Abstract
Parkinson’s disease (PD) is one of the most common neurological diseases in elderly people. The mean age of onset is 55 years of age, and the risk for developing PD increases 5-fold by the age of 70. In PD, there is impairment in both motor and nonmotor (NMS) functions. The strategy of PD motor dysfunction treatment is simple and generally based on the enhancement of dopaminergic transmission by means of the L-dihydroxyphenylalanine (L-dopa) and dopamine (DA) agonists. L-dopa was discovered in the early -60's of the last century by Hornykiewicz and used for the treatment of patients with PD. L-dopa treatment in PD is related to decreased levels of the neurotransmitter (DA) in striatum and ab-sence of DA transporters on the nerve terminals in the brain. L-dopa may also indirectly stimulate the receptors of the D1 and D2 families. Administration of L-dopa to PD patients, especially long-time therapy, may cause side effects in the form of increased toxicity and inflammatory response, as well as disturbances in biothiols metabolism. Therefore, in PD pa-tients treated with L-dopa, monitoring of oxidative stress markers (8-oxo-2’-deoxyguanosine, apoptotic proteins) and in-flammatory factors (high-sensitivity C-reactive protein, soluble intracellular adhesion molecule), as well as biothiol com-pounds (homocysteine, cysteine, glutathione) is recommended. Administration of vitamins B6, B12, and folates along with an effective therapy with antioxidants and/or anti-inflammatory drugs at an early stage of PD might contribute to improvement in the quality of the life of patients with PD and to slowing down or stopping the progression of the disease.
Keywords: Oxidative stress, Immune response, Biothiols, L-dopa, PD.
INTRODUCTION
Parkinson’s disease (PD) is considered a paradigmatic movement disorder. Both motor and nonmotor functions are impaired in PD [1, 2].
It is believed that in pathogenesis of PD there are several mechanisms involved, such as: oxidative stress, mitochondrial dysfunction, DNA damage, protein aggregation, neuroinflammation, excitotoxicity, apoptosis and loss of trophic factors. The most probably is that all factors are represented targets for PD therapy.
The strategy of PD motor function treatment is simple and generally based on the enhancement of dopaminergic transmission by means of L-dihydroxyphenylalanine (L-dopa) and dopamine (DA) agonists [3, 4].
L-dopa was discovered in the 1960's by Hornykiewicz and used for the treatment of patients with PD [4]. The literature data indicate that L-dopa therapy of PD patients may induce oxidative stress by different mechanisms [5-7], and increase the levels of inflammatory markers [8], and leads to abnormal biothiols levels [9], and apoptotic [9] or possibly autophagy cell death [10-13]. Moreover, in PD change levels of biochemical parameters may also lead tomicroglial activation [14], DA oxidation [15] and Lewy’s bodies (LB) deposition [16].
In PD the level of homocysteine (Hcy) is increased, which may in these patients augment the risk of this neurodegenerative disease by toxic effects in dopaminergic neurons [17]. High Hcy concentrations in patients with PD may also lead to impaired cognitive and motor skills and the development of depression and progression of the disease [18].
The exact mechanism of development and progression of PD pathology is not clear. It is known, that a complex interplay of multiple environmental and genetic factors has been involved in pathogenesis of PD and it is possible that PD represents rather a syndrome but not a single disorder.
BASIC CLINICAL SYMPTOMS AND DRUG THERAPY IN PARKINSON’S DISEASE
PD is one of the most common neurological diseases in elderly people. The mean age of onset is 55 years of age, and the risk for developing PD increases 5-fold by the age of 70 [19]. There are two forms of PD, sporadic (SPD), which affects 95% of all patients, and familial (FPD), accounting for about 5-10% of all causes. It is believed that SPD has unknown etiology, and FPD is linked to mutations in genes of the PARK locus. Importantly, unlike FPD which is often is characterized by an early age onset, SPD usually starts in the six or seventh decade of life and progresses over a period of 10 to 20 years [20].
The neuropathological diagnosis of PD requires the presence of LB degeneration with more than 50% dopaminergic neuron loss in the substantia nigra (SN) leading to more than 80% deficiency of striatal DA [21]. The pathology of PD, however, is much more widespread, affecting a multitude of brain areas beyond the nigrostriatal DA system, many of which are not primarily involved in motor control, including brain stem nuclei e.g. raphe nucleus, locus coeruleus, and also extends to the peripheral autonomic nervous system [2].
PD is considered a paradigmatic movement disorder defined by the presence of bradykinesia plus at least one additional motor sign out of rest tremor, rigidity, and impaired postural reflexes, all of which are motor dysfunctions [1]. However, there is also impairment of nonmotor functions.
It is therefore not surprising that nonmotor symptoms (NMS) form an integral part of the clinical spectrum of PD. Overall, 98.6% of the patients with PD reported the presence of one or several NMS. NMS occur more frequent with prolonged disease duration, and dementia, hallucinations, depression, urinary incontinence and orthostatic hypotension were found in 50-80% of the patients with disease duration of more than 15 years [2, 22].
