Abstract
Objectives
Vertebral fractures are an under-recognized problem in children with inflammatory disorders. We studied spine health among 134 children (87 girls) with rheumatic conditions (median age 10 years) within 30 days of initiating glucocorticoid (GC) therapy.
Methods
Children were categorized as follows: juvenile dermatomyositis (juvenile DM, N=30), juvenile idiopathic arthritis (JIA; N=28), systemic lupus erythematosus (SLE) and related conditions (N=26), systemic arthritis (N=22), systemic vasculitis (N=16), and other conditions (N=12). Thoracolumbar spine radiograph and dual energy x-ray absorptiometry for lumbar spine areal bone mineral density (LS BMD) were performed within 30 days of GC initiation. Genant semi-quantitative grading was used for vertebral morphometry. Second metacarpal morphometry was carried out on a hand radiograph. Clinical factors including disease and physical activity, calcium and vitamin D intake, cumulative GC dose, underlying diagnosis, LS BMD Z-score and back pain were analyzed for association with vertebral fracture.
Results
Thirteen vertebral fractures were noted in 9 children (7%). Six patients had a single vertebral fracture and three patients had two to three fractures. Fractures were clustered in the mid-thoracic region (69%). Three vertebral fractures (23%) were moderate (Grade 2); the others were mild (Grade 1). For the entire cohort, mean (±SD) LS BMD Z-score was significantly different from zero (−0.55±1.2, p<0.001) despite a mean height Z-score that was similar to the healthy average (0.02±1.0, p=0.825). Back pain was highly associated with increased odds for fracture (OR 10.6, 95% CI 2.1 to 53.8, p=0.004).
Conclusions
In pediatric rheumatic conditions, vertebral fractures can be present prior to prolonged GC exposure.
Introduction
There is increasing recognition that juvenile-onset, inflammatory rheumatic conditions are associated with adverse effects on the developing skeleton. Reductions in lumbar spine (LS), femoral neck and distal radial bone mineral density (BMD) in children with juvenile idiopathic arthritis (JIA) have been consistently documented among those who have been treated with glucocorticoids (GCs) (1–13) and those who have not (1–6,8,9,12,14–16). Reduced LS BMD has also been shown in children with juvenile dermatomyositis (DM) (17–21) and in juvenile systemic lupus erythematosus (SLE) (3,21–27).
Vertebral and extremity fractures have also been described in pediatric rheumatic disease. An increase in extremity fractures among children with arthritis has been documented through a large, population-based retrospective study (28). Furthermore, vertebral fracture prevalence ranging from 10 to 50% has been shown in cross-sectional studies of children with prolonged rheumatic disease durations and/or GC exposure (11,25,29,30). These cross-sectional reports have been important in highlighting the extent of spine morbidity in the years following diagnosis. However, the timing of vertebral fracture onset remains unknown. In addition, many of the previously reported children with vertebral fractures were treated with GCs and other potentially osteotoxic medications, making it difficult to determine if the observed fractures and reductions in BMD were related to the underlying disease, GC therapy, or other factors.
Our goal was to document the prevalence of vertebral fractures within 30 days of GC initiation in an inception cohort of GC-treated children with rheumatic disorders. In addition, we sought to determine the relationship between vertebral fractures and relevant clinical factors including spine BMD, underlying diagnosis, disease activity, age, pubertal stage, gender, back pain, calcium and vitamin D intake and physical activity.
Subjects and Methods
Patients and Study Design
Patients were recruited through the Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) research initiative, a national research program that studies bone morbidity in children with chronic illnesses. Patients from one month to 17 years of age were enrolled (N = 134) between January 1 2005 and December 31 2007 in 10 participating tertiary care children’s hospitals. Patients were enrolled within 30 days of first-time GC treatment for inflammatory rheumatic conditions, including juvenile DM, juvenile SLE, JIA (all sub-types), systemic pediatric vasculitides (excluding Henoch-Schonlein purpura and Kawasaki Disease), juvenile scleroderma (both systemic and localized), and overlap syndromes (including mixed connective tissue disease). Diagnoses were made by university-affiliated, pediatric rheumatologists.
Children were excluded if GCs had previously been used at any time for treatment of the underlying disease. Patients were also excluded if they had received intravenous or oral GCs for more than 14 consecutive days in the 12 months preceding study enrolment to treat any other medical condition (e.g. asthma). Patients who had received prior medication for osteoporosis were also excluded, as were those who had received previous treatment with calcium and/or vitamin D supplementation that exceeded the Dietary Reference Intake for age (31). Since this study involved radiation from DXA and skeletal radiographs, girls were excluded if they were pregnant or menstruating and unwilling to use medically approved contraception. The study was approved by the Research Ethics Board in each institution and informed consent and/or assent was obtained prior to study enrolment.
Clinical Data
The decision to initiate GCs was made clinically prior to consideration for study enrollment. Demographic and anthropometric data were recorded using standard methods. Height, weight, and body mass index (weight (kg) divided by height (meters2)) raw values were transformed into age- and gender-matched Z-scores according to the United States Centers for Disease Control National Center for Health Statistics normative database (32); for children under 2 years of age, BMI Z-scores were calculated according to the World Health Organization child growth standards (33). Pubertal staging was carried out according to the methods of Marshall and Tanner (34,35). The presence or absence of reported back pain at the time of diagnosis was recorded, and the spine was palpated for tenderness over the posterior spinous processes (T4 to L4). Time since diagnosis and symptom onset to the LS BMD assessment were recorded. Children were divided into sub-groups to facilitate characterization of the cohort, as follows: juvenile DM, juvenile JIA (excluding systemic arthritis), juvenile SLE and related conditions, systemic arthritis, systemic vasculitis and other conditions.
Assessment of Calcium and Vitamin D Intake
Calcium and vitamin D intake were assessed by a validated food frequency questionnaire (36). Intake for each nutrient was expressed as the percent of the Adequate Intake value based on the Dietary Reference Intakes (31). Calcium and vitamin D intake by supplementation was added to the dietary intake to arrive at a total daily intake for both nutrients. The percentage scores were then classified as <50% of the age-related DRI, 50–100% of the DRI, and >100% of the DRI.
Physical Activity Assessment: The Habitual Activity Estimation Scale (HAES)
The HAES is a validated, self/proxy report that provides an estimation of the intensity and duration of physical activity over a single day (37,38). Activity was reported for both a typical weekday and weekend day in the previous three months. Activity classifications were as follows: Inactive (e.g. lying down), Somewhat Inactive (e.g. sitting), Somewhat Active (e.g. walking), and Very Active (e.g. running). Total inactive and total active times were determined by summing the two inactive and the two active categories for each of the weekend and weekday reports
Physician Global Assessment of Disease Activity According to Visual Analogue Scale
There is no assessment tool which has been shown to allow comparison of disease activity across rheumatic conditions. However, the use of visual analogue scales (VAS) completed by a physician who is expert in the assessment of pediatric rheumatic conditions has been validated in a variety of rheumatic conditions in the pediatric setting (39–41). Disease activity was scored according to a VAS by the patients’ attending rheumatologists, measuring Physician Global Assessment of Disease Activity. The VAS was represented by a 10 cm scale, where 0 cm = inactive disease, and 10 cm = extremely active disease. Erythrocyte sedimentation rate (ESR) was also measured, using standard methodology from the local laboratories.
