from Figure 2 were converted to allow a direct comparison of the equipotent sub-maximal antinociceptive effect of fentanyl and morphine.
Table 1.
Efficacy comparison between fentanyl and morphine
| D50 (± 95% C.I.) | Emax (± 95% C.I.) | |||
|---|---|---|---|---|
| β-FNA (μg) | Fentanyl | Morphine | Fentany-4.6 μg | Morphine-10 μg |
| Vehicle | 1.8 (± 0.5) | 3.3 (± 0.8) | 95.8 (± 31.5) | 86.9 (± 17.4) |
| 0.1 | 3.7 (± 2.0)* | 6.9 (± 1.7)* | 64.5 (± 39.3) | 85.2 (± 14.8) |
| 1 | 5.4 (± 2.2)* | 9.1 (± 2.5)* | 49 (± 24.8)* | 56.9 (± 23.6)* |
| 10 | 7.5 (± 3.3)* | 13.2 (± 3.3)* | 34 (± 13.5)* | 53.1 (± 26.6)* |
Notes:
C.I.: confidence interval
p < 0.05 from vehicle
D50 values are presented in μg/0.4 μL
Emax values are presented as %MPE from hotplate latencies
Experiment 2- Tolerance to a single vlPAG microinjection
Acute microinjection of fentanyl (3 μg/0.4 μL) or morphine (5 μg/0.4 μL) into the vlPAG caused a significant and comparable increase in hotplate latency compared to saline pretreated rats (Figure 4; F(3, 60) = 8.48; p < 0.05). Fentanyl microinjection produced a significant increase in hotplate latency compared to saline controls at 3 min (Bonferroni; p < 0.05) and microinjection of morphine produced a significant increase in hotplate latency compared to saline pretreated rats at 30 min (Bonferroni; p < 0.05). At these doses the magnitude of antinociception was similar for fentanyl and morphine treated groups.
Figure 4. Fentanyl and morphine induce comparable antinociception.
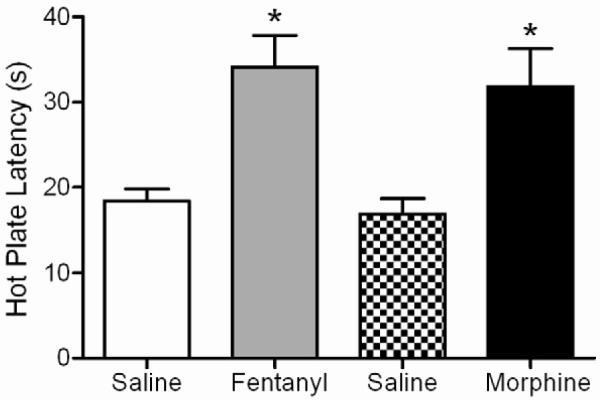
On Trial 1, microinjection of fentanyl (3 μg/0.4 L; n = 16) or morphine (5 μg/0.4 L; n = 15) into the vlPAG caused an increase in hotplate latency compared to the respective saline pretreated control group (n = 16 for each). The magnitude of antinociception produced by these doses of fentanyl and morphine were comparable. Data are shown as mean hotplate latency ± SEM. * p< 0.05.
