Abstract
A click-reaction was site-selectively carried out on 1000 and 12 000 microelectrode arrays and characterized using TOF-SIMS.
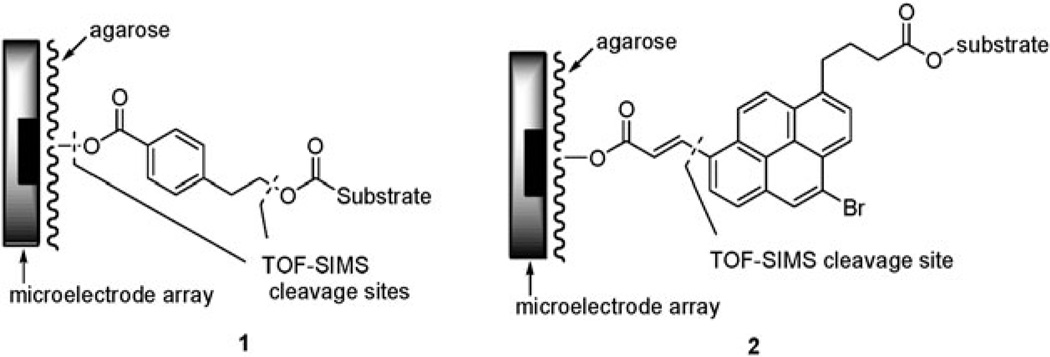
Recently, we have been developing the tools necessary for building molecular libraries on addressable microelectrode arrays that contain between 1024 (a 1K-array) and 12 544 (a 12K-array) electrodes per cm2.1–7 In this work, the microelectrodes are used to effect chemical reactions in a site-selective fashion by generating the reagents or catalysts needed for the synthetic transformations. The reagents or catalysts are then destroyed by a confining agent in solution so that they cannot migrate to non-selected regions of the arrays. In this way, the same chemistry typically used to build molecular libraries on solid-phase supports can in principle be used to assemble them on the arrays. Once the molecules are synthesized on the arrays, the microelectrodes can be used to monitor interactions between the molecules in a library and a biological receptor in ‘‘real-time’’.3a,5a One critical aspect of this work is the development of TOF-SIMS cleavable linkers for attaching molecules to the porous polymer coating the surface of the array.8,9 The use of a linker that cleaves under TOF-SIMS conditions is important for characterizing the molecules built on the arrays. Without a cleavable linker, TOF-SIMS experiments on the arrays only show the polymer coating on the array.8 In other words, the signal to noise of the side-chain relative to the polymer is too small to observe the side-chain. With a cleavable linker, this is not a problem. Since TOF-SIMS experiments can probe surfaces with a resolution of the order of 50 microns and the microelectrodes on a 1K-array are 94 microns and a 12K-array are 45 microns in diameter, a TOF-SIMS cleavable linker enables characterization of the molecules associated with any given electrode in the array. Hence, the use of a cleavable linker provides a method for conducting quality control analyses of libraries built on the arrays. To date, the two linkers shown in Fig. 1 have shown considerable promise along these lines.8,9
Fig. 1.
TOF-SIMS fragmentation sites.
While useful in TOF-SIMS studies, both of these linkers are problematic. They are tricky to prepare, require multiple-step syntheses, and are only available in small quantities. This is especially worrisome because both linkers must be assembled ahead of time, bound to the substrate, and then placed on the array in a separate step. Furthermore, the placement of both linker 1 and linker 2 on an array employs synthetic chemistry that we would like to use in subsequent synthetic steps. Linker 1 is placed on the array using a base-catalyzed addition to an activated ester, a strategy that rules out the use of base-cleavable protecting groups in the substrate. Linker 2 is placed on the array with the use of a Heck reaction, a strategy that rules out the use of Pd(0)-catalyzed reactions to further develop a substrate.