Lumbar Spine BMD by Dual-Energy X-Ray Absorptiometry (DXA)
BMD was measured in the anterior-posterior direction at the LS (L1-L4) by dual-energy x-ray absorptiometry using either Hologic machines (QDR 4500, 3 centers; Discovery, 2 centers; Delphi, 1 center; Hologic, Waltham, MA) or Lunar Prodigy (4 centers; GE Medical Systems, Madison, WI). Machines were cross-calibrated as previously described (42). The primary outcome for the study was LS BMD Z-scores; raw LS BMD results were transformed to chronological age- and gender-specific Z-scores as well as bone-age and gender-matched Z-scores using the Hologic 12.4 normative database provided by the manufacturer, which comprises the full age range of the children enrolled in the study. In vivo precision for LS BMD was available in 8 of 10 centers and ranged from 0.003 to 0.017 gm/cm2.
Bone Age and Second Metacarpal Morphometry
Radiographs of the left hand and wrist for bone age were read independently by two pediatric radiologists (NS, MM) according to Greulich and Pyle (43). If results for the two examiners were within 12 months of each other, the average of the two readings was used. For results that differed by more than 12 months (N=10), a third reader (LMW), blinded to the results of the first two, adjudicated the discrepant reports. The intra-class correlation coefficient (ICC) was 0.99 (95% CI 0.986 to 0.993) between the two initial examiners. The radiographs were also evaluated for the possibility of rickets.
Using the same hand radiographs, a single observer measured the second metacarpal length, mid-shaft periosteal diameter, and inner diameter, as previously described (44), for derivation of the following indices: combined cortical thickness, cortical area, percent cortical area and inner diameter area. Indices were converted into age- and gender-matched Z-scores as previously described (45). The intra-observer reliability scores assessed by ICC were as follows: 1.0 (95% CI 0.999 to 1.0), 0.99 (95% CI 0.986 to 0.997) and 0.89 (95% CI 0.777 to 0.945) for metacarpal length, outer diameter and inner diameter, respectively.
Vertebral Morphometry
The Genant semi-quantitative method for vertebral morphometry was performed in the following manner. Vertebral bodies were first assigned a severity score: grade 0 (normal), grade 1 (mild), grade 2 (moderate) or grade 3 (severe). The morphometric grading corresponded to the extent of the reduction in height ratios when the anterior vertebral height was compared to the posterior height (wedge fracture), the middle height to the posterior height (biconcave fracture), and the posterior height to the posterior height of the adjacent vertebral bodies (crush fracture). The scores corresponded to the following reduction in height ratios: Grade 0: 20% or less; Grade 1: >20 to 25%; Grade 2: >25 to 40%; Grade 3: >40%. Grade 0 was considered to be normal while higher grades were considered to be a fracture. Minimal physiological rounding of vertebral bodies in the mid-thoracic region of the spine, as can be seen in normal children, was assigned a grade 0 score (46).
Vertebral fracture assessment was carried out independently by two radiologists (NS, MM) from T4 to L4 (42,47). Discrepancies between the first two readers were resolved by a third expert radiologist (BL), who was blinded to the results of the other two. The inter-observer reliability for the first two readers according to Cohen’s kappa was 0.44 (95% CI 0.28 to 0.59) when Genant grade 0 scores were compared to Grades 1, 2 and 3 combined. For Grades 0 and 1 combined compared to Grades 2 and 3 combined, the Cohen’s kappa was 0.66 (95% CI 0.46 to 0.87).
Statistical Analyses
Analyses were conducted using SPSS 16.0 (SPSS Inc., Chicago IL). Presented p-values were two-sided. To account for multiple comparisons, a Bonferroni correction was applied to the univariate analyses. Categorical variables were summarized using frequency and percentage. Normally distributed continuous variables were summarized using mean and standard deviation (SD). Non-normally distributed continuous variables were summarized using median and range. Z-score variables were compared against the healthy average (Z-score = 0.0) using one-sample student’s t-test to assess whether the patient population significantly differed from the normal reference values. Proportions and 95% Confidence Intervals (CI) were calculated using the Wilson score method (48). Mann-Whitney or Fisher’s exact test was used to compare patients with and without fracture. The comparison of combined cortical thickness Z-score between patients with and without fracture was adjusted for metacarpal length Z-score using linear regression.
Univariate logistic regressions were performed to identify clinical parameters that were associated with the presence of vertebral fractures. Multiple logistic regression was not performed due to the small number of vertebral fracture events. Univariate linear regressions were similarly performed to identify the factors associated with LS BMD Z-score. To adjust for bone size, height Z-score was included in all linear regression on LS BMD Z-score models (both univariate and multivariate analysis). The following variables were included in a clinically-driven, multiple linear regression model which sought to determine associations between relevant factors and age- and gender-matched LS BMD Z-score: gender, height Z-score, BMI Z-score, pubertal stage (Tanner stage 1 versus 2–5), time since symptom onset, disease activity, cumulative GC dose in prednisone equivalents, diagnosis, and vitamin D intake. The results of this model were then verified using a step-wise model selection procedure which incorporated these same factors as well as age, physical activity, calcium intake, number of days on GCs, and time since diagnosis (log transformed to reduce skewness).
Results
Patient Characteristics
Descriptions of the cohort are provided in Tables 1 a and b. Seventy-five percent of the children were White; the other 25% of children were Black (7%), Aboriginal (5%), South Asian (3%) and Other or Mixed Ethnicity (10%). Height Z-scores were comparable to the healthy average for all disease sub-groups (overall cohort, p=0.825; juvenile DM, p=0.559; JIA, p=0.252; SLE and related conditions, p=0.292, systemic arthritis, p=0.255, systemic vasculitis, p=0.174 and other conditions, p=0.248). Weight was significantly above the healthy average for the overall cohort (p=0.006), and the systemic vasculitis sub-group (p=0.045), while BMI was increased in the overall cohort (p<0.001), the SLE and related conditions sub-group (p=0.017) and in systemic vasculitis (p=0.046).
Table 1a.