In the therapy of PD NMS only three agents were found to treat depressive symptoms: the dopamine agonist pramipexole, and the tricyclics nortriptyline and despiramine, while there was insufficient evidence for efficacy of all selective serotonin reuptake inhibitors tested in PD. Likewise, the cholinesterase inhibitor rivastigmine was the only agent with robust enough evidence for efficacy to treat PD dementia, while evidence for other drugs of this class like donepezil or galantamine, as well as memantine, was insufficient. Other drugs with sufficient evidence for efficacy to treat NMS in PD included clozapine for psychosis, and botulinum toxin and glycopyrrolate to treat sialorrhea, while there was insufficient evidence for efficacy of any intervention to treat such common problems like fatigue, orthostatic hypotension, bladder disturbances, erectile dysfunction, insomnia and excessive daytime sleepiness [2].
The strategy of PD motor function treatment is simple and generally based on the enhancement of DA transmission by means of L-dopa and dopamine agonists [3]. Long-term follow-up of PD therapy improves the parkinsonian symptoms but may lead to fluctuations and dyskinesias, on-off phenomena, and postural instability. Motor fluctuations and dyskinesias are common sequelae of PD that may limit function and quality of life [23].
Future perspectives are disease-modifying therapies which may alter the underlying progression of both motor and nonmotor symptoms.
L-DOPA THERAPY AND OXIDATIVE STRESS IN PARKINSON’S DISEASE
PD was the first neurological disease treated by supplementing a neurotransmitter, DA, the levels of which were reduced as a result of spontaneous degeneration of dopaminergic neurons in SN.
DA is synthesized in dopaminergic neurons from the amino acid tyrosine (Tyr), using the enzyme tyrosine hydroxylase (TH), to L-dopa, followed by use of the enzyme aromatic amino acid decarboxylase (AADC) for metabolism to DA, which is then packaged, released and recovered [24]. However, DA synthesis is altered in PD because 80% and more of DA innervations are reduced in the basal ganglia of these patients. Kostrzewa et al. [25] indicated that the synthesis of DA from exogenous L-dopa is enhanced in patients with PD. However, according to these authors exogenous L-dopa must be administrated in a gram amount per day in order to produce a therapeutic effect in PD. Clinical practice shows that L-dopa is most effective in PD when it is used in combination with a selective inhibitor of peripheral AADC. Under these conditions, a greater amount of peripheral L-dopa can be transported to the brain and transformed into DA in neurons.
There are literature reports indicating that L-dopa may induce oxidative stress (ROS), which affects the development of PD. It is believed that, in the stratum of PD patients, during DA metabolism involving monoamine oxidase (MAO-A), dihydroxyphenylacetic acid (DOPAC) and H2O2 are formed [6]. Moreover, the stratum is reach in iron ions (Fe2+), which can also generate oxidative species, OH∙, via the Fenton reaction. ROS may also be produced by intracellular auto-oxidation of DA and its precursor L-dopa, which generates H2O2 and the more stable DA-quinone [5, 26]. It has been shown that DA-quinone inhibits DA transporter (DAT) function and the TH enzyme, and contributes to the lowering of mitochondrial ATP production [7, 10, 11].
It is known that in PD, toxic species generated by the decrease of mitochondrial complex I function have an important role in the pathogenesis of this disease [27]. Researchers have postulated that depletion of complex I function may lead to production of H2O2 during the metabolism of DA and other oxidants [28]. In PD, oxidants and superoxide radicals are generated by products of oxidative phosphorylation, making mitochondria the main site of ROS generation within the cell. ROS are able to affect mitochondrial DNA (mtDNA), causing modulation in synthesis of electron transport chain (ETC) components and decreased ATP production [29]. However, the presence of an intrinsic sensitivity to complex I defects has been demonstrated in dopaminergic neurons in PD [27, 30].
The hypothesis that oxidative stress is an important mechanism underlying the degeneration of dopaminergic neurons is reinforced by data documenting that high levels of lipid peroxidation, increased oxidation of proteins, and oxidative damage to DNA are observed in postmortem studies of brain tissues of PD patients [31, 32].
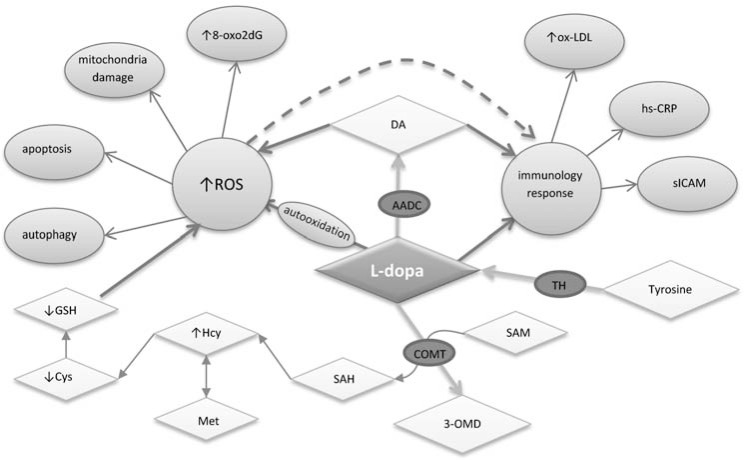
In DNA, this oxidation may affect thymine and guanine, with thymine glycol and 8-oxo-2’-deoxyguanosine (8-oxo2dG) believed to represent markers of oxidative DNA damage (Fig. 1). Oxidative modification of guanine at the C8 position may take place either in nucleic acids or free cellular nucleosides and nucleotides, ready to be incorporated to newly synthesized DNA chains. Incorporation of the modified nucleotide to DNA may result in mutations due to pairing of 8-oxoguanosine with cytosine and adenosine. In the course of pairing with adenosine, 8-oxoguanosine induces GC→AT transversions [33]. 8-Oxoguanine or its nucleoside, 8-oxo2dG, are thus thought to represent markers of oxidative DNA damage in neurodegenerative diseases e.g. PD [9].