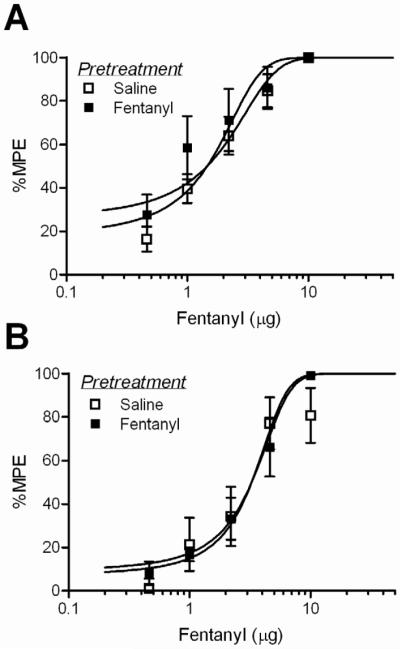
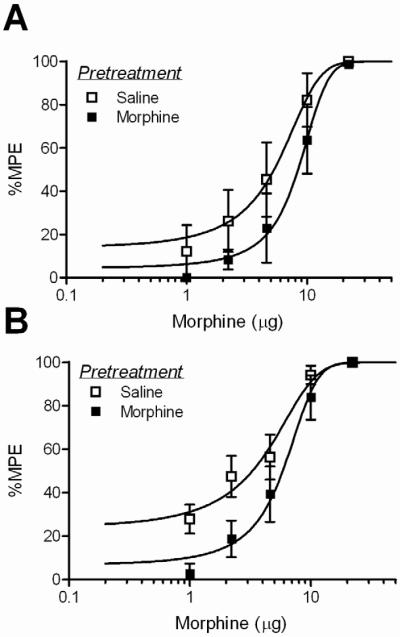
There was no difference between baseline hotplate latencies conducted just prior to cumulative dosing on Trial 2 across drug pretreatments (Table 2; F(3, 54) = 1.31; n.s.) or time tested (F(1, 54) = 0.544; p = n.s.). Tolerance did not develop to fentanyl when assessed at 1 or 18 hours following a single microinjection into the vlPAG. The fentanyl D50s were not significantly different for saline and fentanyl pretreated groups tested at 1 hour (Figure 5A; F(1, 75) = 2.47; n.s.) or 18 hours (Figure 5B; F(1, 86) = 0.88; n.s.). In contrast, tolerance was evident in morphine-pretreated rats (Figure 6). Pretreatment with a single vlPAG microinjection of morphine caused a significant rightward shift in the dose-response curve at 3 (F(1, 58) = 4.34; p < 0.05) and 18 hours (F(1, 86) = 5.95; p < 0.05) following the pretreatment injection. D50 values for fentanyl and morphine are presented in Table 3.
Table 2.
Comparison of baseline hotplate latencies prior to cumulative dosing
| Saline | Fentanyl | Saline | Morphine | |
|---|---|---|---|---|
| 1 or 3 hours | ||||
| 13.1 (± 1.8) s | 12.4 (± 1.1) s | 15.6 (± 1.5) s | 16.3 (± 1.7) s | |
| 18 hours | 13.7 (± 1.0) s | 13.0 (± 1.9) s | 12.9 (± 1.2) s | 14.0 (± 1.3) s |
| 2 day | 15.9 (± 1.4) s | 13.8 (± 1.4) s | 17.1 (± 1.6) s | 17.6 (± 1.4) s* |
| 4 day | 13.7 (± 1.2) s | 13.0 (± 1.9) s | 14.2 (± 1.4) s | 12.2 (± 1.9) s* |
- statistically significant from each other
Figure 5. Lack of acute tolerance to fentanyl microinjections into the vlPAG.
A single microinjection of fentanyl did not cause a shift in the fentanyl dose-response curve measured at A) 1 or B) 18 hours after the pretreatment microinjection.
Figure 6. Acute tolerance to morphine following microinjection in the vlPAG.
A significant rightward shift in the morphine dose-response curve occurred following one morphine microinjection compared to saline pretreated rats when tested A) 3 or B) 18 hours later.
Table 3.
Comparison of D50 values for saline and opioid pretreated groups
| Fentanyl D50 ± C.I (ug) | Morphine D50 ± C.I (ug) | |||
|---|---|---|---|---|
| Pretreatments | Saline | Fentanyl | 1 Saline | Morphine |
| 1 (1 or 3 hrs) | ||||
| 1.7 ± 0.4 | 1.1 ± 0.7 | 5.3 ± 2.1 | 8.4 ± 5.2* | |
| 1 (18 hrs) | 3.1 ± 0.9 | 3.5 ± 0.7 | 3.3± 1.0 | 5.8 ± 1.4* |
| 4 | 1.9 ± 0.5 | 3.9 ± 1.1* | 6.4± 2.5 | 11.4 ± 2.1* |
| 8 | 2.9 ± 1.2 | 6.2 ± 1.5* | 6.9 ± 2.9 | 13.0 ± 6.8* |
Notes:C.I. - 95% confidence interval
- statistically significant from saline
Sample size - 7-9/group
Experiment 3- Tolerance to repeated opioids in the vlPAG
Microinjection of fentanyl or morphine into the vlPAG caused a significant increase in hotplate latency on Trial 1 compared to their respective saline controls (F(3,65) = 16.37; p < 0.05). Fentanyl and morphine produced comparable antinociception when tested at 3 min and 30 min, respectively.
