Abstract
Background/Aim:
Gastric cancer (GC) is considered to be a disease of elderly patients. It has been suggested that GC in young adults has more aggressive clinical and pathologic features than in adults. In this study we aimed to evaluate clinical and pathologic features of GC under age 40 years.
Patients and Methods:
Patients included in this study were those treated and followed up for GC under age 40 years in Ankara Numune Education and Research Hospital from 2002 to 2011.
Results:
Clinical and pathologic features of 82 patients have been evaluated retrospectively. Of the patients 44 were male (54%) and 38 were (46%) female, and the median age was 35 years (min-max: 18-40 years). The tumor was grade 3 in 77% of the patients, 79% had diffuse type tumor, 64% had lymphovascular invasion, and 76% had perineural invasion. Forty-seven patients (57%) were metastatic at the time of diagnosis. The median follow up was 9 (1-101) months. The median overall survival (OS) was 9 months in metastatic patients and 8-year OS was 64% in nonmetastatic patients.
Conclusions:
We observed that young GC patients had more aggressive histopathologic features and more than half was metastatic at the time of diagnosis. We need more studies comparing young and elderly patients to confirm that young patients had more aggressive disease.
Keywords: Gastric cancer, prognosis, young
Gastric cancer (GC) is the fifth most common cancer in Turkey. According to the World Health Organization (WHO) it is the second cause of cancer-related deaths after lung cancer.[1] In the last two decades, with the decreasing incidence of Helicobacter pylori and improvement in hygienic conditions, the incidence of distal-type GC had decreased, but the incidence of proximal type GC had increased.[2] The disease is seen most commonly in fifth and sixth decades of life. Nearly 5-15% of the patients are <40 years and only 1-2% of the patients are <30 years.[3,4,5]
Most of the GC trials include older patients. Trials, directly intended for younger patients are very less, include small number of patients and results are conflicting. In some trials, it has been stated that young GC patients had more poor prognostic factors, diagnosed at more advanced stages and had more rapid progression, whereas this was not reported in some other trials.[6,7]
In this study we aimed to present clinicopathologic outcomes of GC patients under age 40 years.
PATIENTS AND METHODS
This study includes GC patients under age 40 years treated at Ankara Numune Education and Research Hospital between 2002 and 2012. The patients’ data were reviewed retrospectively. The ethical committee approved our study.
The patient's gender, age, smoking habits, place of tumor in the stomach, tumor grades, lymphovascular and perineural invasion status, histopathologic subtypes, and lymph node status were recorded. Histopathologic subtypes were classified according to “Lauren classification.” Also the patient's hematologic parameters, liver and kidney functions tests, tumor markers (carcinoembryonic antigen, alpha-fetoprotein, Ca19-9, Ca125) were recorded. According to the WHO criteria, hemoglobin levels below 13 mg/dL and 12 mg/dL in males and females, respectively, are considered as anemic.
Statistical analyses were performed using the software SPSS for windows, version 13.0 (SPSS, Chicago, IL, USA). Baseline characteristics of groups were compared by χ2 tests (for categorical variables) or two sample t tests (for continuous variables). Tumors with missing values were omitted from the analyses. Kaplan–Meier survival analysis was carried out for disease-free survival (DFS) and overall survival (OS). Survival analysis was based on the date of diagnosis. The log-rank test was used to examine the statistical significance of the differences observed between the groups. Two-sided P < 0.05 were considered statistically significant.
RESULTS
Clinicopathologic characteristics
A total of 1424 patients were diagnosed with GC in our center between 2002 and 2012. One hundred and thirty-three (9.3%) of these patients were under age 40 years and 30 (2.1%) were under age 30 years. Of these, 44 (54%) male and 38 (46%) female patient's data, who were treated and followed up at our institution, were evaluated retrospectively. The median age was 35 years (18-40). Some clinicopathologic characteristics of the patients are shown in Table 1.
Table 1.
