Abstract
Cryptococcus species are fungal pathogens that are a leading cause of mortality. Initial inoculation is through the pulmonary route and, if disseminated, results in severe invasive infection including meningoencephalitis. Macrophages are the dominant phagocytic cell that interacts with Cryptococcus. Emerging theories suggest that Cryptococcus microevolution in macrophages is linked to survival and virulence within the host. In addition, Cryptococcus elaborates virulence factors as well as usurps host machinery to establish macrophage activation states that are permissive to intracellular survival and replication. In this review, we provide an update of the recent findings pertaining to macrophage interaction with Cryptococcus and focus on new avenues for biomedical research.
Keywords: carbohydrate, glycosylation, Cryptococcus, fungi, macrophage, innate immunity, phagosome
Introduction
Cryptococcus species are human yeast pathogens with significant contribution to global mortality in the immunocompromised population (1, 2). Worldwide, the majority of these patients suffer from acquired immunodeficiency syndrome (AIDS) due to the human immunodeficiency virus (HIV), highlighting the critical nature of adaptive immunity to invasive cryptococcal infection. In addition, we care for a growing immunocompromised population directly related to the use of immunomodulatory agents necessary to prevent graft rejection and failure in solid and hematopoietic stem cell transplantation recipients. Moreover, biologic agents are gaining acceptance as first-line treatment regimens for even the most common of autoimmune and rheumatologic diseases. Collectively, AIDS and the increased use of immunomodulatory agents has made encountering serious, life-threatening cryptococcal infections a front-line possibility regardless of the global location or socioeconomic status of our patient populations.
The majority of cryptococcosis is caused by the pathogenic species Cryptococcus neoformans and Cryptococcus gatti. C. neoformans has a ubiquitous, worldwide distribution, whereas C. gatti is typically limited to tropical and subtropical locations, with an exception of an outbreak in the northwest region of the United States and Canada (3–6). C. neoformans typically infects immunocompromised patients whereas C. gatti appears to be hypervirulent infecting seemingly immunocompetent individuals (7). C. neoformans has been isolated from soil and bird guano with high concentration in urban settings. Inhalation of Cryptococcus basidiospores or desiccated yeast cells results in pulmonary infection (8, 9). The initial interaction with the host innate immune system typically results in fungal clearance and elimination whereas in the setting of the immune compromised individual, there is an increased probability for failed pathogen control that may disseminate infection resulting in pneumonia, fungemia, or meningitis. Despite the isolation of Cryptococcus species from nearly every organ, meningoencephalitis is the most common clinical manifestation of invasive cryptococcal disease and underscores the neurotropism of Cryptococcus species (2).
Host immunity to C. neoformans requires a multifactorial, well-coordinated innate and adaptive response (10). The prevalence of disease in patients with AIDS highlights the critical nature of CD4+ T cells. CD4+ T cells are protective, and when profiled both IFN-γ and IL-4 secreting populations are noted in response to Cryptococcus representing Th-1 and Th-2 type CD4+ T cells, respectively. Th-1 type responses are superior in protection, and depletion of Th-2 type CD4+ T cells will accentuate a Th-1 response (11–13). Nonetheless, despite a dominant Th-1 response, mice are not fully protected hinting of another adaptive immune element. In the early 2000, a new T cell subset was described that is primed by IL-23 and produces high levels of IL-17, now termed Th-17 CD4+ T cells (reviewed in (14)). IL-17 deficient mice are highly susceptible to fungal infection and both Th-1 and Th-17 CD4+ T cells are critical for anti-fungal immunity (14–19). In addition, complement, antibody and NK cells play a critical role in anti-cryptococcal immunity (20–22). Naturally, not all host immune responses are similar, and the impact of host genetic diversity including polymorphisms involved in antibody responses as well as receptor-mediated recognition of fungi is growing and requires further clarification (23–26).
While adaptive responses result in long-lasting immunity, innate immune cells play a central role in eliminating the majority of encountered Cryptococci, and trigger adaptive immunity through antigen presentation. Macrophages carry the lion’s share of duty for cryptococcal elimination from the host. Macrophages are highly phagocytic innate cells that are bone marrow derived and found in all tissue types and organs acting as front-line immune surveyors.
This review emphasizes emerging evidence of the importance tissue macrophages play in cryptococcal recognition, phagocytosis, and growth control by tissue macrophages. A deeper understanding of the molecular mechanisms involved in early recognition of Cryptococcal species and subsequent intracellular control provides the critical steps towards development of anti-cryptococcal vaccine and rational immune therapies.