There were no significant differences in baseline hotplate latencies conducted prior to the cumulative dosing procedure between any of the drug pretreatments (Table 2; F(3, 54) = 0.701; n.s.). There was, however, a significant difference in baseline latencies as a result of the different lengths of morphine pretreatment (F(1, 62) = 6.773; p < 0.05). Rats pretreated with morphine for two days had higher baseline hotplate latencies compared to rats pretreated with morphine for four days (Bonferroni; p < 0.05). Although these differences were relatively small, data were converted to %MPE so antinociceptive potency could be compared across groups.
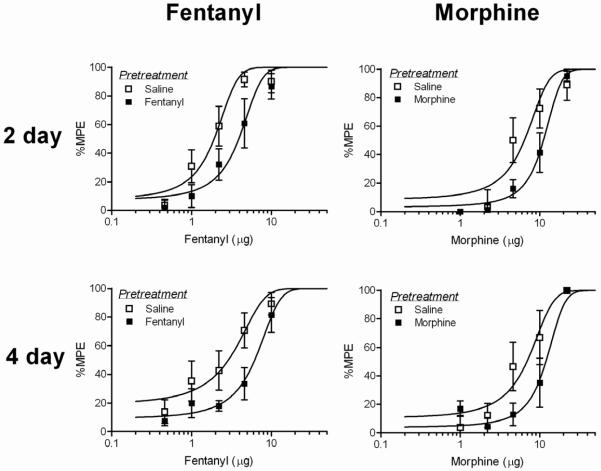
Unlike Experiment 2, repeated microinjections of fentanyl caused a rightward shift in the fentanyl dose-response curve (Figure 7). Four and eight repeated microinjections of fentanyl over two (F(1, 122) = 19.42; p < 0.05) and four days (F(1, 98) = 11.63; p < 0.05) were significantly different from saline pretreated controls (n = 8 or 9). Morphine pretreatment also caused a significant rightward shift in the morphine dose-response curve following two (F(1, 80) = 6.14, p < 0.05) and four days (F(1, 71)= 8.55; p <0.05) of repeated microinjections compared to saline pretreated rats. Changes in D50 values as a result of repeated administration are shown in Table 3.
Figure 7. Tolerance to repeated fentanyl and morphine microinjections into the vlPAG.
A significant rightward shift in the fentanyl dose-response curve was found following four or eight fentanyl microinjections compared to saline pretreated rats. Twice daily microinjections of morphine for two or four days also caused a rightward shift in the morphine dose-response curve.
In order to compare the magnitude of tolerance induced by fentanyl versus morphine, data were transformed to calculate D50s on the same log scale. Two days of fentanyl microinjections caused a rightward shift of 0.36 log units from saline pretreated animals and two days of morphine microinjections caused a 0.25 log shift. There was no significant difference between fentanyl and morphine in the magnitude of this shift (F(1,92) = 0.005; n.s.). Similarly, there was no significant difference in the magnitude of the shift caused by four days of fentanyl or morphine administration (F(1,91) = 0.898; n.s.). Fentanyl caused a rightward shift of 0.41 log units and morphine caused a shift of 0.34 log units from saline treated controls.
Discussion
The present data show that within the vlPAG: 1) fentanyl has similar antinociceptive efficacy to morphine, 2) tolerance develops to repeated, but not a single fentanyl microinjection, 3) a single microinjection of morphine was sufficient to produce tolerance, and 4) the magnitude of tolerance induced by repeated microinjections of fentanyl and morphine are similar. Taken together these experiments confirm that the vlPAG is an important site in the development of tolerance to morphine and other MOPr agonists, and that tolerance develops rapidly and is relatively stable regardless of opioid agonist used or the number of pretreatments.