Description of an Inception Cohort of Children Recently Initiating Glucocorticoids for the Treatment of Rheumatic Disorders
| Clinical Characteristics | Overall Cohort N=134 |
Juvenile DM N=30 |
JIA N=28 |
SLE and Related Conditions N=26 |
Systemic Arthritis N=22 |
Systemic Vasculitis N=16 |
Other Conditions N=12 |
|---|---|---|---|---|---|---|---|
| Demographic Data | |||||||
| Female, N (%) | 87 (65) | 18 (60) | 18 (64) | 22 (85) | 13 (59) | 8 (50) | 8 (67) |
| Age, median (min, max) | 10.0 (1.4, 16.9) | 7.3 (1.9, 15.1) | 12.2 (3.7, 16.9) | 13.6 (5.0, 16.1) | 6.0 (1.4, 16.4) | 12.6 (4.7, 16.9) | 7.7 (3.3, 16.5) |
| Anthropometry | |||||||
| Height Z-score, mean±SD | 0.02±1.0 | −0.11±1.0 | −0.23±1.1 | −0.17±0.8 | 0.28±1.1 | 0.42±1.2 | 0.36±1.0 |
| Weight Z-score, mean±SD | 0.28±1.2 | 0.03±1.2 | 0.07±1.2 | 0.28±1.0 | 0.29±1.0 | 0.77±1.4 | 0.76±1.2 |
| BMI Z-score, mean±SD | 0.37±1.2 | 0.19±1.2 | 0.23±1.1 | 0.46±0.9 | 0.23±1.3 | 0.69±1.3 | 0.76±1.3 |
| Pubertal stage, N (%) | |||||||
| Stage 1 | 69 (53) | 24 (80) | 9 (35) | 7 (27) | 15 (68) | 7 (44) | 7 (64) |
| Stage 2–5 | 62 (47) | 6 (20) | 17 (65) | 19 (73) | 7 (32) | 9 (56) | 4 (36) |
| Bone age, median (min, max) | 10.0 (1.1, 17.5) | 6.8 (1.4, 15.5) | 12.9 (3.0, 16.0) | 14.5 (4.6, 16.5) | 5.9 (1.1, 17.0) | 13.8 (3.3, 17.0) | 7.7 (3.3, 17.5) |
| Age to bone age difference, mean±SD | −0.05±1.0 | 0.07±1.0 | 0.19±1.3 | −0.50±1.0 | 0.06±0.5 | −0.01±1.4 | −0.14±0.8 |
| Rheumatic Conditions Characteristics | |||||||
| Disease activity (10 cm VAS), mean±SD | 5.6±2.8 | 6.2±2.8 | 5.3±2.6 | 4.7±2.9 | 6.3±2.0 | 6.1±3.7 | 4.7±2.4 |
| ESR (mm/hr), median (min, max) | 33.0 (0, 133) | 17.0 (2, 109) | 36.0 (5, 106) | 47.5 (7, 116) | 58.5 (10, 133) | 47.0 (1, 109) | 9.5 (0, 40) |
| Number of days since diagnosis, median (min, max) | 22 (1, 4900) | 19 (1, 357) | 32 (1, 4900) | 16 (1, 235) | 22 (1, 64) | 21 (1, 132) | 61 (8, 1984) |
| Number of days since symptom onset, median (min, max) | 145 (17, 5110) | 124 (27, 742) | 353 (30, 5110) | 67 (17, 765) | 50 (18, 225) | 138 (24, 975) | 298 (155, 2349) |
| Physical Activity Level (the HAES Questionnaire) | |||||||
| Relative physical activity (% of waking hrs), median (min, max) | 46 (0, 97) | 42 (0, 86) | 51 (0, 91) | 38 (0, 97) | 52 (0, 83) | 36 (0, 78) | 67 (29, 90) |
| Very active weekend hours, median (min, max) | 1.0 (0, 17) | 0 (0, 7) | 1.4 (0, 11) | 0 (0, 17) | 0.9 (0, 8) | 0.4 (0, 5) | 4.7 (0, 12) |
| Glucocorticoid Treatment | |||||||
| Cumulative GC dose (mg/m2), mean±SD** | 1404±1690 | 2334±2184 | 460±797 | 1647±1643 | 762±1159 | 1680±1693 | 1477±1417 |
| Number of days on GC, mean±SD | 16.5±8.6 | 17.0±9.5 | 17.9±7.4 | 17.5±9.0 | 16.5±7.7 | 18.4±7.8 | 7.7±6.6 |
| Number of days between initial GC dose and DXA assessment, mean±SD | 16.7±8.7 | 15.8±10.0 | 18.6±7.1 | 16.0±9.4 | 15.5±8.7 | 17.1±7.5 | 19.3±9.5 |
| Lumbar Spine (LS) BMD | |||||||
| LS BMD Z-score, mean±SD | −0.55±1.2 | −1.06±1.0 | −0.71±1.4 | 0.06±1.1 | −0.63±0.8 | −0.70±1.5 | 0.04±0.8 |
| LS BMD Z-score for bone age, mean±SD | −0.60±1.0 | −1.01±0.8 | −0.70±1.2 | −0.22±0.9 | −0.57±1.0 | −0.76±1.3 | −0.02±0.85 |
SD=Standard deviation, BMI=Body mass index, VAS=Visual analogue scale, ESR=Erythrocyte sedimentation rate, BMD= Bone mineral density, GC=Glucocorticoids, JIA = Juvenile Idiopathic Arthritis, DM = Dermatomyositis, SLE = Systemic Lupus Erythematosis
Cumulative GC dose is reported in prednisone equivalents
Table 1b.
Specific Diseases within Diagnostic Sub-Groups
| Diagnosis | n (%) |
|---|---|
| Rheumatic Conditions: Total Cohort | 134 |
| Diagnostic Sub-Groups | |
| Juvenile Dermatomyositis | 30 (22) |
| JIA (Excluding Systemic Arthritis) | 28 (21) |
| Polyarticular Rheumatoid Factor positive arthritis | 5 (18) |
| Polyarticular Rheumatoid Factor negative arthritis | 8 (29) |
| Psoriatic arthritis | 2 (7) |
| Enthesitis-related arthritis | 5 (18) |
| Oligoarticular arthritis | 4 (14) |
| Unclassified | 4 (14) |
| Systemic Lupus Erythematosus and related conditions | 26 (20) |
| Systemic Lupus Erythematosus | 21 (81) |
| Overlap syndromes: mixed connective tissue disease | 5 (19) |
| JIA (Systemic Arthritis) | 22 (16) |
| Systemic Vasculitis (excluding Kawasaki’s disease and Henoch-Schonlein Purpura (HSP)) | 16 (12) |
| Takayasu arteritis | 4 (25) |
| Wegener granulomatosis | 7 (44) |
| Microscopic polyangiitis | 1 (6) |
| Other vasculitis, including: | 4 (25) |
| P-ANCA positive vasculitis with recurrent pericarditis (N=1) | |
| P-ANCA positive vasculitis with Goodpasture syndrome (N=1) | |
| CNS vasculitis (N=1) | |
| P-ANCA positive renal-limited vasculitis (N=1) | |
| Other conditions | 12 (9) |
| Scleroderma - Generalized | 1 (8) |
| Scleroderma - Localized | 10 (84) |
| Eosinophilic Fasciitis | 1 (8) |
Vertebral Fracture and Second Metacarpal Morphometry Status
Nine of the 134 children (7%, 95% CI, 3.6% to 12.3%) were found to have a total of 13 vertebral fractures. These children ranged in age from 6 to 16.5 years (5 boys, 4 girls). Six children had one fracture, two children had two fractures and one child had three fractures. Nine of the fractures were thoracic (four at T6, three at T7, two at T8) and four were lumbar (two at L1 and one each at L2 and L4). Nine of the fractures were mild anterior wedge (Grade 1); three were moderate (Grade 2) wedge fractures and one was a mild (Grade 1) crush fracture. There were 3/30 children with fractures in the juvenile DM sub-group (10%, 95% CI 4% to 26%), 2/22 from the systemic arthritis category (9%, 95% CI 3% to 28%), 2/26 from the SLE and related conditions category (8%, 95% CI 2% to 24%), 1/16 from the systemic vasculitis sub-group (6%, 95% CI 1% to 28%) and 1/12 in the other conditions category (8%, 95% CI 1% to 35%). None of the children with JIA (excluding systemic) manifested vertebral fractures (95% CI 0% to 12%). The three children with moderate (Grade 2) fractures had Wegener granulomatosis, SLE and systemic JIA. The six children with mild (Grade 1) fractures had juvenile DM (N=3), systemic JIA, SLE and scleroderma. Examples of mild and moderate fractures that were representative of the fractures detected in this cohort are presented in the Figure 1. There was no prior history of trauma in any of the patients.
Figure 1.
