Abstract
Systemic Lupus Erythematosus (SLE) is one of the most relevant world-wide autoimmune disorders. The formation of autoantibodies and the deposition of antibody-containing immune complexes in blood vessels throughout the body is the main pathogenic mechanism of SLE leading to heterogeneous clinical manifestations and target tissue damage. The complexity of etiology and pathogenesis in SLE, enclosing genetic and environmental factors, apparently is one of the greatest challenges for both researchers and clinicians. Strong indications for a genetic background in SLE come from studies in families as well as in monozygotic and dizygotic twins, discovering several SLE-associated loci and genes (e.g. IRF5, PTPN22, CTLA4, STAT4 and BANK1). As SLE has a complex genetic background, none of these genes is likely to be entirely responsible for triggering autoimmune response in SLE even if they disclosure a potentially novel molecular mechanisms in the pathogenesis' disease. The clinical manifestations and disease severity varies greatly among patients, thus several studies try to associate clinical heterogeneity and prognosis with specific genetic polymorphisms in SLE associated genes. The continue effort to describe new predisposing or modulating genes in SLE is justified by the limited knowledge about the pathogenesis, assorted clinical manifestation and the possible prevention strategies. In this review we describe newly discovered, as well as the most studied genes associated to SLE susceptibility, and relate them to clinical manifestations of the disease.
Keywords: Autoimmunity, B cells, Clinical manifestations, DSBs, IFN, SLE, SNPs, T cells.
INTRODUCTION
Systemic Lupus Erythematosus (SLE) is a heterogeneous clinical featured disorder characterized by B and T cell hyperactivity, formation of autoantibodies and the deposition of antibody-containing immune complexes in blood vessels throughout the body [1]. In addition SLE diverse presentations range from malar rash and arthritis through anemia and thrombocytopenia to serositis, nephritis, seizures and psychosis [2, 3]. The heterogeneous clinical manifestations in SLE patients led to the establishment of 11 criteria of diagnosis by the American College of Rheumatology (ACR), in which the simultaneously presence of at least four criteria are able to confirm the SLE diagnosis [4]. The pathogenesis of SLE is mainly due to the deficiency of several immunological mechanisms, including inappropriate function of the innate immune system, altered self-tolerance mechanisms and apoptotic cell clearance [3]. Even though the pathogenesis of SLE remains not fully understood, it is known to depend upon the interaction of genetic, environmental and hormonal factors. Indeed, SLE is a complex multisystem autoimmune disorder with a strong sex bias, affecting substantially morewomen (the female-to-male ratio is 15:1) of child-bearing age than any other age group [5].
Nevertheless, the genetic component has a pivotal role in SLE etiology with several genes contributing in disease´s triggering. The majority of genetic variations, including single nucleotide polymorphisms (SNPs), associated with SLE are within immune response–related genes and HLA (Human leukocyte antigen) gene variants, with the last one being the most well-known genetic risk factor not only for SLE but for autoimmune diseases in general [6, 7]. The SNPs associated to SLE can be clustered according to the gene function and their influence on disease´s susceptibility. The Genome Wide Association Studies (GWAS) have been providing an increase in the number of newly associated genes to SLE outside the HLA range and the genetic association studies are powerful tools in confirming these associations, including analysis with disease´s clinical features as depicted in Table 1. In this review we describe SLE old and new susceptibility genes as well as the associations to the disease´s heterogeneous clinical manifestations.
Table 1.
Susceptibility Genes Associated to SLE Clinical Manifestations
| Clinical Manifestations and Susceptibility Genes | |||
|---|---|---|---|
| SLE Features | Definition | Gene | Referencies |
| Skin Lesions | A rash on the face (cheeks and nose), often in a butterfly shape | IL-10, RASGRP3, VDR, CTLA-4, XRCC1, STK17A, TYK2. | [24, 78, 100, 105, 111, 114, 119, 146]. |
| Photosensitivity | Reaction to sunlight leading to a new rash or worsen a present one | XRCC1, TYK2. | [111, 114, 146]. |
| Oral ulcers | Ulcers inside the mouth or nasopharyngeal ulcers. | STAT4. | [159]. |
| Arthritis | Joint pain and swelling of two or more joints | TNFSF4, VDR, STK17A. | [69, 74, 100, 119]. |
| Serositis | Pleuritis or pericarditis | RASGRP3. | [78]. |
| Renal Disorders | Persistent protein or cellular casts in the urine | PDCD1, PTPN22, TNFSF4, VDR, CTLA-4, IRF5,TLRs, STAT4. | [33, 34, 45, 49, 73, 74, 96, 99, 105, 142, 153, 154, 159]. |
| Neurologic disorder | Seizures or psychosis | IL-10, XRCC1, XRCC3, XRCC4. | [21, 25, 107]. |
| Hematological Disorders | Anemia, leukopenia, lymphopenia or thrombocytopenia | FYB. | [61]. |
| Immunologic disorder | Positivity for anti–dsDNA, anti-Sm, or antiphospholipid antibodies | IL-10, PRL, FYB, TNFSF4, RASGRP3, BANK1, VDR, XRCC1, XRCC3 and XRCC4, STK17A, IRF5, STAT4. | [21, 26, 27, 39, 61, 65, 78, 84, 88, 100, 107, 119, 139, 159]. |
| Disease´s Severity and activity | Active phase modulation with symptoms worsening | IFIH1, VDR, IRF5 and STAT4. | [67, 100, 138, 159]. |
THE ROLE B AND T CELLS FUNCTION RELATED GENES IN SLE
The involvement of several genes in SLE etiology has been widely examined and many of those genes that encode relevant proteins for the function of T and B cells have been considered as candidates for susceptibility to SLE and its clinical manifestations. Certain variants in these genes have been identified and may contribute to abnormal lymphocytes function and, as consequence, in autoantibody production and immune complex deposition, being one of the key points in the pathogenesis of SLE.
IL-10
IL-10 (Interleukin-10) is an important immunoregulatory cytokine produced by almost all leukocytes, especially macrophages, dendritic cells (DCS) and T helper (Th) cells [8, 9]. This cytokine inhibits T cell function by suppressing the expression of proinflammatory cytokines such as TNFα, IL-1, IL-6, IL-8, and IL-12 [10, 11] and enhances survival, proliferation, differentiation and antibody production of B cells [12].
The IL-10 gene is located at chromosome 1 (1q31-q32) and a number of genetic polymorphisms at its promoter region have been reported: the microsatellites IL10.G and IL10.R, a CA dinucleotide repeats at position -4000 and -1100 of IL-10 gene, respectively [13, 14] and three single nucleotide polymorphisms (SNPs) located at −1082 (G/A), −819 (C/T) and −592 (C/A) positions. Since IL-10 biosynthesis has been demonstrated to be regulated at transcriptional level, polymorphic forms of its promoter gene may modify binding transcription factors and affect its serum levels [15-17].
Different alleles and haplotypes of these polymorphisms have been reported to be associated with SLE susceptibility both in Caucasian [18-20] as well as in Asiatic [21-23] populations and several studies also revealed the association of these polymorphisms with various clinical manifestations in SLE patients: discoid lesions [24], neurological manifestations development [21, 25], and anti-DNA [26], anti-Smith [27] and anti-cardiolipin antibodies production [21]. We can hypothesize that an abnormal production of IL-10 may cause exacerbated autoantibodies release and inflammatory processes establishment, which may favor SLE and its manifestations incidence.
PDCD1
PDCD1 (Programmed cell death 1), expressed by T and B lymphocytes, is a member of the CD28 family of receptors, which have important function in modulating lymphocyte activation. This receptor has an immunoinhibitory domain in its intracellular tail forming an immunoreceptor tyrosine–based inhibition motif (ITIMs). During cellular activation, tyrosine residues of the ITIMs are phosphorylated by the Src homology 2 protein tyrosine phosphatase 2, leading to deactivation of downstream signaling molecules. Consequently, when PDCD1 binds to its ligands, PD-L1 and PD-L2, which are expressed in many tissues, it attenuates T and B cell responses [28, 29].
PDCD1 is localized at chromosome 2 (2q37.3). Since this gene has an immunoregulatory function, genetic studies have been performed to verify the association between PDCD1 gene polymorphisms and SLE. Several polymorphisms in intron 4 of PDCD1 gene, such as SNPs G>A at 7146 position and C>T at 7209 position, have been reported as associated to SLE: the SNP G>A (7146), also called PD1.3, has been described as contributing to SLE susceptibility in Mexicans [30] and in different populations from Europe [30-32] and also associated to renal manifestations in SLE patients from Sweden [33, 34]. The SNP C>T (7209) demonstrated to be associated to SLE occurrences in populations from Taiwan [35] and Poland [36]. These polymorphisms affect the binding affinity and activity of the transcription factors NFkB and RUNX1 with impact on gene transcription [30]. Therefore, these associations may be due to lower binding affinity of NFkB and RUNX1 and, consequently, decreased expression of PD-1, contributing to deregulated self-tolerance and lymphocyte hyperactivity characteristic of SLE.
PRL
Sex hormones present a key role in regulating the immune response and are often associated to the sex bias in SLE patients. The prolactin (PRL) gene is located at chromosome 6 (6p22.2- p21.3) and encodes the prolactin hormone synthesized mainly by the anterior pituitary gland and by extrapituitary tissues, particularly immune cells, such as T lymphocytes. The PRL gene acts through innate and adaptive immune system by regulating the differentiation of CD4- CD8- thymocytes to CD4+ or CD8+ T cells and its levels are correlated to B and CD4+ T lymphocytes production [37].
A biallelic polymorphism (−1149 G/T) in the promoter gene demonstrated to be responsible to modulate prolactin expression and associated with SLE, including its clinical features. Stevens et al., 2001, showed that G allele consistently causes higher promoter activity, inducing an increment of prolactin mRNA from patients with the G/G genotype in relation to T/T genotype [38]. The G allele of this SNP was also associated with the presence of anti–dsDNA antibodies in serum of Mexican SLE patients [39].
PTPN22
The PTPN22 (Protein tyrosine phosphatase non-receptor type 22) gene, located at chromosome 1 (1p13), encodes a lymphoid-specific tyrosine phosphatase known as Lyp, which is a negative regulator in T cell signaling through direct dephosphorylation of Lck, Fyn and ZAP70 kinases. Lyp also interacts with the tyrosine kinase CSK by binding its first C-terminal poly-proline (P1) region with SH3 domain of CSK [40-43]. A mutation (1858C>T) in the P1 region, which causes an amino acid change from arginine to tryptophan at position 620 (R620W), disrupts this physiological interaction and results in a gain of function that inhibits T cell receptor signaling [44]. Associations between this polymorphism and SLE susceptibility have been reported in different populations from Europe such as Sweden [45], Spain [46], Crete [47] and Poland [48] and also in populations from Egypt [49], North America [50] and Colombia [51]. Furthermore, it was observed the association of the mutation R620W with renal manifestations occurrence in SLE patients from Sweden [45] and Egypt [49]. Another variant (R263Q), located within the catalytic domain of the tyrosine phosphatase and identified as a loss-of-function mutation, was found to reduce the risk of SLE in a multiethnic cohort from Spain, Italy, Argentina and North America [52]. Individuals carrying the variant alleles of PTPN22 are thought to have changes in T cell signaling, affecting disease predisposition by multiple mechanisms, including altered thymic selection, T-helper activity and number/function of regulatory T-cells [53]. A gain of function of PTPN22 could increase the threshold required for effective thymic selection, resulting in the permanence of autoreactive cells during the autotolerance process of immune system, allowing its participation in autoantibodies release. It could increase the formation and deposition of immune complexes, which will trigger an inflammatory response resulting in possible development of SLE and its clinical manifestations. Another mechanism involving PTPN22 gain of function with autoimmunity relates to regulatory T cells function, one of the main mechanisms to prevent autoimmune processes [54]. These cells activity depends on IL2 and a reduced production of this cytokine by peripheral T cells was observed in individuals carrying the variant R620W [55].
Although a regulatory role for Lyp in T-cell activation has been established, the effect of the R620W variant on T lymphocyte responses remains unclear, since functional studies have showed contradictory findings. Zhang et al., 2011, studying knockin mice expressing PEP-R619W, the murine mutant analogous to LYP-R620W, observed the enlargement of spleens and thymuses, expansion of memory/effector T cells and lymphocyte and dendritic cell hyperresponsiveness. Also, the authors found a reduction in PEP protein levels in these mice and observed that both PEP-R619W and LYP-R620W have reduced half-lives and increased sensitivity to calpain-mediated degradation in vitro. The studied knockin mice in the C57BL/6J genetic background did not develop autoimmunity [56]. Dai et al., 2013, also studying PEP-R619W knockin mice, observed some of the findings reported by Zhang et al., such as expansion of effector and memory T cell and lymphocyte hiperresponsiveness. However, they observed that PEP-R619W and LYP-R620W have normal protein stability in vivo and in vitro and aged knockin mice on a mixed C57BL/6J and 129/Sv genetic background developed characteristics of autoimmunity [57].