The optimal TOF-SIMS cleavable linker would avoid these issues by using unique chemistry that rapidly assembles the linker on the array from simple functional groups while tolerating a wide variety of molecules and functional groups. To this end, the use of ‘‘click-chemistry’’ appears ideal.10 ‘‘Click-reactions’’ are compatible with a wide range of substrates and have proven very useful for attaching biomolecules to surfaces.11 Since the chemistry conducted on a microelectrode array is essentially the same as normal solution and solid-phase chemistry (the microelectrodes are used to generate the same catalysts), the use of a ‘‘click-reaction’’ on a microelectrode array should also allow for the attachment of a wide variety of biomolecules to the surface of the array. In addition, one can anticipate the triazole product fragmenting nicely in a TOF-SIMS experiment. ‘‘Click-reactions’’ are well suited for development on a microelectrode array because they are typically accomplished with the use of a Cu(i)-catalyst that is generated in situ by reducing Cu(ii) with sodium ascorbate.10 If the sodium ascorbate is removed from the reaction, then the microelectrodes in the array can in principle be used to reduce Cu(ii) and generate the catalyst selectively at specific locations on the array. This possibility was first recognized by Chidsey, Collman, and co-workers who utilized the ‘‘click-reaction’’ to selectively functionalize a pair of gold interdigitated array band electrodes.12 Selectivity for one electrode over the other was obtained by using one of the electrodes as a cathode in order to generate the desired Cu(i) catalyst from a Cu(ii) precursor in the solution above the electrodes and the second as an anode to destroy any active Cu(i) catalyst above its surface. But how does this chemistry translate to a microelectrode array having a density of over 12 000 microelectrodes per cm2, especially when the arrays do not have the