Abstract
Due to high profile initiatives at the national level, awareness of inadequate pain care affecting many groups in our society has never been greater. Nevertheless, increased awareness of pain disparities and the initiatives to address these disparities have yielded only modest progress, most notably in the form of growing appreciation that pain disparities likely result from multiple factors, including biological, psychological, environmental, health system, and cultural factors. Much less progress has been made in developing interventions that target these multiple determinants to reduce pain management disparities. In this paper we discuss key ethical and methodological challenges that undermine our capacity to investigate and develop meaningful interventions to improve pain outcomes among vulnerable populations. Key challenges in the areas of research engagement, recruitment, design, and measurement are discussed from both scientific and normative standpoints. Specific opportunities within emerging research paradigms to improve designs and measures are also discussed. Finally, we conclude with identifying potential synergies between the pain management disparities research agenda and the broader areas of clinical practice, advocacy, and policy that could help to move the field forward.
Perspective
Researchers studying disparities in pain care face a number of ethical and methodological challenges that must be addressed to advance the field towards eliminating disparities. We discuss these ethical and methodological challenges and propose opportunities for paradigmatic revisions in areas of research engagement, design, measurement, advocacy, and policy.
Keywords: Pain, disparities, research methods, vulnerable populations, advocacy, policy
The year 2010 marked the conclusion of the “Decade of Pain Control and Research.”41 Despite high profile initiatives, pain continues to be a significant clinical problem. Current estimates suggest that at least 100 million adults suffer from chronic pain in the U.S.37 This estimate exceeds the number of people afflicted with cancer, heart disease, and diabetes combined.37 And the economic impact of pain is escalating. In 1998, the estimated costs associated with pain from all causes including health care utilization, loss of productive time, compensation, and litigation were over $100 billion per year.41 In 2011, the annual estimate ranged from $560 to $635 billion.37
Mounting evidence suggests that unrelieved pain results in pathological changes in the central nervous system (central sensitization, cortical reorganization53) as well as functional limitations,19 relational problems,25,26 loss of employment, and depression28 with increasing risk of suicide.2,42,49 The wide-ranging negative physical and psychosocial sequelae and related economic impact are unnecessary because most pain, including cancer pain, can be successfully relieved.36,71
Amidst this backdrop of unrelieved pain and escalating costs across the U.S. population, a growing body of research indicates that pain treatment received by members of racial and ethnic minority groups is more likely to be inadequate.9,11,18,24,30,61 This disproportionate suffering has led to increasing interest in pain management disparities as a distinct area of inquiry for researchers and an area of policy concern for advocates and policymakers.
The field of pain management disparities is now in its third decade of formal existence as a nexus of scientific inquiry, advocacy, and policy. In its first 2 decades, much emphasis was placed on documenting disparities in pain care and pain outcomes in disadvantaged groups, particularly among ethnic minorities. However, increased awareness of pain disparities and the initiatives to address these disparities have yielded only modest progress, most notably in the form of growing appreciation that pain disparities result from multiple factors, including biological, psychological, environmental, health system, and cultural factors.9,18,30,61 Much less progress has been made in identifying the individual contribution of these factors or how they interact to create and maintain the pain management disparities we see today. And perhaps most importantly, little research has been aimed at developing interventions that target these multiple determinants to reduce or eliminate pain management disparities.
Increasing our capacity to conduct research on the causes of pain management disparities and the mechanisms through which disparities may be eliminated is critical for advancing this field of inquiry. In this paper, we highlight ethical and methodological challenges in conducting pain management disparities research with minority, underserved, and other vulnerable populations. Additionally, we consider ways that this area of inquiry can be positively influenced by greater input from practitioners, advocates, and policymakers who are often best positioned to identify knowledge gaps and pose novel research questions aimed at reducing pain care disparities. Fig 1 depicts interrelationships between ethics, research methods, practice, advocacy, and policy issues to be discussed in this paper.
Figure 1.
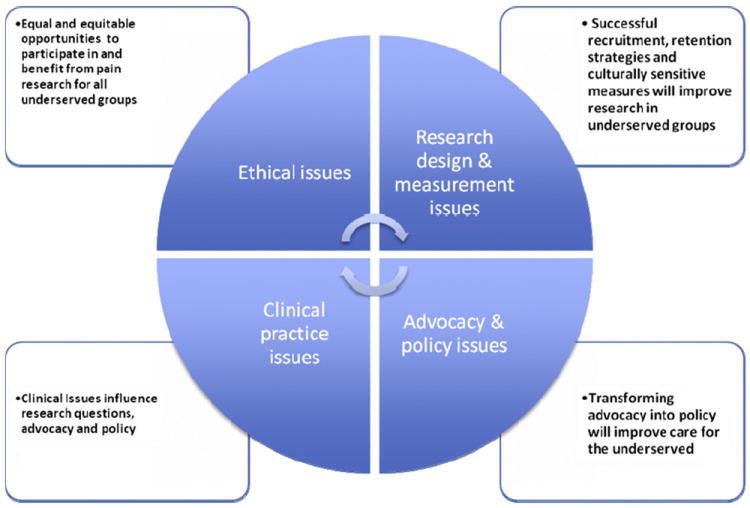
Links between research, ethical issues, clinical practice, and advocacy and policy.