FYB
Another gene involved in T cell signal transduction pathway is FYB (Fyn binding protein) gene, located at chromosome 5 (5p13.1) and encoding an adaptor protein implied in positive regulation of T cells [58, 59]. This gene has been shown to be downregulated in active and inactive SLE patients [60] and to have different SNPs associated to SLE and its clinical manifestations. Addobbati et al., 2013 reported the association of rs6863066 (C>T) and rs358501 (T>C) SNPs with increased risk for SLE in a population from Southern Brazil. These SNPs, located at the 5' and 3' region of FYB, respectively, may affect the mRNA synthesis or stability and consequently protein expression. Additionally it was demonstrated the association between rs379707 (A>C) and rs2161612 (A>G) SNPs and anti-dsDNA production and protection to hematological alterations, respectively. The SNP rs379707, in exon 15 of FYB gene, causes the substitution of a phenylanine to a valine amino acid, which could cause an impaired function of the Fyb protein, leading to anti-DNA antibodies production, while SNP rs2161612 is located in the 5’UTR of the gene and may protect SLE patients from excessive inflammation [61].
TNFSF4
TNFSF4 (Tumor necrosis factor ligand superfamily, member 4), also known as TNFRSF4/OX40 ligand, belongs to the tumor necrosis factor ligand family and is found to be involved in proliferation and differentiation of T and B lymphocytes. Moreover, it has a crucial role in regulating the development and survival of CD4+ T cells at sites of inflammation and in inducing proinflammatory cytokines production [62-64]. Considering the role of TNFSF4 in the immune system and its possible involvement in autoimmunity process, various genetic studies have been performed to evaluate the association between TNFSF4 gene polymorphisms and SLE. Several SNPs in the upstream region of the gene (rs2205960, rs844648, rs844644, rs10489265 and rs1234315) have been reported to be associated with increased risk of SLE development in Asiatic [65-69], Caucasian [70, 71] and African-American [72] populations. Additionally, it has been found an association of rs2205960 SNP with both anti-Ro antibodies production [65] and renal disorders occurrence [73] in Chinese and European-derived SLE patients, respectively, and the association between rs1234315 SNP and arthritis development in Asiatic patients [69]. Since these polymorphisms are upstream of the promoter region of TNFSF4, they could be involved in alterations of gene regulation and, in consequence, predispose to SLE. Rajabi et al., 2012 also found that mRNA expression level in SLE patients was significantly higher than in healthy controls and demonstrated the association between this increased transcripts number and the development of nephritis, arthritis and atherosclerosis in SLE patients [74].
RASGRP3
RASGRP3 (Ras guanyl nucleotide releasing proteins 3), expressed in B cells, is one of the proteins responsible for the regulation of Ras, a small membrane-bound GTPase implicated in immune B cell receptor signaling. Conversion of Ras-GDP to Ras-GTP leads to stimulation of various downstream pathways such as the Raf-Erk kinase cascade, with ensuing changes in transcription and other cellular responses [75]. RasGRP3–deficient B cells having reduced basal and BCR-regulated Ras-Erk signaling in vitro indicated modestly impaired proliferation in response to stimulation with anti-IgM antibodies and costimulation with anti-CD40 antibodies [76]. The polymorphism rs13385731, located in an intronic region of RASGRP3 gene, showed association with SLE susceptibility in both populations from China [67] and Sweden [77]. Moreover, He et al., 2010 reported the association of this SNP with malar rash, discoid lesion and serositis occurrence and antinuclear antibody (ANA) release in Chinese SLE patients [78].
BANK 1
Another protein involved in B cell signaling pathway is BANK1 (B-cell scaffold protein with ankyrin repeats 1), an adaptor protein that is phosphorylated upon BCR stimulation and thereafter promotes phosphorylation of inositol 1,4,5-trisphosphate receptors (IP3R) by the Src tyrosine kinase Lyn, leading to Ca2+ mobilization from intracellular stores [79, 80]. In addition the stimulation of the B cell receptor enhances the binding of BANK1 and BLK, an interaction mediated by cellular kinases [81]. Three BANK1 gene polymorphisms (rs10516487, rs17266594 and rs3733197) were firstly reported to be associated to SLE in a Swedish population and in a combined analysis with populations from Scandinavia, Argentina, Germany, Italy and Spain [82]. This result has been replicated in African-Americans [83], Asians [64, 83] and other European populations [84, 85]. SNP rs10516487, in exon 2 of BANK1 gene, leads to a non-synonymous substitution of arginine by histidine at position 61 (R61H) of the protein. The above mentioned amino acid change influences mRNA splicing and, consequently, the quantity of protein and causes a protein isoform with increased potential for multimerization [81, 86]. SNP rs17266594, located in a putative branch-point site at intron 1, is able to modify the splicing efficiency of BANK1; and SNP rs3733197 causes an amino acid substitution in the ankyrin domain (A383T), affecting cytoplasmic calcium mobilization [82].
The SNPs rs10516487 and rs17266594 were associated with high-title of ANA and anti-SSA antibodies production in SLE patients from a Chinese Han population [83]. SNP rs10516487 also showed to be associated to anti-DNA antibody production in European-derived SLE patients [87]. Another BANK1 gene polymorphism associated with SLE is the rs4522865 SNP, which is located at its first intron and may have a role in the expression of the gene [65, 68]. BANK1 mutations may contribute to sustained B-cell receptor signaling and subsequent B-cell hyperactivity, contributing to SLE development and autoantibodies production.
VDR
Vitamin D receptor (VDR) gene was firstly described more than three decades ago and since then over 50 targets has been identified for vitamin D, providing a broader role for this vitamin function [89]. The immunologic effect of vitamin D includes: downregulation of Th1 immune responses, depressing activated B cells proliferation, up regulation of regulatory T cells and particularly, preserving immune response [90]. Decreased levels of vitamin D are associated to immune disorders including SLE in several populations as well as its clinical manifestations [91-94]. Vitamin D acts through various immune cells, among them T and B lymphocytes and dendritic cells. These cells express VDR and 1α- hydroxylase and are able to produce vitamin D locally, which makes its deficiency a key factor in the immune system balance. When vitamin D complexes with VDR leads to pro-inflammatory cytokines suppression, such as IFN-γ and IL-12, which are interestedly elevated in SLE patient serum levels [95].
VDR gene is highly polymorphic but only four SNPs are frequently studied: BmsI (rs1544410), ApaI (rs7975232), TaqI (rs731236) and FokI (rs2228570). The functional role of BmsI, ApaI and TaqI polymorphisms is associated with increased stability of the messenger RNA. FokI polymorphism can alter VDR transcription start site and synthesizes a truncated version of the VDR protein with less three amino acids [90, 95]. Studies associating VDR polymorphisms with SLE provided heterogeneous results mainly due cohort ethnical differences. Luo et al., 2012, tested the association between VDR gene BsmI polymorphism and the genetic susceptibility to SLE in a Chinese population. The results indicated that the B allele was influencing disease susceptibility. Additionally, VDR B allele was associated with the development of lupus nephritis and the downregulation of VDR mRNA expression in SLE [96]. Renal disorders are a common feature in SLE patients and are considered as an indicator of diseases' activity [97]. This might be due to the deposition of anti-dsDNA, which is the main pathogenic event in renal failure in SLE patients [98]. VDR FokI (rs2228570) polymorphism is a T>C change producing a truncated VDR protein, is one of the most studied SNPs and has been associated with renal manifestation [99]. De Azevêdo Silva, et al., 2013, assessed the possible association of five Tag-SNPs, which covered most of VDR gene by linkage disequilibrium (LD), and SLE as well as its clinical manifestations´ susceptibility in a Brazilian Population [100]. The authors described association of rs11168268 with cutaneous alterations, rs3890733 with arthritis, rs2248098 with immunological alterations and the rs4760648 with anti-dsDNA antibody. However, the study lacked association to the disease’ susceptibility itself.
CTLA-4
Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) is a cell surface molecule involved in the regulation of T cells through a pathway of proliferative unresponsiveness and tolerance. CTLA-4 signaling mediates antigen-specific apoptosis of T cells and suppresses autoreactive proliferation of T lymphocytes. Therefore, this immunomodulatory protein is able to regulate as well as to maintain self-tolerance [101]. CTLA-4 shares sequence homology with T cell co-stimulatory protein CD28 and both bind to the same ligands, B7.1 (CD80) and B7.2 (CD86), however with opposing functions. While CD28 promotes T-cell activity, CTLA-4 is a negative regulator of T cell responses [102]. The human CTLA-4 gene is located at chromosome 2 (2q33) [103] and several studies have reported an association between CTLA-4 gene polymorphisms and autoimmune diseases including SLE.
CTLA-4 SNPs have been associated to SLE in several populations and according to Ulker et al., 2009, one of the most studied SNP in CTLA-4 exon 1 at position +49 A/G has been associated with SLE susceptibility but not with disease activity or clinical manifestations in Turkish population [104]. The study performed by Sugimoyo et al., 2008, presented interesting data about SLE clinical manifestations and CTLA-4 at exon1 (+49 A/G). Two Japanese families with SLE were studied: in family 1, a boy (5-years old at SLE diagnosis) showed cardiovascular complications associated with heart failure, and his mother, also with clinically active SLE, presented lupus nephritis. In family 2, a boy (13-years old at SLE diagnosis) developed severe renal complications and peripheral vasculitis, along with skin alterations in the lower extremities. CTLA-4 polymorphism analysis indicated that both boys and the mother of family 2 presented G/G genotype in CTLA-4 exon 1 at +49 with a 106-bp fragment length of the 3’ untranslated region (UTR) in exon 4 [105]. Ahmed et al., 2001, observed that CTLA-4 exon 1 at +49 SNP is associated to SLE but not CD28 SNPs, suggesting that CTLA-4 SNPs might be directly linked to SLE developmental pathway [106].
DNA REPAIR GENES IN SLE
Many environmental stimuli have been associated to SLE triggering, in particular ultraviolet light exposure, which might be capable to produce Double Strand Breaks (DSBs) and DNA oxidative damage [107]. The DNA molecule itself is poorly immunogenic, however DSBs might induce immunogenicity and lead to immune response. Particularly, defects in the repair of DSBs may cause accumulation of genomic alterations and promote apoptosis [108]. In SLE patients, DSBs are ineffectively repaired which lead to apoptosis and once the clearance mechanism is not efficiently performed in SLE patients, it may contribute to the self-tolerance breakdown [107, 109]. In addition, polymorphic sites in DNA repair genes were associated with a predisposition of SLE clinical manifestations and anti-DNA antibody, supporting the role of DNA repair genes in autoimmunity [107, 110].
XRCC1, 3 and 4
Proteins encoded by the X-ray repair cross-complementing (XRCC) gene family protect DNA against damage from ionizing radiation [111]. Alterations in XRCC gene have been shown to be associated not only to SLE, but with other autoimmune disorders due to its presence at DNA damage sites [112]. XRCC1 gene is located at chromosome 19 (19q13.2–13.3), it encodes a scaffold protein to complex with several enzymes involved with DNA repair, being the most studied XRCC family member on SLE [113]. Even though there are over 300 SNPs described in XRCC1 gene, only three are frequently studied: the amino acid substitutions at codon 194 (at position 26304 on exon 6, rs1799782), codon 280 (at position 27466 on exon 9, rs25489) and codon 399 (at position 28152 on exon 10, rs25487), these non-conservative amino acid changes may alter XRCC1 function [113]. These three particular SNPs may lead to a diminished repair kinetic influencing on the immune response balance. The XRCC1 Arg399Gln minor allele showed relatively high frequency in the Polish population and is associated to SLE increased risk. Interestedly, the SLE clinical manifestations associated to XRCC1 Arg399Gln are malar rash and photosensitivity, features expressed and/or exacerbated after sun light exposure and related to the reduced ability to respond UV irradiation [111]. In the Chinese Han population, the XRCC1 Arg399Gln also confers risk to SLE development and to the same clinical manifestations observed in the Polish population: malar rash and photosensitivity [114].