capability of employing some microelectrodes as cathodes and others as anodes at the same time? Is there a confining agent that can be added to the solution above an array to stop the migration of the Cu(i)-catalyst generated at one microelectrode to the neighboring microelectrodes? If the reactions can be run site-selectively on a microelectrode array, then will the linkers cleave cleanly in TOF-SIMS experiments in a manner that allows for the observation of molecules on the surface of the array? We report here that ‘‘click-reactions’’ can be run site-selectively on both 1K- and 12K-microelectrode arrays with the use of air as a confining agent, and that the triazole product generated is a useful TOF-SIMS cleavable linker.
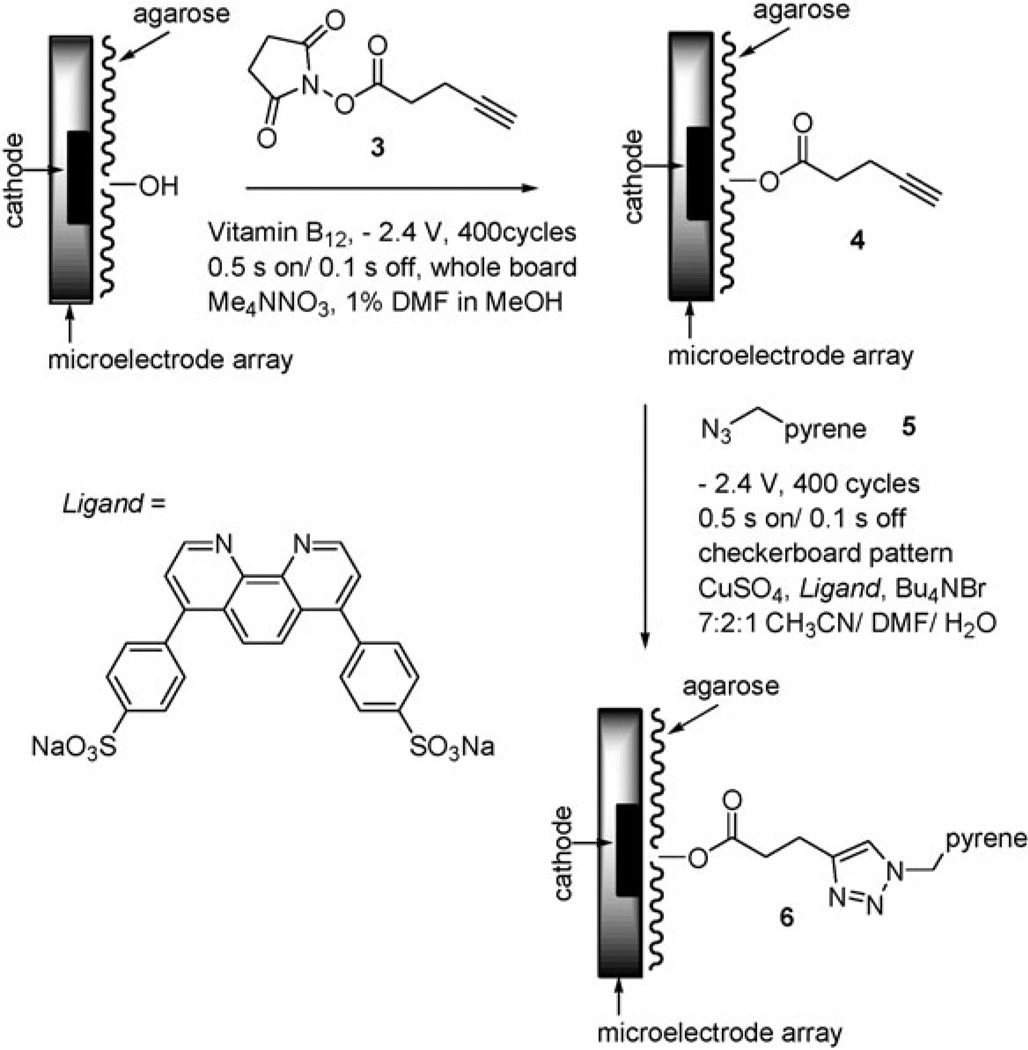
The feasibility of the microelectrode array reaction was first examined using a 1K-array. To this end, the activated ester 3 was placed on the surface of the array proximal to the electrodes using the previously developed electro-generated base conditions (Scheme 1).2–7 The base was generated by using the microelectrodes in the array as cathodes in order to reduce vitamin B12. This was done using every microelectrode in the array.
Scheme 1.
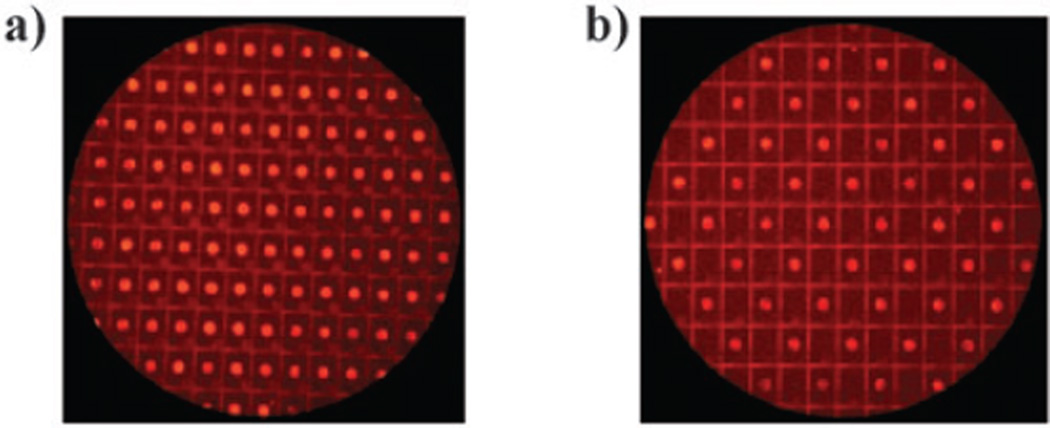
The functionalized array was then inserted into a 7 : 2 : 1 acetonitrile–dimethylformamide–water solution containing pyrene methylazide 5, copper sulfate, tetrabutylammonium bromide, and disodium bis(bathophenanthroline) disulfonate ligand.13 In order to confirm that the reaction described above placed the acetylene by each microelectrode in the array, the ‘‘click-reaction’’ was first performed by cycling every microelectrode in the array on for 0.5 s at −2.4 V relative to a remote Pt wire anode and off again for 0.1 s. Four hundred cycles were completed. As expected, this reaction led to the generation of Cu(i)-catalyst and hence a successful ‘‘click-reaction’’ at each microelectrode (Fig. 2a).