Key Challenges in Conducting Pain Management Disparities Research
Limited Engagement in Research by Minority and Underserved Populations
Identifying the multiple, interrelated determinants of pain management disparities has moved the field closer to framing appropriate research questions. However, most researchers who conduct research in universities and academic medical centers must contend with the research gap that exists between research institutions and minority and underserved populations that are the focus of pain disparities research. This research gap is characterized by low research literacy and distrust of researchers,20,39 and limited efforts by researchers to improve research literacy in the broader communities in which research institutions are situated. Research literacy encompasses the degree to which an individual understands key research concepts and procedures necessary for informed consent.27,29 Low primary literacy (ie, limited ability to read and write) can contribute to low research literacy; however, even among literate individuals, knowledge of the research process is frequently low, and research on interventions to improve research literacy remains limited.27
One consequence of the research gap is low participation by groups who may have the most to gain from advances in clinical research. Another consequence is lack of opportunity to validate theories developed on typical study samples (ie, largely Caucasian and often middle income) with understudied populations (eg, racial, ethnic, and lower income groups). Both of these consequences limit the ecological validity of research findings. For pain disparities research this means that the theories and concepts related to pain coping and outcomes go untested among understudied racial and ethnic groups.14 The research gap is also a barrier to evaluating pain measures for validity across different populations and their relation to the intrapersonal, interpersonal, and systems processes that ultimately contribute to the persistence of disparities.
Ethical Challenges
People suffering from chronic pain can experience negative effects of pain on their sense of humanity, intelligence, and autonomy.54 Among minority and underserved individuals disproportionately affected by chronic pain, these vulnerabilities are often compounded by the effects of poverty, discrimination, and other markers of social disadvantage. Vulnerable individuals volunteering to participate in pain research in hopes of receiving relief may put themselves at risk for little to no benefit and may even take on undue harm.67 However, exclusion of these pain sufferers from research would hinder progress toward addressing persistent disparities in pain care. The ethical obligation for pain disparities researchers, therefore, is to ensure that the research process is fair and does not place undue burden on vulnerable populations.
We would argue that this fair and just standard might best be achieved by making research methods and ethical issues in pain management disparities research the actual focus of investigation. Studies that aim to identify research methods that are problematic for vulnerable populations are needed to modify those methods to create equal and equitable opportunities for underserved groups to participate in and benefit from pain research (Fig 1). Using study design as an example, as pain disparities research transitions from primarily focusing on quantifying disparities to developing interventions to eliminate disparities, researchers will be increasingly challenged to employ control group designs to answer their research questions. Current international recommendations suggest that control group subjects in investigations of diagnostic, therapeutic, or preventive interventions should receive an “established effective intervention.”21 In addition, the use of a placebo or “no treatment” is permissible only when doing so will expose control subjects to “temporary discomfort or delay in relief of symptoms.”21
The use of control group designs is complicated further by lack of consensus among researchers and across Institutional Review Boards regarding acceptable wait time and risk of discomfort to potential participants, especially when “no-treatment” control designs are used.10,23,33 Given the link between untreated pain, nervous system changes that may perpetuate pain,53 and reduced quality of life and psychological well-being,44 it will be important for pain disparities researchers to examine whether it is ever appropriate to not offer or to delay potentially effective treatment.
In addition to the design issues discussed above, it will also be important for pain disparities researchers to study other aspects of research, such as recruitment/retention methods and the informed consent process. Ultimately, research on the full spectrum of ethical issues in pain disparities research is needed to identify fair and just practices that promote greater inclusion of minority and underserved populations in pain management disparities research.
Recruitment Challenges
In addition to the ethical issues emphasized above, researchers must also contend with the practical challenges related to promoting inclusion and ensuring access to research. One of the most challenging aspects of pain management disparities research is recruiting participants from among populations most at risk for experiencing disparities in pain care and pain outcomes. These populations include racial and ethnic minority groups, the socioeconomically disadvantaged, and the medically underserved living in both urban and rural settings. While these populations are often described as separate groups, the reality is that there is considerable overlap. Many people experiencing pain disparities fall into more than 1 category and are subject to the compound effects of multiple risk factors for worse pain outcomes. When disparity populations become the focus of research efforts, these multiple risk factors coalesce to produce a number of barriers to recruitment and research participation that must be overcome (Fig 1).
Even among minority and underserved individuals willing to participate in research, several primary and secondary access barriers may limit their capacity to participate.67,68 Primary access issues include proximity of research site to the populations of interest and associated transportation, financial, and time demands associated with study participation.15 Secondary access relates to factors that influence willingness to participate when primary barriers do not exist.15 In the pain management research context, secondary access issues might include perceived relevance of the research to current clinical pain care needs (ie, research is seen as a distraction from needed pain care).