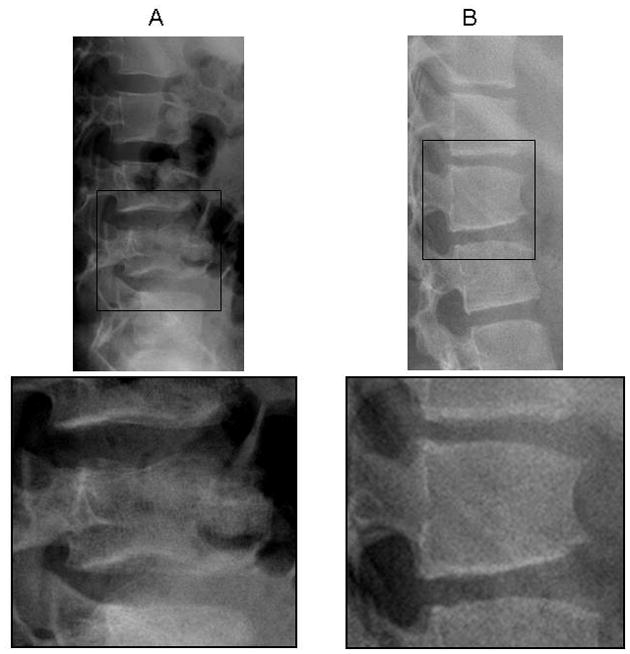
A, 12-year-old boy with systemic juvenile idiopathic arthritis. Shown is a grade 1 crush fracture at L4, plus loss of endplate parallelism and endplate interruption. The lumbar spine bone mineral density (BMD) Z score was _1.4. B, 15-year-old girl with systemic lupus erythematosus. Shown is a grade 2 wedge fracture at L1, plus loss of endplate parallelism and anterior cortical buckling. The lumbar spine BMD Z score was _1.7.
Table 2 shows a comparison of children with vertebral fractures and those without. Back pain was reported in seven of nine (78%) children with fractures compared to 25% (31/125) of those without (p=0.002). A sub-set of patients (131/134) also underwent palpation of the T4 to L4 posterior spinous processes; only 8 of these 131 children reported pain on palpation and none of these 8 children manifested vertebral fractures. Children with fractures had a mean ± SD LS BMD Z-score of −1.2±1.0 compared to −0.5±1.2 among those without (p=0.082). For the children with fractures, the mean combined second metacarpal cortical thickness Z-score was −0.23±0.9 compared to 0.31±1.0 among those without (p=0.061), following adjustment for metacarpal length Z-score. In Table 3, univariate logistic regression against prevalent vertebral fracture revealed that back pain was highly associated with increased odds for fracture (OR 10.6, 95% CI 2.1 to 53.8, p=0.004).
Table 2.
Comparison of Children With and Without Vertebral Fractures
| Clinical Characteristics | Children Without Vertebral Fracture N = 125 |
Children With Vertebral Fractures N = 9 |
*pΨ |
|---|---|---|---|
| Demographic Data | |||
| A Girls, N (%) | 83 (66) | 4 (44) | 0.277 |
| Age, median (min, max) | 10.0 (1.4, 16.9) | 14.3 (6.4, 16.5) | 0.043 |
| Anthropometry | |||
| Height Z-score, mean±SD | 0.04±1.0 | −0.29±0.9 | 0.267 |
| Weight Z-score, mean±SD | 0.31±1.2 | −0.20±0.9 | 0.154 |
| BMI Z-score, mean±SD | 0.40±1.2 | −0.13±1.1 | 0.213 |
| A Pubertal stage, N (%) | |||
| Stage 1 | 65 (53) | 4 (50) | 1.000 |
| Stage 2 – 5 | 58 (47) | 4 (50) | |
| Bone age, median (min, max) | 10.0 (1.1, 17.5) | 13.5 (6, 16) | 0.103 |
| Second Metacarpal Morphometry | |||
| Metacarpal length Z-score, mean±SD | 0.35±1.1 | 0.50±0.9 | 0.553 |
| Percent cortical area Z-score, mean±SD | 0.21±0.9 | 0.15±0.6 | 0.628 |
| Combined cortical thickness Z-score, mean±SD | 0.31±1.0 | −0.23±0.9 | B0.061 |
| Rheumatic Conditions Characteristics | |||
| Disease activity (10 cm VAS), mean±SD | 5.6±2.7 | 5.5±3.4 | 0.887 |
| ESR (mm/hr), median (min, max) | 40.5 (31.2) | 27.9 (24.5) | 0.234 |
| Number of days since diagnosis, median (min, max) | 23 (1, 4900) | 22 (3, 1902) | 0.407 |
| Number of days since symptom onset, median (min, max) | 146 (17, 5110) | 93 (19, 2268) | 0.771 |
| Back Pain | |||
| A Yes, N (%) | 31 (25) | 7 (77.8) | 0.002# |
| Lumbar Spine BMD | |||
| Lumbar spine BMD Z-score, mean±SD | −0.51±1.2 | −1.2±1.0 | 0.082 |
| Glucocorticoid Treatment | |||
| Cumulative GC dose (mg/m2), mean±SD | 1410±1600 | 1320±2755 | 0.244 |
| Number of days on GC, mean±SD | 16.7±8.5 | 14.1±10.4 | 0.376 |
| CTotal Calcium and Vitamin D Intake (Diet & Supplement) | |||
| Total calcium daily intake, mean± SD % of the DRI | |||
| < 50 (N = 4 with fractures, N = 0 without) | 31±19 | NA | NA |
| 50 – 100 (N = 12 with fractures, N = 1 without) | 68±13 | 70 (NA) | 0.923 |
| >= 100 (N = 104 with fractures, N = 8 without) | 273±164 | 178±60 | 0.042 |
| Total vitamin D daily intake, mean± SD % of the DRI | |||
| < 50 (N = 30 with fractures, N = 2 without) | 24±15 | 30±25 | 0.734 |
| 50 – 100 (N = 18 with fractures, N = 1 without) | 74±14 | 74 (NA) | 0.737 |
| >= 100 (N = 73 with fractures, N = 6 without) | 170±65 | 163±57 | 0.868 |
| HAES Activity Levels | |||
| Very active weekend hours, median (min, max) | 1.1 (0, 17) | 0 (0, 4) | 0.046 |
SD=Standard deviation, BMI=Body mass index, VAS=Visual analogue scale, ESR=Erythrocyte sedimentation rate, BMD= Bone mineral density, GC=Glucocorticoids, DRI=Dietary Reference Intake
Cumulative glucocorticoid dose is reported in prednisone equivalents
Statistical significance determined by non-parametric test (Mann-Whitney U with 2 independent samples)
Statistical significance determined by Chi-squared test or Fisher’s Exact Test
p-value with adjustment for metacarpal length Z-score by linear regression
Intake grouped into three groups based on the percent relative to the Dietary Recommended Intake for age
Level of significance after Bonferroni correction = 0.002
Significant at P ≤0.002
Table 3.