Fig. 2.
(a) A ‘‘click-reaction’’ with every microelectrode turned on, on a 1K-array. (b) A site-selective ‘‘click-reaction’’ on a 1K-array.
With the control experiment in place, a second array (functionalized in an identical manner to the first) was then inserted into an identical 7 : 2 : 1 acetonitrile–dimethyl-formamide– water solution containing pyrene methylazide 5, copper sulfate, disodium bis(bathophenanthroline) disulfonate ligand,13 and tetrabutylammonium bromide. In this case, the reaction was not degassed and not protected from the air in the hope that oxygen would serve as a scavenger for any Cu(i) generated at selected microelectrodes during the reaction. A checkerboard pattern of electrodes in the array was then used to reduce the Cu(ii)-species in solution by cycling the microelectrodes on for 0.5 s at −2.4 V relative to a remote Pt wire anode and off again for 0.1 s. Four hundred cycles were completed. Microelectrodes that were not selected for the experiment were left off for the entire reaction. The microelectrode array was then washed with water and 95% ethanol and examined using a fluorescence microscope to give the image in Fig. 2b. From this image, it is clear that the reaction only occurred at microelectrodes that were turned on. No reaction was observed at microelectrodes not used for the experiment, microelectrodes that were proximal to acetylene substrate. Hence, the use of oxygen to confine the reaction was very successful and no ‘‘bleed-over’’ of Cu(i) from the selected microelectrodes to neighboring electrodes was found.
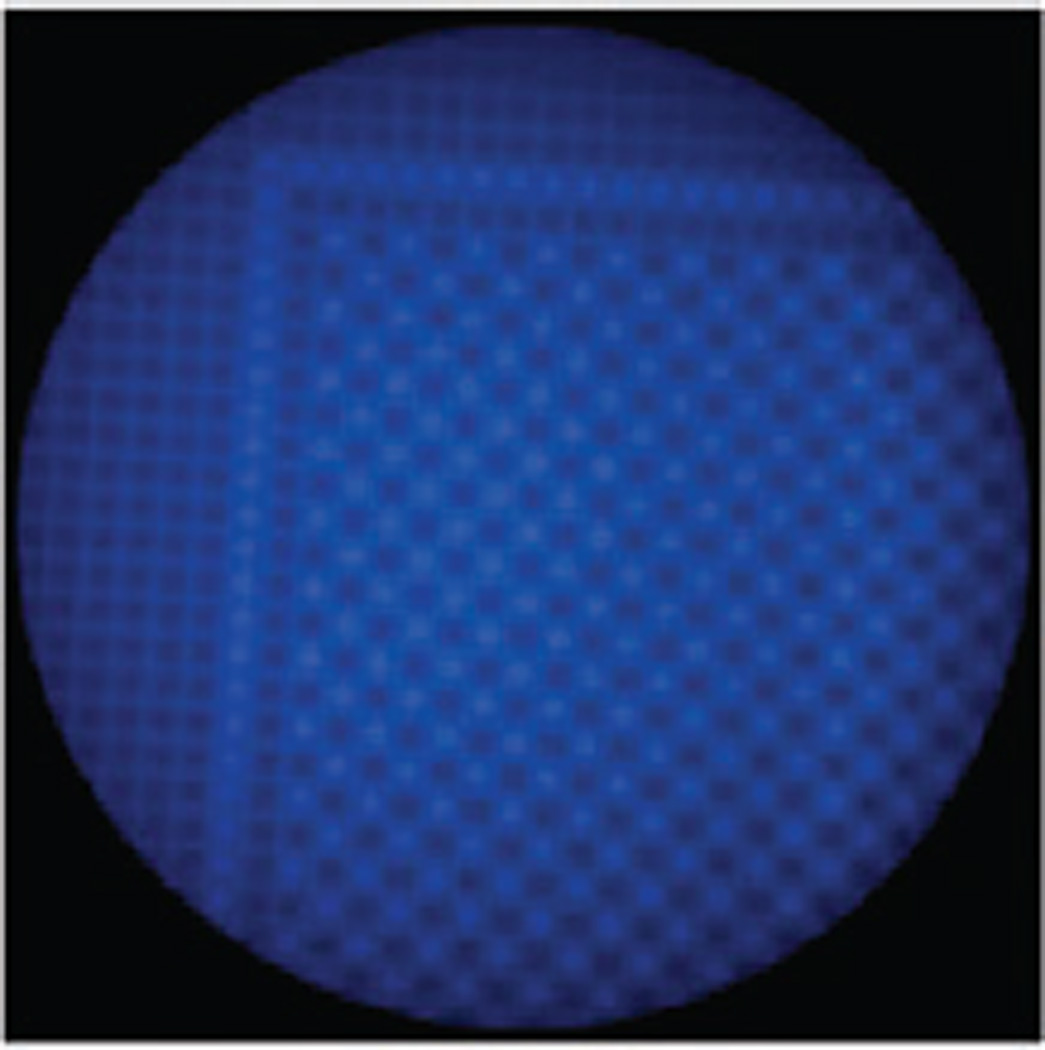
The exact same set of conditions could be utilized to perform a site-selective ‘‘click-reaction’’ on a 12K-array (Fig. 3). In this case, a checkerboard inside of a box pattern of microelectrodes was used for generating the Cu(i)-catalyst. Once again, the oxygen present in the solution over the microelectrode array served as an excellent confining agent for the reaction. No reaction was observed at microelectrodes that were not used as cathodes.
Fig. 3.
A site-selective reaction on a 12K-array.
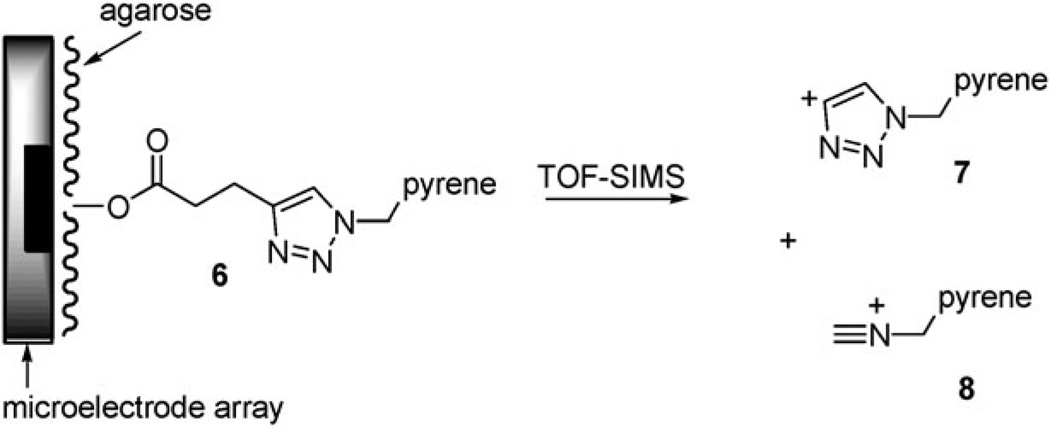
With the site-selective ‘‘click-reaction’’ in place, attention was turned toward examining the utility of the triazole linker prepared. First, the cleavage properties of the triazole linker under TOF-SIMS conditions were examined. When the functionalized array 6 was placed in a primary ion beam at 25 keV (generated using a Binm+ (n = 1–7, m = 1, 2) liquid metal ion gun) and the positive ions generated collected for analysis, it was found that the triazole linker cleaved along two major pathways (Scheme 2).
Scheme 2.
