Abstract
It has been 13 years since the first outbreak of West Nile Virus (WNV) occurred in the Americas. Since then, thousands of human cases have been reported in the United States. In contrast, there has not yet been an outbreak of WNV in any Latin American countries, including Mexico where <20 cases have been reported. We aimed to review publications to gather the main theories related to the fact that not all the countries of the continent reported human cases or that they have reported few cases since the introduction of WNV in the Western Hemisphere. We identified relevant publications using the PubMed database. Furthermore, we present on-line published information from Mexico. We found that researchers have tried to explain this phenomenon using several theories, like pre-existing antibodies against a heterotypical virus that have conferred cross protection in the population. Another explanation is that the strains circulating in Latin America are attenuated or that they came from a different origin of introduction in the continent. Another theory is that a conclusive diagnostic in regions where more than one Flavivirus is circulating results in cross-reaction in serological tests. Probably the sum of factors described by researchers in these theories in order to explain the behavior of the virus has resulted in the low number of reported cases in Latin America.
Keywords: Latin American countries, Theories, West Nile Virus
INTRODUCTION
The first known contact of West Nile Virus (WNV) in the Western Hemisphere took place in the fall of 1999 in the city of New York (NY), USA.[1,2,3,4] Since then, the virus has spread widely across the rest of the country. By 2003, almost all the states had reported cases. Exceptions include Oregon, Washington and Alaska in the north, Hawaii in the far west, and Maine on the east coast. Then, in 2004 and 2006, Oregon and Washington reported cases, respectively.[5,6,7] According to the virus spreading theories, migratory birds are responsible for spreading the virus to new areas.[8,9] In this case, the next obvious paths that the virus could have taken would have been northward and southward dispersion patterns (Canada and Mexico). Indeed, by 2001, the first report from birds seropositive for WNV came from Canada, followed by several reports of human and horse cases in that country in 2002.[10,11]
Just as in Canada, in the summer of 2002, reports of encephalitis-like illness started in Mexico (birds and horses in the northern states of the country: Tamaulipas, Coahuila and Chihuahua).[12,13] By 2003, isolation of the virus was performed in a Common Raven (Corvus corax) from the southeast state of Tabasco.[12] In 2004, isolation in mosquitoes resulted from a single pool of Culex quinquefasciatus from the northern state of Nuevo Leon and a virus isolation from a human, a 62-year-old woman from the state of Sonora.[14] In 2009, a fatal human case was reported in the state of Nuevo Leon.[15] Recently, in the southernmost state of the country (Chiapas), WNV circulation was demonstrated in two positive mosquito pools (Culex nigripalpus and Culex interrogator) and 17 domestic animals with antibodies against the virus.[16]
Since its introduction in the Western Hemisphere, the virus has been circulating in many other countries of Latin America. There are several reports of WNV activity in birds, horses, or mosquitoes. The initial report came from the Cayman Islands in 2001, reporting a human West Nile neurological disease.[17] In 2002, the Caribbean countries of Dominican Republic, Jamaica and Guadeloupe reported neutralizing antibodies against the virus in resident birds.[18,19,20,21] In addition, horses seropositive for WNV were identified in Guadeloupe.[18] The Bahamas and Cuba confirmed a local amplification cycle in 2003 by detecting antibodies to WNV in a human (as well as horses in Cuba,[22] El Salvador,[23] and Belize[24]). In 2004, antibodies to WNV were detected in resident and migratory birds in Puerto Rico[23] and by 2007 three human samples of blood donors showed evidence to the virus.[25,26,27] Also, in 2004, Haiti reported two human cases after a surveillance that started due the hurricane Jeanne;[28] and Costa Rica, Guatemala, Colombia recorded seropositive horses in the same year,[29,30,31] and horses and birds in Trinidad and Tobago.[32] In Venezuela, birds and horses in 2005 resulted positive to serological tests.[33]
In 2006, a case was reported of a Spanish missionary living in Nicaragua with antibodies against WNV, although he was not diagnosed as positive to WNV in Nicaragua. Symptoms persisted, however and 13 days later, he was transferred to a hospital in Madrid, Spain. It was not until he was admitted to the Tropical Medicine Unit in Madrid, 160 days after the onset of initial symptoms,[34] that they finally had a diagnosis. The last report from South America came from Brazil, where neutralizing antibodies against the virus were found in horses in 2009 and in birds in 2010,[35,36] nevertheless no human cases were found after a screening of patients that year.[37] For some reason, there are no reports in several countries from Central and South America and also for some Caribbean Islands; although, all have the variables that could favor the circulation of the virus. There are also reports of WNV circulation in neighboring countries. Interestingly, Argentina reported horses, birds and human cases, thus concluding that the viruses’ cycle exists in the southernmost area of continent[38,39][Table 1 and Figure 1].