Another secondary access issue is the distrust that exists between medical researchers and groups that have traditionally been underserved.20 This distrust has its roots in past egregious research and medical misconduct such as the often-cited Tuskegee Syphilis Study38 and the decades-long sterilization programs carried out upon vulnerable populations throughout the U.S.62 Finally, if study participation requires rigid adherence to a schedule or has a high demand for time, undue burden may be placed on the participant, and recruitment and retention can be negatively affected.31,69
Additional barriers may exist for research studies evaluating medical or psychosocial pain interventions in pain patients. In addition to long-standing suspicion and mistrust of medically related research, the pain experience itself and associated emotional distress can often increase participant burden and reduce motivation for participating in a research study when participants perceive a low likelihood for direct pain control benefits.13 Finally, in psychosocial pain research there is the additional secondary access barrier of social stigma associated with psychological services, including concerns that seeking such services may be perceived as a sign of weakness or mental instability.
Several methods have been used to reduce barriers to access. Strategies such as the provision of babysitting, taxicab fare or bus vouchers to the research site, flexible scheduling, and the use of incentives have been used with limited success.34 More successful strategies for recruiting minority populations are those borrowed from ethnography.12 Success depends upon the relationships researchers develop with the underserved population and the degree to which community members are involved in critical phases of the research project such as design, recruitment, data collection, and dissemination.
Successful strategies for recruitment published in the literature include 1) crafting a strategic plan that uses nontraditional means to attract, recruit, and retain ethnic minorities34; 2) allowing sufficient time to identify community leaders and build trusting relationships4; 3) involving bilingual/bicultural medical clinic staff in the recruiting process5; 4) partnering with community leaders or their designees as cultural brokers8; and 5) including cultural brokers as legitimate members of the research team.45 Cultural brokers will warrant particular attention as pain disparities researchers look for ways to make research accessible to populations at greatest risk for undertreatment of pain. Brokers are often community health workers who speak the language (eg, promotoras) and reside in the community of interest. Their role has traditionally been to assist in recruitment, but cultural brokers also provide feedback on the order and language of survey questions, advise on methods of data collection, and may be trained to participate in the actual collection of data.8,45
Ultimately, successful recruitment and retention of underserved groups in pain management research will require greater use of cultural brokers and other strategies shown to be successful, as well as identification of new approaches to promote research participation. In other words, resarchers are called upon to make better use of existing knowledge in this area and to create new knowledge for the next generation of researchers to employ in order to advance the field.
Measurement Challenges
The importance of measurement to pain management disparities research cannot be overstated (Fig 1). As the field begins to focus increasingly on reducing or eliminating disparities in pain care, the ability to measure change will become increasingly important. Research examining the psychometric equivalence of the most widely used measures of self-reported pain and related variables (eg, pain coping) across different racial and ethnic groups remains limited. The existing literature provides some support for cross-cultural equivalence with a subset of measures of pain and pain coping, with most research comparing Caucasians and African Americans.32,57,63
Future research must also consider whether existing measures demonstrate adequate reliability and validity in other populations, such as among diverse Latino cultural groups.14 To move toward this goal there is a need for greater refinement in defining racial, ethnic, and cultural groups. It has been argued that broad definitions of these terms produces an “ethnic gloss”64 that undermines our capacity to identify relevant intra- and intergroup differences, including the moderating effects of national origin, immigration, and adaptation.43
A noteworthy advance in pain measurement research is the development of pain domains in the Patient Reported Outcomes Measurement Information System (PROMIS), a multicenter cooperative project initiated by the National Institutes of Health (NIH) in 2004.50 We highlight PROMIS because of its potential utility for pain management disparities research. PROMIS consists of a network of clinicians, clinical researchers, and measurement experts collaborating across 13 primary research sites. It is hoped that PROMIS will facilitate research in a number of relevant areas in pain care including symptom and function monitoring, provider-patient communication, and patient self-management.52 PROMIS has 3 domains relevant to pain research: pain intensity, pain interference, and pain behavior. Importantly, instruments for the pain intensity and pain interference domains have been translated into Spanish.
PROMIS, as a measurement model, is unique owing to its advantages in the areas of 1) comparability (ie, common standardized metrics to allow comparisons across domains and conditions); 2) strong psychometric properties; 3) flexibility (ie, ability to administer in static or adaptive forms); and 4) inclusion of individuals regardless of literacy, language, or physical function. This latter property of inclusiveness makes the PROMIS model particularly attractive to pain disparities researchers. Yet, despite this theoretical advantage, evidence suggests that further efforts are needed to make the PROMIS model truly inclusive. For instance, in the development of pain-related item banks, the sample remains overwhelmingly Caucasian, and several racial and ethnic groups are underrepresented compared to their population representation in the U.S.7,56 Furthermore, there are limited data available on the socioeconomic characteristics of the sample. Thus, while PROMIS represents a notable advance in pain research methodology, further refinements are needed to optimize its applicability to pain management disparities research.
Another advancement in pain-related measurement is the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT).66 The goal of the IMMPACT initiative was to identify a comprehensive set of outcomes to characterize both the statistical and clinical significance of interventions used in pain trials, facilitate comparison of data across studies, and enable clinicians to make informed decisions regarding the utility of treatment.22,65 Six core domains for chronic pain efficacy and effectiveness clinical trials were recommended65 and appropriate measures corresponding to each of the 6 core domains were identified.22 The 6 domains included 1) pain; 2) physical functioning; 3) emotional functioning; 4) participant ratings of improvement and satisfaction with treatment; 5) symptoms and adverse events; and 6) participant disposition (eg, adherence to the treatment and reasons for withdrawal).