Microinjection of fentanyl or morphine into the vlPAG produced maximal antinociception on the hot plate test. The magnitude of this antinociception was reduced in a stepwise manner following pretreatment with increasing doses of the irreversible opioid receptor antagonist, β-FNA. This decrease in antinociception was comparable for fentanyl and morphine indicating equal antinociceptive efficacy for these agonists when microinjected into the vlPAG. This finding is surprising given that previous studies have shown that systemically administered fentanyl has greater antinociceptive efficacy than morphine 8,20. Sufentanil, a fentanyl analog, also has been shown to have greater antinociceptive efficacy and potency than morphine following intrathecal injections 24,36. The decrease in efficacy to fentanyl is matched by a reduction in relative potency compared to morphine following microinjection into the vlPAG. That is, the antinociceptive D50 for fentanyl (5.7 nmol) and morphine (13.2 nmol) is similar following vlPAG microinjections, but fentanyl (151 nmol) is approximately 100 times more potent than morphine (13.2 μmol) following systemic administration 21,31. The D50 doses of other MOPr agonists classified as high efficacy, such as DAMGO, are much lower when microinjected in the vlPAG (0.9 nmol) 23. Spinal cord administration also shows a greater potency difference between high efficacy MOPr agonists (0.2 – 0.5 nmol) compared to morphine (3 – 6 nmol) than found in the current study 24,37. Taken together, this reduction in fentanyl potency and efficacy with vlPAG administration suggests there are fewer spare receptors or different MOPrs in the vlPAG compared to other structures24,36. This finding is important because it shows that ligand specific efficacy varies depending on the site of action.
The development of tolerance to morphine following vlPAG microinjections is not surprising given that morphine tolerance develops under many different experimental conditions, ranging from a single injection to continuous infusion 7,8,10,20. The present finding that acute tolerance develops to morphine microinjection into the vlPAG also is consistent with other studies showing tolerance to morphine after a single intrathecal, systemic, or intracerebroventricular injection 7,10,33. In contrast, tolerance was not evident at 1 or 18 hours after a single injection of fentanyl. Other studies have reported acute tolerance following a single systemic injection of fentanyl 22, however the doses in the current study were equal to D50 doses determined for antinociception induced with microinjections into the vlPAG 2, whereas other studies have used doses 50-100 times greater than ED50 doses 22,34. Thus, acute tolerance to vlPAG administration may occur with a higher dose of fentanyl, but solubility problems caused by the small microinjection volume (0.4 μl) precludes assessment. The short duration of antinociception following fentanyl microinjection into the vlPAG 2 may also limit the development of tolerance, but acute tolerance has been reported with systemic administration of fentanyl 22. A more likely explanation is that ligand specific signaling differences between fentanyl and morphine 22 may prevent acute tolerance to fentanyl.
Tolerance to repeated microinjections of morphine into the vlPAG has been reported numerous times previously 14,25,27,35,39. The current data extend this finding by showing that the magnitude of tolerance is similar regardless of the number of pretreatments. Although a slight increase in the magnitude of tolerance measured by a shift in log units was evident for both fentanyl and morphine, the shift in the morphine dose-response curve is not significantly different whether rats received a single or eight pretreatments. Tolerance also was evident following repeated microinjections of fentanyl into the vlPAG, and the magnitude of this tolerance was comparable to that produced by morphine.
These data add to a growing body of evidence revealing the importance of the vlPAG in tolerance to MOPr agonists. Microinjection of MOPr agonists into the vlPAG produces tolerance 23,25,39, and inactivation of the vlPAG attenuates tolerance to systemically administered morphine 18. The surprising finding is that comparable tolerance occurs to both MOPr agonists, whereas an inverse relationship between efficacy and tolerance has been reported with systemic administration 8,20. The difference is that we found comparable antinociceptive efficacy for fentanyl and morphine when microinjected into the vlPAG. Thus, the similar development of tolerance with repeated administration of fentanyl and morphine is consistent with the efficacy/tolerance relationship. However, the lack of tolerance to a single injection of fentanyl is not consistent with the lower antinociceptive efficacy of fentanyl when injected into the vlPAG.
Our experiments using fentanyl may differ from previous studies because of the intermittent administration paradigm. For example, the magnitude of tolerance to morphine is much greater than to higher efficacy agonists when these opioids are continuously infused, but this difference is less pronounced with intermittent injections 8,28,29,34.The development of tolerance to repeated microinjection of fentanyl is in contrast to systemic administration where little or no tolerance is reported 8,28,29,34. However, closer examination of these studies reveals that tolerance does develop to repeated systemic administration of fentanyl, but the magnitude of tolerance is less than to morphine. Moreover, many of these studies assess tolerance by measuring the magnitude of cross-tolerance to morphine 20. Given that different mechanisms appear to underlie tolerance to morphine and fentanyl 12,22, tolerance could occur to fentanyl even in the absence of cross-tolerance to morphine.