The XRCC4 is an important player in genome stability, and XRCC3 is suggested to play a pivotal role in the development of carcinogenesis [115-117]. Even though their functions are so important in DNA stability, these genes are not enough studied regarding autoimmune disorder, in particular SLE. In the Brazilian population, a study evaluated SNPs from XRCC1, 3 and 4 genes and SLE susceptibility and its clinical manifestations. Bassi et al., 2008, also investigated the efficiency of SLE peripheral blood leucocytes in repairing DNA damage induced by ionizing radiation. SLE patients’ leucocytes present decreased efficiency of DNA repair after irradiation and XRCC1 Arg399Gln SNP was associated with the presence of anti-dsDNA antibody. In addition, the three assessed SNPs taken together (XRCC1 Arg399Gln, XRCC3 Thr241Met and XRCC4 Ile401Thr) were associated with the presence of neuropsychiatric disorders and antiphospholipid antibody syndrome [107]. Furthermore, in SLE patients even with presence of XRCC4, one of the most important protein in Non Homologous End Joining (NHEJ), the action of this protein is delayed when compared with healthy individuals [110]. Finally, the UV irradiation may damage DNA and decreased DNA repair may induce an autoimmune response; DNA damage might produce nucleoprotein complexes containing stimulate autoreactive T lymphocytes and so induce SLE in susceptible individuals [108, 111].
STK17A
Another study, based on microarray data analysis, pointed towards three DNA repair genes (LIG4, RAD52 and STK17A) as associated to SLE development [60]. Although no association was found between LIG4 and RAD52 and SLE susceptibility [118], STK17A was associated not only to SLE but to several clinical features including arthritis, cutaneous and immunological alterations [119]. STK17A, localized at chromosome 7 (7p13), is involved in the regulation of nuclear processes, DNA damage response, positive regulation of apoptotic process, intracellular protein kinase cascade [120] and regulation of reactive oxygen species (ROS) metabolic process [121]. STK17A can be activated in reply to the external stimuli such as UV radiation and certain drugs.The activity of serine/threonine kinase has been requested by the DNA PKs (DNA dependent protein kinase catalytic subunit) for DNA repair of double strand breaks (DSBs) [122], consequently STK17A may have a central role in DSBs repair, caused by endogenous damage and/or environmental agents [120, 123]. Thus, DNA repair genes are potential SLE markers since their altered function are so closely related not only to the disease clinical features but in the maintenance of immune balance in those patients.
INTERFERON REGULATORY GENES
When increased expression of type I IFN regulated genes, known as IFN signature, was first described in lupus [124, 125], the interest for the potential involvement of type I IFN system in SLE etiopathogenesis raised in the scientific community. Later studies revealed that the IFN signature is not exclusive for SLE, but can also be found in others autoimmune diseases. Since IFN-α therapy can induce autoimmunity and considering that other autoimmune disorders have a noticeable IFN signature, it has been accepted that the type I IFN system has a pivotal role in the etiopathogenesis of these diseases [126]. IFN I include interferon alpha (IFN-α) and interferon beta (IFN-β), and both of them signal through type I interferon receptor [127]. The IFNs are cytokines which mediate the Th1 response, sustain activated T cells and B cells survival [1]. Th1 responses are able to broadcast proinflammatory cytokines, contributing to chronic inflammation and tissue damage [1, 127]. High IFN-α levels are associated with more a severe disease and presence of particular autoantibodies and a variety of genetic polymorphisms in genes related to IFN-α pathway have been associated with SLE susceptibility and activity.
IFIH1
The Interferon-induced Helicase C domain 1 (IFIH1) gene is located at chromosome 2 (2q24) and the encoded protein participates in the activation of apoptosis in viral dsRNA infected cells, modulating type 1 IFN response, production of pro-inflammatory cytokines and apoptotic processes [128]. The erroneous activation of these virus-sensitive proteins by self-derived and intracellular nucleic acids might be able to alter immune balance. IFN-1 plays a key role on SLE pathogenesis enhancing autoimmune processes and disease’s complications [129, 130].
Studies on the IFIH1 gene are limited, although those involving INF-induced genes could be used to comprehend the regulatory mechanisms involved in activity and development of SLE [131]. The deletion of the ATP-binding domain, which includes the SNP rs10930046, is associated to apoptosis in melanoma cells. The other SNP frequently studied in autoimmune diseases is rs1990760, which is located at HNF-3b binding site within exon 15, encoding an alanine to threonine change. This protein region is highly conserved in mammals and may have other unknown functions or may influence the active domains through effects on tertiary structure [131]. Rs10930046 studies in autoimmune diseases have been inconclusive because this SNP is in linkage disequilibrium (LD) with other IFIH1 gene polymorphisms (rs2068330, rs2111485 and rs984971) and also because of the heterogeneity of frequency distribution in different populations [129, 131].
Gateva, et al., 2009, reported the first association between IFIH1 SNPs and susceptibility to SLE: the authors identified the altered risk A946T polymorphism (rs1990760) for SLE susceptibility in Sweden and US populations [70]. The results were confirmed by a study of Han et al., 2009 [67]. The rs1990760 was also associated to IFN-α increased levels from SLE patients with positivity for anti-dsDNA antibodyand the meta-analysis performed by Moura et al., 2013, reinforced the association of this SNP to SLE onset [132]. The rs1990760 is the most associated IFIH1 SNP in SLE and it increases gene expression, which may lead to an IFN cascade initiated by nucleic acids which is highly related to SLE pathogenesis and disease´s severity.
IRF-5
Interferon regulatory factor (IRF) 5, a member of the IRF family, is a transcription factor that regulates the expression of a wide range of genes. IRF-5 is located at chromosome 7 (7q32) and expressed in B and dendritic cells. IRF-5 is able to induce transcription of IFN-α mRNA and is involved in the induction of IFN and proinflammatory cytokines [133]. Some studies report that IRF5 gene polymorphisms are a risk factor for SLE patients in different populations [134, 135].
The first association study with IRF5 and SLE was performed in Nordic patients (Swedish, Finnish and Icelandic) by using the SNP rs2004640 [136]. Thereafter, another study replicated the association of this SNP with SLE in cohorts with different ancestry. The rs2004640 risk allele indicated a new splice site for alternate splicing of the first exon (1A, 1B, and 1C) and was the unique polymorphism responsible for mRNAs containing exon 1B [137]. The rs2004640 forms a haplotype with the SNPs rs2004640, rs10954213 and rs10499631 and is associated to IRF5 expression levels. These SNPs are within the IRF5-SLE risk haplotype, which consists of two copies of the 4xCGGGG promoter indel, the T-allele of SNP rs2004640, the A-allele of SNP rs10954213, and C-allele of SNP rs10488631, which is associated to disease's activity [138].
The genetic variations in IRF5 are associated with risk of developing SLE and closely related with anti-Ro autoantibodies, which are among the most frequently detected autoantibodies against nuclear antigens and also associated with SLE Sjögren's syndrome (SS) [139]. The formation of autoantibodies in SLE anticipates the disease´s active phase once it may stimulate IFNα production in humans in vivo via activation of the endosomal TLR system. In addition, subjects who present anti-Ro antibodies but have no clinical autoimmunity also have no high serum levels of IFNα [140]. SLE patients’ homozygotes or heterozygotes for IRF5 risk haplotype have higher serum levels of INF-α than the ones with the protective haplotype and this effect is more noticeable in patients positive for anti-ribosomal P antibody (anti-RBP) or anti-dsDNA autoantibodies [140]. Autoantibodies play a crucial role in the induction of glomerular inflammation, especially anti-dsDNA antibodies, which have been frequently associated with lupus nephritis (LN) [141]. Lupus nephritis (LN) is a common feature in SLE clinical manifestations and one of the worst organ damage in SLE leading to worse prognosis. The study performed by Qin et al., 2010, in a Chinese population was the first one to describe the association between IRF5 rs2004640 and lupus nephritis [142].
TYK2
Tyrosine Kinase 2 (TYK2) is located at chromosome 19 (19p13.2), a linkage locus for SLE. TYK2 phosphorylates the receptor subunits of cytokine receptors, including type-I IFN receptors, leading to increased production of IFN-1 responsive genes [130]. Serum levels of IFN-α in SLE patients follow the flares of disease activity and expression of external manifestations, such as skin rash and fever [143]. TYK2 is part of the Janus kinase (JAK) which binds to the interferon (IFN)-α receptor, IFNAR, on the cell surface of IFN-producing cells. This binding leads to phosphorylation and TYK2 activation. Active TYK2 then phosphorylates IFNAR to allow binding of STAT3 and STAT5. The production of type I IFN and the regulation of IFN- inducible genes play a crucial role in SLE etiology. In addition, an increased level of IFN- α is a well-known phenotype in SLE patients and is strongly correlated with both disease activity and severity [143].
In the meta-analysis performed by Lee et al., 2012, the authors investigated four TYK2 SNPs namely: rs2304256, rs12720270, rs280519 and rs12720356 in SLE. In the meta-analysis, the TYK2 rs2304256 presented a significant association with SLE. However, when stratifying by ethnicity the study identified a significant association with SLE in Europeans, but not in Asians. In addition, no association was found for TYK2 rs12720270, rs280519 and rs12720356 polymorphisms and SLE susceptibility by the meta-analysis [144]. Importantly, most of the studies were performed in European descent populations, and only two studies [143, 144] were conducted on Asians populations.
Very few studies aimed at correlating TYK2 polymorphisms with the SLE disease´s clinical manifestations. Nevertheless, the study of Li et al., 2011, investigated the role of TYK2 SNPs including rs12720270, rs2304356 and rs2304255 without finding any association with SLE susceptibility, however associations of rs2304256 and rs12720270 with photosensitivity and discoid rash in SLE patients have been observed [146].
TLR7, 8 and 9
The Toll like Receptor (TLR) family plays a pivotal role in the mammalian innate immune system and has been considered in autoimmunity balance since activates autoreactive B cells and plasmacytoid dendritic cells by TLR ligands [147]. TLRs are very well studied pattern-recognition receptors and TLR7/8/9 gene alterations are frequently associated to SLE mainly because their activation of INF-α production as an antiviral response. TLR7 and TLR8 recognize RNA and TLR9 recognize DNA [148, 149]. TLR7 and 9 are expressed in B and plasmacytoid dendritic cells (pDC) and TLR8 is expressed in monocyte-derived cells, such as macrophages and myeloid DC (mDC) [148].
TLR7 gene is located at X chromosome (Xp22.3-p22.2) and contains the SNP rs179008, resulting in a change from a Gln (A allele) to a Leu (T allele) at position 11 in the peptide. This exchange abbreviates the TLR7 protein N region and extends the hydrophobic region within the signal sequence, altering TLR7 processing. TLR8 is located at chromosome X, 16kb from TLR7, and appears to be a linkage disequilibrium between them [150]. TLR8 encodes two splice variants (TLR8v1 and TLR8v2) with alternative translation start sites. In fact, TLR7 and TLR8 are homologues and have TLR9 as their closest evolutionary relative [147, 150]. The TLR9 gene is located at chromosome 3 (3p21.3) and has two polymorphisms able to form the four common TLR9 haplotypes: rs5743836 and rs352140 [151]. TLR9 protein expression is regulated by rs3764880, this particular SNP leads to an A to G exchange at the first codon, and the G allele is responsible for increasing TLR8v1 translation with no changes in mRNA levels or even protein function [152].
TLRs recognizing self-derived nucleic acids may contribute to the pathogenesis of autoimmune diseases including SLE and particularly lupus nephritis. TLRs are expressed within the kidney cells of patients diagnosed with SLE. In lupus nephritis, immunologic injury, originated by autoantibodies attack, immune complex deposition and cell-mediated injury, leads to kidneys infiltration by leukocytes. The study performed by Papadimitraki et al., 2009, assessed the expression of TLR9 in the renal tissue of patients with lupus in comparison with healthy individuals. The study showed for the first time the up-regulation of TLR9 within the glomerulus from lupus nephritis patients [153]. In the review of Conti et al., 2011, it was shown that TLRs signaling are key components in lupus nephritis in several animal models [154].
STAT4
STAT4 (Signal Transducer and Activator of Transcription 4) belongs to the STAT family and is expressed in T and B lymphocytes, monocytes, macrophages, natural killer cells and dendritic cells. Its expression may be related to the differentiation of immune cells to inflammatory subsets, production of inflammatory cytokines and autoantibodies, prevention of apoptosis and presentation of autoantigens, which may stimulate the development of autoimmune diseases [155]. STAT4 gene lies adjacent to STAT1 at chromosome 2 (2q32.2–2q32.3). In murine lupus model, STAT4-deficiency is associated with acute renal disease and increased mortality, but with protective effects for arthritis in knockout mice [156].
Remmers et al., 2007, identified STAT4 as a new susceptibility gene for rheumatoid arthritis (RA) and SLE [157]. In addition, STAT4 gene polymorphisms have been shown to be associated with other autoimmune diseases such as Systemic Sclerosis (SSc) and primary Sjogren’s Syndrome (pSS), suggesting that STAT4 polymorphisms share a common pathway for multiple autoimmune diseases [158]. One of the most associated SNPs in STAT4 gene is rs7574865, which is located within the third intron and shows the strongest association with autoimmunity. There is no evidence indicating that this intronic SNP is responsible for any gene changes, instead any other from the many SNPs in linkage disequilibrium (LD) with it could be one explaining the disease´s association [158]. STAT4 rs7574865 is not only associated to SLE susceptibility but with a more severe SLE clinical manifestations, particularly nephritis and the production of autoantibodies against dsDNA. In addition, in this study STAT4 rs7574865 was associated to severe nephritis, renal disease and oral ulcers [159].