While the set of outcomes is comprehensive, there remains a need to characterize these identified domains for disparities populations as well as to compare how the available normative data and measures compare with regard to psychometric properties across subgroups. This will be important to ensure that findings have not only a common metric but also shared meanings and understandings across subpopulations.16
Clinical Practice and Pain Management Disparities Research
Pain disparities research does not occur in silos separate from clinical practice (Fig 1). Many forces that drive clinical practice can directly or indirectly influence the pain disparities research agenda. For instance, the “demise” and trending down of multidisciplinary pain centers in the U.S.59 may discourage generation of scientific knowledge related to potentially effective interventions in ameliorating disparities.46 Because pain is a biopsychosocial phenomenon, multidisciplinary pain programs are considered an optimal paradigm in pain care.35 It has been argued that the poor and minorities disproportionately have conditions (eg, greater emotional responses to pain, higher levels of physical and psychological comorbidities and pain-related distress, and less control and task persistence) that may benefit from several components of multidisciplinary pain care.46 However, it is not surprising that little research exists on the effect of multidisciplinary pain programs on vulnerable populations. Scientists may not be keen on testing interventions that have little viability with regard to reimbursement and third party payment. Thus, a clinical practice trend driven by market forces may create a vacuum in generating scientific knowledge on pain care disparities. Researchers must be cognizant of clinical and economic realities that may influence the pain care disparities research agenda.
The realities of clinical practice influence not only what is studied (drug efficacy, behavioral interventions, etc) but increasingly how it is studied or toward what end (eg, dissemination into real-world clinical settings and uptake by clinicians). With a greater emphasis on translational research, federal research funding agencies (eg, NIH and Agency for Healthcare Quality and Research) are challenging researchers to conduct science that produces high-quality data48,58 that are timely and meaningful for clinical practice.70
Given that high-quality data are an important component of translational science, we wish to briefly note some of the issues surrounding data quality and to link these issues to pain care disparities research. Quality evidence is evidence that is appraised, synthesized, ranked, and representative of the current state of science.48,58 Conventional approaches to ranking of the quality of evidence places synthesis or meta-analysis of data from randomized clinical trials (RCTs) and/or data from a single RCT as the highest level of evidence.60 However, it can be argued that utilization of current best evidence will not help to eliminate pain management disparities because, more often than not, RCT study designs create homogeneous samples, control for (ie, eliminate) potentially relevant participant and environmental variables, and use the group average as the unit of analysis. Real-world clinical practice does not mimic a controlled environment; individual reactions to an intervention may differ from that of the average group member; and RCTs lack representation from disadvantaged, underserved, or minority participants, thus limiting applicability of findings from RCTs or systematic reviews to this large and ever-growing portion of the U.S. population.
In response to the limited utility of RCTs for addressing pain management disparities, it can be argued that there is a need to broaden our idea of high-quality evidence to encompass generalizability to disparity populations. One solution to the limitations of RCTs is use of practical controlled trials (PCTs). PCTs study the impact of the therapeutic ritual and other contextual variables on disease and quality of life.55 PCTs are designed to capture the nuances of clinical expertise and patient preferences/beliefs when comparing clinically relevant interventions within diverse populations over varied settings.
Another solution to the limitations of RCTs is data mining of electronic health records (EHRs) with adequate representation of disparity populations.52 Health information technology allows for the standardization of electronic data collection in health care settings.1 EHRs store real-time data relevant to clinical encounters including race, ethnicity, insurance status, residential zip code, reason for visit, diagnosis, treatment, and outcomes. Using EHRs, researchers can design large observational and innovative quasi-experimental studies52 that will provide real-time, clinically relevant data related to pain disparities. Data extracted from diverse practice settings can be compared and disparities in pain management can be identified using geographic information system technology.
PCTs and large studies mining data from electronic health records have the potential to 1) greatly expand research productivity relevant to pain management disparities; 2) better inform practice; 3) assess real-world application of translational research; 4) study the process of staff and clinician uptake of empirically validated approaches to improving care, when such approaches are identified; and 5) move research beyond efficacy to effectiveness research.
Advocacy and Pain Management Disparities Research
Advocacy is action in support of a cause or in favor of a particular policy.3 In recent years, pain advocacy efforts have become increasingly visible, culminating in the Institute of Medicine report on pain37; however, a concerted agenda to ameliorate pain disparities is not explicitly or consistently identified in the advocacy mission of the prominent pain advocacy groups in the U.S.47 The Institute of Medicine report37 put forth several recommendations with direct relevance to advocacy and pain management disparities research. At the national level, the report recommends that NIH designate a lead institute responsible for pain research, and that the scope of and support for the NIH Pain Consortium be expanded. The report also recommends the development of a comprehensive strategy for population research addressing pain prevention, treatment, and management.