Univariate Logistic Regression Analysis of Factors Potentially Associated with the Presence of Vertebral Fractures
| Clinical Parameter | Children with Rheumatic Disorders N=134 |
|
|---|---|---|
| Odds Ratio (95% CI) | pΨ | |
| Age | 1.2 (1.0, 1.4) | 0.062 |
| Gender (girls vs. boys) | 0.4 (0.1, 1.6) | 0.195 |
| Height Z-score | 0.7 (0.4, 1.4) | 0.334 |
| BMI Z-score | 0.7 (0.4, 1.2) | 0.185 |
| Back pain | 10.6 (2.1, 53.8) | 0.004‡ |
| Disease activity (10 cm VAS) | 1.0 (0.8, 1.3) | 0.887 |
| Diagnosis (Juvenile DM vs. Other Diagnoses) | 1.8 (0.4, 7.7) | 0.420 |
| Number of days since diagnosis (log transformed) | 0.9 (0.6, 1.5) | 0.693 |
| Total vitamin D daily intake (≥ 100% of DRI vs. < 100% of DRI) | 1.3 (0.3, 5.5) | 0.708 |
| Total calcium daily intake (≥ 100% of DRI vs. < 100% of DRI) | 1.3 (0.2, 11.1) | 0.806 |
| Cumulative GC dose (g/m2) | 1.0 (0.6, 1.5) | 0.876 |
| Lumbar spine BMD Z-score | 0.6 (0.4,1.1) | 0.090 |
CI=Confidence interval, BMI=Body mass index, BMD=Bone mineral density, VAS=Visual analogue scale, GC=Glucocorticoids, DM=Dermatomyositis, DRI=Dietary Reference Intake
Level of significance after Bonferroni correction = 0.004
Significant at P ≤ 0.004
Bone Densitometry
LS BMD Z-scores for the entire cohort are presented in Table 1. There was no significant difference between bone age and chronological age (p=0.610). Similarly, LS BMD Z-scores were no different when bone age was substituted for chronological age (p=0.331). The mean LS BMD Z-scores were significantly below the healthy average for the entire cohort (p<0.001) and for the following diagnostic sub-groups: juvenile DM sub-group (p<0.001), JIA (excluding systemic JIA, p=0.015), and systemic arthritis (p=0.002). Such differences were not observed in the SLE and related conditions sub-group (p=0.768), in systemic vasculitis (p=0.089) and in the other conditions category (p=0.875). There was no significant difference in the mean (±SD) LS BMD Z-score between those without fractures (−0.51±1.23; N=125) compared to those with mild (−0.76±0.92; N=6) and moderate vertebral fractures (−2.1±0.70; N=3; p=0.079).
The following variables were significant in a clinically-driven linear regression model which sought to determine associations between relevant factors and age- and gender-matched LS BMD Z-score: gender (β 0.67; 95% CI 0.25 to 1.08; p=0.002), height Z-score (β 0.32; 95% CI 0.13 to 0.50; p=0.001) and BMI Z-score (β 0.43; 95% CI 0.26 to 0.60; p<0.001). The results of this model were confirmed using a step-wise model selection procedure, which produced the same results and explained 31% of the variability in the LS BMD Z-score.
Discussion
Our work highlights novel observations about bone morbidity in pediatric rheumatic conditions, since this prospective study evaluated vertebral fracture status early in the course of GC exposure. We have documented a prevalent vertebral fracture rate of 7% in our inception cohort, with rates of 10% in juvenile DM, 9% in systemic arthritis, 8% in SLE and related conditions, 6% in systemic vasculitis, and 8% in the other conditions sub-group. While fractures were not observed in the JIA (excluding systemic) sub-group, our data suggest the potential for up to 12% of children with JIA to manifest vertebral fracture if the results were inferred to a larger population of children with JIA. Given that agreement between the radiologists on vertebral fracture assignment was fair to moderate, the protocol used in our study to assign vertebral fractures (which required agreement by two of three radiologists before a vertebra was considered fractured) would tend to under-estimate the prevalence of fracture; therefore, the fracture prevalence rate may have been even slightly higher in these disease groups.
The observations in this study have important clinical implications. First, children with rheumatic conditions can manifest clear evidence of bone fragility (i.e. vertebral fractures) early in their disease course and exposure to GCs. Second, back pain is a highly associated clinical feature (though not universal, since 2 of the 9 children with fractures did not report such pain). That vertebral fractures can be present in the absence of back pain has been described in women with post-menopausal osteoporosis (49), in children with long-standing histories of rheumatic conditions (11), and in childhood acute lymphoblastic leukemia (ALL) (42). Overall, these results highlight that vertebral fractures are an under-recognized problem in children who have recently initiated GC therapy for rheumatic disorders.
We found that vertebral fractures were clustered in the mid-thoracic and upper lumbar regions, similar to reports in men and women with osteoporosis (50–53), as well as recent studies in children with rheumatic conditions (29) and leukemia (42). It is suggested that this fracture pattern results from the mechanical stresses induced by the natural kyphosis-lordosis of the spine (54). The location of fractures in areas for which there is a known predilection adds credence to our method of fracture determination. The fact that wedge deformity was the most common morphological finding is further in keeping with observations in large populations of adults with vertebral fractures (54) and in children with leukemia (42).
The few studies in the literature that have assessed vertebral fractures status in children with rheumatic conditions have been conducted at time-points more distant in their disease course compared to our study. Specifically, these reports have been cross-sectional or retrospective, often in the face of long-term GC exposure, and have shown vertebral fracture prevalence rates ranging from 10 to 50% (11,25,29,30). Our study stands unique for its timing of patient evaluation, within 30 days of GC initiation. The only other study conducted early in the course of the illness was by Rouster-Stevens et al (18), who assessed spine areal BMD by DXA in 37 children with untreated juvenile DM. They found that 6 of 33 (18%) evaluable patients had LS areal BMD Z-scores less than −1.5, and that the LS BMD Z-score was related to disease duration. Vertebral fracture status was not evaluated in this cohort of patients. Interestingly, we did not find a link between disease duration and either vertebral fracture or LS BMD Z-score. Disease activity indices also showed a lack of association. These findings may reflect lack of sufficient power to detect an association, a relatively short duration from both the time since diagnosis and symptom onset for most patients, and/or confounding effects of both underlying disease and short-term exposure to GCs in our cohort.
When children with vertebral fractures in our study were compared to those without, the only variable that showed a strong relationship to fractures was back pain. Of particular note is the borderline relationship between the presence of vertebral fractures and LS BMD Z-score. In contrast, children with newly diagnosed leukemia demonstrate a strong relationship between LS BMD and vertebral fractures, with the LS BMD Z-score lower in those with fractures, and falling as the grade of fracture worsens (42). These disparate observations may be the result of lower power due to the smaller number of fracture events in rheumatic conditions soon after GC initiation compared to leukemia (7% versus 16%); on the other hand, the more fulminate effect of the leukemic process on bone may have greater impact on LS BMD in the short term compared to the typically more insidious inflammatory state in recently diagnosed rheumatic conditions.
Our study has two limitations which merit further consideration. First, back pain by report was determined but the location of the self-reported pain and the timing of pain onset were not specified. We found that such pain was highly correlated with vertebral fracture; however, without additional information as to the precise location of the reported pain or the timing of onset, we could not further correlate such parameters with the presence of vertebral fracture or with the initiation of GC therapy. A sub-set of patients (131/134) underwent palpation of the posterior spinous processes. While only 8 of the 131 children reported pain on palpation, none of these 8 children manifested vertebral fractures. Given the small number of children with palpation tenderness, we were unable to draw further conclusions as to the relationships among spine palpation tenderness, reported back pain and vertebral fractures. At the present time, the clinical significance of back pain and vertebral fractures in the absence of localized vertebral tenderness in this population remains unclear, particularly since the underlying disorders may also be associated with back pain and tenderness.
The second limitation arises from the study design. While our overall research program is predicated upon within-subject change over time in key parameters such as vertebral morphometery, this inaugural description of an inception cohort is based on uncontrolled, cross-sectional evaluation of spine status in relation to relevant clinical parameters. The lack of a control group gives rise to two issues in data interpretation. First, our spine BMD and anthropometric Z-scores have been generated through comparison to historical, published normative data, which may serve to under-estimate such indices given the rise in secular trends (55). Secondly, the frequency of mild (Grade 1) vertebral deformity in healthy children and thereby the clinical significance of mild changes in chronic illness remains unknown. In post-menopausal women, mild prevalent vertebral fractures are associated with an increased risk of future vertebral and hip fractures (56,57), with prevalent vertebral fracture severity being the strongest independent risk factor. The relationship between prevalent Grade 1 vertebral deformity at baseline in children with rheumatic conditions and the potential for development of new or worsening fractures will be assessed through further longitudinal study of this cohort.