Cryptococcal capsular molecules
Cryptococcus species uniquely possess a large, thick polysaccharide capsule primarily composed of glucuronoxylomannan (GXM) including the minor components glucuronoxylomannogalactan (GXMGal) and mannoproteins (27). Cryptococcus can enlarge or thin the GXM capsule depending on environmental cues (10). The capsule has profound anti-phagocytic properties and masks underlying innate immune ligands on the Cryptococcus surface (27). In fact, in the absence of either complement or antibody opsonization of the capsule, Cryptococcus is not ingested (28). In addition, soluble molecules can be found associated with the capsule, which often are released or shed, such as mannoprotein species (29–32). Recently, secreted C. neoformans heat shock protein-70 (hsp70) was described to have immune modulating properties (33). C. neoformans hsp70 bound to the surface of macrophages competing with GXM, therefore suggesting hsp70 recognition is through TLR4, TLR2, CD18 and/or CD14, all known receptors in the recognition of GXM (33, 34). Macrophage exposure to C. neoformans hsp70 resulted in decreased fungal killing as well as nitric oxide (NO) production and may represent a novel virulence factor. Vaccine or pharmaceutical strategies that interrupt Cryptococcus hsp70 function may offer promising options for the treatment and prevention of invasive cryptococcosis.
In vivo intracellular adaptation
Cryptococcus species are facultative intracellular organisms with extra- and intracellular growth phases with the ability to persist for years in infected individuals. Several recent studies highlight the ability of C. neoformans to adapt to in vivo conditions. Using a in vivo rodent model of chronic meningoencephalitis, the isolation of C. neoformans over a period of time suggests that older generations of yeast have undergone microevolution resulting in enhanced resistance to stress and phagocytic killing within macrophages (35). Interestingly, there is an extension of this observation to human disease. C. neoformans was isolated from cerebrospinal fluid collected from patients with cryptococcal meningitis. Cryptococcus isolates from patients with poor clinical outcomes had enhanced ability to survive within macrophages and undergo intraphagosomal replication, supporting the possibility of in vivo microevolution and selection towards a more fit phenotype (36). The microevolution of C. neoformans over time appears to have, in part, a molecular explanation in the composition of the capsule. A comparison of the biophysical and chemical profiles of the capsule between younger and older generations showed uniquely distinct carbohydrate moieties. C. neoformans isolates from later generations demonstrate enhanced anti-phagocytic properties suggesting that Cryptococcal capsules have a unique plasticity that allow adaptation to the host environment for prolonged survival (37). The precise intracellular cues that govern cryptococcal microevolution through altering capsular carbohydrate composition require further investigation.
The epidemiology of cryptococcal meningitis shows infection to be more common in men. The effect of gender differences on macrophage-specific control of C. neoformans was recently explored. Male-derived macrophages isolated from patients infected with Cryptococcus were found to be more permissive for intracellular C. neoformans. Compared to female controls, male macrophages allowed higher rates of replication and had higher fungal burdens (38) suggesting gender-specific host elements such as hormones may play a role in modulating host macrophage fungicidal activity.
Mounting evidence suggests that evolutionary adaptation for intracellular survival developed by Cryptococcus originate from the predation of environmental organisms such as acanthamoeba (soil protozoa). Indeed, C. neoformans gene expression following ingestion by amoeba and macrophages reveals similar patterns of gene expression (39). In fact, multiple stress gene response elements are linked to Cryptococcus-specific environmental sensors and function through common kinase signaling pathways. PKH2-02, a homolog to mammalian PDK1 kinase, in C. neoformans is required for MAPK activation in response to cell wall and oxidative stress (40). Cryptococcal mutants lacking PKH2-02 are hypovirulent in a Galleria invertebrate model and are more susceptible to the anti-fungal agents, fluconazole and amphotericin B when compared with wild-type controls (40).
While there is mounting evidence that C. neoformans has undergone evolutionary selective pressure through interactions with soil amoeba over years, it is remarkable that Cryptococcus species remain heterogeneous with a broad adaptability permitting accelerated evolutionary adaptation once faced with in vivo host conditions.
Macrophage state of activation
The activation state of resident macrophages can influence their fungicidal activity. Classically activated macrophages generated under the influence of Th-1 CD4+ T cell cytokines such as IFN-γ have enhanced capacity to generate reactive species such as NO for intracellular killing. On the other hand, alternatively activated macrophages skewed under the influence of Th-2 CD4+ T cells secreting IL-4 and IL-13 have poor ability to contain fungal pathogen growth (10, 27). At a tissue or organ level, a balance exists between both classically and alternatively activated tissue macrophage populations. Furthermore, recent data demonstrates that these populations are not committed to their activation phenotype but can transition fluidly between both activation states depending on the inflammatory mileu (41). The use of genetic engineering to alter cytokine profiles and influence macrophage activation states for enhanced C. neoformans control has been explored. A mouse with a selective IL-4 receptor deficient macrophage lineage using a LysM-Cre IL-4Rα system, which therefore allowed development of a predominantly classically activated macrophage population (lack of Th-2 influence) shows near complete protection to C. neoformans pulmonary challenge with significantly reduced fungal burdens (42). Similar to C. neoformans, enhancing classically activated macrophages also appear to be effective for controlling other intracellular pathogens such as Leishmania species through reactive species generation such as NO (42, 43).