Recommendations involving agencies at various levels (ie, national, state, regional, local) include improving data collection and reporting on pain, especially with subpopulations at risk for undertreatment; and developing strategies to reduce barriers to pain care.37 A subsequent report by pain disparities researchers identified a comprehensive set of opportunities within recent legislative initiatives to overcome advocacy, policy, and research barriers to effective pain treatment in the U.S.47 Specific to advocacy, the authors reviewed the mission statements of all leading pain organizations in the U.S. and concluded that a pain disparities agenda should be specifically articulated in the missions of leading pain advocacy organizations. The report proposed recommendations for advocacy models that aggressively engage key stakeholders in generating solutions.47
One such innovative advocacy model is the The Pain Action Initiative: A National Strategy (PAINS). The PAINS initiative was launched by the Center for Practical Bioethics to assess the capacity and readiness of individual leaders and organizations across the country to develop a national strategy to improve treatment for those living with chronic pain. In late 2010 this initiative convened 5 regional meetings (held in Seattle, Boston, Chicago, Tampa, and San Diego) to hear from pain leaders across the country.17
Across the regional meetings, advocates articulated a number of issues that are relevant to pain management disparities. Issues noted included the need for 1) more guidance on working with diverse patient groups; 2) improvements in medical education and continuing education to include more training on cultural factors that may promote pain care disparities; 3) challenges of providing high-quality pain care to rural populations; 4) limitations of evidence-based medicine for meeting the needs of patients having pain; and 5) the need for greater interdisciplinary collaboration among researchers and agencies.17 The ideas coming out of these regional meetings suggest that advocacy communities across the country have been underutilized in pain management disparities research to date. Pain care advocates are well positioned to identify the many research gaps that exist and can be important partners in refining research foci to transform advocacy into policy17,37 (Fig 1).
Policy and Pain Management Disparities Research
Pain policy is distinct from advocacy but is often shaped by advocacy efforts. Policy refers to a set of basic principles and associated guidelines formulated and enforced by a governing body for the purpose of directing actions in pursuit of long-term goals. Pain advocacy efforts have produced numerous recommendations for policy with the potential to impact disparities in pain care, but very few of the existing recommendations have been translated into policy (Fig 1). This is due, in part, to a paucity of research to guide policy development, implementation, and evaluation. In this section we highlight policies relevant to pain care disparities and ideas for further translation of advocacy into policy through research.
The Commonwealth Fund, a proponent for the development of policies to eliminate racial and ethnic disparities, recommended establishing state standards, routine collection of racial and ethnic data by health care providers, evaluating disparities reduction programs, establishing minimum standards for cultural and linguistic competency, and increasing minorities in the workforce. Following this and other advocacy efforts, recent legislative and regulatory changes have resulted in new policies for pain care but without addressing disparities. For example, in July 2011, SB 1083 was signed into law limiting the practice of “Fail first” or “Step therapy” for people suffering from chronic pain. This practice, encouraged by insurance companies, forced physicians to prescribe the cheapest drug first even if the alternative was a better option for the patient. As a result of this practice, patients had to undergo a process of trial and error to find the medication that worked best for them.
State policies governing pain management practices can serve to either facilitate or inhibit research on pain management disparities. The Pain & Policy Study Group (PPSG) evaluates state pain policies using the balanced policy approach and assigns grades to states based on their ability to achieve a balance between positive and negative policy provisions.51 The PPSG report51 reported that state pain policies have steadily improved since 2000 and continue to make gains. According to this report, 88% of states currently scored above average (better than a C) in achieving a balance between negative and positive provisions in their pain policies. Interestingly, while the latest PPSG report acknowledges unrelieved pain as a particular burden to minorities (among other vulnerable groups), it does not explicitly link pain policy to pain management disparities. However, we would argue that pain policies are an important contextual factor to consider in pain management disparities research.
Policies governing collection of ethnic identifiers are also relevant for conducting pain management disparities research. Among the many uses of these data, the most important is their utility in documentation of progress toward eliminating disparities in pain management. Based on a previous legal challenge in California, it should be noted by pain management disparities researchers that future access to these data cannot be assumed.6 Also, the effect of these policies on equitable pain care has yet to be seen. Researchers should make every effort to make use of available data to demonstrate its current utility to policymakers and to recommend refinements to how these data are collected to further increase their usefulness in understanding, monitoring, and reducing disparities in pain care.6
Importantly, disparities in pain and other health issues are garnering new attention as an economic challenge that must be addressed. The economic realities of pain disparities could help to spur pain disparities research that will support policy development. In a recent report, the Joint Center for Political and Economic Studies reported that 30% of direct medical costs incurred by African Americans, Hispanics, and Asian Americans over a 3-year period from 2003 to 2006 were excess costs owing to health inequities and that these inequities cost more than $230 billion over the period evaluated.40 Furthermore, when indirect costs related to these inequities were taken into account (ie, lost productivity, premature death), the total was estimated at $1.24 trillion. As was noted earlier, the costs of untreated pain are considerable and the current and future economic climate may provide the backdrop to help amplify the message that timely, appropriate, and effective pain care is not only morally sound but also economically sound.