Despite similarities in the development and magnitude of tolerance following vlPAG microinjections, the signaling proteins underlying tolerance to morphine and fentanyl have been reported to differ. Blockade of G-protein receptor kinase prevents tolerance to fentanyl but not morphine, whereas knocking out β-arrestin 2 or blocking c-Jun-N terminal kinase attenuates tolerance to morphine, but not fentanyl 12,22,32. These different signaling pathways provide a plausible explanation for differences in tolerance to fentanyl and morphine. Our previous finding that tolerance occurs to repeated DAMGO microinjections into the vlPAG in addition to inducing cross-tolerance to morphine 23 suggests that DAMGO activates the same signaling pathways as morphine despite reports showing that DAMGO is a higher efficacy agonist 1,15,16. Current studies in our lab are examining whether fentanyl and morphine engage similar or different tolerance mechanisms.
These findings have several clinical implications. The lack of tolerance to acute administration of fentanyl reinforces the value of using fentanyl to relieve pain during surgery or other transient pain, although greater tolerance may occur with systemic administration. Moreover, tolerance will develop with repeated administration of either fentanyl or morphine. Once induced, the magnitude of tolerance is relatively consistent regardless of the number of pretreatments. Although opioid microinjection into the vlPAG provides an opportunity to examine the molecular mechanisms underlying tolerance, these results may not generalize to systemic administration of opioids used clinically which produce antinociception and tolerance at multiple sites and mechanisms. However, our data are consistent with clinical data showing that opioid tolerance can develop after a single injection and remain relatively stable following prolonged administration 4,5,6,9,11.
Acknowledgements
RAH conducted the efficacy experiments and ENB conducted the tolerance experiments. Financial support was provided by NIH grant DA015498 and by funds provided for medical and biological research by the State of Washington Initiative Measure No. 171.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures.
The authors have no conflicts of interest or financial disclosures related to the work presented in this manuscript.
References
- [1].Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. Mu-opioid receptor desensitization in mature rat neurons: Lack of interaction between damgo and morphine. J Neurosci. 2003;23:10515–10520. doi: 10.1523/JNEUROSCI.23-33-10515.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bodnar R, Paul D, Pasternak GW. Synergistic analgesic interactions between the periaqueductal gray and the locus coeruleus. Brain Res. 1991;558:224–230. doi: 10.1016/0006-8993(91)90772-n. [DOI] [PubMed] [Google Scholar]
- [4].Buntin-Mushock C, Phillip L, Moriyama K, Palmer PP. Age-dependent opioid escalation in chronic pain patients. Anesth Analg. 2005;100:1740–1745. doi: 10.1213/01.ANE.0000152191.29311.9B. [DOI] [PubMed] [Google Scholar]
- [5].Chia YY, Liu K, Wang JJ, Kuo MC, Ho ST. Intraoperative high dose fentanyl induces postoperative fentanyl tolerance. Can J Anaesth. 1999;46:872–877. doi: 10.1007/BF03012978. [DOI] [PubMed] [Google Scholar]
- [6].Collett BJ. Opioid tolerance: The clinical perspective. Br J Anaesth. 1998;81:58–68. doi: 10.1093/bja/81.1.58. [DOI] [PubMed] [Google Scholar]
- [7].Dighe SV, Madia PA, Sirohi S, Yoburn BC. Continuous morphine produces more tolerance than intermittent or acute treatment. Pharmacol Biochem Behav. 2009;92:537–542. doi: 10.1016/j.pbb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- [8].Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- [9].Eisenberg E, McNicol ED, Carr DB. Efficacy and safety of opioid agonists in the treatment of neuropathic pain of nonmalignant origin: Systematic review and meta-analysis of randomized controlled trials. Jama. 2005;293:3043–3052. doi: 10.1001/jama.293.24.3043. [DOI] [PubMed] [Google Scholar]
- [10].Fairbanks CA, Wilcox GL. Spinal plasticity of acute opioid tolerance. J Biomed Sci. 2000;7:200–212. doi: 10.1007/BF02255467. [DOI] [PubMed] [Google Scholar]