Fig. (1).
Contribution of L-dopa in generation of oxidative stress and immunological response in PD. 3-OMD, 3-O-methyldopa; 8-oxo2dG, 8-oxo-2`-deoxyguanosine; AADC, aromatic amino acid decarboxylase; COMT, catechol-O-methyl transferase; Cys, cysteine; DA, dopamine; GSH, glutathione; Hcy, homocysteine; hs-CRP, high-sensitivity C-reactive protein; L-dopa, Ldihydroxyphenylalanine; Met, methionine; ox-LDL, oxidized low density lipoproteins; ROS, reactive oxygen species; SAH, Sadenosylhomocysteine; SAM, S-adenosylmethionine; sICAM, soluble intracellular adhesion molecule; TH, tyrosine hydroxylase. (↑) An increase and (↓) a decrease of biochemical parameters levels.
The study by Dorszewska and Kozubski [9] has shown that L-dopa can modify the level of oxidative DNA damage (8-oxo2dG) in the peripheral blood cells of PD patients. On the other hand, it is interesting that in PD a significant increase of DNA damage has been observed in the 4th stage of disease development (according to Hoehn and Yahr scale), even though 8-oxo2dG levels are increased between the stages 1 and 3 of the disease evolution. As shown by the literature [9, 34], the elevated level of oxidized guanine in DNA (8-oxo2dG) in PD also reflects pharmacotherapy with L-dopa preparations. The study by Dorszewska and Kozubski [9] has shown that the levels of 8-oxo2dG in the patients treated with L-dopa preparations reflect the duration of administration of the drug. In this study, patients were most exposed to oxidative stress, resulting from L-dopa administration, during the first 5 years of treatment with the preparation and following its long-term (over 10 years) administration. According to Spencer et al. [34], the augmented oxidative stress in patients treated with L-dopa might have resulted from lowered levels of antioxidants (glutathione, GSH), disturbed mitochondrial transport, and from excessive oxidation of DA.
It seems that the reason for increased levels of oxidative processes altering nucleic acids in PD is thought to involve overproduction of ROS as well as decreased levels of enzymatic and non-enzymatic antioxidants, and less effective repair mechanisms. For example, AD patients have been found to have decreased activity of the specific 8-oxoguanine glycosylase 1, OGG1 [35, 36].
In the patients suffering from PD, DA level-controlled deposition of ubiquitin- and α-synuclein-positive inclusion bodies, LB, is found in the cytoplasm of dopaminergic neurons [37]. Deposition of pathological proteins in the brains of patients affected by neurodegenerative diseases results in pronounced neurotoxic effects on the central nervous system (CNS). Moreover, in PD, inclusions (LB with α-synuclein) are now known to be comprised of filamentous polymers of α-synuclein, which may generate oxidative stress in the brains of PD patients [38, 39]. This phenomenon could result from several mechanisms, such as depletion of antioxidants, defects in mitochondrial electron transport, neurotoxin exposure, and excessive oxidation of DA in the patients treated with L-dopa. Conway et al. [40] have shown that DA or L-dopa inhibits the fibrillization of α-synuclein filaments by stabilization of their structure.
However, Alves Da Costa et al. [41] have shown that α-synuclein drastically lowered caspase-3 activity and p53 protein expression and transcriptional activity, which are proteins controlling the apoptotic cascade. Blandini et al. [42] and Dorszewska and Kozubski [9] have shown that apoptotic proteins, such as Bcl-2 family proteins and PARP, are involved in the pathogenesis of PD as well.
The study by Dorszewska and Kozubski [9] demonstrated that PD patients treated with L-dopa had significantly increased level of PARP protein and significantly decreased levels of Bax and Bcl-2 proteins. The 85-kDa fragment was significantly elevated compared to PD patients not treated with L-dopa. However, this study suggests that long-term (more than 5 years) therapy of L-dopa in PD patients probably leads to apoptosis of peripheral cells, because elevated levels of Bax:Bcl-2 ratio and 85-kDa fragment were found in patients compared to the controls. It seems that pharmacological treatment of PD patients with L-dopa has a major role in modulating the levels of some apoptotic proteins in lymphocytes, which are important for this process. The study by Hirsch et al. [49] has indicated that dopaminergic neurons are degenerated by apoptosis in PD. Another process probably leading to cell death in PD is autophagy [12, 13].
Autophagy is a process in which cytoplasmic proteins and organelles accumulate in autophagy vacuoles and then are transported into lysosomes [44]. Munoz et al. [13] indicated that in PD there is a relationship between the oxidation of DA to aminochrome, the precursor of neuromelanin, and that there is autophagy dysfunction in dopaminergic neurons containing neuromelanin. Moreover, it has been shown that in PD aminochrome induces the formation of α-synuclein protofibrils that inactivate chaperone-mediated autophagy and adducts with α- and β-tubulin, which induce the aggregation of the microtubules required for the fusion of autophagy vacuoles and lysosomes. All molecules may be altered by oxidative modifications in the development of this disease.