Bolstered by this economic argument, pain management disparities researchers have new opportunities to enhance the translation potential of their work. Collaboration with health economists will become increasingly important to formulate research questions and enhance study methodology to capture important information on the costs of disparities in pain care and the cost-effectiveness of policies to reduce or eliminate these disparities. Research examining the impact of new policies on socially disadvantaged groups will be helpful to ensure that systemic and individual provider biases are not being perpetuated. Furthermore, examining innovative models of care and their effectiveness on diverse populations will help facilitate the development of targeted, successful, and cost-effective therapies.
Summary and Conclusions
Due to high profile initiatives at the national level, awareness of pain management disparities has never been higher. Yet despite this progress in increasing awareness, solutions for eliminating pain management disparities remain elusive. In this paper, we discussed challenges that pain management disparities researchers encounter in advancing the field. We also discussed links between pain management disparities research and the clinical practice, advocacy, and policy arenas. We believe these links must be strengthened in order to turn awareness and advocacy into research, research into policy, and policy into real progress toward closing the pain management disparities gap.
Acknowledgments
We would like to thank Dr. Barbara Hastie and Dr. Cathy Campbell for thoughtful feedback on this manuscript.
Supported in part by Award Number R15NR012190 (K.R.) from the National Institute of Nursing Research (NINR).
Footnotes
The authors have no conflicts of interest to report.
References
- 1.Agency for Healthcare Research and Quality: Health Information Technology. [January 2, 2011]; Available at: http://healthit.ahrq.gov/portal/server.pt/community/ahrq_national_resource_center_for_health_it/650.
- 2.Akechi T, Okamura H, Nishiwaki Y, Uchitomi Y. Predictive factors for suicidal ideation in patients with unresectable lung carcinoma. Cancer. 2002;95:1085–1093. doi: 10.1002/cncr.10769. [DOI] [PubMed] [Google Scholar]
- 3.Alliance for Justice: Immigrant Advocacy Toolkit. [November 8, 2011]; Available at: http://www.afj.org/for-nonprofits-foundations/immigrant-advocacy-toolkit/what-is-advocacy.pdf.
- 4.Alvarez RA, Vasquez E, Mayorga CC, Feaster DJ, Mitrani VB. Increasing minority research participation through community organization outreach. West J Nurs Res. 2006;28:541–560. doi: 10.1177/0193945906287215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Pain Foundation: Position Statement on Access to Care. [July 11, 2011]; Available at: http://www.painfoundation.org/about/position-statements/access-to-pain-care-august2010.html.
- 6.American Pain Society, Organizing Committee of the Pain and Disparities Special Interest Group: Racial and Ethnic Identifiers in Pain Management. The Importance to Research, Clinical Practice, and Public Health Policy. APS Bulletin. 2005:7–10. [Google Scholar]
- 7.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, Cella D, Rothrock N, Keefe F, Callahan L, Lai JS. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anders R, Olson T, Robinson K, Wiebe J, DiGregorio R, Solis G, Albrechtsen J, Bean N, Ortiz M. A health survey of a colonia located on the West Texas, US/Mexico border. J Immigr Minor Health. 2008;12:361–369. doi: 10.1007/s10903-008-9186-7. [DOI] [PubMed] [Google Scholar]
- 9.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: Causes and consequences of unequal care. J Pain. 2009;10:1187–1204. doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Bautmans I, Van Arken J, Van Mackelenberg M, Mets T. Rehabilitation using manual mobilization for thoracic kyphosis in elderly postmenopausal patients with osteoporosis. J Rehabil Med. 2010;42:129–135. doi: 10.2340/16501977-0486. [DOI] [PubMed] [Google Scholar]
- 11.Bonham VL. Race, ethnicity, and pain treatment: Striving to understand the causes and solutions to the disparities in pain treatment. J Law Med Ethics. 2001;29:52–68. doi: 10.1111/j.1748-720x.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 12.Burns NK, Gove SK. Qualitative research issues, in The Practice of Nursing Research: Appraisal, Synthesis, and Generation of Evidence. St Louis, MO: Saunders Elsevier; 2009. pp. 543–546. [Google Scholar]
- 13.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54:399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 14.Campbell LC, Andrews N, Scipio C, Flores BD, Feliu M, Keefe FJ. Pain coping in Latino populations. J Pain. 2009;10:1012–1019. doi: 10.1016/j.jpain.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Campbell LC, Warner TD. Accessibility of health care. In: Jackson Y, editor. Encyclopedia of Multicultural Psychology. Thousand Oaks, CA: Sage Publications; 2006. pp. 3–5. [Google Scholar]
- 16.Cella D, Gershon R, Lai JS, Choi S. The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Qual Life Res. 2007;16(Suppl 1):133–141. doi: 10.1007/s11136-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 17.Center for Practical Bioethics. Pain Action Initative: A National Strategy (PAINS) [March 11, 2011]; Available at: http://www.practicalbioethics.org/
- 18.Cintron A, Morrison RS. Pain and ethnicity in the United States: A systematic review. J Palliat Med. 2006;9:1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 19.Cleeland CS, Nakamura Y, Mendoza TR, Edwards KR, Douglas J, Serlin RC. Dimensions of the impact of cancer pain in a four country sample: New information from multidimensional scaling. Pain. 1996;67:267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 20.Corbie-Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Int Med. 2002;162:2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 21.Council for International Organisations of Medical Sciences: 2002 International Ethical Guidelines for Biomedical Research Involving Human Subjects. [February 6, 2009]; Available at: http://www.cioms.ch/ [PubMed]