CONCLUDING REMARKS
SLE is quite known for being the prototype of the autoimmune diseases. Indeed, it displays a sophisticated intricate pathway, yet to be fully understood, leading to autoimmunity triggering and disease´s establishment. In this review we report several genes in SLE etiology encoding relevant proteins for T and B cells proper function. Actually, the most striking immune alterations in lupus patients rely on T and B cells. Therefore, the genes involved somehow to its perfect action and balance are often associated to autoimmune disorders in general. In this regard, from the “newly” associated genes batch in this review, VDR, for instance, seems to be a promising genetic marker in SLE and its clinical manifestations. VDR is very tangled in SLE etiology once it is a hormone able to modulate the immune response it can be linked to the mainly lupus symptoms in skin and kidney. The association with FYB gene revealed the importance of the T cell activation cascade to the autoimmunity development, since the altered activation may result in an inefficient autotolerance process and, in consequence, in the autoreactive cells release, a major event in SLE.
Far from less important, the genes associated to IFN pathway are often responsible for SLE activity and worsen of clinical conditions. However, the IFN signature is not exclusive for SLE patients being frequent in other autoimmune diseases. Most of the IFN pathway genes are already well known, but IFIH1 raises as a prominent genetic marker in autoimmune disorders. Environmental factors such as UV light exposition and stress are also a major aspect to be aware of. Additionally, genes related to DNA repair are often not working properly in SLE patients, leading to DSB accumulation, recognition of the DNA as a potential immunogenic molecule and pulling the trigger of autoimmune attack in those susceptible individuals. In this review, we described newly associated STK17A gene which plays a key role in DSBs repair and also a promising genetic marker in SLE. Finally, we casted for this review several genes, clustered by their function, role in SLE and most importantly, their association with SLE clinical manifestations. In conclusion, the key message in this review is that several genes are not merely associated to SLE but to its clinical manifestations as well, what may strengthens the standpoint on those genes not only to researchers but to clinicians as well toward patient´s benefit.
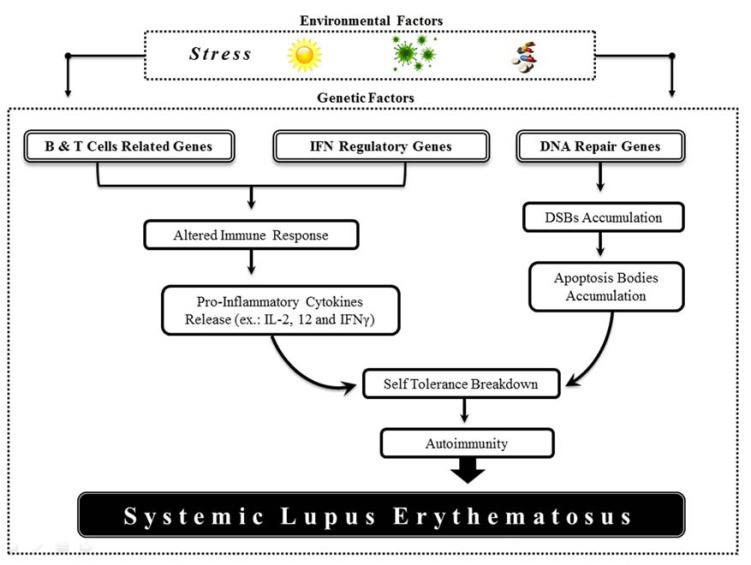
Fig. (1).
Environmental factors are able to trigger the immune response and the genetic factors play a key role in this activation. The gene clusterization regarding their role in immune system indicates the major functions and general path leading to self-tolerance breakdown, autoimmunity and SLE development.
ACKNOWLEDGEMENTS
Research in our lab is supported by the following funding agencies: CAPES, CNPq and FACEPE.
De Azevêdo Silva, J. wrote the manuscript and prepared the tables and illustration; Addobbati, C. wrote the manuscript and provided insightful suggestions to the manuscript; Sandrin-Garcia, P. read and corrected the manuscript; Crovella, C. read, corrected and provided major suggestions to this manuscript.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Guerra SG, Vyse TJ, Cunninghame Graham D S. The Genetics of Lupus: A Functional Perspective. Arthritis Res. Tther. 2012;14:211. doi: 10.1186/ar3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chai HC, Phipps ME, Chua KH. Genetic Risk Factors of Systemic Lupus Erythematosus in the Malaysian Population: a Minireview. Clin. Dev. Immunol. 2012;2012:963730. doi: 10.1155/2012/963730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman A, Isenberg DA. Systemic Lupus Erythematosus. 2008;358(9):929–939. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;9:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 5.Murphy G, Isenberg D. Effect of Gender on Clinical Presentation in Systemic Lupus Erythematosus. Rheu-matology (Oford England) 2013 doi: 10.1093/rheumatology/ket160. [DOI] [PubMed] [Google Scholar]
- 6.Tsokos G C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011;365:2110–21. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 7.Criswell LA. The Genetic Contribution to Systemic Lupus Erythematosus. Bull. NYU. Hosp. Jt. Dis. 2008;3:176–83. [PubMed] [Google Scholar]
- 8.Rhodes K A, Andrew E M, Newton D J, Tramonti D, Carding S R. A Subset of IL-10-producing Gammadelta T Cells Protect the Liver from Listeria-elicited, CD8(+) T Cell-mediated Injury. Eur. J. Immunol. 2008;38:2274–83. doi: 10.1002/eji.200838354. [DOI] [PubMed] [Google Scholar]
- 9.Yanaba K, Bouaziz J D, Matsushita T, Tsubata T, Tedder T F. The Development and Function of Regulatory B Cells Expressing IL-10 (B10 Cells) Requires Antigen Receptor Diversity and TLR Signals. J. Immunol. 2009;182:7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malefyt D W, Haanen J, Spits H, Koncarolo M, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, Vries J E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J. Exp. Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell.IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989;170:2081– 95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousset F, Garcia E, Defrance T, Péronne C, Vezzio N, Hsu D H, Kastelein R, Moore K W, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1890–93. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskdale J, Gallagher G. A Polymorphic Dinucleotide Repeat in the Human IL-10 Promoter. Immunogenetics. 1995;42:444–5. doi: 10.1007/BF00179416. [DOI] [PubMed] [Google Scholar]
- 14.Eskdale J, Kube D, Gallagher G. A Second Polymorphic Dinucleotide Repeat in the 5’ Flanking Region of the Human IL10 Gene. Immunogenetics. 1996;45:82–83. doi: 10.1007/s002510050174. [DOI] [PubMed] [Google Scholar]
- 15.Kube D, Platzer C, von Knethen A, Straub H, Bohlen H, Hafner M, Tesch H. Isolation of the human interleukin 10 promoter.Characterization of the promoter activity in Burkitt’s lymphoma cell lines. Cytokine. 1995;7:1–7. doi: 10.1006/cyto.1995.1001. [DOI] [PubMed] [Google Scholar]
- 16.Lim S, Crawley E, Woo P, Barnes P J. Haplotype Associated with Low Interleukin-10 Production in Patients with Severe Asthma. Lancet. 1998;352:113. doi: 10.1016/S0140-6736(98)85018-6. [DOI] [PubMed] [Google Scholar]
- 17.Turner D M, Williams D M, Sankaran D, Lazarus M, Sinnott P J, Hutchinson H I. An Investigation of Polymorphism in the Interleukin-10 Gene Promoter. Immunogenetics. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 18.D’Alfonso S, Rampi M, Bocchio D, Colombo G, Scorza-Smeraldi R, Momigliano-Richardi P. Systemic Lupus Erythematosus Candidate Genes in the Italian Population: Evidence for a Significant Association with Interleukin-10. Arthritis Tther. 2000;43:120–8. doi: 10.1002/1529-0131(200001)43:1<120::AID-ANR15>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Sobkowiak A, Lianeri M, Wudarski M, Lacki JK, Jagodzinski PP. Genetic Variation in the Interleukin-10 Gene Promoter in Polish Patients with Systemic Lupus Erythematosus. Rheumatol. Int. 2009;29:921–5. doi: 10.1007/s00296-008-0776-4. [DOI] [PubMed] [Google Scholar]
- 20.Rosado S, Rua-Figueroa I, Vargas Ja, Garcia-Laorden MI, Losada-Fernandez I, Martin-Donaire T, Perez-Chacon G, Rodriguez-Gallego C, Naranjo Hernandez, Ojeda-Bruno S, Citores MJ, Perez-Aciego P. Interleukin-10 Promoter Polymorphisms in Patients with Systemic Lupus Erythematosus from the Canary Islands. Int. J. Immunogenetics. 2008;35:235–42. doi: 10.1111/j.1744-313X.2008.00762.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen J Y, Wang C M, Lu S C, Chou Y H, Luo S F. Association of Apoptosis-related Microsatellite Polymorphisms on Chromosome 1q in Taiwanese Systemic Lupus Erythematosus Patients. Clin. Exp. Immunol. 2006;143:281–7. doi: 10.1111/j.1365-2249.2005.02984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong WP, Ip WK, Wong WHS, Lau CS, Chan TM, Lau YL. Association of Interleukin-10 Promoter Polymorphisms with Systemic Lupus Erythematosus. Genes Immun. 2004;5:484–92. doi: 10.1038/sj.gene.6364119. [DOI] [PubMed] [Google Scholar]
- 23.Khoa P D, Sugiyama T, Yokochi T. Polymorphism of Interleukin-10 Promoter and Tumor Necrosis Factor Receptor II in Vietnamese Patients with Systemic Lupus Erythematosus. Clin. Rheumat. 2005;24:11–3. doi: 10.1007/s10067-004-0952-1. [DOI] [PubMed] [Google Scholar]
- 24.Suárez A, López P, Mozo L, Gutiérrez C. Differential Effect of IL10 and TNF{alpha} Genotypes on Determining Susceptibility to Discoid and Systemic Lupus Erythematosus. Ann. Rheum. Dis. 2005;64:1605–10. doi: 10.1136/ard.2004.035048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rood M J, Keijsers V, van der Linden MW, Tong T Q, Borggreve SE, Verweij C L, Breedveld F C, Huizinga T W. Neuropsychiatric Systemic Lupus Erythematosus Is Associated with Imbalance in Interleukin 10 Promoter Haplotypes. Ann. Rheum. Dis. 1999;58:85–9. doi: 10.1136/ard.58.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu L J, Liu Z H, Zeng C H, Chen Z H, Yu C, Li L S. Association of Interleukin-10 Gene -592 A/C Polymorphism with the Clinical and Pathological Diversity of Lupus Nephritis. Clin. Exp. Rheum. 2005;23:854–60. [PubMed] [Google Scholar]
- 27.Schotte H, Gaubitz M, Willeke P, Tidow N, Assmann G, Domschke W, Schlüter B. Interleukin-10 Promoter Microsatellite Polymorphisms in Systemic Lupus Erythematosus: Association with the anti-Sm Immune Response. Rheumatol. 2004;43:1357–63. doi: 10.1093/rheumatology/keh353. [DOI] [PubMed] [Google Scholar]
- 28.Khoury S J, Sayegh M H. The Roles of the New Negative T Cell Costimulatory Pathways in Regulating Autoimmunity. Immunity. 2004;20:529–538. doi: 10.1016/s1074-7613(04)00116-5. [DOI] [PubMed] [Google Scholar]
- 29.Francisco L M, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prokunina L, Castillejo-López C, Oberg F, Gunnarsson I, Berg L, Magnusson V, Brookes AJ, Tentler D, Kristjansdóttir H, Gröndal G, Bolstad AI, Svenungsson E, Lundberg I, Sturfelt G, Jönssen A, Truedsson L, Lima G, Alcocer-Varela J, Jonsson R, Gyllensten U B, Harley JB, Alarcón-Segovia D, Steinsson K, Alarcón-Riquelme ME. A Regulatory Polymorphism in PDCD1 Is Associated with Susceptibility to Systemic Lupus Erythematosus in Humans. Nature Genet. 2002;32:666–9. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 31.Bertsias GK, Nakou M, Choulaki C, Raptopoulou A, Papadimitraki E, Goulielmos G, Kritikos H, Sidiropoulos P, Tzardi M, Kardassis D, Mamalaki C, Boumpas DT. Genetic, Immunologic, and Immunohistochemical Analysis of the Programmed Death 1/programmed Death Ligand 1 Pathway in Human Systemic Lupus Erythematosus. Arthr. Rheum. 2009;60:207–18. doi: 10.1002/art.24227. [DOI] [PubMed] [Google Scholar]