Studies investigating the effects of oxidative stress on the nerve cells in PD are contradictory. Generally, it has been shown that the auto-oxidation of DA and L-dopa leads to neuronal damage in culture [45]. The work of Floor and Wetzel [31] in SN of PD patients treated with L-dopa has shown a 2-fold higher corbonyl modification level compared to the basal ganglia and prefrontal cortex of healthy subjects. On the other hand, it has been indicated that L-dopa administration in vivo is not toxic to neurons [46]. Moreover, it has been also shown that both DA and L-dopa at sub-toxic concentrations can have neuroprotective and neurotrophic effects [47].
L-DOPA THERAPY AND INFLAMMATORY PROCESS IN PARKINSON’S DISEASE
Neuroinflammation and oxidative damage are implicated in the pathogenesis of neurodegenerative diseases. Moreover, in PD degradation of dopaminergic neurons is associated with microglial activation via an unknown mechanism [14, 48]. Activation of microglia in PD by oxidative stress from DA metabolism may confirm e.g. the presence of antibodies against proteins modified by DA oxidation products [15] and complement proteins associated with filamentous inclusions (LB) [16]. Both molecules are modified by oxidative stress, and melanin released from dying dopaminergic neurons is able to activate microglia, which may lead to increased dopaminergic toxicity through the production of cytokines and ROS [49]. It seems that neuroinflammatory processes in the PD brain most likely increase secondary to a primary insult and may participate in the neurodegenerative process, and might be associated with the applied therapy [48].
The study by Andican et al. [8] has shown that, in PD patients treated with L-dopa, increased plasma levels of both oxidized-low density lipoproteins (ox-LDL) and soluble intracellular adhesion molecule (sICAM) may be found, as compared to controls (Fig. 1). In this study, the duration of L-dopa treatment of PD patients had no effect on ox-LDL level. In PD patients, it seems that sustained stress during treatment with L-dopa may be the cause of an elevated risk of atherosclerotic events, such as acute coronary syndromes [50]. However, Andican et al. [8] have shown that PD patients in stage 2 demonstrated higher levels of both high-sensitivity C-reactive protein (hs-CRP) and sICAM than patients at all other stages. The authors suggest that L-dopa treatment may be effective in the inflammatory response in PD at later stages.
It also appears that, in PD patients treated with L-dopa, the ox-LDL, hs-CRP and sICAM are biomarkers for systemic inflammation and oxidative damage. However, anti-inflammatory treatment for PD patients might have beneficial effects in the progression if this disease (Fig. 1) [8].
Establishing the roles of these pathological processes in PD might be the key to early stage effective therapy with antioxidants and/or anti-inflammatory drugs.
BIOTHIOLS AND L-DOPA THERAPY IN PARKINSON’S DISEASE
Elevated Hcy level is a risk factor for vascular diseases, cognitive impairment and dementia, as well as neurodegenerative diseases (e.g. PD). It is also known that vascular dementia and cognitive impairment worsen the prognosis of PD patients, and it is important to minimize the risk of their occurrence as much as possible. Gorell et al. [51] have indicated that patients with PD have shown an increased risk for cardiovascular disease and stroke.
It is believed that high levels of Hcy in PD may increase the risk of this neurodegenerative disease by toxic effects in dopaminergic neurons. In vitro studies conducted on human dopaminergic neurons have shown a significant increase in neurotoxicity associated with high Hcy levels [17]. High Hcy concentrations in patients with PD may also lead to impaired cognitive and motor skills and the development of depression [18].
Reports in the literature [9, 52] indicate that plasma Hcy levels in PD have also been affected by pharmacotherapy with L-dopa. It is demonstrated that PD patients who are initiating L-dopa therapy have elevated Hcy levels within six weeks to a few months after L-dopa initiation [53]. The study of Miller et al. [54] indicates that L-dopa may induce elevated levels of Hcy during its methylation to 3-O-methyldopa (3-OMD) with the involvement of COMT (catechol O-methyltransferase), both in peripheral blood leukocytes and in nigrostriatal neurons. In the course of the reaction, COMT in the presence of magnesium ions induces in parallel the transition of SAM to SAH and further hydrolysis of SAH to Hcy (Fig. 1).
Elevated levels of Hcy in the SN of PD patients have been demonstrated after just 3 months of L-dopa treatment [55]. Long-term administration of L-dopa is thought to promote benign vascular lesions in patients with PD and may result in cognitive disturbances or dementia in these patients, particular at late stages of treatment with the preparation [56].
The study by Dorszewska et al. [57] showed that elevated plasma levels of Hcy in PD possibly might have developed due to altered processes of Hcy remethylation to Met and transsulfuration to Cys. Furthermore, a decreased concentration of Met has been observed in PD patients, paralleled by elevated levels of Cys and a decreased ratio of Met and Cys to Hcy. The demonstrated decrease in Met to Hcy ratio may be linked to transformation of Hcy to thiolactone in endothelial cells. According to one of the more recent hypotheses, sulfonic sulfur of thiol compounds may be involved in the development of Hcy-induced arteriosclerotic lesions [58]. Additionally, the demonstrated increased plasma Cys level in PD may result from intensified release of the amino acid from proteins, due to substitution by the circulating Hcy or due to diminished transformation of Cys to GSH, which is important for maintenance of redox homeostasis in the body. Moreover, GSH is believed to act as a chelator of free metal ions [59], including the previously mentioned Fe2+ generating ROS by Fenton reaction. GSH in the brain is responsible also for detoxication of the DA auto-oxidation product DA-O-quinone by forming a conjunct, 5-S-glutathionyldopamine (5-S-Glu-DA), which undergoes further metabolism end excretion [13, 60]. The protective role of GSH to neurons subjected to excessive concentrations of DA has been shown by Allen et al. [61]. In this study, the increased synthesis of GSH was induced by administration of DA alone, as well as L-dopa, while AADC remained active. However, after inhibition of AADC, the GSH level was not raised by L-dopa.