In conclusion, we have shown that children with a variety of GC-treated rheumatic conditions can manifest vertebral fractures around the time of GC initiation, and that back pain is a highly correlated feature. Whether the fractures will undergo reshaping or deterioration with ongoing GC treatment will be determined through longitudinal study.
Acknowledgments
This study was primarily funded by an operating grant from the Canadian Institutes for Health Research. Additional funding for this work has been provided by the Canadian Institutes for Health Research New Investigator Program (to Dr. Leanne Ward), the Canadian Child Health Clinician Scientist Career Enhancement Program (to Dr. Leanne Ward), the Children’s Hospital of Eastern Ontario and Women and Children’s Health Research Institute, University of Alberta.
The Canadian STOPP Consortium would like to thank the following individuals:
The children and their families who participated in the study and without whom the STOPP study would not have been possible.
Research Associates who managed the study at the co-ordinating center (the Children’s Hospital of Eastern Ontario Ottawa, Ontario): Elizabeth Sykes (STOPP Project Manager), Maya Scharke (STOPP Data Analyst and Database Manager), Victor Konji (STOPP Publications and Presentations Committee Liaison and hand morphometry measurements), Steve Anderson (Children’s Hospital of Eastern Ontario Pediatric Bone Health Program Research Manager), Catherine Riddell (STOPP National Study Monitor).
Research Associates who took care of the patients from the following institutions: Alberta Children’s Hospital, Calgary, Alberta: Eileen Pyra; British Columbia Children’s Hospital, Vancouver British Columbia: Terry Viczko; Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Amanda George, Catherine Riddell; Children’s Hospital of Western Ontario, London, Ontario: Leila MacBean; McMaster Children’s Hospital, Hamilton, Ontario: Susan Docherty-Skippen; IWK Health Center, Halifax, Nova Scotia: Aleasha Warner; Montréal Children’s Hospital, Montréal, Québec: Diane Laforte, Mayito St-Pierre; Ste. Justine Hospital, Montréal, Québec: Claude Belleville, Stéphanie Pellerin, Natacha Gaulin Marion; Stollery Children’s Hospital, Edmonton, Alberta: Deborah Olmstead, Melissa Gabruck, Linda Manasterski; ; Toronto Hospital for Sick Children, Toronto, Ontario: Julie Lee, Karen Whitney; Winnipeg Children’s Hospital, Winnipeg, Manitoba: Dan Catte, Erika Bloomfield.
The Research Nurses, Support Staff and all the STOPP collaborators from the various Divisions of Nephrology, Oncology, Rheumatology and Radiology who have contributed to the care of the children enrolled in the study.
Abbreviations
- BMI
Body mass index
- BMD
Bone mineral density
- CI
Confidence interval
- DM
Dermatomyositis
- DRI
Dietary reference intake
- DXA
Dual-energy x-ray absorptiometry
- JIA
Juvenile idiopathic arthritis
- LS
Lumbar spine
- SD
Standard deviation
- SLE
Systemic lupus erythematosus
- VAS
Visual analogue scale
The Canadian STeroid-associated Osteoporosis in the Pediatric Population (STOPP) Consortium (a pan-Canadian, pediatric bone health working group)
Co-ordinating Center
Children’s Hospital of Eastern Ontario, Ottawa, Ontario: Leanne M. Ward#,*,§ (Study Principal Investigator), Janusz Feber*,§ (Nephrology), Isabelle Gaboury*,§ (Biostatistics, Chalmers Research Group), Jacqueline Halton*,§ (Oncology), MaryAnn Matzinger (Radiology, Central Radiograph Analyses), David Moher*,§ (Research Methods, Chalmers Research Group), Johannes Roth (Rheumatology), Nazih Shenouda§ (Radiology, Central Radiograph Analyses)
Participating Centers
Alberta Children’s Hospital, Calgary, Alberta: David Stephure (Site Principal Investigator), Reinhard Kloiber (Radiology), Victor Lewis (Oncology), Julian Midgley (Nephrology), Paivi Miettunen (Rheumatology)
British Columbia Children’s Hospital, Vancouver, British Columbia: David Cabral* (Site Principal Investigator), David B. Dix (Oncology), Kristin Houghton (Rheumatology), Helen R. Nadel (Radiology), Colin White (Nephrology)
British Columbia Women’s Hospital and Health Sciences Center, Vancouver, British Columbia: Brian C. Lentle§ (Radiology)
Brock University, Faculty of Applied Health Sciences, St. Catharines, Ontario: John Hay§ (Physical Activity Measurements)
Children’s Hospital of Western Ontario, London, Ontario: Cheril Clarson and Robert Stein (Site Principal Investigators), Elizabeth Cairney (Oncology), Guido Filler (Nephrology), Joanne Grimmer (Nephrology), Keith Sparrow (Radiology)
IWK Health Center, Halifax, Nova Scotia: Elizabeth Cummings (Site Principal Investigator), Conrad Fernandez (Oncology), Adam M. Huber§ (Rheumatology), Bianca Lang*,§ (Rheumatology), Kathy O’Brien (Radiology), Andrew Ross (Radiology)
McMaster Children’s Hospital, Hamilton, Ontario: Stephanie Atkinson*,§ (Site Principal Investigator), Steve Arora (Nephrology), Ronald Barr§ (Oncology), Craig Coblentz (Radiology), Peter B. Dent (Rheumatology), Colin Webber* (DXA Methodology), Montréal Children’s Hospital, Montréal, Québec: Celia Rodd§ (Site Principal Investigator), Sharon Abish (Oncology), Lorraine Bell (Nephrology), Rosie Scuccimarri (Rheumatology)
Shriners Hospital for Children, Montréal, Québec: Frank Rauch*,§ (Co-Chair, Publications and Presentations Committee), Francis Glorieux* (Chair, Ancillary Studies Committee)
Ste. Justine Hospital, Montréal, Québec: Nathalie Alos* (Site Principal Investigator), Josée Dubois (Radiology), Caroline Laverdière (Oncology), Véronique Phan (Nephrology), Claire Saint-Cyr (Rheumatology)
Stollery Children’s Hospital, Edmonton, Alberta: Robert Couch* (Site Principal Investigator), Janet Ellsworth (Rheumatology), Claire Leblanc (Rheumatology), Maury Pinsk (Nephrology), Kerry Siminoski§ (Radiology), Beverly Wilson (Oncology)
Toronto Hospital for Sick Children, Toronto, Ontario: Ronald Grant* (Site Principal Investigator), Martin Charron (Radiology), Diane Hebert (Nephrology)
Winnipeg Children’s Hospital, Winnipeg, Manitoba: Shayne Taback§ (Site Principal Investigator), Tom Blydt-Hansen (Nephrology), Sara Israels (Oncology), Kiem Oen (Rheumatology), Martin Reed (Radiology)
Footnotes
Principal Investigator;
Executive Committee Member;
Publications and Presentations Committee Member
References
- 1.Lien G, Selvaag AM, Flato B, Haugen M, Vinje O, Sorskaar D, Dale K, Egeland T, Forre O. A two-year prospective controlled study of bone mass and bone turnover in children with early juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(3):833–40. doi: 10.1002/art.20963. [DOI] [PubMed] [Google Scholar]