- 32.Ferreiros-Vidal I, Gomez-Reino JJ, Barros F, Carracedo A, Carreira P, Gonzalez-Escribano F, Liz M, Martin J, Ordi J, Vicario JL, Gonzalez A. Association of PDCD1 with Susceptibility to Systemic Lupus Erythematosus: Evidence of Population-specific Effects. Arthr. Rheum. 2004;50:2590–7. doi: 10.1002/art.20436. [DOI] [PubMed] [Google Scholar]
- 33.Johansson M, Arlestig L, Möller B, Rantapää-Dahlqvist S. Association of a PDCD1 Polymorphism with Renal Manifestations in Systemic Lupus Erythematosus. Arthr. Rheum. 2005;52:1665–9. doi: 10.1002/art.21058. [DOI] [PubMed] [Google Scholar]
- 34.Prokunina L, Gunnarsson I, Sturfelt G, Truedsson L, Seligman V A, Olson J L, Seldin M F, Criswell L A, Alarcón-Riquelme M E. The Systemic Lupus Erythematosus-associated PDCD1 Polymorphism PD1. A in Lupus Nephritis. Arthr. Rheum. 2004;50:327–8. doi: 10.1002/art.11442. [DOI] [PubMed] [Google Scholar]
- 35.Wang S C, Chen Y J, Ou T T, Wu C C, Tsai W C, Liu H W, Yen J H. Programmed Death-1 Gene Polymorphisms in Patients with Systemic Lupus Erythematosus in Taiwan. J. Clin. Immunol. 2006;26:506–11. doi: 10.1007/s10875-006-9048-9. [DOI] [PubMed] [Google Scholar]
- 36.Mostowska M, Wudarski M. The Programmed Cell Death 1 Gene 7209 C>T Polymorphism Is Associated with the Risk of Systemic Lupus Erythematosus in the Polish Population. Clin. Exp. Rheum. 2008;26:457–60. [PubMed] [Google Scholar]
- 37.Shelly S, Boaz M, Orbach H. Prolactin and autoimmunity. Autoimmun. Rev. 2012:a465–a470. doi: 10.1016/j.autrev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 38.Stevens A, Ray D, Alansari A, Hajeer A, Thomson W, Donn R, Ollier W E, Worthington J D J, Davis J R. Characterization of a Prolactin Gene Polymorphism and Its Associations with Systemic Lupus Erythematosus. Arthritis Rheum. 2001:2358–66. doi: 10.1002/1529-0131(200110)44:10<2358::aid-art399>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 39.Montoya-Díaz E, Cervera-Castillo H, Chávez-Sánchez L, Legorreta-Haquet M V, Sánchez-González L, Chávez-Rueda K, Blanco-Favela F. Prolactin Promoter Polymorphism (-1149 G/T) Is Associated with anti-DNA Antibodies in Mexican Patients with Systemic Lupus Erythematosus. Immunol. Invest. 2011;40:614–26. doi: 10.3109/08820139.2011.570402. [DOI] [PubMed] [Google Scholar]
- 40.Cloutier J F, Veillette A. Cooperative Inhibition of T-cell Antigen Receptor Signaling by a Complex Between a Kinase and a Phosphatase. J. Exp. Med. 1999;189:111–21. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, Katrekar A, Honigberg L, Smith A M, Conn M T, Tang J, Jeffery D, Mortara K, Sampang J, Williams S R, Buggy J, Clark J M. Identification of Substrates of Human Protein-tyrosine Phosphatase PTPN22. J. Biol. Chem. 2006;281:11002–10. doi: 10.1074/jbc.M600498200. [DOI] [PubMed] [Google Scholar]
- 42.Ghose R, Shekhtman A, Goger M J, Ji H, Cowburn D. A Novel, Specific Interaction Involving the Csk SH3 Domain and Its Natural Ligand. Nat. Struc. Biol. 2001;8:998–1004. doi: 10.1038/nsb1101-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manjarrez-Orduño N, Marasco E, Chung S A, Katz M S, Kiridly J F, Simpfendorfer K R, Freudenberg J, Ballard D H, Nashi E, Hopkins T J, Cunninghame Graham D S, Lee A T, Coenen M J, Franke B, Swinkels D W, Graham R R, Kimberly R P, Gaffney P M, Vyse T J, Behrens T W, Criswell L A, Diamond B, Gregersen P K. CSK regulatory polymorphism is associated with systemic lupus erythematosus and influences B-cell signaling and activation. Nat. Genet. 2012;11:1227–30. doi: 10.1038/ng.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vang T, Congia M, Macis M D, Musumeci L, Orrú V, Zavattari P, Nika K, Tautz L, Taskén K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated Lymphoid Tyrosine Phosphatase Is a Gain-of-function Variant. Nat. Genet. 2005:1317–9. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 45.Reddy M V, Johansson M, Sturfelt G, Jönsen A, Gunnarsson I, Svenungsson E, Rantapää-Dahlqvist S, Alarcón-Riquelme M E. The R620W C/T Polymorphism of the Gene PTPN22 Is Associated with SLE Independently of the Association of PDCD1. Genes Immun. 2005;6:658–62. doi: 10.1038/sj.gene.6364252. [DOI] [PubMed] [Google Scholar]
- 46.Orozco G, Sánchez E, González-Gay M A, López-Nevot M A, Torres B, Cáliz R, Ortego-Centeno N, Jiménez-Alonso J, Pascual-Salcedo D, Balsa A, de Pablo R, Nuñez-Roldan A, González-Escribano MF, Martín J. Association of a Functional Single-nucleotide Polymorphism of PTPN22, Encoding Lymphoid Protein Phosphatase, with Rheumatoid Arthritis and Systemic Lupus Erythematosus. Arthritis Rheum. 2005;52:219–24. doi: 10.1002/art.20771. [DOI] [PubMed] [Google Scholar]
- 47.Eliopoulos E, Zervou MI, Andreou A, Dimopoulou K, Cosmidis N, Voloudakis G, Mysirlaki H, Vazgiourakis V, Sidiropoulos P, Niewold T B, Boumpas D T, Goulielmos G N. Association of the PTPN22 R620W polymorphism with increased risk for SLE in the genetically homogeneous population of Crete. Lupus. 2011;20:501–6. doi: 10.1177/0961203310392423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piotrowski P, Lianeri M, Wudarski M, Lacki J K, Jagodzinski P P. Contribution of the R620W Polymorphism of Protein Tyrosine Phosphatase Non-receptor 22 to Systemic Lupus Erythematosus in Poland. Clin. Exp. Rheumatol. 2008;26:1099–102. [PubMed] [Google Scholar]
- 49.Moez P, Soliman E. Association of PTPN22 Gene Polymorphism and Systemic Lupus Erythematosus in a Cohort of Egyptian Patients: Impact on Clinical and Laboratory Results. Rheumatol. Int. 2012;32:2753–8. doi: 10.1007/s00296-011-2063-z. [DOI] [PubMed] [Google Scholar]
- 50.Kyogoku C, Langefeld C D, Ortmann W A, Lee A, Selby S, Carlton V E, Chang M, Ramos P, Baechler E C, Batliwalla F M, Novitzke J, Williams A H, Gillett C, Rodine P, Graham R R, Ardlie K G, Gaffney P M, Moser K L, Petri M, Begovich A B, Gregersen P K, Behrens T W. Genetic Association of the R620W Polymorphism of Protein Tyrosine Phosphatase PTPN22 with Human SLE. Am. J. Hum. Genet. 2004;75:504–7. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez L M, Anaya J M, Gonzalez C I, Pineda-Tamayo R, Otero W, Arango A, Martín J. PTPN22 C1858T Polymorphism in Colombian Patients with Autoimmune Diseases. Genes Immun. 2005;6:628–31. doi: 10.1038/sj.gene.6364261. [DOI] [PubMed] [Google Scholar]
- 52.Arnett F C, Wu H, Gonzalez-Gay M A, Tsao B P, Pons-Estel B, Alarcon-Riquelme M E, He Y, Zhang Z Y, Allayee H, Chen X S, Martin J, Bottini N, Orrú V, Tsai S J, Rueda B, Fiorillo E, Stanford S M, Dasgupta J, Hartiala J, Zhao L, Ortego-Centeno N, D’Alfonso S. Italian Collaborative Group.A Loss-of-function Variant of PTPN22 Is Associated with Reduced Risk of Systemic Lupus Erythematosus. Hum. Mol. Genet. 2009;18:569–79. doi: 10.1093/hmg/ddn363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siggs O M, Miosge L A, Yates A L, Kucharska E M, Sheahan D, Brdicka T, Weiss A, Liston A, Goodnow C C. Opposing Functions of the T Cell Receptor Kinase ZAP-70 in Immunity and Tolerance Differentially Titrate in Response to Nucleotide Substitutions. Immunity. 2007;27:912–26. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gregersen P K. Gaining insite into PTPN22 and autoimmunity. Nat. Genet. 2005;22:1300–13002. doi: 10.1038/ng1205-1300. [DOI] [PubMed] [Google Scholar]
- 55.Vang T, Congia M, Macis M D, Musumeci1 L, Orru V, Zavattari P, Nika K, Tautz L, Taskén K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat. Gen. 2005;12:1317–19. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 56.Zhang J, Zahir N, Jiang Q, Miliotis H, Heyraud S, Meng X, Dong B, Xie G, Qiu F, Hao Z, McCulloch C A, Keystone E C, Peterson A C, Siminovitch K A. The autoimmune disease-associated PTPN22 variant promotes calpain-mediated Lyp/Pep degradation associated with lymphocyte and dendritic cell hyperresponsiveness. Nat. Genet. 2011;9:902–907. doi: 10.1038/ng.904. [DOI] [PubMed] [Google Scholar]
- 57.Dai X, James R G, Habib T, Singh S, Jackson S, Khim S, Moon RT, Liggitt D, Wolf-Yadlin A, Buckner JH, Rawlings DJ. A disease-associated PTPN22 variant promotes systemic autoimmunity in murine models. J. Clin. Invest. 2013;5:2024–2036. doi: 10.1172/JCI66963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peterson E J, Woods M L, Dmowski S A, Derimanov G, Jordan MS, Wu J N, Myung P S, Liu Q H, Pribila J T, Freedman B D, Shimizu Y, Koretzky G A. Coupling of the TCR to Integrin Activation by Slap-130/Fyb. Science. 2001;293:2263–5. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 59.Griffiths EK, Krawczyk C, Kong YY, Raab M, Hyduk SJ, Bouchard D, Chan VS, Kozieradzki I, Oliveira-Dos-Santos A J, Wakeham A, Ohashi PS, Cybulsky MI, Rudd CE, Penninger JM. Positive Regulation of T Cell Activation and Integrin Adhesion by the Adapter Fyb/Slap. Science. 2001;293:2260–3. doi: 10.1126/science.1063397. [DOI] [PubMed] [Google Scholar]
- 60.Sandrin-Garcia P, Junta CM, Fachin AL, Mello SS, Baião AM, Rassi DM, Ferreira MC, Trevisan GL, Sakamoto-Hojo ET, Louzada-Júnior P, Passos GA, Donadi EA. Shared and Unique Gene Expression in Systemic Lupus Erythematosus Depending on Disease activity. Ann. NY Acad. Sci. 2009:493–500. doi: 10.1111/j.1749-6632.2009.04636.x. [DOI] [PubMed] [Google Scholar]