Patient's characteristics and hematologic and biochemical parameters
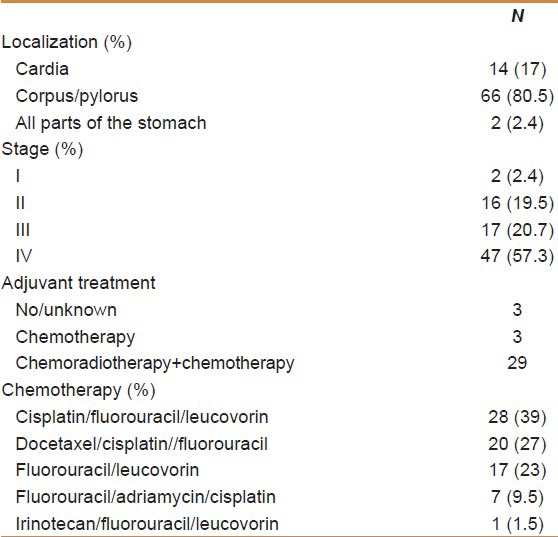
At the time of diagnosis, 26 patients (32%) were smokers, 50 patients (61%) were nonsmokers, and 6 (7%) patients were exsmokers. Primary tumor was at cardia in 14 patients (17%), at corpus and pylorus in 66 patients (80.5%), and at whole stomach in two patients (2.4%). The most common presenting symptoms were abdominal pain (67.0%), weight loss (51.2%), nausea (30.4%), dysphagia (25.6%), and early satiety (23.1%). Two (2.4%) of these patients were Stage I, 16 patients (19.5%) were Stage II, 17 of the patients (20.7%) were stage III and 47 patients (57.3%) were Stage IV according to the TNM staging system 7th edition. Thirty-two patients (39%) with locally advanced tumor had R0 resection, two patients (2.4%) had R1 resection, and one patient (1.2%) had R2 resection. Eighteen of the 47 patients with metastatic disease had undergone palliative surgery.
The source of gastric tissue for histopathologic diagnosis in 53 patients (35 nonmetastatic patients and 18 metastatic patients who had undergone palliative surgery) was surgery and the source was endoscopy in 29 patients. The tumor was of diffuse type in 31 patients (79%) and intestinal type in eight patients (21%), and with histopathologic evaluation lymphovascular invasion was present in 27 of 42 patients (64%) and perineural invasion was present in 31 of 41 patients (76%). Five of the 69 patients (7%) had Grade 1 tumor, 11 had (16%) Grade 2 tumor, and 53 had (77%) Grade 3 tumor. Twenty-four (77.4%) of 31 patients with diffuse type had Grade 3 tumor, whereas only one of eight patients with intestinal-type GC had Grade 3 tumor (P < 0.0001).
Seven patients continued their treatments at other centers and two patients had refused chemotherapy. A total of 32 patients (44%) had received adjuvant chemotherapy and 41 patients (56%) had received palliative chemotherapy. Cisplatin/fluorouracil/leucovorin were administered to 28 (39%) patients, fluorouracil/leucovorin to 17 (23%) patients, docetaxel/cisplatin//fluorouracil were administered to 20 (27%) patients, fluorouracil/adriamycin/cisplatin to seven (9.5%) patients, and irinotecan/fluorouracil/leucovorin to one (1.5%) patient. Twenty nine patients with locally advanced disease were also treated with fluoropirimidin-based chemoradiotherapy.
Survival outcomes
The median follow-up time was 9 months (1-101 months). In the follow up period, 26 (74%) of the 35 nonmetastatic patients were in remission, four had local recurrences, and five had distant metastasis. Eight-year DFS was 63% in nonmetastatic patients and progression-free survival (PFS) was 6 months in metastatic patients (P = 0.001). The median OS was 9 months in metastatic patients and 8-year OS was 64% in nonmetastatic patients (P = 0.013) [Figure 1]. After the multivariate analysis, the only significant parameter for DFS/PFS or OS, was being metastatic at the time of diagnosis. Tumor grade, histopathologic subtype, lymphovascular and perineural invasion, gender, and presenting with anemia had not found to have prognostic value.
Figure 1.