Table 1.
West Nile Virus reports in the Western Hemisphere, since its introduction
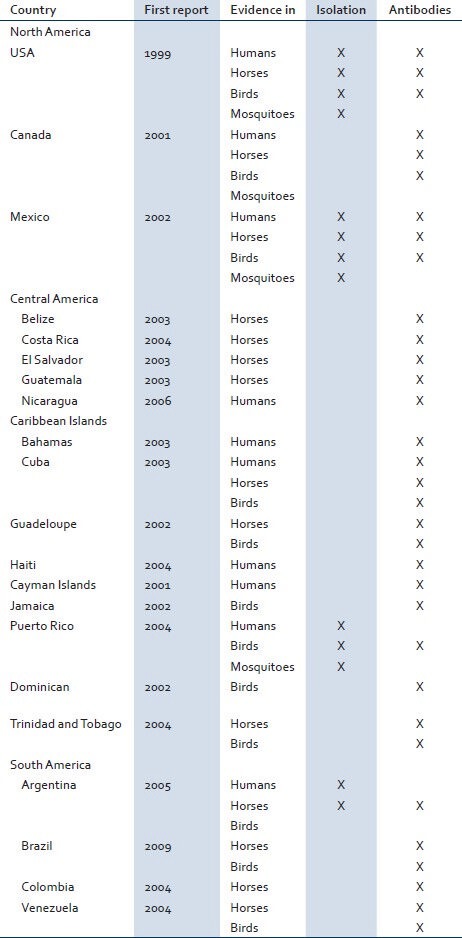
Figure 1.
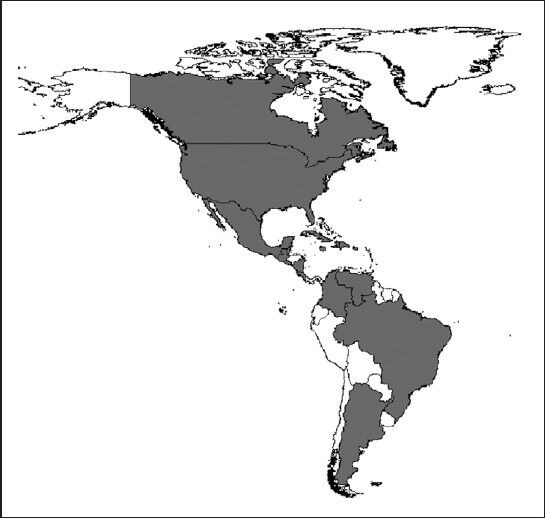
New World Countries with West Nile Virus evidence until 2012 (grey areas). Few areas have not reported virus activity (Central America: Honduras and Panama; West Indies: Barbados and Grenada; South America: Bolivia, Ecuador, French Guinea, Guyana, Paraguay, Peru, Suriname and Uruguay
AIM
The information presented herein includes peer-reviewed publications recorded through almost 13 years since the introduction of WNV in the Western Hemisphere. We reviewed publications in order to gather the main theories related to the fact that not all the countries of the continent reported human cases or they have reported only few cases. Furthermore, we are presenting on-line published information from Mexico.
METHODS
Search strategy
We reviewed publications to track the existence of WNV in the Americas, based on case reports from 1999 until now and also theories related to the fact that not all the countries of the continent reported human cases or that they have reported only few cases. The search was performed in the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed) and also directly in open access on-line Journals: Center for Disease Control and Prevention (CDC) Emerging Infectious Diseases, Plos Neglected Tropical Diseases, and World Health Organization Bulletin. The key words used in this literature search were WNV, crossed with the different countries of the continent. As an example, we searched in PubMed for publications using the key words WNV Mexico, resulting in 99 publications; out of these, we select 23 for review [Table 2].