- 61.Addobbati C, Brandão L A, Guimarães R L, Pancotto J A, Donadi E A, Crovella S, Segat L, Sandrin-Garcia P. FYB Gene Polymorphisms Are Associated with Susceptibility for Systemic Lupus Erythemathosus (SLE). Hum. Immunol. 2013;74:1009–14. doi: 10.1016/j.humimm.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 62.Ito T, Wang Y H, Duramad O, Hori T, Delespesse G J, Watanabe N, Qin F X, Yao Z, Cao W, Liu YJ. TSLP-activated Dendritic Cells Induce an Inflammatory T Helper Type 2 Cell Response Through OX40 Ligand. J. Exp. Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linton P J, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick R C, Bradley L M. Costimulation via OX40L Expressed by B Cells Is Sufficient to Determine the Extent of Primary CD4 Cell Expansion and Th2 Cytokine Secretion in Vivo. J. Exp. Med. 2003;197:875–83. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Godfrey WR, Fagnoni F F, Harara M A, Buck D, Engleman EG. Identification of a human OX-40 ligand, a costimulator of CD4+ T cells with homology to tumor necrosis factor. J. Exp. Med. 1994;180:757–62. doi: 10.1084/jem.180.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang Y K, Yang W, Zhao M, Mok C C, Chan T M, Wong R W S, Lee KW, Mok MY, Wong S N, Ng I O, Lee T L, Ho M H, Lee P P, Wong WH, Lau C S, Sham P C, Lau Y L. Association of BANK1 and TNFSF4 with Systemic Lupus Erythematosus in Hong Kong Chinese. Genes Immun. 2009;10:414–20. doi: 10.1038/gene.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou X, Lu X, Nath S K, Lv J, Zhu S, Yang H, Qin L, Zhao M, Su Y, Shen N, Li Z G, Zhang H. International Consortium on the Genetics of Systemic Lupus Erythematosus.Gene-gene Interaction o BLK TNFSF4, TRAF1, TNFAIP3, and REL in Systemic Lupus Erythematosus. Arthritis Rheum. 2012; 64:222–31. doi: 10.1002/art.33318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han J W, Zheng HF, Cui Y, Sun LD, Ye DQ, Hu Z, Xu J H, Cai Z M, Huang W, Zhao G P, Xie H F, Fang H, Lu Q J, Xu J H, Li X P, Pan YF, Deng D Q, Zeng FQ, Ye Z Z, Zhang XY, Wang QW, Hao F, Ma L, Zuo XB, Zhou F S, Du WH, Cheng Y L, Yang J Q, Shen S K, Li J, Sheng Y J, Zuo X X, Zhu W F, Gao F, Zhang P L, Guo Q, Li B, Gao M, Xiao FL, Quan C, Zhang C, Zhang Z, Zhu K J, Li Y, Hu D Y, Lu W S, Huang J L, Liu S X, Li H, Ren Y Q, Wang ZX, Yang C J, Wang P G, Zhou W M, Lv Y M, Zhang A P, Zhang S Q, Lin D, Li Y, Low H Q, Shen M, Zhai Z F, Wang Y, Zhang F Y, Yang S, Liu J J, Zhang X J. Genome-wide Association Study in a Chinese Han Population Identifies Nine New Susceptibility Loci for Systemic Lupus Erythematosus. Nature Genet. 2009;41:1234–7. doi: 10.1038/ng.472. [DOI] [PubMed] [Google Scholar]
- 68.Yang W, Shen N, Ye D Q, Liu Q, Zhang Y, Qian X-X, Hirankarn N, Ying D, Pan H F, Mok C C, Chan T M, Wong R W, Lee K W, Mok MY, Wong SN, Leung AM, Li X P, Avihingsanon Y, Wong C M, Lee T L, Ho MH, Lee P P, Chang Y K, Li P H, Li R J, Zhang L, Wong W H, Ng I O, Lau C S, Sham P C, Lau Y L. sian Lupus Genetics Consortium.Genome-wide Association Study in Asian Populations Identifies Variants in ETS1 and WDFY4 Associated with Systemic Lupus Erythematosus. . PLoS Genet. 2010;6:e1000841. doi: 10.1371/journal.pgen.1000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang S Q, Han J W, Sun L D, Lu W S, Yin X Y, Zhang X J, Yang S. A Single-nucleotide Polymorphism of the TNFSF4 Gene Is Associated with Systemic Lupus Erythematosus in Chinese Han Population. Rheum. Int. 2011;31:227–31. doi: 10.1007/s00296-009-1247-2. [DOI] [PubMed] [Google Scholar]
- 70.Gateva V, Sandling JK, Hom G, Taylor KE, Chung SA, Sun X, Ortmann W, Kosoy R, Ferreira RC, Nordmark Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jönsen A, Bengtsson AA, Rantapää-Dahlqvist S, Baechler EC, Brown EE, Alarcón GS, Edberg JC, Ramsey-Goldman R, McGwin G, Reveille JD, Vilá LM, Kimberly RP, Manzi S, Petri MA, Lee A, Gregersen PK, Seldin MF, Rönnblom L, Criswell LA, Syvänen AC, Behrens TW, Graham RR. A Large-scale Replication Study Identifies TNIP1 PRDM1 JAZF1 UHRF1BP1 and IL10 as Risk Loci for Systemic Lupus Erythematosus. Nat. Genet. 2009;41:1228–33. doi: 10.1038/ng.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cunninghame Graham D S, Graham R R, Manku H, Wong A K, Whittaker J C, Gaffney P M, Moser K L, Rioux JD, Altshuler D, Behrens T W, Vyse TJ. Polymorphism at the TNF Superfamily Gene TNFSF4 Confers Susceptibility to Systemic Lupus Erythematosus. Nat. Gen. 2008;40:83–9. doi: 10.1038/ng.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaffney P M, Jacob C O, Niewold T B, Richardson B C, Harley J B, Alarcón-Riquelme M E, Sawalha A H, Sánchez E, Comeau M E, Freedman B I, Kelly J A, Kaufman K M, Langefeld C D, Brown E E, Alarcón G S, Kimberly R P, Edberg J C, Ramsey-Goldman R, Petri M, Reveille J D, Vilá L M, Merrill J T, Tsao B P, Kamen D L, Gilkeson G S, James J A, Vyse T J. International Consortium on the Genetics of Systemic Lupus Erythematosus.Identification of novel genetic susceptibility loci in African American lupus patients in a candidate gene association study. Arthritis Rheum. 2011;63:3493–501. doi: 10.1002/art.30563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez E, Nadig A, Richardson B C, Freedman B I, Kaufman K M, Kelly J A, Niewold T B, Kamen D L, Gilkeson G S, Ziegler J T, Langefeld C D, Alarcón G S, Edberg J C, Ramsey-Goldman R, Petri M, Brown E E, Kimberly R P, Reveille J D, Vilá L M, Merrill J T, Anaya J M, James J A, Pons-Estel B A, Martin J, Park S Y, Bang S Y, Bae S C, Moser K L, Vyse T J, Criswell L A, Gaffney P M, Tsao B P, Jacob C O, Harley J B, Alarcón-Riquelme M E, Sawalha A H. BIOLUPUS and GENLES, Phenotypic associations of genetic susceptibility loci in systemic lupus erythematosus. Ann. Rheum. Dis. 2011;70:1752–7. doi: 10.1136/ard.2011.154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rajabi P, Alaee M, Mousavizadeh K, Samadikuchaksaraei A. Altered Expression of TNFSF4 and TRAF2 mRNAs in Peripheral Blood Mononuclear Cells in Patients with Systemic Lupus Erythematosus: Association with Atherosclerotic Symptoms and Lupus Nephritis. Inflamm. Res. 2012;61:1347–54. doi: 10.1007/s00011-012-0535-6. [DOI] [PubMed] [Google Scholar]
- 75.Stone J C. Regulation and Function of the RasGRP Family of Ras Activators in Blood Cells. Genes Can. 2011;2:320–34. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coughlin J J, Stang S L, Dower N A, Stone J C. RasGRP1 and RasGRP3 Regulate B Cell Proliferation by Facilitating B Cell receptor-Ras Signaling. J. Immunol. 2005;175:7179–84. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 77.Wang C, Ahlford A, Järvinen T M, Nordmark G, Eloranta M L, Gunnarsson I, Svenungsson E, Padyukov L, Sturfelt G, Jönsen A, Bengtsson A A, Truedsson L, Eriksson C, Rantapää-Dahlqvist S, Sjöwall C, Julkunen H, Criswell L A, Graham R R, Behrens T W, Kere J, Rönnblom L, Syvänen A C, Sandling J K. Genes Identified in Asian SLE GWASs Are Also Associated with SLE in Caucasian Populations. Eur. J. Hum. Genet. 2012:1–6. doi: 10.1038/ejhg.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He C F, Liu Y S, Cheng Y L, Gao J P, Pan T M, Han J W, Quan C, Sun L D, Zheng H F, Zuo X B, Xu S X, Sheng Y J, Yao S, Hu W L, Li Y, Yu Z Y, Yin X Y, Zhang X J, Cui Y, Yang S. TNIP1, SLC15A4, ETS1, RasGRP3 and IKZF1 Are Associated with Clinical Features of Systemic Lupus Erythematosus in a Chinese Han Population. Lupus. 2010;19:1181–6. doi: 10.1177/0961203310367918. [DOI] [PubMed] [Google Scholar]
- 79.Yokoyama K, Su Ih I, Tezuka T, Yasuda T, Mikoshiba K, Tarakhovsky A, Yamamoto T. BANK Regulates BCR-induced Calcium Mobilization by Promoting Tyrosine Phosphorylation of IP(3) Receptor. EMBO J. 2002;21:83–92. doi: 10.1093/emboj/21.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kurosaki T. Regulation of B-cell Signal Transduction by Adaptor Proteins. Nat. Rev. Immunol. 2002:354–63. doi: 10.1038/nri801. [DOI] [PubMed] [Google Scholar]
- 81.Castillejo-Lopez C, Delgado-Vega AM, Wojcik J, Kozyrev SV, Thavathiru E, Wu YY, Sánchez E, Pöllmann D, López-Egido JR, Fineschi S, Domínguez N, Lu R, James JA, Merrill JT, Kelly JA, Kaufman KM, Moser KL, Gilkeson G, Frostegård J, Pons-Estel BA, D'Alfonso S, Witte T, Callejas J L, Harley JB, Gaffney PM, Martin J, Guthridge J M, Alarcón-Riquelme ME. Genetic and physical interaction of the B-cell systemic lupus erythematosus-associated genes BANK1 and BLK. Ann. Rheum. Dis. 2012;71:136–142. doi: 10.1136/annrheumdis-2011-200085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kozyrev SV, Abelson AK, Wojcik J, Zaghlool A, Linga Reddy MV, Sanchez E, Gunnarsson I, Svenungsson E, Sturfelt G, Jönsen A, Truedsson L, Pons-Estel BA, Witte T, D'Alfonso S, Barizzone N, Danieli MG, Gutierrez C, Suarez A, Junker P, Laustrup H, González-Escribano M F, Martin J, Abderrahim H, Alarcón-Riquelme ME. Functional Variants in the B-cell Gene BANK1 Are Associated with Systemic Lupus Erythematosus. Nat. Genet. 2008;40:211–6. doi: 10.1038/ng.79. [DOI] [PubMed] [Google Scholar]
- 83.Sánchez E, Rasmussen A, Riba L, Acevedo-Vasquez E, Kelly J A, Langefeld C D, Williams A H, Ziegler J T, Comeau M E, Marion M C, García-De La Torre I, Maradiaga-Ceceña M A, Cardiel M H, Esquivel-Valerio JA, Rodriguez-Amado J, Moctezuma J F, Miranda P, Perandones C E, Castel C, Laborde H A, Alba P, Musuruana J L, Goecke I A, Anaya J M, Kaufman K M, Adler A, Glenn S B, Brown E E, Alarcón G S, Kimberly R P, Edberg J C, Vilá L M, Criswell L A, Gilkeson G S, Niewold T B, Martín J, Vyse T J, Boackle S A, Ramsey-Goldman R, Scofield R H, Petri M, Merrill J T, Reveille J D, Tsao B P, Orozco L, Baca V, Moser K L, Gaffney P M, James J A, Harley J B, Tusié-Luna T, Pons-Estel B A, Jacob C O, Alarcón-Riquelme M E. Impact of genetic ancestry and so-ciodemographic status on the clinical expression of systemic lupus erythematosus in American Indian-European populations. Arthritis Rheum. J. 2012;11:3687–3694. doi: 10.1002/art.34650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guan M, Yu B, Wan J, Zhang X, Wu Z, Zhong Q, Zhang W, Zou H. Identification of BANK1 Polymorphisms by Unlabelled Probe High Resolution Melting: Association with Systemic Lupus Erythematosus Susceptibility and Autoantibody Production in Han Chinese. Rheumatology. 2011;50:473–80. doi: 10.1093/rheumatology/keq353. [DOI] [PubMed] [Google Scholar]
- 85.Järvinen T M, Hellquist A, Zucchelli M, Koskenmies S, Panelius J, Hasan T, Julkunen H, D’Amato M, Kere J. Replication of GWAS-identified Systemic Lupus Erythematosus Susceptibility Genes Affirms B-cell Receptor Pathway Signalling and Strengthens the Role of IRF5 in Disease Susceptibility in a Northern European Population. Rheumatology. 2012;51:87–92. doi: 10.1093/rheumatology/ker263. [DOI] [PubMed] [Google Scholar]