IL-33 is a cytokine expressed by the epithelial cells of the lung, gut, and brain and is released in pulmonary tissue following C. neoformans infection (44). The effect of IL-33 on macrophage activation state was recently described. In wild-type mice, IL-33 resulted in polarization of Th-2 type CD4+ T cells and the induction of alternatively activated alveolar macrophages. IL-33 receptor deficient mice had decreased numbers of alternatively activated macrophages and reduced hallmarks of Th-2 inflammation, specifically IgE and alveolar eosinophils. Following C. neoformans infection, IL-33 deficient mice had a lower fungal burden both locally in the lung as well disseminated to brain. IL-33 deficient mice show increased survival compared to wild-type supporting improved fungicidal control with classically activated macrophages as the effector innate immune cells (44).
Cryptococcus elaborates virulence factors capable of skewing macrophage activation state to promote survival. Laccase, an enzyme responsible for production of prostaglandin and melanin pigments from C. neoformans, has multiple roles in immune evasion (45). Historically, laccase was known to be crucial for the production of C. neoformans melanin pigment, which shields the yeast from killing by quenching reactive radical species (46). Recently, laccase has been shown to be essential for the production of prostaglandins, which regulate the activation of host macrophages. Prostaglandins promote alternatively activated macrophages therefore promoting migration and carriage of intracellular cryptococcal organisms resulting in dissemination. A laccase deficient C. neoformans mutant demonstrates a shift towards a classically activated macrophage phenotype with increased expression of MHC class II, IFN-γ, TNF-α, IL-17A but has decreased expression of IL-10 and IL-4 (45). Furthermore, laccase deficiency blocked dissemination to the central nervous system (CNS) suggesting that prostaglandin-mediated Th-2 phenotype with alternatively activated macrophages is required for disseminated Cryptococcus infection.
In addition to yeast-specific virulence factors, Cryptococcus is capable of utilizing host proteins for immune evasion. Scavenger receptors comprise recognize fungal cell wall components, specifically β-1,3-glucan, and are expressed on macrophages, dendritic cells, and endothelial cells (47). SCARF1 and CD36, both scavenger receptors, recognize and bind C. neoformans (47). CD36 deficient mice are more susceptible to C. neoformans infection as compared to wild-type controls (47). More recently, the role of the scavenger family member, scavenger receptor A (SRA), towards C. neoformans immunity was elucidated. In contrast to CD36, SRA deficient mice subjected to a pulmonary model of C. neoformans infection had reduced fungal burdens and improved survival (48). Analysis of cytokines from lungs of Cryptococcus infected SRA deficient mice revealed lower levels of IL-4 and IL-13. There was also a reduction in eosinophil numbers and levels of IgE. In fact, the upregulation of Th-1 CD4+ type cytokines in SRA deficient mice suggests that classical activation of macrophages is favored compared to alternatively activated alveolar macrophages (48). The mechanisms responsible for SRA-dependent skewing of macrophage activation state do not appear to involve direct fungal interaction and require further investigation.
Novel approaches to immunologically “mimic” Th-1 type activation and favor the induction of classically activated macrophages show dramatic improvement in survival and reductions in fungal load. Using a C. neoformans strain that autonomously produces IFN-γ H99γ in a pulmonary mouse model demonstrates an absence of alternatively activated macrophages, but instead elevated expression of iNOS, IL-17, and IFN-γ (49). Impressively, use of H99γ results in near sterilization of fungal burden compared to wild-type C. neoformans (49) highlighting a potential therapeutic strategy to prime classically activated macrophage population for pulmonary fungal elimination.
Macrophage phagocytosis and phagosome maturation
The Cryptococcus capsule is well known to possess anti-phagocytic properties. In fact, in the absence of opsonization with either antibody or complement, the phagocytosis of C. neoformans by phagocytes is quite poor (27). There are several theories to why the capsule prevents phagocytosis including continued shedding of capsule components therefore inhibiting firm capture and engulfment at the macrophage surface. Capsule concealment of fixed ligands such as mannans or β-glucans from receptors such as the mannose receptor or Dectin-1, respectively (27) is another possibility. In support of this concept is the observation that acapsular mutants are readily phagocytosed in the absence of opsonins. Other modifications of the Cryptococcus capsule, such as galactofuranose, appear to have no effect on phagocytosis or virulence (50). Rare populations of Cryptococcus termed titan cells, often measuring up to 100 microns in diameter, are formed in infected hosts (51). The enlarged size of a titan cell was thought to prevent engulfment, but cross-resistance to phagocytosis of smaller cryptococci and even inert beads has been demonstrated suggesting that titan cells modulate the host immune repertoire (52).