- [11].Farrar JT, Messina J, Xie F, Portenoy RK. A novel 12-week study, with three randomized, double-blind placebo-controlled periods to evaluate fentanyl buccal tablets for the relief of breakthrough pain in opioid-tolerant patients with noncancer-related chronic pain. Pain Med. 2010;11:1313–1327. doi: 10.1111/j.1526-4637.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- [12].Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. The effect of protein kinase c and g protein-coupled receptor kinase inhibition on tolerance induced by mu-opioid agonists of different efficacy. J Pharmacol Exp Ther. 2010;332:1127–1135. doi: 10.1124/jpet.109.161455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974;185:1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- [14].Jacquet YF, Lajtha A. The periaqueductal gray: Site of morphine analgesia and tolerance as shown by 2-way cross tolerance between systemic and intracerebral injections. Brain Res. 1976;103:501–513. doi: 10.1016/0006-8993(76)90448-0. [DOI] [PubMed] [Google Scholar]
- [15].Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- [16].Kelly E, Bailey CP, Henderson G. Agonist-selective mechanisms of gpcr desensitization. Br J Pharmacol. 2008;153(Suppl 1):S379–388. doi: 10.1038/sj.bjp.0707604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Knoll J, Kelemen K, Knoll B. Experimental studies on the higher nervous activity of animals. I. A method for the elaboration of a non-extinguishable conditioned reflex in the rat. Acta Physiol Hung. 1955;8:327–344. [Google Scholar]
- [18].Lane DA, Patel PA, Morgan MM. Evidence for an intrinsic mechanism of antinociceptive tolerance within the ventrolateral periaqueductal gray of rats. Neuroscience. 2005;135:227–234. doi: 10.1016/j.neuroscience.2005.06.014. [DOI] [PubMed] [Google Scholar]
- [19].Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- [20].Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1673-6. [DOI] [PubMed] [Google Scholar]
- [21].Meert TF, Vermeirsch HA. A preclinical comparison between different opioids: Antinociceptive versus adverse effects. Pharmacol Biochem Behav. 2005;80:309–326. doi: 10.1016/j.pbb.2004.12.002. [DOI] [PubMed] [Google Scholar]
- [22].Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-jun n-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U S A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the mu-opioid agonist damgo into the ventrolateral periaqueductal gray. Neuropharmacology. 2007;52:1580–1585. doi: 10.1016/j.neuropharm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mjanger E, Yaksh TL. Characteristics of dose-dependent antagonism by beta-funaltrexamine of the antinociceptive effects of intrathecal mu agonists. J Pharmacol Exp Ther. 1991;258:544–550. [PubMed] [Google Scholar]
- [25].Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- [26].Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- [27].Morgan MM, Tierney BW, Ingram SL. Intermittent dosing prolongs tolerance to the antinociceptive effect of morphine microinjection into the periaqueductal gray. Brain Res. 2005;1059:173–178. doi: 10.1016/j.brainres.2005.08.024. [DOI] [PubMed] [Google Scholar]
- [28].Paronis CA, Holtzman SG. Development of tolerance to the analgesic activity of mu agonists after continuous infusion of morphine, meperidine or fentanyl in rats. J Pharmacol Exp Ther. 1992;262:1–9. [PubMed] [Google Scholar]
- [29].Pawar M, Kumar P, Sunkaraneni S, Sirohi S, Walker EA, Yoburn BC. Opioid agonist efficacy predicts the magnitude of tolerance and the regulation of mu-opioid receptors and dynamin-2. Eur J Pharmacol. 2007;563:92–101. doi: 10.1016/j.ejphar.2007.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. Academic Press; Sydney: 2005. [Google Scholar]
- [31].Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female sprague-dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- [32].Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rodriguez-Munoz M, de la Torre-Madrid E, Gaitan G, Sanchez-Blazquez P, Garzon J. Rgs14 prevents morphine from internalizing mu-opioid receptors in periaqueductal gray neurons. Cell Signal. 2007;19:2558–2571. doi: 10.1016/j.cellsig.2007.08.003. [DOI] [PubMed] [Google Scholar]
- [34].Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513–519. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (pag) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- [36].Stevens CW, Yaksh TL. Potency of infused spinal antinociceptive agents is inversely related to magnitude of tolerance after continuous infusion. J Pharmacol Exp Ther. 1989;250:1–8. [PubMed] [Google Scholar]
- [37].Stevens CW, Yaksh TL. Time course characteristics of tolerance development to continuously infused antinociceptive agents in rat spinal cord. J Pharmacol Exp Ther. 1989;251:216–223. [PubMed] [Google Scholar]
- [38].Tallarida RJ. Drug synergism and dose-effect data analysis. Chapman and Hall/CRC; 2000. [Google Scholar]
- [39].Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]