- 86.Suarez-Gestal M, Calaza M, Endreffy E, Pullmann R, Ordi-Ros J, Sebastiani G D, Ruzickova S, Jose Santos M, Papasteriades C, Marchini M, Skopouli F N, Suarez A, Blanco F J, D'Alfonso S, Bijl M, Carreira P, Witte T, Migliaresi S, Gomez-Reino J J, Gonzalez A. European Consortium of SLE DNA Collections.Replication of Recently Identified Systemic Lupus Erythematosus Genetic Associations: a Case-control Study. Arthritis Res. Ther. 2009;11:R69. doi: 10.1186/ar2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kozyrev S V, Bernal-Quirós M, Alarcón-Riquelme M E, Castillejo-López C. The dual effect of the lupus-associated polymorphism rs10516487 on BANK1 gene expression and protein localization. Genes Immun. 2012;13:129–38. doi: 10.1038/gene.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung S A, Taylor K E, Graham R R, Nititham J, Lee A T, Ortmann W A, Jacob C O, Alarcón-Riquelme M E, Tsao B P, Harley J B, Gaffney P M, Moser K L, SLEGEN; Petri M, Demirci F Y, Kamboh M I, Manzi S, Gregersen P K, Langefeld C D, Behrens T W, Criswell L A. Differential Genetic Associations for Systemic Lupus Erythematosus Based on anti-dsDNA Autoantibody Production. PLoS Genet. 2011;7:e1001323. doi: 10.1371/journal.pgen.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Uitterlinden A G, Fang Y, Van Meurs J B, Pols H A, Van Leeuwen J P. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;2:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 91.Mory D B, Rocco E R, Miranda W L, Kasamatsu T, Crispim F, Dib S A. Prevalence of Vitamin D Receptor Gene Polymorphisms FokI and BsmI in Brazilian Individuals with Type 1 Diabetes and Their Relation to Beta-cell Autoimmunity and to Remaining Beta-cell Function. Human Immun. 2009;70:447–51. doi: 10.1016/j.humimm.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Luo X Y, Wu L J, Chen L, Yang M H, Liao T, Liu N T, Ku-Er B, Xie C M, Shi R G, Tang Z, Zhao Y, Zeng X F Y G. The Association of Vitamin D Receptor Gene ApaI and BsmI Polymorphism with Systemic Lupus Erythematosus. Zhonghua Nei. Ke. Za. Zhi. 2012;51:131–135. [PubMed] [Google Scholar]
- 93.Pani M A, Knapp M, Donner H, Braun J, Baur M P, Usadel K H, Badenhoop K. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diaetes. 2000; 49:505–7. doi: 10.2337/diabetes.49.3.504. [DOI] [PubMed] [Google Scholar]
- 94.Panierakis C, Goulielmos G, Mamoulakis D, Petraki E, Papavasiliou E, Galanakis E. Vitamin D Receptor Gene Polymorphisms and Susceptibility to Type 1 Diabetes in Crete, Greece. Clin. Immunol. 2009;133:276–81. doi: 10.1016/j.clim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 95.Monticielo O A, Teixeira T D M, Chies J A B, Brenol J C T, Xavier R M. Vitamin D and Polymorphisms of VDR Gene in Patients with Systemic Lupus Erythematosus. Clin. Rheum. 2012;31:1411–21. doi: 10.1007/s10067-012-2021-5. [DOI] [PubMed] [Google Scholar]
- 96.Luo X Y, Yang M H, Wu F X, Wu L J, Chen L, Tang Z, Liu N T, Zeng X F, Guan J L, Yuan G H. Vitamin D Receptor Gene BsmI Polymorphism B Allele, but Not BB Genotype, Is Associated with Systemic Lupus Erythematosus in a Han Chinese Population. Lupus. 2012;21:53–9. doi: 10.1177/0961203311422709. [DOI] [PubMed] [Google Scholar]
- 97.Borchers A T, Leibushor N, Naguwa S M, Cheema G S, Shoenfeld Y, Gershwin M E. Lupus Nephritis: A Critical Review. Autoimmun. Rev. 2012;12:174–194. doi: 10.1016/j.autrev.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 98.Li P H, Wong W H, Lee T L, Lau C S, Chan T M, Leung A M, Tong K L, Tse N K, Mo C C, Wong S N, Lee K W, Ho M H, Lee P P, Chong C Y, Wong R W, Mok M Y, Ying S K, Fung S K, Lai W M, Yang W, Lau Y L. Relationship Between Autoantibody Clustering and Clinical Subsets in SLE?: Cluster and Association Analyses in Hong Kong Chinese. Rheum. 2012;52:337–45. doi: 10.1093/rheumatology/kes261. [DOI] [PubMed] [Google Scholar]
- 99.Mostowska A, Lianeri M, Wudarski M, Olesinska M, Jagodzinski P P. Vitamin D Receptor Gene BsmI, FokI, ApaI and TaqI Polymorphisms and the Risk of Systemic Lupus Erythematosus. Mol. Biol. Rep. 2013;40:803–10. doi: 10.1007/s11033-012-2118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Azevêdo Silva J, Fernandes KM, Pancotto JAT, Fragoso TS, Donadi EA, Crovella C, Sandrin-Garcia P. Vitamin D receptor (VDR) gene Polymorphisms and Susceptibility to Systemic Lupus Erythematosus Clinical Manifestations. Lupus. 2013;1:1110–7. doi: 10.1177/0961203313500549. [DOI] [PubMed] [Google Scholar]
- 101.Chistiakov D A, Turakulov R I. CTLA-4 and Its Role in Autoimmune Thyroid Disease. J. Mol. Endocrinol. 2003;31:21–36. doi: 10.1677/jme.0.0310021. [DOI] [PubMed] [Google Scholar]
- 102.Kimkong I, Nakkuntod J, Sae-Ngow S, Snabboon T, Avihingsanon Y, Hirankarn N. Association Between CTLA-4 Polymorphisms and the Susceptibility to Systemic Lupus Erythematosus and Graves’ Disease in Thai Population. Asian Pac. J. Allergy Immunol. 2011;29:229–35. [PubMed] [Google Scholar]
- 103.Dariavach P, Mattéi M G, Golstein P, Lefranc M P. Human Ig Superfamily CTLA-4 Gene: Chromosomal Localization and Identity of Protein Sequence Between Murine and Human CTLA-4 Cytoplasmic Domains. Eur. J. Immunol. 1988;18:1901–5. doi: 10.1002/eji.1830181206. [DOI] [PubMed] [Google Scholar]
- 104.Ulker M, Yazisiz V, Sallakci N, Avci a B, Sanlioglu S, Yegin O, Terzioglu E. CTLA-4 Gene Polymorphism of Exon 1(+49 A/G) in Turkish Systemic Lupus Erythematosus Patients. Int. J. Immunogenet. 2009;36:245–50. doi: 10.1111/j.1744-313X.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- 105.Sugimoto K, Fujita S, Yanagida H, Shimada Y, Tabata N, Yagi K, Okada M, Takemura T. Clinical Manifestations and Analyses of the Cytotoxic T-lymphocyte Associated-4 Gene in Two Japanese Families with Systemic Lupus Erythematosus. Clin. Exp. Nephrol. 2008;12:149–54. doi: 10.1007/s10157-007-0019-0. [DOI] [PubMed] [Google Scholar]
- 106.Ahmed S, Ihara K, Kanemitsu S, Nakashima H, Otsuka T, Tsuzaka K, Takeuchi T, Hara T. Association of CTLA-4 but Not CD28 Gene Polymorphisms with Systemic Lupus Erythematosus in the Japanese Population. Rheum. 2001;40:662–7. doi: 10.1093/rheumatology/40.6.662. [DOI] [PubMed] [Google Scholar]
- 107.Bassi C, Xavier DJ, Palomino G, Nicolucci P, Soares C, Sakamoto-Hojo E, Donadi E. Efficiency of the DNA Repair and Polymorphisms of the XRCC1, XRCC3 and XRCC4 DNA Repair Genes in Systemic Lupus Erythematosus. Lupus. 2008;17:988–95. doi: 10.1177/0961203308093461. [DOI] [PubMed] [Google Scholar]
- 108.Takeda Y, Dynan S. Autoantibodies Against DNA Double-Strand Break repair proteins. Front. Biosci. 2001;6:1412–1422. doi: 10.2741/takeda. [DOI] [PubMed] [Google Scholar]
- 109.Pavlovic M, Kats A, Cavallo M, Chen R, Hartmann J X, Shoenfeld Y. Pathogenic and Epiphenomenal Anti-DNA Antibodies in SLE. Autoimmune Dis. 2011:462841. doi: 10.4061/2010/462841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davies R C, Pettijohn K, Fike F, Wang J, Nahas S A, Tunuguntla R, Hu H, Gatti R. a; McCurdy, D. Defective DNA Double-strand Break Repair in Pediatric Systemic Lupus Erythematosus. Arthritis Rheum. 2012;64:568–78. doi: 10.1002/art.33334. [DOI] [PubMed] [Google Scholar]
- 111.Warchol T, Mostowska A, Lianeri M, Lacki J K, Jagodzinski P P. XRCC1 Arg399Gln Gene Polymorphism and the Risk of Systemic Lupus Erythematosus in the Polish Population. DNA Cell Biol. 2012;31:50–6. doi: 10.1089/dna.2011.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee K J, Dong X, Wang J, Takeda Y, Dynan W S. Identification of Human Autoantibodies to the DNA Ligase IV/XRCC4 Complex and Mapping of an Autoimmune Epitope to a Potential Regulatory Region. J. Immunol. 2002;169:3413–21. doi: 10.4049/jimmunol.169.6.3413. [DOI] [PubMed] [Google Scholar]
- 113.Lamerdin JE, Montgomery M, Stilwagen SA, Scheidecker LK, Tebbs RS, Brookman KW, Thompson LH, Carrano AV. Genomic Sequence Comparison of the Human and Mouse XRCC1 DNA Repair Gene Regions. Genomics. 1995;25:547–54. doi: 10.1016/0888-7543(95)80056-r. [DOI] [PubMed] [Google Scholar]
- 114.Lin Y J, Wan L, Huang C M, Chen S Y, Huang Y C, Lai C H, Lin W Y, Liu H P, Wu Y S, Chen C M, Tsai Y H, Tsai C H, Sheu J J, Tsai F J. Polymorphisms in the DNA Repair Gene XRCC1 and Associations with Systemic Lupus Erythematosus Risk in the Taiwanese Han Chinese Population. Lupus. 2009;18:1246–51. doi: 10.1177/0961203309345777. [DOI] [PubMed] [Google Scholar]
- 115.Fasching P A, Gayther S, Pearce L, Schildkraut J M, Goode E, Thiel F, Chenevix-Trench G, Chang-Claude J, Wang-Gohrke S, Ramus S, Pharoah P, Berchuck A. OCAC (Ovarian Cancer Association Consortium).Role of genetic polymorphisms and ovarian cancer susceptibility. Mol. Ocol. 2009;2:171–81. doi: 10.1016/j.molonc.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tseng RC, Hsieh FJ, Shih CM, Hsu HS, Chen CY, Wang YC. Lung cancer susceptibility and prognosis associated with polymorphisms in the nonhomologous end-joining pathway genes: a multiple genotype-phenotype study. Cancer. 2009;13:2939–48. doi: 10.1002/cncr.24327. [DOI] [PubMed] [Google Scholar]
- 117.Schildkraut JM, Iversen ES, Wilson MA, Clyde MA, Moorman PG, Palmieri RT, Whitaker R, Bentley RC, Marks JR, Berchuck A. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;4:e10061. doi: 10.1371/journal.pone.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Azevêdo Silva J, Pancotto JAT, Donadi EA, Crovella C, Sandrin-Garcia P. LIG4 and RAD52 DNA repair genes polymorphisms and Systemic Lupus Erythematosus. Mol. Bio. Rep. 2013;1 doi: 10.1007/s11033-014-3076-y. [DOI] [PubMed] [Google Scholar]
- 119.Fonseca AMS, Pancotto JAT, Donadi EA, Segat L, Crovella S, Sandrin-Garcia P. Polymorphisms in STK17A gene are associated to Systemic Lupus Erythematosus and its Clinical Manifestations. Gene. 2013;2:435–9. doi: 10.1016/j.gene.2013.06.074. [DOI] [PubMed] [Google Scholar]
- 120.Sanjo H, Kawai T, Akira S. DRAKs, Novel Serine/threonine Kinases Related to Death-associated Protein Kinase That Trigger Apoptosis. J. Biol. Chem. 1998;273:29066–71. doi: 10.1074/jbc.273.44.29066. [DOI] [PubMed] [Google Scholar]