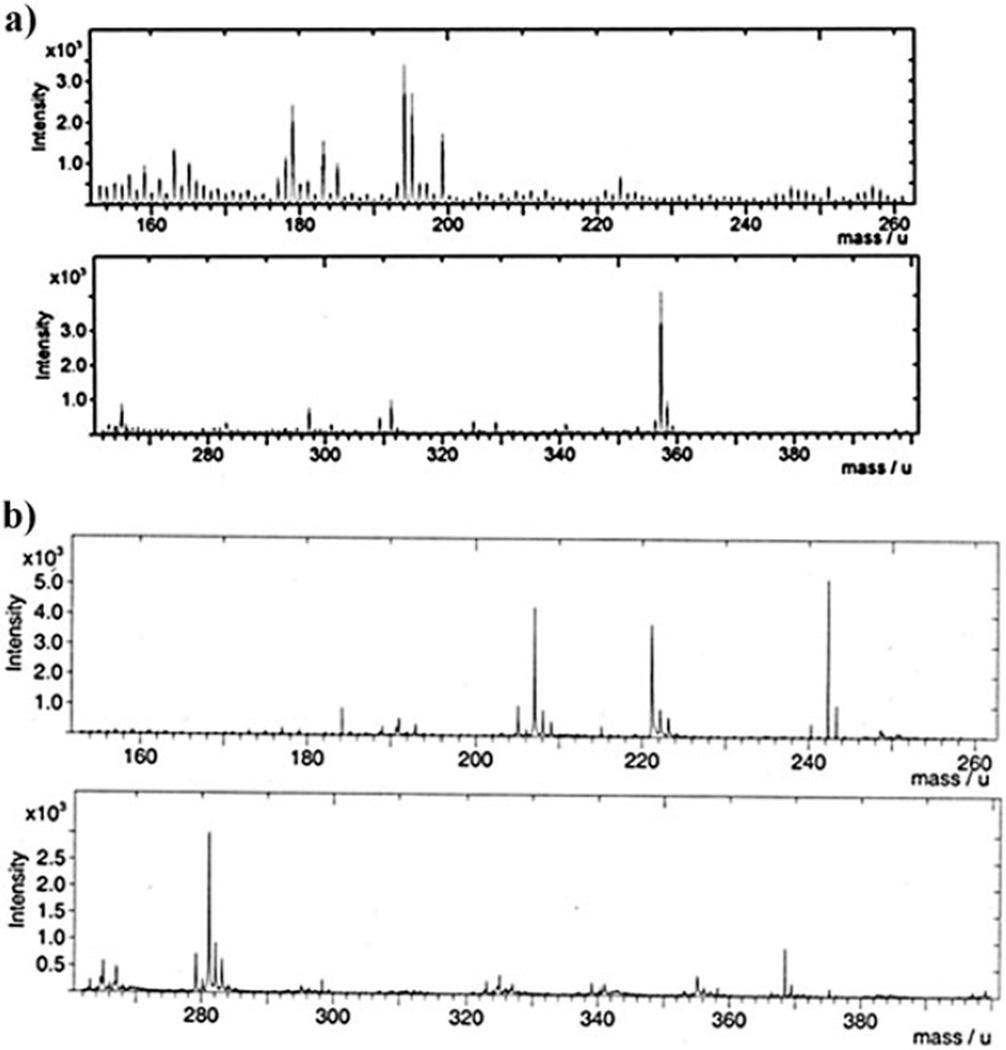
The first observed cleavage pathway (7, m/z = 281) resulted from a β-elimination of the triazole ring from the ester attaching the linker to the agarose polymer. This cleavage may well be a characteristic of the specific substrate used on the array for the ‘‘click-reaction’’. The second fragmentation pathway (8, m/z = 242) resulted from a retro [3+2]-cycloaddition involving the triazole itself. This fragmentation should be independent of the alkyl substituents on the triazole and hence represent a general cleavage mechanism for the linker that can be used to characterize molecules placed or constructed on the surface of the array. Cleavage of the linker along both pathways allowed for the presence of the pyrene group on the surface of the array to be detected in the presence of the agarose polymer coating the array (Fig. 4).
Fig. 4.
(a) TOF-SIMS for agarose. (b) TOF-SIMS for agarose functionalized with compound 6. For full spectra see the ESI†.
In conclusion, the ‘‘click-reaction’’ provides an ideal method for attaching molecules to the surface of an addressable microelectrode array. The reactions can be performed site-selectively on both 1K- and 12K-arrrays using the same reagents employed in solution phase reactions and oxygen as a confining agent. Furthermore, the triazole product formed is readily cleaved in TOF-SIMS experiments and can therefore be used to gather information about molecules attached to the surface of the array. At the present time, it appears clear that the use of ‘‘click-chemistry’’ derived linkers will play a pivotal role in the development of future addressable microelectrode array supported molecular libraries.