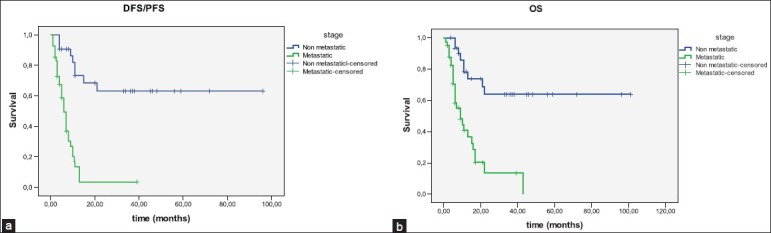
(a) Disease-free survival/progression-free survival of patients according to the stages; (b) overall survival of patients according to the stages.
The median PFS was 11 months in patients with metastatic disease and had partial remission with chemotherapy, 8 months in patients with metastatic disease and had stable disease with chemotherapy and 3 months in patients with metastatic disease and had progressive disease with chemotherapy (P < 0.0001). There was no progression of disease in eight (19.5%) of 41 metastatic patients, while 33 patients (80.5%) had progression. The metastasis were liver in 10 (31%) patients, intra-abdominal lymph nodes in 10 (31%) patients, lung in one (3%) patient, other sites in five (16%) patients, and six (19%) patients had multiorgan metastasis.
There was no correlation between tumor grade, histopathologic subtype, lymphovascular and perineural invasion, and DFS and PFS. There was no DFS and PFS difference in terms of chemotherapy regimens. There was no correlation between tumor markers and DFS/PFS.
The OS was 7 months in females and 15 months in males (P > 0.05). In the metastatic group, the median OS was 5 months in patients whose tumor were at corpus and cardia and 11 months in patients whose tumor were at pylorus (P = 0.023). There was no correlation between tumor grade, histopathologic subtype, lymphovascular/perineural invasion, and OS. There was no OS difference in terms of chemotherapy regimens. In nonmetastatic group, the median OS was 6 months in patients who had progressive disease after chemotherapy and 22 months who were in remission (P = 0.004). The median OS was 16 months in patients with metastatic disease and had partial remission with chemotherapy, 9 months in patients who had stable disease with chemotherapy and 5 months in patients who had progressive disease with chemotherapy (P = 0.01). There was no correlation between tumor markers and OS.
DISCUSSION
Despite decreasing frequency, GC is the fifth common cancer around the world. Most of the patients have advanced age. In the literature, the percentage of patients under age 40 years is 2-15% and male/female ratio is 1,5-2/1 in all patients and 1-1,5/1 in young patients.[3,4,5] In our study, 9.3% of patients were under age 40 years and 46% were female.
Environmental factors such as smoking, infections, and dietary habits are more important in the etiology of stomach cancer in advanced age.[8,9,10] This may be one cause of the GC to be present more frequently in males in advanced age. Genetic causes and hormonal status are more important in their etiology in young GC patients.[11,12] In the study by Kath et al., female patient ratio was 75% in GC patients under age 30 years.[5] Also it has been seen that all female patients had a pregnancy history within 24 months before the GC diagnosis. The relationship between hormonal status and GC development was also observed in the study by Chung et al.[4]
Although it was not observed that young female GC patients have more aggressive disease than males, it has been shown that young female GC patients were diagnosed in more advanced stages of the disease.[5,13] Especially during the pregnancy period, nausea, vomiting, and stomach ache may be more frequent. For this reason many females do not go in for further examination. In our study, 53% of the female patients and 47% of the male patients were diagnosed in the metastatic stage. From this study it is understood that females who have gastric symptoms and risk factors for GC should have further examination so that diagnosis in early stages may be possible.
In the last two decades, the incidence of distal-type GC has decreased slowly with the improvement in hygienic conditions and more effective therapies for H. pylori.[2] In our study, primary tumor was at cardia in 17% patients (n = 14) and at corpus and pylorus in 81% patients (n = 66). These results are comparable with advanced-age GC patients in developed countries. Another finding from our study supporting genetic etiology is, 79% of patients have diffuse-type and 21% patients have intestinal-type GC. Although lymphovascular and perineural invasion were more frequent in diffuse type, it has no negative effect on survival time.