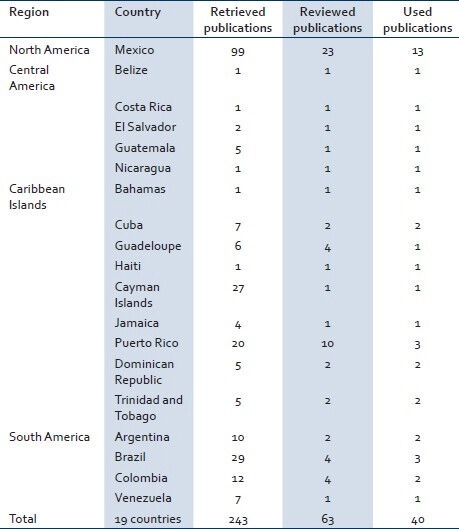
Table 2.
Number of retrieved articles in PubMed database by country, using as keywords the name of each country crossed with West Nile Virus
We also are presenting on-line published information from the health departments of USA: CDC (http://www.cdc.gov/), US Department of Agriculture (http://www.aphis.usda.gov/); Canada: Public Health Agency of Canada (http://www.phac-aspc.gc.ca); and Mexico: National Center of Epidemiological Surveillance (http://www.cenave.gob.mx).
Selection
We selected for review all retrieved publications for each Latin American country. Inclusion criteria were: Title or abstract resulting from the search, showing WNV circulation evidence specifically in the search country and the article summarized theories formulated by researchers of these publications. Exclusion criteria were: We did not include papers or websites that used previously described information regarding virus circulation evidence.
RESULTS
For Latin American countries, the search in PubMed retrieved 243 articles, of which 180 were excluded because they did not meet inclusion criteria, 63 were selected for full-text review and out of them 40 were selected to be included in the review [Table 2].
Spread theories over the continent
The introduction of the virus in the Western Hemisphere was in NY. Lanciotti et al. compared 33 WNV strains through phylogenetic analysis and showed that the strain circulating in this state (WN-NY99) was most closely related to a strain circulating in Israel in 1998. The higher pathogenicity in birds than in humans may be due to background human immunity to WNV in Israel.[1] Likely due to the lack of this immunity in USA, the strain that started the outbreak in NY eventually caused more than 30,000 human cases and 1,200 fatalities in the past 13 years.
Owing to the similarities in strains, the main theories of introduction of WNV not only involved intercontinental viremic migratory birds carrying the virus from the old world to the Western Hemisphere, but also zoo, pet or domestic birds introduced illegally to USA. There is also the possibility of a commercial flight transporting an infected mosquito into the country.[3,40,41] Once the virus established its endemic cycle in NY, it started to spread through the rest of the country, south and westward. By 2011, 326 bird species, including native and exotic, were reported as positive for evidence of WNV infection by the CDC,[42] and by 2010, there were 25,889 cases in horses.[43]
In Mexico, it has been speculated that there could have been at least two entry routes; the first one, in the southeast of the country in the state of Tabasco, probably by migratory birds from the southeastern United States or Caribbean Islands. The other route could have been from the southern United States to Northern Mexico.[44] For the rest of the continent, WNV probably took different routes. It could have arrived by one of the potential methods suggested for USA; nevertheless, it could have been carried from the USA by birds, one of the suspected carriers and amplifiers of the disease, using one of the three major migratory flyaway routes (Atlantic, Central or Pacific).[3,45]
WNV in Mexico
The first efforts at confirming the presence of the virus in Mexico Began in March 2000 through collaboration between the Autonomous University of Yucatan and Colorado State University (CSU), who sampled blood from birds in Yucatan State in Southeastern Mexico. A total of 8,611 birds were collected, and 21 of them had serologic evidence of Flavivirus infection. Of these, 8 had antibodies to WNV by enzyme-linked immunosorbent assay, but only 5 of them confirmed the infection by plaque reduction neutralization assay; sampling was performed during March 2000 and April 2003.[46] At the same time, they sampled asymptomatic mammals, birds and reptiles in the Yucatan Peninsula of Mexico, in the states of Campeche (in farmed crocodiles), Quintana Roo (two locations on Cozumel Island) and Yucatan (Merida, Zoo), where antibodies against the virus were detected in all the sites of collection. Interestingly, results of seroprevalence on crocodiles were overwhelming (86%) and also high for horses on Cozumel Island (52%). Seroprevalence for WNV in birds and mammals in the Merida Zoo was 2.7% and 3.8%, respectively.[47]
Simultaneously in the northeast of the country, Fernαndez-Salas et al. and Blitvich et al. in 2003, reported evidence of virus circulation; they reported antibodies to WNV on nine birds (1.13%) out 796 by December 2001[13] and 15 horses sera (62.5%) out of 24 samples, confirmed by plaque neutralization assay. However, the rate of asymptomatic seropositivity was high, with 10 (67.7%) of 15 WNV-infected horses showing no signs of illness.[48] These reports from both regions, showed that the virus was circulating by December 2001 and December 2002, north and south of Mexico respectively.