- 121.Mao P, Hever M P, Niemaszyk L M, Haghkerdar J M, Yanco E G, Desai D, Beyrouthy M J, Kerley-Hamilton J S, Freemantle S J, Spinella M J. Serine/threonine Kinase 17A Is a Novel P53 Target Gene and Modulator of Cisplatin Toxicity and Reactive Oxygen Species in Testicular Cancer Cells. J. Biol. Chem. 2011;286:19381–91. doi: 10.1074/jbc.M111.218040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ding Q, Reddy Y V R, Wang W, Woods T, Douglas P, Ramsden D A, Lees-miller S P, Meek K. Autophosphorylation of the Catalytic Subunit of the DNA-Dependent Protein Kinase is Required for Efficient End Processing During DNA Double-Strand Break Repair. Mol. Cell. Biol. 2003:5836–48. doi: 10.1128/MCB.23.16.5836-5848.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohnishi T, Mori E, Takahashi A. DNA Double-strand Breaks: Their Production, Recognition, and Repair in Eukaryotes. Mutation Res. 2009;669:8–12. doi: 10.1016/j.mrfmmm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 124.Baechler E C, Batliwalla F M, Karypis G, Gaffney P M, Ortmann W A, Espe K J, Shark K B, Grande W J, Hughes K M, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible Gene Expression Signature in Peripheral Blood Cells of Patients with Severe Lupus. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2610–5. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bengtsson A, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Rönnblom L. Activation of Type I Interferon System in Systemic Lupus Erythematosus Correlates with Disease Activity but Not with Antiretroviral Antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 126.Rönnblom L, Alm G V, Eloranta M-L. The Type I Interferon System in the Development of Lupus. Sem. Immunol. 2011;23:113–21. doi: 10.1016/j.smim.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 127.Salloum R, Niewold T B. Interferon Regulatory Factors in Human Lupus Pathogenesis. Transl. Res. 2011;157:326–31. doi: 10.1016/j.trsl.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chistiakov D A. Interferon Induced with Helicase C Domain 1 (IFIH1) and Virus-induced Autoimmunity: a Review. Vir. Immunol. 2010;23:3–15. doi: 10.1089/vim.2009.0071. [DOI] [PubMed] [Google Scholar]
- 129.Lenert P. Nucleic Acid Sensing Receptors in Systemic Lupus Erythematosus.Development of Novel DNA- And/or RNA-like Analogues for Treating Lupus. Clin. Exp. Immunol. 2010;161:208–22. doi: 10.1111/j.1365-2249.2010.04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cunninghame Graham D S, Morris D L, Bhangale T R, Criswell L A, Syvänen A-C, Rönnblom L, Behrens T W, Graham R R, Vyse T J. Association of NCF2, IKZF1, IRF8, IFIH1, and TYK2 with Systemic Lupus Erythematosus. PLoS Genetics. 2011;7:e1002341. doi: 10.1371/journal.pgen.1002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cen H, Leng R, Wang W, Zhou M, Feng C, Zhu Y, Yang X, Yang M, Zhai Y, Li B, Liu J, Pan HF, Ye DQ. Association Study of IFIH1 rs1990760 Polymorphism with Systemic Lupus Erythematosus in a Chinese Population. Inflammation. 2013;2:444–48. doi: 10.1007/s10753-012-9564-0. [DOI] [PubMed] [Google Scholar]
- 132.Moura R, De Azevêdo Silva J, Pancotto J AT, Donadi EA, Brandão LAC, Crovella S, Sandrin-Garcia P. Interferon-Induced with Helicase C Domain 1 (IFIH1) gene polymorphisms and meta-analysis study in Systemic Lupus Erythematosus. J. Mol. Biol. Rep. 2013:1. [Google Scholar]
- 133.Kawasaki A, Kyogoku C, Ohashi J, Miyashita R, Hikami K, Kusaoi M, Tokunaga K, Takasaki Y, Hashimoto H, Behrens T W, Tsuchiya N. Association of IRF5 Polymorphisms with Systemic Lupus Erythematosus in a Japanese Population: Support for a Crucial Role of Intron 1 Polymorphisms. Arthritis Rheum. 2008;58:826–34. doi: 10.1002/art.23216. [DOI] [PubMed] [Google Scholar]
- 134.Cham C M, Ko K, Niewold T B. Interferon Regulatory Factor 5 in the Pathogenesis of Systemic Lupus Erythematosus. Clin. Dev. Immunol. 2012;2012:780436. doi: 10.1155/2012/780436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Alarcón-Riquelme M E, Prokunina L. Finding Genes for SLE: Complex Interactions and Complex Populations, Journal of Autoimmunity. J. Autoimmun. 2003;21:117–120. doi: 10.1016/s0896-8411(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 136.Sigurdsson S, Nordmark G, Göring H H H, Lindroos K, Wiman A-C, Sturfelt G, Jönsen A, Rantapää-Dahlqvist S, Möller B, Kere J, Koskenmies S, Widén E, Eloranta ML, Julkunen H, Kristjansdottir H, Steinsson K, Alm G, Rönnblom L, Syvänen AC. Polymorphisms in the Tyrosine Kinase 2 and Interferon Regulatory Factor 5 Genes Are Associated with Systemic Lupus Erythematosus. J. Hum. Genet. 2005;76:528–37. doi: 10.1086/428480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Graham R R, Hom G, Ortmann W, Behrens T W. Review of Recent Genome-wide Association Scans in Lupus. J. Int. Med. 2009;265:680–8. doi: 10.1111/j.1365-2796.2009.02096.x. [DOI] [PubMed] [Google Scholar]
- 138.Stone R C, Du P, Feng D, Dhawan K, Rönnblom L, Eloranta M-L, Donnelly R, Barnes B J. RNA-Seq for Enrichment and Analysis of IRF5 Transcript Expression in SLE. Plo One. 2013; 8:e54487. doi: 10.1371/journal.pone.0054487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cherian T S, Kariuki S N, Franek B S, Buyon J P, Clancy R M, Niewold T B. Brief Report: IRF5 Systemic Lupus Erythematosus Risk Haplotype Is Associated with Asymptomatic Serologic Autoimmunity and Progression to Clinical Autoimmunity in Mothers of Children with Neonatal Lupus. Arthritis Rheum. 2012;64:3383–7. doi: 10.1002/art.34571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niewold T B, Adler J E, Glenn S B, Lehman T J A, Harley J B, Crow M K. Age- and Sex-Related Patterns of Serum Interferon-a Activity in Lupus Families. Arthritis Rheum. 2009;58:2113–2119. doi: 10.1002/art.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ntali S, Karim M Y. Autoantibodies as Markers for Detecting Concurrent Disease Activity in Systemic Lupus Erythematosus. Transl. Res. 2010;156:317–9. doi: 10.1016/j.trsl.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Qin L, Lv J, Zhou X, Hou P, Yang H, Zhang H. Association of IRF5 Gene Polymorphisms and Lupus Nephritis in a Chinese Population. Nephrol. 2010;15:710–3. doi: 10.1111/j.1440-1797.2010.01327.x. [DOI] [PubMed] [Google Scholar]
- 143.Järvinen T M, Hellquist A, Koskenmies S, Einarsdottir E, Koskinen L L E, Jeskanen L, Berglind L, Panelius J, Hasan T, Ranki A, Kere J, Saarialho-Kere U. Tyrosine Kinase 2 and Interferon Regulatory Factor 5 Polymorphisms Are Associated with Discoid and Subacute Cutaneous Lupus Erythematosus. Exp. Dermatol. 2010;19:123–31. doi: 10.1111/j.1600-0625.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 144.Lee Y H, Choi S J, Ji J D, Song G G. Associations Between PXK and TYK2 Polymorphisms and Systemic Lupus Erythematosus: a Meta-analysis. Inflamm. Res. 2012;61:949–54. doi: 10.1007/s00011-012-0486-y. [DOI] [PubMed] [Google Scholar]
- 145.Kyogoku C, Morinobu A, Nishimura K, Sugiyama D, Hashimoto H, Tokano Y, Mimori T, Terao C, Matsuda F, Kuno T. Kumagai, S. Lack of Association Between Tyrosine Kinase 2 (TYK2) Gene Polymorphisms and Susceptibility to SLE in a Japanese Population. Mod. Rheumatol. 2009;19:401–6. doi: 10.1007/s10165-009-0173-1. [DOI] [PubMed] [Google Scholar]
- 146.Li P, Chang Y U K K, Shek K A W A I, Lau Y U L. Lack of Association of TYK2 Gene Polymorphisms in Chinese Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2011;38:1–3. doi: 10.3899/jrheum.100424. [DOI] [PubMed] [Google Scholar]
- 147.Dos Santos B P, Valverde J V, Rohr P, Monticielo O A, Brenol J C T, Xavier R M, Chies J A B. TLR7/8/9 Polymorphisms and Their Associations in Systemic Lupus Erythematosus Patients from Southern Brazil. Lupus. 2012;21:302–9. doi: 10.1177/0961203311425522. [DOI] [PubMed] [Google Scholar]
- 148.Xu Y, Fan H, Li X, Sun L, Hou Y. 17ß-Estradiol Enhances Response of Mice Spleen B Cells Elicited by TLR9 Agonist. Cell. Immunol. 2012;278:125–35. doi: 10.1016/j.cellimm.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 149.Haas T, Metzger J, Schmitz F, Heit A, Müller T, Latz E, Wagner H. The DNA Sugar Backbone 2’ Deoxyribose Determines Toll-like Receptor 9 Activation. Immun. 2008;28:315–23. doi: 10.1016/j.immuni.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 150.Møller-Larsen S, Nyegaard M, Haagerup A, Vestbo J, Kruse T A, Børglum A D. Association Analysis Identifies TLR7 and TLR8 as Novel Risk Genes in Asthma and Related Disorders. Thorax. 2008;63:1064–9. doi: 10.1136/thx.2007.094128. [DOI] [PubMed] [Google Scholar]
- 151.Lazarus R, Klimecki W T, Raby B A, Vercelli D, Palmer L J, Kwiatkowski D J, Silverman E K, Martinez F, Weiss S T. Single-nucleotide Polymorphisms in the Toll-like Receptor 9 Gene (TLR9): Frequencies, Pairwise Linkage Disequilibrium, and Haplotypes in Three U.. Ethnic Groups and Exploratory Case-control Disease Association studies. Genomics. 2003;81:85–91. doi: 10.1016/s0888-7543(02)00022-8. [DOI] [PubMed] [Google Scholar]
- 152.Gantier M P, Irving A T, Kaparakis-Liaskos M, Xu D, Evans V A, Cameron P U, Bourne J A, Ferrero R L, John M, Behlke M A. Williams, B. Genetic Modulation of TLR8 Response Following Bacterial Phagocytosis.. Hum. Mutation. 2010;31:1069–79. doi: 10.1002/humu.21321. [DOI] [PubMed] [Google Scholar]
- 153.Papadimitraki E D, Tzardi M, Bertsias G, Sotsiou E, Boumpas D T. Glomerular Expression of Toll-like Receptor-9 in Lupus Nephritis but Not in Normal Kidneys: Implications for the Amplification of the Inflammatory Response. Lupus. 2009;18:831–5. doi: 10.1177/0961203309103054. [DOI] [PubMed] [Google Scholar]
- 154.Conti F, Spinelli F R, Alessandri C, Valesini G. Toll-like Receptors and Lupus Nephritis. Clin. Rev. Allerg. Immu. 2011;40:192–8. doi: 10.1007/s12016-010-8208-0. [DOI] [PubMed] [Google Scholar]
- 155.Piotrowski P, Lianeri M, Wudarski M, Olesinska M, Jagodzinski P P. Contribution of STAT4 Gene Single-nucleotide Polymorphism to Systemic Lupus Erythematosus in the Polish Population. Mol. Biol. Rep. 2012;39:8861–6. doi: 10.1007/s11033-012-1752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacob CO, Zang S, Li L, Ciobanu V, Mizutani A, Satoh M, Koss M, Quismorio F. Pivotal role of Stat4 and Stat6 in the pathogenesis of the lupus-like disease in the New Zealand mixed 2328 mice. J. Immunol. 2003;171:1564–71. doi: 10.4049/jimmunol.171.3.1564. [DOI] [PubMed] [Google Scholar]
- 157.Remmers E F, Plenge R M, Lee A T, Graham R R, Hom G, Behrens T W, Bakker P I W de, Le J M, Lee H-S, Batliwalla F, Li W, Masters SL, Booty MG, Carulli JP, Padyukov L, Alfredsson L, Klareskog L, Chen WV, Amos CI, Criswell LA, Seldin MF, Kastner DL. Gregersen, P STAT4 and the Risk of Rheumatoid Arthritis and Systemic Lupus Erythematosus. . N. Engl. J. Med. 2007;357:977–86. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng J, Yin J, Huang R, Petersen F, Yu X. Meta-analysis Reveals an Association of STAT4 Polymorphisms with Systemic Autoimmune Disorders and anti-dsDNA Antibody. Hum. Immunol. 2013;74:986–92. doi: 10.1016/j.humimm.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 159.Taylor KE, Remmers EF, Lee AT, Ortmann WA, Plenge RM, Tian C, Chung S, Nititham J, Hom G, Kao AH, Demirci FY, Kamboh MI, Petri M, Manzi S, Kastner DL, Seldin MF, Gregersen PK, Behrens TW, Criswell LA. Specificity of the STAT4 Genetic Association for Severe Disease Manifestations of Systemic Lupus Erythematosus. PLoS Genet. 2008;4:e1000084. doi: 10.1371/journal.pgen.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]