- 2.Cetin A, Celiker R, Dincer F, Ariyurek M. Bone mineral density in children with juvenile chronic arthritis. Clin Rheumatol. 1998;17(6):551–3. doi: 10.1007/BF01451301. [DOI] [PubMed] [Google Scholar]
- 3.Kashef S, Saki F, Karamizadeh Z, Kashef MA. Bone mineral density in children wth systemic lupus erythematosus and juvenile rheumatoid arthritis. Ann Saudi Med. 2007;27(6):427–31. doi: 10.5144/0256-4947.2007.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lien G, Flato B, Haugen M, Vinje O, Sorskaar D, Dale K, Johnston V, Egeland T, Forre O. Frequency of osteopenia in adolescents with early-onset juvenile idiopathic arthritis: a long-term outcome study of one hundred five patients. Arthritis Rheum. 2003;48(8):2214–23. doi: 10.1002/art.11097. [DOI] [PubMed] [Google Scholar]
- 5.Celiker R, Bal S, Bakkaloglu A, Ozaydin E, Coskun T, Cetin A, Dincer F. Factors playing a role in the development of decreased bone mineral density in juvenile chronic arthritis. Rheumatol Int. 2003;23(3):127–9. doi: 10.1007/s00296-002-0265-0. [DOI] [PubMed] [Google Scholar]
- 6.Pepmueller PH, Cassidy JT, Allen SH, Hillman LS. Bone mineralization and bone mineral metabolism in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1996;39(5):746–57. doi: 10.1002/art.1780390506. [DOI] [PubMed] [Google Scholar]
- 7.Kotaniemi A, Savolainen A, Kautiainen H, Kroger H. Estimation of central osteopenia in children with chronic polyarthritis treated with glucocorticoids. Pediatrics. 1993;91(6):1127–30. [PubMed] [Google Scholar]
- 8.Kotaniemi A, Savolainen A, Kroger H, Kautiainen H, Isomaki H. Weight-bearing physical activity, calcium intake, systemic glucocorticoids, chronic inflammation, and body constitution as determinants of lumbar and femoral bone mineral in juvenile chronic arthritis. Scand J Rheumatol. 1999;28(1):19–26. doi: 10.1080/03009749950155733. [DOI] [PubMed] [Google Scholar]
- 9.Falcini F, Trapani S, Civinini R, Capone A, Ermini M, Bartolozzi G. The primary role of steroids on the osteoporosis in juvenile rheumatoid patients evaluated by dual energy X-ray absorptiometry. J Endocrinol Invest. 1996;19(3):165–9. doi: 10.1007/BF03349860. [DOI] [PubMed] [Google Scholar]
- 10.Hartman C, Shamir R, Eshach-Adiv O, Iosilevsky G, Brik R. Assessment of osteoporosis by quantitative ultrasound versus dual energy X-ray absorptiometry in children with chronic rheumatic diseases. J Rheumatol. 2004;31(5):981–5. [PubMed] [Google Scholar]
- 11.Valta H, Lahdenne P, Jalanko H, Aalto K, Makitie O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. J Rheumatol. 2007;34(4):831–6. [PubMed] [Google Scholar]
- 12.Pereira RM, Corrente JE, Chahade WH, Yoshinari NH. Evaluation by dual X-ray absorptiometry (DXA) of bone mineral density in children with juvenile chronic arthritis. Clin Exp Rheumatol. 1998;16(4):495–501. [PubMed] [Google Scholar]
- 13.Brik R, Keidar Z, Schapira D, Israel O. Bone mineral density and turnover in children with systemic juvenile chronic arthritis. J Rheumatol. 1998;25(5):990–2. [PubMed] [Google Scholar]
- 14.Henderson CJ, Specker BL, Sierra RI, Campaigne BN, Lovell DJ. Total-body bone mineral content in non-corticosteroid-treated postpubertal females with juvenile rheumatoid arthritis: frequency of osteopenia and contributing factors. Arthritis Rheum. 2000;43(3):531–40. doi: 10.1002/1529-0131(200003)43:3<531::AID-ANR8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Henderson CJ, Cawkwell GD, Specker BL, Sierra RI, Wilmott RW, Campaigne BN, Lovell DJ. Predictors of total body bone mineral density in non-corticosteroid-treated prepubertal children with juvenile rheumatoid arthritis. Arthritis Rheum. 1997;40(11):1967–75. doi: 10.1002/art.1780401108. [DOI] [PubMed] [Google Scholar]
- 16.Polito C, Strano CG, Rea L, Alessio M, Iammarrone CS, Todisco N, Marotta A, Iaccarino E, Pirozzi M. Reduced bone mineral content and normal serum osteocalcin in non-steroid-treated patients with juvenile rheumatoid arthritis. Ann Rheum Dis. 1995;54(3):193–6. doi: 10.1136/ard.54.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro TC, Terreri MT, Szejnfeld VL, Len C, Fonseca AS, Hilario MO. Bone mineral density of Brazilian girls with juvenile dermatomyositis. Braz J Med Biol Res. 2005;38(2):309–13. doi: 10.1590/s0100-879x2005000200020. [DOI] [PubMed] [Google Scholar]
- 18.Rouster-Stevens KA, Langman CB, Price HE, Seshadri R, Shore RM, Abbott K, Pachman LM. RANKL:osteoprotegerin ratio and bone mineral density in children with untreated juvenile dermatomyositis. Arthritis Rheum. 2007;56(3):977–83. doi: 10.1002/art.22433. [DOI] [PubMed] [Google Scholar]
- 19.Stewart WA, Acott PD, Salisbury SR, Lang BA. Bone mineral density in juvenile dermatomyositis: assessment using dual x-ray absorptiometry. Arthritis Rheum. 2003;48(8):2294–8. doi: 10.1002/art.11211. [DOI] [PubMed] [Google Scholar]
- 20.Santiago RA, Silva CA, Caparbo VF, Sallum AM, Pereira RM. Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use. Scand J Rheumatol. 2008;37(1):40–7. doi: 10.1080/03009740701687226. [DOI] [PubMed] [Google Scholar]
- 21.Alsufyani KA, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, Nadel H, Malleson PN. Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol. 2005;32(4):729–33. [PubMed] [Google Scholar]
- 22.Compeyrot-Lacassagne S, Tyrrell PN, Atenafu E, Doria AS, Stephens D, Gilday D, Silverman ED. Prevalence and etiology of low bone mineral density in juvenile systemic lupus erythematosus. Arthritis Rheum. 2007;56(6):1966–73. doi: 10.1002/art.22691. [DOI] [PubMed] [Google Scholar]
- 23.Lilleby V, Lien G, Frey Froslie K, Haugen M, Flato B, Forre O. Frequency of osteopenia in children and young adults with childhood-onset systemic lupus erythematosus. Arthritis Rheum. 2005;52(7):2051–9. doi: 10.1002/art.21115. [DOI] [PubMed] [Google Scholar]
- 24.Trapani S, Civinini R, Ermini M, Paci E, Falcini F. Osteoporosis in juvenile systemic lupus erythematosus: a longitudinal study on the effect of steroids on bone mineral density. Rheumatol Int. 1998;18(2):45–9. doi: 10.1007/s002960050056. [DOI] [PubMed] [Google Scholar]
- 25.Regio P, Bonfa E, Takayama L, Pereira R. The influence of lean mass in trabecular and cortical bone in juvenile onset systemic lupus erythematosus. Lupus. 2008;17(9):787–92. doi: 10.1177/0961203308089446. [DOI] [PubMed] [Google Scholar]
- 26.Castro TC, Terreri MT, Szejnfeld VL, Castro CH, Fisberg M, Gabay M, Hilario MO. Bone mineral density in juvenile systemic lupus erythematosus. Braz J Med Biol Res. 2002;35(10):1159–63. doi: 10.1590/s0100-879x2002001000008. [DOI] [PubMed] [Google Scholar]
- 27.Coimbra IB, Costallat LT. Bone mineral density in systemic lupus erythematosus and its relation to age at disease onset, plasmatic estradiol and immunosuppressive therapy. Joint Bone Spine. 2003;70(1):40–5. doi: 10.1016/s1297-319x(02)00009-x. [DOI] [PubMed] [Google Scholar]