Once phagocytosed, an ingested Cryptococcus is placed into a subcellular organelle termed a phagosome that is formed through the invagination of the surface membrane. The phagosome is a dynamic compartment where continued pathogen sensing and sampling occurs. Recent work suggests that the Toll-like receptors (TLR) including TLR-9 and tetraspanins (CD63, CD82) are recruited to the phagosome for pathogen-sensing purposes through an as of yet undefined mechanism (53–55). Unlike other phagocytes such as neutrophils, macrophages do not rely heavily on reactive species for killing but instead use acidification and degradative enzymes to achieve pathogen elimination. Phagosome maturation is a process that recruits the host machinery to the phagosome such as lysosomes to produce an acidified, enzymatically-contained compartment where fungicidal activity occurs (56, 57). In contrast to the strategies of facultative intracellular pathogens such as Candida glabrata, Mycobacterium tuberculosis, or Coxiella burnetii to inhibit phagosomal function, C. neoformans does not interrupt phagolysosomal maturation, but has evolved to survive in the harsh environment of the phagosome (10).
Host macrophages and Cryptococcus both utilize metals for survival and biologic function. Iron is critical for Cryptococcus neoformans survival. Cig1 an extracellular Cryptococcus mannoprotein has hemophore activity assisting with heme uptake. Loss of Cig1 results in attenuated Cryptococcus virulence (58). Copper has antimicrobial properties and is concentrated in macrophage phagosomes. Recent studies show that C. neoformans has the ability to detoxify copper through the use of metallothionein proteins, which results in increased host expression of the macrophage phagosomal copper transporter, Ctr1 (59, 60). Cryptococcus strains lacking the ability to up regulate copper detoxifying proteins have attenuated virulence properties (59, 60).
The histopathology of disseminated Cryptococcus demonstrates that yeast often replicate as cryptococcomas or collections of fungi in the extracellular space raising the question of cryptococcal escape from macrophages. Cryptococcus can kill and directly rupture host phagocytes but evidence of non-lytic escape from macrophages has been described. Non-lytic escape appears to be dependent on specific phagosomal conditions such as pH (61, 62). Recent studies suggest that the autophagy pathway, a process involved in the recycling of host proteins and organelles but implicated in host defense against pathogens, may affect non-lytic escape. Macrophages with reduced expression of ATG5 (autophagy-related 5), one of several major constituents to the formation of the autophagosome, show impaired ability to phagocytose C. neoformans (63). Mice deficient in ATG5 had fewer alternatively activated macrophages, indicating the autophagy pathway plays a role at multiple points in the interaction of Cryptococcus macrophage interaction. Other evidence shows Cryptococcus serotype-specific interference with host macrophage signaling pathways, specifically NF-κB resulting in decreased macrophage proliferation by affecting the cell cycle at S and G2/M phases as well as induction of apoptosis (64).
The molecular mechanism of cryptococcal dissemination from the lungs remains unclear. Cryptococcus species may require phagocytosis and intracellular transport in macrophages to permit extra-pulmonary dissemination as well as crossing through the tightly regulated blood-brain barrier. Indeed, these macrophage “vectors”, or Trojan horses, appear to be alternatively activated not classically activated macrophages. In support of this hypothesis, the role of pulmonary surfactant, an opsonizing immune molecule found in healthy lungs, and illustrates that surfactant-mediated uptake of Cryptococcus by macrophages was crucial for virulence. Mice deficient in surfactant protein D (SP-D) and challenged with pulmonary C. neoformans had lower fungal burden, and improved survival (65). The addition of exogenous surfactant at the time of cryptococcal challenge resorted virulence suggesting that C. neoformans uses host surfactant as a virulence factor. When examined, SP-D-treated Cryptococcus is efficiently phagocytosed suggesting that the yeast uses SP-D to promote uptake and intracellular carriage (65). Despite data supporting a macrophage vector system, a recent study using a mouse brain transmigration assessment with tagged C. neoformans demonstrates that Cryptococcus itself is capable of performing transcellular migration into the CNS raising the possibility that both macrophage-dependent and –independent routes to CNS entry occur as part of invasive cryptococcal pathogenesis (66). Cryptococcus elaborates a number of virulence factors that make it well suited for intraphagosomal survival allowing use of phagocytosis for extra-pulmonary dissemination.
Future studies
The interaction between tissue macrophages and Cryptococcal species is complex. The ability to decipher the precise molecular mechanisms responsible for the 1) surface receptor interaction, 2) protein repertoire recruited to Cryptococcus-containing phagosomes, 3) steps leading to intracellular elimination versus escape, and 4) virulence factors resulting in classically or alternatively activated macrophage phenotypes will be critical to developing more effective anti-Cryptococcus therapies. A major barrier to achieving these goals is the complexity of the host-pathogen interactions; not only is the capsule composed of complex dynamic carbohydrate structures, but one must account for the secreted virulence factors, and microevolution of the pathogen. One proposed strategy is to utilize a bottom-up approach and generate fungal pathogens with defined surface glycoepitopes from fungal cell walls. This technique has been recently used to synthesize fungal-like particles that mimic the size and shape of Candida albicans. Macrophages recognized the fungal particles as they would C. albicans, producing inflammatory cytokines and undergoing normal phagolysosomal maturation (figure 1) (67, 68). It is certainly possible to mimic other fungal pathogens including C. neoformans, which would allow the microscopic visualization and biochemical evaluation of capsular components interaction with host cells in a step-wise fashion. Another approach is to use genetically modified fungal strains (a top-down strategy) although this method also has limitations because certain fungal components are essential for viability. Given the intricacy of host-fungal interactions, it is clear that multiple complimentary scientific approaches will be required to dissect the complexity of macrophage Cryptococcus interactions.