Autonomous University of Nuevo Leon, in collaboration with CSU, sampled from 2001 through 2006 in the Mexican states bordering Texas (Nuevo Leon, Coahuila and Tamaulipas). Sampling was performed on birds, horses and mosquitoes. We found 90 (58 resident and 32 migratory) out of 1,686 birds (5.3 %) were seropositive to WNV. Sampling of horses in Nuevo Leon and Coahuila found 116 of 498 (23.2%) seropositive to the virus (unpublished data) and from 238 pools of mosquitoes, only a single pool of Cx. quinquefasciatus, designated as NL-54 (0.4%), resulted in a virus isolation in the state of Nuevo Leon; the same publication showed a virus isolation from a 62-year-old woman from the western state of Sonora.[14]
The first regional workshop for strengthening and training in surveillance, control and prevention activities for WNV in Mexico was held in 2005. Health authorities in Mexico performed the sampling in all the states of the country and they found evidence of virus circulation for 2002-2004. Sampling was performed on birds, horses and humans in all 32 states and the presence of the virus was detected in 31 of these states (277 municipalities out from 587) Sampling in these years accumulated a total of 983 humans with presumptive encephalitis symptoms, but only eight were seropositive for WNV (0.8%). Of the 23,596 birds sampled, only 452 (1.9%) were seropositive for WNV and of the 10,275 horses sampled, 3,690 (35.9%) were seropositive for WNV.[49]
Because of these numbers, the health department implemented in 2005 the national committee for epidemiological surveillance of WNV in collaboration with state universities and animal health organizations such as Animal Health division SAGARPA, SEMARNAT, etc. In 2006, 1,586 horses were sampled and 553 of them were seropositive (34.8%). In addition, 943 birds were sampled and 113 were seropositive (11.9%). Of the 203 humans tested, only one (0.4%) was seropositive (http://www.cenave.gob.mx/von/default.asp?id=24) accessed 09 September 2011.