GSH in the brain is believed to be synthesized more rapidly by glial cells, mostly astrocytes. The study of Makar et al. [62] has shown higher concentrations of total GSH, its synthesizing enzymes, and other potent antioxidants in in vitro cultures of chick astrocytes, as compared to the forebrain chick embryo neurons of similar age. Taking into account that intracellularly synthesized GSH might be released to the tissue fluid and then absorbed by cells possessing the membrane-bound gamma-glutamyl transpeptidase [63], this observation might indicate a protective role of astrocytes as producers of brain GSH that is subsequently absorbed by neurons [64]. The safeguard role of GSH on neurons during treatment with L-dopa might be augmented by administration of exogenous substances playing the role of substrates for GSH production or imitating its in vivo activity. One such molecule is N-acetyl-cysteine, which over past ten years has been investigated as a putative neuroprotective antioxidant agent [65].
Reports in the literature [9, 52] have indicated that plasma Hcy levels in PD have also been affected by pharmacotherapy with L-dopa. The study by Dorszewska and Kozubski [9] has shown that the levels of the sulfuric amino acid were also affected by the duration of pharmacotherapy. The patients that are most exposed to the neurotoxic effects of Hcy have seemed to be those in their first 5 years L-dopa treatment, while its continued administration has resulted in stably elevated Hcy level, suggesting a constant disturbance in the metabolism of Hcy to Met and Cys.
Treatment with L-dopa preparations seems to be a potential risk factor for vascular diseases in PD patients by disturbances to Hcy metabolism [18].
COMBINATION THERAPY IN PARKINSON’S DISEASE
Hinz et al. [66] have indicated that side effects and/or adverse reactions tend to be L-dopa dose dependent and apply to the administration of this medicament only. It seems that including AADC inhibitors (carbidopa), DA agonists, anticholinergics, MAO-B inhibitors and COMT inhibitors to PD therapy may give better results with a potentially augmented action of L-dopa in PD.
COMT has a broad detoxification potential in humans. Two compounds are currently available, entacapone and tolcapone, which are peripheral and central inhibitors of COMT, respectively. COMT inhibition is also under investigation to prevent motor complications and seems to have a beneficial effect on L-dopa-related hyperhomocysteinemia [67, 68]. Some animal studies have shown that COMT inhibition may eliminate L-dopa-induced hyperhomocysteinemia, but not all previous studies confirm this [25].
CONCLUSION
L-dopa treatment in PD is related to lower levels of the neurotransmitter DA in the striatum and absence of DAT on the nerve terminals in the brain. L-dopa may also indirectly stimulate the receptors of the D1 (D1, D5 receptors) and D2 (D2, D3, D4 receptors) families.
Administration of L-dopa in PD patients, especially long-term therapy, may cause side effects in the form of increased toxicity and inflammatory responses, and disturbances in biothiols metabolism. However, therapy with L-dopa leads to an increase in the level of factors induced in oxidative stress (8-oxo2dG) and apoptosis (Bax:Bcl-2, 85-kDa fragment), as well as changes in the concentrations of risk factors of vascular diseases, such as Hcy, Cys, ox-LDL, hs-CRP, sICAM.
Therefore, in PD patients treated with L-dopa, monitoring of the oxidative stress markers (8-oxo2dG, apoptotic proteins) as well as inflammatory factors (hs-CRP, sICAM), and levels of biothiol compounds (Hcy, Cys) is recommended. In patients with PD, administration of vitamins B6, B12 and folates together with effective therapy by antioxidants and/or anti-inflammatory drugs at an early stage of the disease may contribute to an improvement in the quality of the life of patients with PD and to slowing down or stopping the progression of the disease.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(4):861–870. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 2.Hussl A, Seppi K, Poewe W. Nonmotor symptoms in Parkinson's disease. Expert Rev. Neurother. 2013;13(6):581–583. doi: 10.1586/ern.13.53. [DOI] [PubMed] [Google Scholar]
- 3.Hermanowicz N. Drug therapy for Parkinson's disease. Semin. Neurol. 2007;27(2):97–105. doi: 10.1055/s-2007-971177. [DOI] [PubMed] [Google Scholar]
- 4.Ehringer H, Hornykiewicz O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin. Wochenschr. 1960;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- 5.Sulzer D, Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox. Res. 2000;1(3):181–195. doi: 10.1007/BF03033289. [DOI] [PubMed] [Google Scholar]
- 6.Gesi M, Santinami A, Ruffoli R, Conti G, Fornai F. Novel aspects of dopamine oxidative metabolism (confounding outcomes take place of certainties). Pharmacol. Toxicol. 2001;89(5):217–224. doi: 10.1034/j.1600-0773.2001.d01-151.x. [DOI] [PubMed] [Google Scholar]