Figure 1.
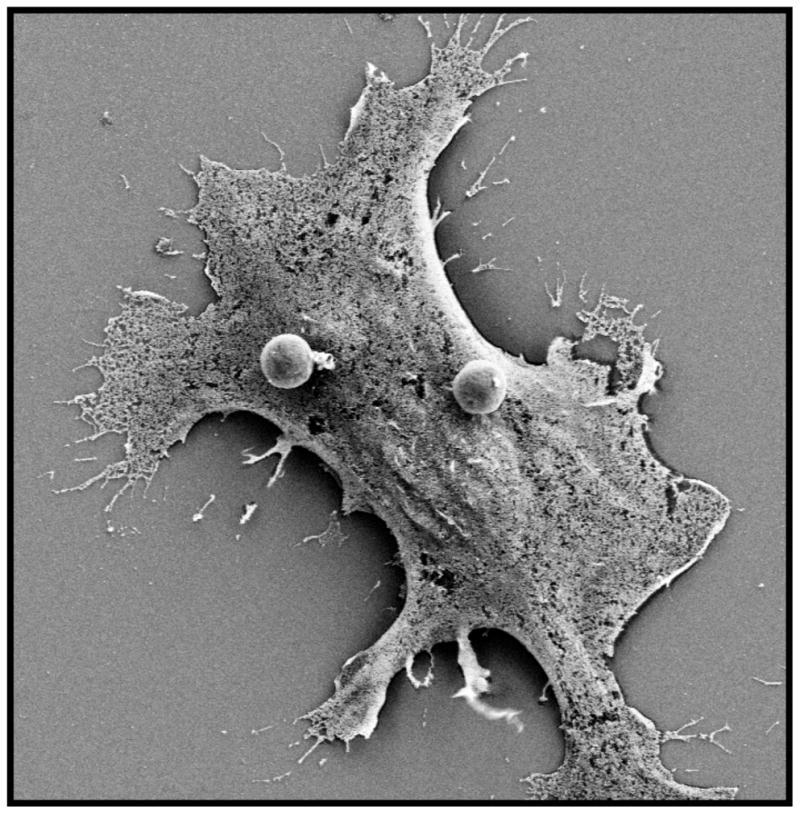
Scanning electron micrograph of a macrophage interacting with synthetic fungal-like β-glucan particles (spheres, two visualized on surface) following brief incubation period. (Authors’ data, unpublished)
Acknowledgments
This work was supported by National Institutes of Health Grants NIAID T32-AI007061-35 (M. K. M., J. L. R.), NIAID 1R01-AI092084 (J. M. V.) and 1R01-AI097519 (J.M.V.).
Footnotes
Conflict of Interest
MK Mansour declares no conflicts of interest.
JL Reedy declares no conflicts of interest.
JM Tam declares no conflicts of interest.
JM Vyas declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Denning DW, Gow NAR, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Science Translational Medicine. 2012;4(165):165rv13. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 3.Kronstad JW, Attarian R, Cadieux B, Choi J, D’souza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9(3):193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrnes EJ, Li W, Lewit Y, Ma H, Voelz K, Ren P, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 2010;6(4):e1000850. doi: 10.1371/journal.ppat.1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdougall L, Kidd SE, Galanis E, Mak S, Leslie MJ, Cieslak PR, et al. Spread of Cryptococcus gattii in British Columbia, Canada, and detection in the Pacific Northwest, USA. Emerg Infect Dis. 2007;13(1):42–50. doi: 10.3201/eid1301.060827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta K, Bartlett KH, Baer R, Byrnes E, Galanis E, Heitman J, et al. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185–91. doi: 10.3201/eid1508.081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437(7063):1360–4. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 8.Botts MR, Hull CM. Dueling in the lung: how Cryptococcus spores race the host for survival. Curr Opin Microbiol. 2010;13(4):437–42. doi: 10.1016/j.mib.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giles SS, Dagenais TRT, Botts MR, Keller NP, Hull CM. Elucidating the Pathogenesis of Spores from the Human Fungal Pathogen Cryptococcus neoformans. Infect Immun. 2009;77(8):3491–500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho C, Bocca A, Casadevall A. The Intracellular Life of Cryptococcus neoformans. Annu Rev Pathol Mech Dis. 2013 doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis JN, Casazza JP, Stone HH, Meintjes G, Lawn SD, Levitz SM, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. Journal of Infectious Diseases. 2013;207(12):1817–28. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackstock R, Murphy JW. Role of interleukin-4 in resistance to Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 2004;30(1):109–17. doi: 10.1165/rcmb.2003-0156OC. [DOI] [PubMed] [Google Scholar]
- 13.Steele C, Wormley FL. Immunology of fungal infections: lessons learned from animal models. Current Opinion in Microbiology. 2012;15(4):413–9. doi: 10.1016/j.mib.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11(5):425–35. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Zielinski CE, Lanzavecchia A. Human Th17 subsets. Eur J Immunol. 2012;42(9):2215–20. doi: 10.1002/eji.201242741. [DOI] [PubMed] [Google Scholar]
- 16.Wozniak KL, Hardison SE, Kolls JK, Wormley FL. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS ONE. 2011;6(2):e17204. doi: 10.1371/journal.pone.0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wang F, Tompkins KC, Mcnamara A, Jain AV, Moore BB, et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. The American Journal of Pathology. 2009;175(6):2489–500. doi: 10.2353/ajpath.2009.090530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Santos N, Huppler AR, Peterson AC, Khader SA, Mckenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunology. 2013;6(5):900–10. doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladiator A, Wangler N, Trautwein-Weidner K, Leibundgut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. Journal of immunology (Baltimore, Md : 1950) 2013;190(2):521–5. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 20.Price MS, Perfect JR. Host defenses against cryptococcosis. Immunol Invest. 2011;40(7–8):786–808. doi: 10.3109/08820139.2011.605196. [DOI] [PubMed] [Google Scholar]