Supplementary Material
Acknowledgments
This work is generously supported by the National Science Foundation (CHE-9023698). We also gratefully acknowledge the Washington University High Resolution NMR facility, partially supported by NIH grants RR02004, RR05018, and RR07155, and the Washington University Mass Spectrometry Resource Center, partially supported by NIHRR00954, for their assistance.
Footnotes
Electronic supplementary information (ESI) available: Experimental procedures for microelectrode array reactions, TOF-SIMS and synthesis and characterization of all compounds used. See DOI: 10.1039/b910577h
Notes and references
- 1.For a description of the chips used here see: Dill K, Montgomery DD, Wang W, Tsai JC. Anal. Chim. Acta. 2001;444:69–78.. 1K chips: electrode diameter = 92 µm; distance between the Pt-electrodes (rectangular cells) = 245.3 µm and 337.3 µm; 12K slide: diameter = 44 µm; distance between the Pt-electrodes (square cells) = 33 µm.
- 2.For alternative approaches see: Sullivan MG, Utomo H, Fagan PJ, Ward MD. Anal. Chem. 1999;71:4369–4375. doi: 10.1021/ac990331y. Zhang S, Zhao H, John R. Anal. Chim. Acta. 2000;421:175–187. Hintsche R, Albers J, Bernt H, Eder A. Electroanalysis (N. Y.) 2000;12:660–665.
- 3.For Pd(ii) reactions: Tesfu E, Roth K, Maurer K, Moeller KD. Org. Lett. 2006;8:709–712. doi: 10.1021/ol052891r. Tesfu E, Maurer K, Ragsdale SR, Moeller KD. J. Am. Chem. Soc. 2004;126:6212–6213. doi: 10.1021/ja0489036. Tesfu E, Maurer K, McShae A, Moeller KD. J. Am. Chem. Soc. 2006;128:70–71. doi: 10.1021/ja057155x.
- 4.For Pd(0) reactions: Tian J, Maurer K, Tesfu E, Moeller KD. J. Am. Chem. Soc. 2005;127:1392–1393. doi: 10.1021/ja042448w. Tang F, Chen C, Moeller KD. Synthesis. 2007:3411–3420.
- 5.For examples of the site-selective generation of base: Stuart M, Maurer K, Moeller KD. Bioconjugate Chem. 2008;19:1514–1517. doi: 10.1021/bc800025z. Maurer K, McShea A, Strathmann M, Dill K. J. Comb. Chem. 2005;7:637–640. doi: 10.1021/cc0498175.
- 6.For the site-selective generation of acid: Kesselring D, Maurer K, Moeller KD. Org. Lett. 2008;10:2501–2504. doi: 10.1021/ol8007827.
- 7.For the use of CAN in a site-selective fashion: Kesselring D, Maurer K, Moeller KD. J. Am. Chem. Soc. 2008;130:11290–11291. doi: 10.1021/ja804661h.
- 8.Chen C, Nagy G, Walker AV, Maurer K, McShae A, Moeller KD. J. Am. Chem. Soc. 2006;128:16020–16021. doi: 10.1021/ja067194o. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Lu P, Walker A, Maurer K, Moeller KD. Electrochem. Commun. 2008;10:973–976. [Google Scholar]
- 10.Kolb HC, Finn MG, Sharpless KB. Angew. Chem., Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Devaraj NK, Collman JP. QSAR Comb. Sci. 2007;26:1253–1260. [Google Scholar]
- 12.Devaraj NK, Dinolfo PH, Chidsey CED, Collman JP. J. Am. Chem. Soc. 2006;128:1794–1795. doi: 10.1021/ja058380h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis WG, Magallon FG, Fokin VV, Finn MG. J. Am. Chem. Soc. 2004;126:9152–9153. doi: 10.1021/ja048425z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.