- 7.Khan FH, Saha M, Chakrabarti S. Dopamine induced protein damage in mitochondrial-synaptosomal fraction of rat brain. Brain Res. 2001;895(1-2):245–249. doi: 10.1016/s0006-8993(00)03284-4. [DOI] [PubMed] [Google Scholar]
- 8.Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiina S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson's disease. Acta Neurol. Belg. 2012;112(2):155–159. doi: 10.1007/s13760-012-0015-3. [DOI] [PubMed] [Google Scholar]
- 9.Dorszewska J, Kozubski W. Oxidative DNA damage and the level of biothiols, and L-dopa therapy in Parkinson’s Disease. In: Rana A.Q., editor. Etiology and Pathophysiology of Parkinson's Diease In Tech. 2011. pp. 349–372. [Google Scholar]
- 10.Berman SB, Hastings TG. Dopamine oxidation alters mitochondrial respiration and induces permeability transition in brain mitochondria: implications for Parkinson's disease. J. Neurochem. 1999;73(3):1127–1137. doi: 10.1046/j.1471-4159.1999.0731127.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn DM, Arthur RE, Thomas DM, Elferink LA. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: possible relevance to Parkinson's disease. J. Neurochem. 1999;73(3):1309–1317. doi: 10.1046/j.1471-4159.1999.0731309.x. [DOI] [PubMed] [Google Scholar]
- 12.Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. The role of autophagy in Parkinson's disease. Cold Spring Harb. Perspect Med. 2012;2(4):a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinsons Dis. 2012:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 15.Rowe DB. Antibodies from patients with Parkinson's disease react with protein modified by dopamine oxidation. J. Neurosci. Res. 1998;53(5):551–558. doi: 10.1002/(SICI)1097-4547(19980901)53:5<551::AID-JNR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, McGeer PL, McGeer EG. Lewy bodies in Parkinson's disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84(1):100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- 17.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson's disease. J. Neurochem. 2002;80(1):101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn W, Roebroek R, Blom H, van Oppenraaij D, Przuntek H, Kretschmer A, Büttner T, Woitalla D, Müller T. Elevated plasma levels of homocysteine in Parkinson's disease. Eur. Neurol. 1998;40(4):225–227. doi: 10.1159/000007984. [DOI] [PubMed] [Google Scholar]
- 19.Hornykiewicz , Kish SJ. Biochemical pathophysiology of Parkinson's disease. Adv. Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- 20.Martinez M, Brice A, Vaughan JR, Zimprich A, Breteler MM, Meco G, Filla A, Farrer MJ, Bétard C, Hardy J, De Michele G, Bonifati V, Oostra B, Gasser T, Wood NW, Dürr A. French Parkinson's Disease Genetics Study Group; European Consortium on Genetic Susceptibility in Parkinson's Disease.Genome-wide scan linkage analysis for Parkinson's disease: the European genetic study of Parkinson's disease. J. Med. Genet. 2004;41(12):900–907. doi: 10.1136/jmg.2004.022632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 2002;175(2):303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 22.Armañanzas R, Bielza C, Chaudhuri KR, Martinez-Martin P, Larrañaga P. Unveiling relevant non-motor Parkinson's disease severity symptoms using a machine learning approach. Artif. Intell. Med. 2013;58(3):195–202. doi: 10.1016/j.artmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Wright BA, Waters CH. Continuous dopaminergic delivery to minimize motor complications in Parkinson's disease. Expert Rev. Neurother. 2013;13(6):719–729. doi: 10.1586/ern.13.47. [DOI] [PubMed] [Google Scholar]
- 24.Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J. Biol. Chem. 1962;237:89–93. [PubMed] [Google Scholar]
- 25.Kostrzewa RM, Nowak P, Kostrzewa JP, Kostrzewa RA, Brus R. Peculiarities of L: -DOPA treatment of Parkinson's disease. Amino Acids. 2005;28(2):157–164. doi: 10.1007/s00726-005-0162-4. [DOI] [PubMed] [Google Scholar]
- 26.Pattison DI, Dean RT, Davies MJ. Oxidation of DNA, proteins and lipids by DOPA, protein-bound DOPA, and related catechol(amine)s. Toxicology. 2002;177(1):23–37. doi: 10.1016/s0300-483x(02)00193-2. [DOI] [PubMed] [Google Scholar]
- 27.Sherer TB, Betarbet R, Greenamyre JT. Environment, mitochondria, and Parkinson's disease. Neuroscientist. 2002;8(3):192–197. doi: 10.1177/1073858402008003004. [DOI] [PubMed] [Google Scholar]
- 28.Gluck M, Ehrhart J, Jayatilleke E, Zeevalk GD. Inhibition of brain mitochondrial respiration by dopamine: involvement of H(2)O(2) and hydroxyl radicals but not glutathione-protein-mixed disulfides. J. Neurochem. 2002;82(1):66–74. doi: 10.1046/j.1471-4159.2002.00938.x. [DOI] [PubMed] [Google Scholar]
- 29.Reale M, Pesce M, Priyadarshini M, Kamal MA, Patruno A. Mitochondria as an easy target to oxidative stress events in Parkinson's disease. CNS Neurol. Disord. Drug Targets. 2012;11(4):430–438. doi: 10.2174/187152712800792875. [DOI] [PubMed] [Google Scholar]