- 21.LeibundGut-Landmann S, Wüthrich M, Hohl TM. Immunity to fungi. Curr Opin Immunol. 2012;24(4):449–58. doi: 10.1016/j.coi.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li SS, Kyei SK, Timm-Mccann M, Ogbomo H, Jones GJ, Shi M, et al. The NK Receptor NKp30 Mediates Direct Fungal Recognition and Killing and Is Diminished in NK Cells from HIV-Infected Patients. Cell Host & Microbe. 2013;14(4):387–97. doi: 10.1016/j.chom.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Chai LYA, de Boer MGJ, van der Velden WJFM, Plantinga TS, van Spriel AB, Jacobs C, et al. The Y238X stop codon polymorphism in the human β-glucan receptor dectin-1 and susceptibility to invasive aspergillosis. Journal of Infectious Diseases. 2011;203(5):736–43. doi: 10.1093/infdis/jiq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361(18):1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Netea MG. Toward Identification of the Genetic Risk Profile for Cryptococcal Disease in HIV-Infected Patients. mBio. 2013;4(5) doi: 10.1128/mBio.00798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohatgi S, Gohil S, Kuniholm MH, Schultz H, Dufaud C, Armour KL, et al. Fc gamma receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. mBio. 2013;4(5):e00573–13. doi: 10.1128/mBio.00573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnston SA, May RC. Cryptococcus interactions with macrophages: evasion and manipulation of the phagosome by a fungal pathogen. Cellular Microbiology. 2013;15(3):403–11. doi: 10.1111/cmi.12067. [DOI] [PubMed] [Google Scholar]
- 28.Levitz SM. Innate recognition of fungal cell walls. PLoS Pathog. 2010;6(4):e1000758. doi: 10.1371/journal.ppat.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levitz SM, Nong S, Mansour MK, Huang C, Specht CA. Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans. Proc Natl Acad Sci USA. 2001;98(18):10422–7. doi: 10.1073/pnas.181331398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mansour MK, Yauch LE, Rottman JB, Levitz SM. Protective efficacy of antigenic fractions in mouse models of cryptococcosis. Infect Immun. 2004;72(3):1746–54. doi: 10.1128/IAI.72.3.1746-1754.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansour MK, Schlesinger LS, Levitz SM. Optimal T cell responses to Cryptococcus neoformans mannoprotein are dependent on recognition of conjugated carbohydrates by mannose receptors. J Immunol. 2002;168(6):2872–9. doi: 10.4049/jimmunol.168.6.2872. [DOI] [PubMed] [Google Scholar]
- 32.Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol. 2006;176(5):3053–61. doi: 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- 33•.Silveira CP, Piffer AC, Kmetzsch L, Fonseca FL, Soares DA, Staats CC, et al. The heat shock protein (Hsp) 70 of Cryptococcus neoformans is associated with the fungal cell surface and influences the interaction between yeast and host cells. Fungal Genet Biol. 2013 doi: 10.1016/j.fgb.2013.08.005. This article shows that Cryptococcus heat-shock protein localizes to the capsule and decreases macrophage fungicidal activity. [DOI] [PubMed] [Google Scholar]
- 34.Yauch LE, Mansour MK, Levitz SM. Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun. 2005;73(12):8429–32. doi: 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Bouklas T, Pechuan X, Goldman DL, Edelman B, Bergman A, Fries BC. Old Cryptococcus neoformans Cells Contribute to Virulence in Chronic Cryptococcosis. mBio. 2013;4(4) doi: 10.1128/mBio.00455-13. This article illustrates that Cryptococcus undergoes in vivo microevolution to a improved survival phenotype. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alanio A, Desnos-Ollivier M, Dromer F. Dynamics of Cryptococcus neoformans-Macrophage Interactions Reveal that Fungal Background Influences Outcome during Cryptococcal Meningoencephalitis in Humans. MBio. 2011;2(4) doi: 10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordero RJB, Pontes B, Guimarães AJ, Martinez LR, Rivera J, Fries BC, et al. Chronological aging is associated with biophysical and chemical changes in the capsule of Cryptococcus neoformans. Infect Immun. 