For some reason not elucidated to date, no evidence on avian mortality has been reported in Latin American countries, in such proportions as reported in USA. Only few dead birds were reported in Mexico (Sonora, Tamaulipas, Tabasco and Nuevo Leon).[50]
No human cases in Mexico (Latin America theories)
The most important question that has puzzled the researchers was the fact that in Mexico, not a single report indicated there was a human outbreak. Several hypotheses resulted in order to explain this phenomenon, which in fact was also observed elsewhere in Latin America since most of the other countries reported birds and horses, but not humans, seropositive for WNV.[50] Because of this, one hypothesis was that viral strains circulating in the USA probably were different from those circulating in most of the states of Mexico (and elsewhere in Latin America). This derived from genetic studies that showed some differences of nucleotide (nt) sequences indicating mutations at 9 nt of the Mexican raven isolated in 2003 in Tabasco state, compared with the prototype NY99 strain. However, comparison with sequences in 2002 from Texas strains, showed only one shared mutation, supporting the hypothesis regarding a different entry route of the virus into Mexico.[44]
Furthermore, this hypothesis could be supported by a recent publication from Colombia where the virus was isolated from apparently healthy birds, suggesting that probably this strain could be attenuated (also it was more closely related to Louisiana isolates from 2001).[51]
The most plausible hypothesis is that pre-existing neutralizing antibodies against another Flavivirus in the population, such as dengue virus (DENV) or Saint Louis encephalitis virus (SLEV), common mosquito-transmitted viruses throughout Latin America, might offer partial protection from WNV disease. This is supported by studies of heterologous Flavivirus on hamsters.[52,53]
Another theory is based on the delay between the beginning of cases reported on fauna and humans as in the USA. There was a delay of 3 years from the first reports in NY in 1999 until the first major outbreak appeared with more than 100 human cases in 2002.[54]
Furthermore, health services in Mexico may fail to recognize the clinical signs of WNV disease as some of the symptoms are similar to those caused by DENV. Another consideration is the difficulty to perform serological diagnose in areas where more than one Flavivirus is circulating, with the high probability of finding people previously infected, which can produce a cross reaction in serological tests.[14,55,56]
In order to explain the low incidence of WNV illness in Northern Mexico, another group of researchers in the state of Nuevo Leon carried out a clinical and serological investigation on three groups of individuals: Patients with unspecific fever (n = 588), encephalitic patients (n = 44) and asymptomatic blood donors (n = 800). From the total (1432 individuals), none was positive to WNV immunoglobulin (Ig) IgM test, but there was a range from 40% to 59% of IgG against Flavivirus, where DENV was the responsible for most of the cases. From these patients, only six were seropositive for WNV, concluding that probably the large proportion of the population previously infected with DENV is resistant or less susceptible to severe WNV.[57]
DISCUSSION
It is interesting to note how WNV has been circulating in USA since 1999 and in Canada since 2001, causing human cases every year in different states, in contrast to the rest of the continent, which never developed epidemic outbreaks. Nevertheless, the most important thing is that there is no proven explanation for this phenomenon.
For instance, in Mexico, there are areas or states where DENV is not present. Thus, it could be expected that the population was susceptible to WNV. In these states, vector mosquitoes are present, but no outbreak has occurred. Even more, the confirmed human case in 2004 in the state of Sonora highlighted the precedent that WNV can coexist in an endemic DENV zone since this state is considered high risk for dengue by public health authorities (http://www.cenave.gob.mx/dengue/default.asp?id=32) accessed 18 November 2011; and more recently the fatal human case in the state of Nuevo Leon where DENV also circulates year by year.
On one hand, it could have made more sense to consider the attenuated Latin American strains theory supported by the publication of WNV isolated from healthy birds from Colombia. Following this idea, we could speculate that due to attenuated strains, avian mortality it was not present in Latin American countries. Furthermore, the number of infected horses with disease symptoms or that died in Latin America, was not as high as the number reported in USA (where in 2002, there were 15,275 confirmed cases, which was the highest number of cases since the introduction of the virus to Western Hemisphere).[43] It is also known that in horses infection can be sub-clinical and not necessarily show symptoms.[58] What we could think is that probably horse sampling performed in Latin American horses was not as exhaustive to determine the actual number in comparison to USA.
Therefore, this theory would lead us to think that if the number of horse disease cases were greater than that reported to date in Latin America and if a random sampling in human population living in risk areas were also performed, then the percentage of seropositive people also would be greater. These people would have had either a sub-clinical infection or misdiagnosis.
On the other hand, considering cross protection due to heterologous specific antibodies against another Flavivirus,[52] one should also consider that a very high proportion of the Latin American population has been infected with at least one Flavivirus in their lives as concluded by the report of the serological surveillance in Nuevo Leon state, where the proportion of seropositive people for Flavivirus infection was very high. Furthermore, we could speculate about a new undiscovered Flavivirus, such as T’Ho virus, found in Cx. quinquefasciatus mosquitoes from the Merida Zoo. This is a novel Flavivirus that has a close genetic and phylogenetic relationship and therefore probably a close antigenic relationship to WNV, which could provide cross protection.[59]
However using the example of dengue infection as the main circulating virus in Mexico, the disease is mainly in urban areas, leaving rural areas with antibody-free populations against DENV, which are therefore prone to acquire the WNV infection. It is also relevant to note that DENV does not circulate in all the states of Mexico.