- 22.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz N, Kerns RK, Stucki G, Allen RR. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Ehni HJ, Wiesing U. International ethical regulations on placebo-use in clinical trials: A comparative analysis. Bioethics. 2008;22:64–74. doi: 10.1111/j.1467-8519.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 24.Ezenwa MO, Ameringer S, Ward SE, Serlin RC. Racial and ethnic disparities in pain management in the United States. J Nurs Scholarsh. 2006;38:225–233. doi: 10.1111/j.1547-5069.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 25.Flor H, Turk DC, Scholz OB. Impact of chronic pain on the spouse: Marital, emotional and physical consequences. J Psychosom Res. 1987;31:63–71. doi: 10.1016/0022-3999(87)90099-7. [DOI] [PubMed] [Google Scholar]
- 26.Flor H, Turk DC, Rudy TE. Relationship of pain impact and significant other reinforcement of pain behaviors: The mediating role of gender, marital status and marital satisfaction. Pain. 1989;9:38–45. doi: 10.1016/0304-3959(89)90071-7. [DOI] [PubMed] [Google Scholar]
- 27.Flory J, Emanual E. Interventions to improve research participants’ understanding in informed consent for research: A systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 28.Gagliese L, Gauthier LR, Rodin G. Cancer pain and depression: A systematic review of age-related patterns. Pain Res Manag. 2007;12:205–211. doi: 10.1155/2007/150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman MS, Dias JJ, Stafford JD. Increasing research literacy in minority communities: CARES fellows training program. J Empir Res Hum Res Ethics. 2010;5:33–41. doi: 10.1525/jer.2010.5.4.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kalauokalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH. The unequal burden of pain: Confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AR, Mokusu N, Hughes C, Tortolero-Luna G, Risendal B, Ho RCS, Prewitt TE, McCaskill-Stevens WJ. Participation of minorities in cancer research: The influence of structural, cultural, and linguistic factors. Ann Epidemiol. 2000;10(Supp 1):S22–S34. doi: 10.1016/s1047-2797(00)00195-2. [DOI] [PubMed] [Google Scholar]
- 32.Hastie BA, Riley JL, Fillingim RB. Ethnic differences in pain coping: Factor structure of the coping strategies questionnaire and coping strategies questionnaire-revised. J Pain. 2004;5:304–316. doi: 10.1016/j.jpain.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Huang DT, Hadian M. Bench-to-bedside review: Human subjects research – are more standards needed? Crit Car. 2006;10:244–249. doi: 10.1186/cc5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hulme PA. Cultural considerations in evidence-based practice. J Transcult Nurs. 2010;21:271–280. doi: 10.1177/1043659609358782. [DOI] [PubMed] [Google Scholar]
- 35.International Association for the Study of Pain. Task Force on Guidelines for Desirable Characteristics for Pain Treatment Facilities. [March 27, 2011]; Available at: http://www.iasp-pain.org/desirabl.html.
- 36.Institute for Clinical Systems Improvement. Assessment and Management of Chronic Pain. Bloomington, MN: Institute for Clinical Systems Improvement; 2008. [Google Scholar]
- 37.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 38.Jones JH. The Tuskeegee Syphilis Experiment. New York, NY: Free Press; 1993. Bad Blood. [Google Scholar]
- 39.Kripilani S, Bengtzen R, Henderson LE, Jacobsen TA. Clinical research in low-literacy populations: Using teach-back to assess comprehension in informed consent and privacy information. IRB. 2008;30:13–19. [PubMed] [Google Scholar]
- 40.LaVeist TA, Gaskin DJ, Richard P. The Economic Burden of Health Inequalities in the United States. Washington, DC: Report of the Joint Center for Political and Economic Studies; 2009. [Google Scholar]
- 41.Lippe PM. The decade of pain control and research. Pain Med. 2000;1:286. doi: 10.1046/j.1526-4637.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 42.Llorente MD, Burke M, Gregory GR, Bosworth HB, Grambow SC, Horner RD, Golden A, Olsen EJ. Prostate cancer: A significant risk factor for late-life suicide. Am J Geriatr Psychiatry. 2005;13:195–201. doi: 10.1176/appi.ajgp.13.3.195. [DOI] [PubMed] [Google Scholar]
- 43.Lin S, Kelsey JL. Use of race and ethnicity in epidemiological research: Concepts, methodological issues and suggestions for research. Epidemiol Rev. 2000;22:187–202. doi: 10.1093/oxfordjournals.epirev.a018032. [DOI] [PubMed] [Google Scholar]
- 44.Lynch ME, Campbell F, Clark AJ, Dunbar MJ, Goldstein D, Peng P, Stinson J, Tupper H. A systematic review of the effect of waiting for treatment of chronic pain. Pain. 2008;136:97–116. doi: 10.1016/j.pain.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 45.Marquez MA, Muhs JM, Tosomeen A, Riggs BL, Melton LJ. Costs & strategies for minority recruitment for osteoporosis research. J Bone Miner Res. 2003;18:3–8. doi: 10.1359/jbmr.2003.18.1.3. [DOI] [PubMed] [Google Scholar]
- 46.Meghani SH. Corporatization of Pain Medicine: Implications for Widening Pain Care Disparities. Pain Med. 2011;12:634–644. doi: 10.1111/j.1526-4637.2011.01074.x. [DOI] [PubMed] [Google Scholar]
- 47.Meghani SH, Polomano RC, Tait RC, Vallerand AH, Anderson KO, Gallagher RM. Advancing a National Agenda to Eliminate Disparities in Pain Care: Directions for Health Policy, Education, Practice, and Research. Pain Med. 2012;13:5–28. doi: 10.1111/j.1526-4637.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 48.Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 49.Monsivais JJ, Robinson K. Psychological profile and work status of a predominantly Hispanic worker’s compensation population with chronic limb pain. HAND. 2008;3:352–358. doi: 10.1007/s11552-008-9123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Institutes of Health. [November 30, 2011]; Available at: http://www.Nihpromis.org/measures/domainframework.