According to the “Surveillance, Epidemiology and End Results (SEER)” database, 38% of patients in all age groups and 53% of patients under age 45 years were diagnosed in the metastatic stage.[3] In the study by Santoro et al., the percentage of metastatic disease in patients under age 45 years was 49%.[14] In our study, this ratio was 57.3%. Although there was no screening program about GC in Turkey, it may be suggested that young patients with gastric symptoms, especially resistant to antiacid therapies, should have further examination.
We did not observe a survival difference between male and female patients. Although we did not make a comparison between young and older patients, the survival time was commensurate with advanced-age GC patients in the literature. So we may say that age is not a poor prognostic factor for GC. There was also no difference in survival between chemotherapy regimens.
Although most of the GC patients are in advanced ages, GC may be diagnosed in young patients, especially in females. Individuals who have multiple risk factors, should be recommended upper gastrointestinal system endoscopy immediately. Young GC patients have more pathologically poor prognostic factors such as diffuse type, lymphovascular and perineural invasion, high tumor grade, and diagnosed in more advanced stages. But we do not have enough evidence to say that more young GC patients have advanced stages and have worse prognosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. 1. [DOI] [PubMed] [Google Scholar]
- 2.Zhu AL, Sonnenberg A. Is gastric cancer again rising? J Clin Gastroenterol. 2012;46:804–6. doi: 10.1097/MCG.0b013e3182604254. [DOI] [PubMed] [Google Scholar]
- 3.Al-Refaie WB, Hu CY, Pisters PW, Chang GJ. Gastric adenocarcinoma in young patients: A population-based appraisal. Ann Surg Oncol. 2011;18:2800–7. doi: 10.1245/s10434-011-1647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol. 2010;16:256–63. doi: 10.3748/wjg.v16.i2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kath RFJ, Schneider CP, Höffken K. Gastric cancer in very young adults: Apropos four patients and a review of the literature. J Cancer Res Clin Oncol. 2000;126:233–7. doi: 10.1007/s004320050038. [DOI] [PubMed] [Google Scholar]
- 6.Lai IR, Lee WJ, Chen CN, Lee PH, Chang KJ, Chang-Yu S, et al. Gastric cancer in the young. Hepatogastroenterology. 1997;44:1641–5. [PubMed] [Google Scholar]
- 7.Bloss RS, Miller TA, Copeland EM., 3rd Carcinoma of the stomach in the young adult. Surg Gynecol Obstet. 1980;150:883–6. [PubMed] [Google Scholar]
- 8.Tredaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: Review and meta-analysis. Int J Cancer. 1997;72:565–73. doi: 10.1002/(sici)1097-0215(19970807)72:4<565::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.The EUROGAST Study Group. An international association between Helicobacter pylori infection and gastric cancer. Lancet. 1993;341:1359–62. [PubMed] [Google Scholar]
- 10.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: Review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura K, Ueyama T, Yao T, Xuan ZX, Ambe K, Adachi Y, et al. Pathology and prognosis of gastric carcinoma. Findings in 10,000 patients who underwent primary gastrectomy. Cancer. 1992;70:1030–7. doi: 10.1002/1097-0142(19920901)70:5<1030::aid-cncr2820700504>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira C, Seruca R, Carneiro F. Genetics, pathology, and clinics of familial gastric cancer. Int J Surg Pathol. 2006;14:21–33. doi: 10.1177/106689690601400105. [DOI] [PubMed] [Google Scholar]
- 13.Maeta M, Yamashiro H, Oka A, Tsujitani S, Ikeguchi M, Kaibara N. Gastric cancer in the young, with special reference to 14 pregnancy-associated cases: Analysis based on 2,325 consecutive cases of gastric cancer. J Surg Oncol. 1995;58:191–5. doi: 10.1002/jso.2930580310. [DOI] [PubMed] [Google Scholar]
- 14.Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young european adults. Br J Surg. 2007;94:737–42. doi: 10.1002/bjs.5600. [DOI] [PubMed] [Google Scholar]