- 30.Subramaniam SR, Chesselet MF. Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Prog. Neurobiol. 2013;106:17–32. doi: 10.1016/j.pneurobio.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998;70(1):268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi A, Takeda A, Onodera H, Kimpara T, Hisanaga K, Sato N, Nunomura A, Castellani RJ, Perry G, Smith MA, Itoyama Y. Systemic increase of oxidative nucleic acid damage in Parkinson's disease and multiple system atrophy. Neurobiol. Dis. 2002;9(2):244–248. doi: 10.1006/nbdi.2002.0466. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T. Repair system of 7, 8-dihydro-8-oxoguanine as a defense line against carcinogenesis. J. Radiat. Res. 2008;49(4):329–340. doi: 10.1269/jrr.08049. [DOI] [PubMed] [Google Scholar]
- 34.Spencer JP, Jenner P, Halliwell B. Superoxide-dependent depletion of reduced glutathione by L-DOPA and dopamine.Relevance to Parkinson's disease. Neuroreport. 1995;6(11):1480–1484. doi: 10.1097/00001756-199507310-00004. [DOI] [PubMed] [Google Scholar]
- 35.Dorszewska J, Florczak J, Rozycka A, Jaroszewska-Kolecka J, Trzeciak WH, Kozubski W. Polymorphisms of the CHRNA4 gene encoding the alpha4 subunit of nicotinic acetylcholine receptor as related to the oxidative DNA damage and the level of apoptotic proteins in lymphocytes of the patients with Alzheimer's disease. DNA Cell. Biol. 2005;24(12):786–794. doi: 10.1089/dna.2005.24.786. [DOI] [PubMed] [Google Scholar]
- 36.Dorszewska J, Kempisty B, Jaroszewska-Kolecka J, Rozycka A, Florczak J, Lianeri M, Jagodzinski PP, Kozubski W. Expression and polymorphisms of gene 8-oxoguanine glycosylase 1 and the level of oxidative DNA damage in peripheral blood lymphocytes of patients with Alzheimer's disease. DNA Cell. Biol. 2009;28(11):579–588. doi: 10.1089/dna.2009.0926. [DOI] [PubMed] [Google Scholar]
- 37.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 38.Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000;157(2):401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou JX, Zhang HB, Huang Y, He Y, Zheng Y, Anderson JP, Gai WP, Liang ZG, Wang Y, Ren XM, Wang Q, Gong XL, Yang J, Wang X, Halliday G, Wang XM. Tenuigenin Attenuates a-Synuclein-Induced Cytotoxicity by Down-Regulating Polo-Like Kinase 3. CNS Neurosci. Ther. 2013;19(9):688–94. doi: 10.1111/cns.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conway KA, Rochet JC, Bieganski RM, Lansbury PT. Kinetic stabilization of the alpha-synuclein protofibril by a dopamine-alpha-synuclein adduct. Science. 2001;294(5545):1346–1349. doi: 10.1126/science.1063522. [DOI] [PubMed] [Google Scholar]
- 41.Alves Da Costa C, Paitel E, Vincent B, Checler F. Alpha-synuclein lowers p53-dependent apoptotic response of neuronal cells.Abolishment by 6-hydroxydopamine and implication for Parkinson's disease. J. Biol. Chem. 2002;277(52):50980–50984. doi: 10.1074/jbc.M207825200. [DOI] [PubMed] [Google Scholar]
- 42.Blandini F, Cosentino M, Mangiagalli A, Marino F, Samuele A, Rasini E, Fancellu R, Tassorelli C, Pacchetti C, Martignoni E, Riboldazzi G, Calandrella D, Lecchini S, Frigo G, Nappi G. Modifications of apoptosis-related protein levels in lymphocytes of patients with Parkinson's disease.The effect of dopaminergic treatment. J. Neural. Transm. 2004;111(8):1017–1030. doi: 10.1007/s00702-004-0123-1. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch EC, Hunot S, Faucheux B, Agid Y, Mizuno Y, Mochizuki H, Tatton WG, Tatton N, Olanow WC. Dopaminergic neurons degenerate by apoptosis in Parkinson's disease. Mov. Disord. 1999;14(2):383–385. doi: 10.1002/1531-8257(199903)14:2<383::aid-mds1037>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 44.Matsui Y, Kyoi S, Takagi H, Hsu CP, Hariharan N, Ago T, Vatner SF, Sadoshima J. Molecular mechanisms and physiological significance of autophagy during myocardial ischemia and reperfusion. Autophagy. 2008;4(4):409–415. doi: 10.4161/auto.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clement MV, Long LH, Ramalingam J, Halliwell B. The cytotoxicity of dopamine may be an artefact of cell culture. J. Neurochem. 2002;81(3):414–421. doi: 10.1046/j.1471-4159.2002.00802.x. [DOI] [PubMed] [Google Scholar]
- 46.Mytilineou C, Walker RH, JnoBaptiste R, Olanow CW. Levodopa is toxic to dopamine neurons in an in vitro but not an in vivo model of oxidative stress. J. Pharmacol. Exp. Ther. 2003;304(2):792–800. doi: 10.1124/jpet.102.042267. [DOI] [PubMed] [Google Scholar]
- 47.Jia Z, Zhu H, Misra BR, Li Y, Misra HP. Dopamine as a potent inducer of cellular glutathione and NAD(P)H:quinone oxidoreductase 1 in PC12 neuronal cells: a potential adaptive mechanism for dopaminergic neuroprotection. Neurochem. Res. 2008;33(11):2197–2205. doi: 10.1007/s11064-008-9670-4. [DOI] [PubMed] [Google Scholar]