- 28.Burnham JM, Shults J, Weinstein R, Lewis JD, Leonard MB. Childhood onset arthritis is associated with an increased risk of fracture: a population based study using the General Practice Research Database. Ann Rheum Dis. 2006;65(8):1074–9. doi: 10.1136/ard.2005.048835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakhla M, Scuccimarri R, Duffy KN, Chedeville G, Campillo S, Duffy CM, Azouz EM, Shenouda N, Sharma AK, Rodd C. Prevalence of Vertebral Fractures in Children with Chronic Rheumatic Diseases at Risk for Osteopenia. J Pediatr. 2009;154:438–43. doi: 10.1016/j.jpeds.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Varonos S, Ansell BM, Reeve J. Vertebral collapse in juvenile chronic arthritis: its relationship with glucocorticoid therapy. Calcif Tissue Int. 1987;41(2):75–8. doi: 10.1007/BF02555248. [DOI] [PubMed] [Google Scholar]
- 31.Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, Vitamin D, fluoride. Washington, D.C: National Academy Press; 1997. [PubMed] [Google Scholar]
- 32.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. World Health Organization; Geneva: 2006. pp. 229–300. [Google Scholar]
- 34.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45(239):13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musgrave KO, Giambalvo L, Leclerc HL, Cook RA, Rosen CJ. Validation of a quantitative food frequency questionnaire for rapid assessment of dietary calcium intake. J Am Diet Assoc. 1989;89(10):1484–8. [PubMed] [Google Scholar]
- 37.Hay J. Development and validation of the Habitual Activity Estimation Scale (HAES) Children and Exercise. 1997;XIX II:125–9. [Google Scholar]
- 38.Wells GD, Wilkes DL, Schneiderman-Walker J, Elmi M, Tullis E, Lands LC, Ratjen F, Coates AL. Reliability and validity of the habitual activity estimation scale (HAES) in patients with cystic fibrosis. Pediatr Pulmonol. 2008;43(4):345–53. doi: 10.1002/ppul.20737. [DOI] [PubMed] [Google Scholar]
- 39.Marks SD, Pilkington C, Woo P, Dillon MJ. The use of the British Isles Lupus Assessment Group (BILAG) index as a valid tool in assessing disease activity in childhood-onset systemic lupus erythematosus. Rheumatology (Oxford) 2004;43(9):1186–9. doi: 10.1093/rheumatology/keh284. [DOI] [PubMed] [Google Scholar]
- 40.Falcone A, Cassone R, Rossi E, Pistorio A, Martini A, Ravelli A. Inter-observer agreement of the physician’s global assessment of disease activity in children with juvenile idiopathic arthritis. Clin Exp Rheumatol. 2005;23(1):113–6. [PubMed] [Google Scholar]
- 41.Rider LG, Feldman BM, Perez MD, Rennebohm RM, Lindsley CB, Zemel LS, Wallace CA, Ballinger SH, Bowyer SL, Reed AM, Passo MH, Katona IM, Miller FW, Lachenbruch PA. Development of validated disease activity and damage indices for the juvenile idiopathic inflammatory myopathies: I. Physician, parent, and patient global assessments. Juvenile Dermatomyositis Disease Activity Collaborative Study Group. Arthritis Rheum. 1997;40(11):1976–83. doi: 10.1002/art.1780401109. [DOI] [PubMed] [Google Scholar]
- 42.Halton J, Gaboury I, Grant R, Alos N, Cummings EA, Matzinger M, Shenouda N, Lentle B, Abish S, Atkinson S, Cairney E, Dix D, Israels S, Stephure D, Wilson B, Hay J, Moher D, Rauch F, Siminoski K, Ward LM. Advanced vertebral fracture among newly diagnosed children with acute lymphoblastic leukemia: results of the Canadian Steroid-Associated Osteoporosis in the Pediatric Population (STOPP) research program. J Bone Miner Res. 2009;24(7):1326–34. doi: 10.1359/jbmr.090202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford University Press; Stanford, California: 1959. [Google Scholar]
- 44.Spencer RP, Sagel SS, Garn SM. Age changes in five parameters of metacarpal growth. Invest Radiol. 1968;3(1):27–34. doi: 10.1097/00004424-196801000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Garn SM, Poznanski AK, Larson K. Metacarpal lengths, cortical diameters and areas from the 10-state nutrition survey. In: ZFGJ, editor. Proceedings of the first workshop on bone morphometry. University of Ottawa Press; Ottawa, Canada: 1976. pp. 367–391. [Google Scholar]
- 46.Keats TE, Smith TH. Year Book of Medical Publishers. 1977. An Atlas of Normal Developmental Anatomy. [Google Scholar]
- 47.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–48. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 48.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Statistics in Medicine. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(Suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- 50.Rea JA, Chen MB, Li J, Blake GM, Steiger P, Genant HK, Fogelman I. Morphometric X-ray absorptiometry and morphometric radiography of the spine: a comparison of prevalent vertebral deformity identification. J Bone Miner Res. 2000;15(3):564–74. doi: 10.1359/jbmr.2000.15.3.564. [DOI] [PubMed] [Google Scholar]
- 51.Vallarta-Ast N, Krueger D, Wrase C, Agrawal S, Binkley N. An evaluation of densitometric vertebral fracture assessment in men. Osteoporos Int. 2007;18(10):1405–10. doi: 10.1007/s00198-007-0381-5. [DOI] [PubMed] [Google Scholar]
- 52.Wu C, van Kuijk C, Li J, Jiang Y, Chan M, Countryman P, Genant HK. Comparison of digitized images with original radiography for semiquantitative assessment of osteoporotic fractures. Osteoporos Int. 2000;11(1):25–30. doi: 10.1007/s001980050002. [DOI] [PubMed] [Google Scholar]
- 53.Jackson SA, Tenenhouse A, Robertson L. Vertebral fracture definition from population-based data: preliminary results from the Canadian Multicenter Osteoporosis Study (CaMos) Osteoporos Int. 2000;11(8):680–7. doi: 10.1007/s001980070066. [DOI] [PubMed] [Google Scholar]
- 54.Ismail AA, Cooper C, Felsenberg D, Varlow J, Kanis JA, Silman AJ, O’Neill TW. Number and type of vertebral deformities: epidemiological characteristics and relation to back pain and height loss. European Vertebral Osteoporosis Study Group. Osteoporos Int. 1999;9(3):206–13. doi: 10.1007/s001980050138. [DOI] [PubMed] [Google Scholar]
- 55.Tremblay MS, Katzmarzyk PT, Willms JD. Temporal trends in overweight and obesity in Canada, 1981–1996. Int J Obes Relat Metab Disord. 2002;26(4):538–43. [PubMed] [Google Scholar]
- 56.Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures. Study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14(5):821–8. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 57.Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, Adachi JD. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33(4):522–32. doi: 10.1016/s8756-3282(03)00241-2. [DOI] [PubMed] [Google Scholar]