- 51.Pain & Policy Studies Group. Achieving Balance in State Pain Policy: A Progress Report Card. Madison, WI: University of Wisconsin Paul P. Carbone Comprehensive Cancer Center; 2008. [Google Scholar]
- 52.Perlin JB, Kupersmith J. Information technology and the inferential gap. Health Aff. 2007;26:w192–w194. doi: 10.1377/hlthaff.26.2.w192. [DOI] [PubMed] [Google Scholar]
- 53.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facillitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 54.Post LF, Blustein J, Gordon E, Dubler NN. Pain: Ethics, culture and informed consent to relief. J Law Med Ethics. 1996;24:348–359. doi: 10.1111/j.1748-720x.1996.tb01878.x. [DOI] [PubMed] [Google Scholar]
- 55.Rakel D. Practical controlled trials: Researching the therapeutic ritual and all its parts. [March 9, 2011];Bulletin. Available at: http://www.ampainsoc.org/pub/bulletin/fall06/research1.htm.
- 56.Revicki DA, Chen WH, Harnam N, Cook KF, Amtmann D, Callahan LF, Jensen MP, Keefe FJ. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–169. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riley JL, Robinson ME, Geisser ME. Empirical subgroups of the Coping Strategies Questionnaire-Revised: A multi-sample study. Clin J Pain. 1999;15:111–116. doi: 10.1097/00002508-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Sackett DL. Evidence-based medicine. Semin Perinatol. 1997;21:3–5. doi: 10.1016/s0146-0005(97)80013-4. [DOI] [PubMed] [Google Scholar]
- 59.Schatman ME. Ethical issues in chronic pain management. New York, NY: Informa Healthcare; 2007. The demise of the multidisciplinary chronic pain management clinic: Bioethical perspectives on providing optimal treatment when ethical principles collide. [Google Scholar]
- 60.Selgelid MJ. Module four: Standards of care and clinical trials. Dev World Bioeth. 2005;5:55–72. doi: 10.1111/j.1471-8847.2005.00102.x. [DOI] [PubMed] [Google Scholar]
- 61.Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U. S. adult population. J Health Care Poor Underserved. 2010;21:177–220. doi: 10.1353/hpu.0.0255. [DOI] [PubMed] [Google Scholar]
- 62.Stern AM. Sterilized in the name of public health. Race, immigration, and reproductive control in modern California. Am J Pub Health. 2005;95:1128–1138. doi: 10.2105/AJPH.2004.041608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 64.Trimble JE. Ethnic specification, validation prospects and the future of drug abuse research. Int J Addict. 1990-1991;25:149–169. doi: 10.3109/10826089009071038. [DOI] [PubMed] [Google Scholar]
- 65.Turk DC, Dworkin RH, Allen RR, Bellamy N, Brandenburge N, Carr D, Cleeland C, Dionne R. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 66.Turk DC, Dworkin RH, Burke LB, Gershon R, Rothman M, Scott J, Allen RR, Hampton Atkinson J, Chandler J, Cleeland C. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125:208–215. doi: 10.1016/j.pain.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 67.Viens AM. Socio-economic status and inducement to participate. Am J Bioethics. 2001;1:1–2. [Google Scholar]
- 68.Wendler D, Kington R, Madans J, Van Wye G, Christ-Schmidt H, Pratt LA. Are racial and ethnic minorities less willing to participate in health research? PLOS Med. 2005 Dec 6; doi: 10.1371/journal.pmed.0030019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wilbur J, McDevitt J, Wang E, Dancy B, Briller J, Ingram D, Nicola T, Lee H, Zenk SN. Recruitment of African American women to a walking program: Eligibility, ineligibility, and attrition during screening. Res Nurs Health. 2006;29:176–189. doi: 10.1002/nur.20136. [DOI] [PubMed] [Google Scholar]
- 70.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299:211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 71.World Health Organization. With a Guide to Opioid Availability. Geneva: WHO; 1996. Cancer Pain Relief. [Google Scholar]