- 48.Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann. Neurol. 2003;53(Suppl 3 ):49–58. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- 49.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson's disease: is there a causal link? Exp. Neurol. 2005;193(2):279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Quadri R, Comino I, Scarzella L, Cacioli P, Zanone MM, Pipieri A, Bergamasco B, Chiandussi L. Autonomic nervous function in de novo parkinsonian patients in basal condition and after acute levodopa administration. Funct. Neurol. 2000;15(2):81–86. [PubMed] [Google Scholar]
- 51.Gorell JM, Johnson CC, Rybicki BA. Parkinson's disease and its comorbid disorders: an analysis of Michigan mortality data, 1970 to 1990. Neurology. 1994;44(10):1865–1868. doi: 10.1212/wnl.44.10.1865. [DOI] [PubMed] [Google Scholar]
- 52.Miller JW, Selhub J, Nadeau MR, Thomas CA, Feldman RG, Wolf PA. Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology. 2003;60(7):1125–1129. doi: 10.1212/01.wnl.0000055899.24594.8e. [DOI] [PubMed] [Google Scholar]
- 53.O'Suilleabhain PE, Bottiglieri T, Dewey RB, Sharma S, Diaz-Arrastia R. Modest increase in plasma homocysteine follows levodopa initiation in Parkinson's disease. Mov. Disord. 2004;19(12):1403–1408. doi: 10.1002/mds.20253. [DOI] [PubMed] [Google Scholar]
- 54.Miller JW, Shukitt-Hale B, Villalobos-Molina R, Nadeau MR, Selhub J, Joseph JA. Effect of L-Dopa and the catechol-O-methyltransferase inhibitor Ro 41-0960 on sulfur amino acid metabolites in rats. Clin. Neuropharmacol. 1997;20(1):55–66. doi: 10.1097/00002826-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Yasui K, Nakaso K, Kowa H, Takeshima T, Nakashima K. Levodopa-induced hyperhomocysteinaemia in Parkinson's disease. Acta Neurol. Scand. 2003;108(1):66–67. doi: 10.1034/j.1600-0404.2003.00135.x. [DOI] [PubMed] [Google Scholar]
- 56.Müller T, Werne B, Fowler B, Kuhn W. Nigral endothelial dysfunction, homocysteine, and Parkinson's disease. Lancet. 1999;354(9173):126–127. doi: 10.1016/s0140-6736(99)01660-8. [DOI] [PubMed] [Google Scholar]
- 57.Dorszewska J, Florczak J, Rozycka A, Kempisty B, Jaroszewska-Kolecka J, Chojnacka K, Trzeciak WH, Ko-zubski W. Oxidative DNA damage and level of thiols as related to polymorphisms of MTHFR, MTR, MTHFD1 in Alzheimer's and Parkinson's diseases. Acta Neurobiol. Exp. (Wars). 2007;67(2):113–129. doi: 10.55782/ane-2007-1639. [DOI] [PubMed] [Google Scholar]
- 58.Borowczyk K, Tisonczyk J, Jakubowski H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: protective role of bleomycin hydrolase. Amino Acids. 2012;43(3):1339–1348. doi: 10.1007/s00726-011-1207-5. [DOI] [PubMed] [Google Scholar]
- 59.Ballatori N. Glutathione mercaptides as transport forms of metals. Adv. Pharmacol. 1994;27:271–298. doi: 10.1016/s1054-3589(08)61036-4. [DOI] [PubMed] [Google Scholar]
- 60.Shen XM, Xia B, Wrona MZ, Dryhurst G. Synthesis, redox properties, in vivo formation, and neurobehavioral effects of N-acetylcysteinyl conjugates of dopamine: possible metabolites of relevance to Parkinson's disease. Chem. Res. Toxicol. 1996;9(7):1117–1126. doi: 10.1021/tx960052v. [DOI] [PubMed] [Google Scholar]
- 61.Allen GF, Ullah Y, Hargreaves IP, Land JM, Heales SJ. Dopamine but not l-dopa stimulates neural glutathione metabolism.Potential implications for Parkinson's and other dopamine deficiency states. Neurochem. Int. 2013;62(5):684–694. doi: 10.1016/j.neuint.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 62.Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ. Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J. Neurochem. 1994;62(1):45–53. doi: 10.1046/j.1471-4159.1994.62010045.x. [DOI] [PubMed] [Google Scholar]
- 63.Griffith OW, Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. U S A. 1979;76(11):5606–5610. doi: 10.1073/pnas.76.11.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gegg ME, Clark JB, Heales SJ. Co-culture of neurones with glutathione deficient astrocytes leads to increased neuronal susceptibility to nitric oxide and increased glutamate-cysteine ligase activity. Brain Res. 2005;1036 (1-2):1–6. doi: 10.1016/j.brainres.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 65.Martínez-Banaclocha MA. N-acetyl-cysteine in the treatment of Parkinson's disease. What are we waiting for? Med. Hypotheses. 2012;79(1):8–12. doi: 10.1016/j.mehy.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Hinz M, Stein A, Uncini T. Amino acid management of Parkinson's disease: a case study. Int. J. Gen. Med. 2011;4:165–174. doi: 10.2147/IJGM.S16621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Müller T. Possible treatment concepts for the levodopa-related hyperhomocysteinemia. Cardiovasc. Psychiatry Neurol. 2009;9:1–5. doi: 10.1155/2009/969752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nevrly M, Kanovsky P, Vranova H, Langova K, Hlustik P. Effect of entacapone on plasma homocysteine levels in Parkinson's disease patients. Neurol. Sci. 2010;31(5):565–569. doi: 10.1007/s10072-010-0262-0. [DOI] [PubMed] [Google Scholar]