2011;79(12):4990–5000. doi: 10.1128/IAI.05789-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mcclelland EE, Hobbs LM, Rivera J, Casadevall A, Potts WK, Smith JM, et al. The Role of Host Gender in the Pathogenesis of Cryptococcus neoformans Infections. PLoS ONE. 2013;8(5):e63632. doi: 10.1371/journal.pone.0063632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39••.Derengowski LdS, Paes HC, Albuquerque P, Tavares AHFP, Fernandes L, Silva-Pereira I, et al. The transcriptional response of Cryptococcus neoformans to ingestion by Acanthamoeba castellanii and macrophages provides insights into the evolutionary adaptation to the mammalian host. Eukaryotic Cell. 2013;12(5):761–74. doi: 10.1128/EC.00073-13. This study uses gene expression to demonstrate Cryptococcus gene expressiuon is similar in response when interacting with macrophage and ameoba supporting amoeba as the evolutionary selection pressure for intracellular adaptation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chabrier-Roselló Y, Gerik KJ, Koselny K, DiDone L, Lodge JK, Krysan DJ. Cryptococcus neoformans phosphoinositide-dependent kinase 1 (PDK1) ortholog is required for stress tolerance and survival in murine phagocytes. Eukaryotic Cell. 2013;12(1):12–22. doi: 10.1128/EC.00235-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, et al. Macrophage M1/M2 Polarization Dynamically Adapts to Changes in Cytokine Microenvironments in Cryptococcus neoformans Infection. mBio. 2013;4(3) doi: 10.1128/mBio.00264-13. This study demonstrates that macrophage activation state is not a fixed phenotype with an ability to switch between classically to alternatively states depending on inflammatory conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Müller U, Stenzel W, Piehler D, Grahnert A, Protschka M, Köhler G, et al. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing T(h)2 response. International Immunology. 2013;25(8):459–70. doi: 10.1093/intimm/dxt003. [DOI] [PubMed] [Google Scholar]
- 43.Hölscher C, Arendse B, Schwegmann A, Myburgh E, Brombacher F. Impairment of alternative macrophage activation delays cutaneous leishmaniasis in nonhealing BALB/c mice. Journal of immunology (Baltimore, Md : 1950) 2006;176(2):1115–21. doi: 10.4049/jimmunol.176.2.1115. [DOI] [PubMed] [Google Scholar]
- 44•.Flaczyk A, Duerr CU, Shourian M, Lafferty EI, Fritz JH, Qureshi ST. IL-33 Signaling Regulates Innate and Adaptive Immunity to Cryptococcus neoformans. Journal of immunology (Baltimore, Md : 1950) 2013;191(5):2503–13. doi: 10.4049/jimmunol.1300426. This article describes the influence of IL-33 on promoting alternatively activated macrophages that poorly control Cryptococcus. The elimination of IL-33 results in improved survival following Cryptococcus challenge. [DOI] [PubMed] [Google Scholar]
- 45•.Qiu Y, Davis MJ, Dayrit JK, Hadd Z, Meister DL, Osterholzer JJ, et al. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS ONE. 2012;7(10):e47853. doi: 10.1371/journal.pone.0047853. This study highlights Cryptococcus laccase a critical virulence factor for promoting alternatively activated macrophages and enhacing dissemination. Cryptococcus straing deficient in laccase resulted in stimulation of Th-17 T cell and improved animal survival following challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez BL, Nosanchuk JD. Melanin and fungi. Curr Opin Infect Dis. 2003;16(2):91–6. doi: 10.1097/00001432-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Means TK, Mylonakis E, Tampakakis E, Colvin RA, Seung E, Puckett L, et al. Evolutionarily conserved recognition and innate immunity to fungal pathogens by the scavenger receptors SCARF1 and CD36. J Exp Med. 2009;206(3):637–53. doi: 10.1084/jem.20082109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu Y, Dayrit JK, Davis MJ, Carolan JF, Osterholzer JJ, Curtis JL, et al. Scavenger Receptor A Modulates the Immune Response to Pulmonary Cryptococcus neoformans Infection. Journal of immunology (Baltimore, Md : 1950) 2013;191(1):238–48. doi: 10.4049/jimmunol.1203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Hardison SE, Herrera G, Young ML, Hole CR, Wozniak KL, Wormley FL. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. Journal of immunology (Baltimore, Md : 1950) 2012;189(8):4060–8. doi: 10.4049/jimmunol.1103455. This study demonstrates the protective efficiacy of using IFN-γ expressing Cryptococcus to skew a classically activated macrophage state. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heiss C, Skowyra ML, Liu H, Klutts JS, Wang Z, Williams M, et al. Unusual galactofuranose modification of a capsule polysaccharide in the pathogenic yeast Cryptococcus neoformans. Journal of Biological Chemistry. 2013;288(16):10994–1003. doi: 10.1074/jbc.M112.441998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr Opin Microbiol. 2013;16(4):409–13. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryotic Cell. 2012;11(6):820–6. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Artavanis-Tsakonas K, Love JC, Ploegh HL, Vyas JM. Recruitment of CD63 to Cryptococcus neoformans phagosomes requires acidification. Proc Natl Acad Sci USA. 2006;103(43):15945–50. doi: 10.1073/pnas.0607528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Artavanis-Tsakonas K, Kasperkovitz PV, Papa E, Cardenas ML, Khan NS, Van der Veen AG, et al. The tetraspanin CD82 is specifically recruited to fungal and bacterial phagosomes prior to acidification. Infection and Immunity. 2011;79(3):1098–106. doi: 10.1128/IAI.01135-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasperkovitz PV, Khan NS, Tam JM, Mansour MK, Davids PJ, Vyas JM. Toll-like receptor 9 modulates macrophage antifungal effector function during innate recognition of Candida albicans and Saccharomyces cerevisiae. Infect Immun. 2011;79(12):4858–67. doi: 10.1128/IAI.05626-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nature reviews Immunology. 2012 doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9(10):781–95. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cadieux B, Lian T, Hu G, Wang J, Biondo C, Teti G, et al. The Mannoprotein Cig1 supports iron acquisition from heme and virulence in the pathogenic fungus Cryptococcus neoformans. Journal of Infectious Diseases. 2013;207(8):1339–47. doi: 10.1093/infdis/jit029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Ding C, Festa RA, Chen Y-L, Espart A, Palacios Ò, Espín J, et al. Cryptococcus neoformans copper detoxification machinery is critical for fungal virulence. Cell Host Microbe. 2013;13(3):265–76. doi: 10.1016/j.chom.2013.02.002. This study illustrates the use of copper for fungicidal acitivity by host macrophages and the virulence factors elaborated by Cryptococcus neoformans for phagosomal copper detoxification. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waterman SR, Park Y-D, Raja M, Qiu J, Hammoud DA, O’Halloran TV, et al. Role of CTR4 in the Virulence of Cryptococcus neoformans. mBio. 2012;3(5) doi: 10.1128/mBio.00285-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–60. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 62.Nicola AM, Robertson EJ, Albuquerque P, Derengowski LDS, Casadevall A. Nonlytic Exocytosis of Cryptococcus neoformans from Macrophages Occurs In Vivo and Is Influenced by Phagosomal pH. mBio. 2011;2(4):e00167–11-e-11. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicola AM, Albuquerque P, Martinez LR, Dal-Rosso RA, Saylor C, De Jesus M, et al. Macrophage autophagy in immunity to Cryptococcus neoformans and Candida albicans. Infect Immun. 2012;80(9):3065–76. doi: 10.1128/IAI.00358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ben-Abdallah M, Sturny-Leclère A, Avé P, Louise A, Moyrand F, Weih F, et al. Fungal-induced cell cycle impairment, chromosome instability and apoptosis via differential activation of NF-κB. PLoS Pathog. 2012;8(3):e1002555. doi: 10.1371/journal.ppat.1002555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65•.Geunes-Boyer S, Beers MF, Perfect JR, Heitman J, Wright JR. Surfactant protein D facilitates Cryptococcus neoformans infection. Infect Immun. 2012;80(7):2444–53. doi: 10.1128/IAI.05613-11. This study illustrates the essential role of the host protein surfactant protein-D to Cryptococcus pathogensis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tseng H-K, Liu C-P, Price MS, Jong AY, Chang J-C, Toffaletti DL, et al. Identification of genes from the fungal pathogen Cryptococcus neoformans related to transmigration into the central nervous system. PLoS ONE. 2012;7(9):e45083. doi: 10.1371/journal.pone.0045083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Mansour MK, Tam JM, Khan NS, Seward M, Davids PJ, Puranam S, et al. Dectin-1 activation controls maturation of β-1,3-glucan-containing phagosomes. Journal of Biological Chemistry. 2013;288:16043–16054. doi: 10.1074/jbc.M113.473223. This article describes the control exerted by the surface regnition receptor, Dectin-1, on maturation of fungal phagosomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tam JM, Mansour MK, Khan NS, Yoder NC, Vyas JM. Use of fungal derived polysaccharide-conjugated particles to probe Dectin-1 responses in innate immunity. Integr Biol. 2012;4(2):220–7. doi: 10.1039/c2ib00089j. [DOI] [PMC free article] [PubMed] [Google Scholar]