Using Mexico as a Latin American example, where limited financial resources are a barrier to comprehensive testing and coverage, public health authorities of the Institute of Diagnosis and Epidemiological Reference say that WNV tests in humans can only be covered (financially) and be carried out when there are symptoms of encephalopathy. Therefore, they do not apply the test on patients that were negatively diagnosed for DENV, even if a patient presented febrile symptoms typical of infection by some other Flavivirus. Interestingly, there have been samples of patients with symptoms of dengue fever-like illness, which turns out to be WNV. There have also been patients with WNV symptoms that were in fact dengue, but these samples have not been reported (Carmen Guzmαn, personal communication, October 2010).
Therefore, the laboratory misdiagnosis theory could be considered, which does not mean that the disease is not WNV. A negative diagnosis for DENV could be positive to WNV. These would be the most valid theories, taking as reference the case reported in Nicaragua, where it was observed that disease diagnosis is difficult in regions where several viruses of the same family are circulating at the same time. With the case of the Spanish missionary, diagnosis was not confirmed until more than 5 months after the patient first became infected. Cross-reaction in serologic tests of dengue and tick-borne encephalitis virus was found. Nevertheless, antibody titers found in both tests did not vary much with the ones found in WNV, not to mention that there was only IgM specific to WNV. In the absence of IgM’s for the other two Flavivirus, the diagnosis resulted positive for WNV. Furthermore, it was mentioned that the missionary probably had been previously infected with dengue.
The sum of factors described by researchers through all these years in order to explain the behavior of the virus in Latin America, mainly Mexico (being the neighbor country of USA where the dispersion of WNV began) probably has resulted in the low number of reported cases in the rest of the Americas. Therefore, we add the following factors: WNV strains circulating in Latin America are attenuated, there is a preexistence of heterologous antibodies in some part of the population and the fact that there are not sufficient resources to perform tests result in the fact that the actual disease incidence cannot be determined in the human population. We thereby conclude and recommend that more attention should be paid to cases of patients looking for medical attention in health institutions, when they are presenting typical signs and symptoms of DENV infection but have a negative test results. They should be tested for similar diseases. Tests should not solely be performed on people presenting some type of encephalopathy.
Without considering the strain, WNV origin or its dispersion patterns in the Western Hemisphere, we need to keep in mind that WNV is an enzootic disease and should therefore be under epidemiological surveillance that looks for seroconversion in domestic animals and not only in wild fauna. It is easier and more economical to sample sentinel fauna (for example using zoo or farmed animals as was used in the Mexican Yucatan Peninsula), instead of investing great amounts of resources collecting wild birds.
In this case, as in any other vector-borne disease, it is enough to have the coincidence of all the appropriate conditions or variables, so it can turn from an outbreak into an epidemic, just as it is with another virus like dengue. Regarding the strains of WNV that are circulating in Latin America and which probably come from the virus of the United States, they managed to attenuate themselves.[60] However, nothing assures that they cannot change again, turning more aggressive and leading to infection of humans.
In conclusion, since the virus first appeared in Mexico and after subsequent reports from Central and South America of a considerable number of wild fauna and even domestic animals seropositive to WNV, public health authorities should monitor the disease in human patients, mainly in those who present a febrile phase similar to dengue and are negative to this disease. The main reasons presented in this paper for the need to carry-out studies on human subjects are: (1) It is well-known that the cycle is already established in the fauna, (2) existing evidence that WNV can coexist in regions where other Flavivirus are circulating and (3) since WNV can be confused with these other diseases, it is probable that the virus has been misdiagnosed and therefore, its real incidence is unknown.
ACKNOWLEDGEMENTS
This paper was supported by the grant SALUD-161864. We would like to thank Dr. Kristen Eckart, senior editor at Strategic Solutions and Dr. Kirk Allen from CIATEJ/Peace Corps, for their valuable remarks in editing the English of the manuscript.
Footnotes
Source of Support: This paper was supported by the grant SALUD-161864.
Conflict of Interest: None declared.
REFERENCES
- 1.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, et al. Origin of the west nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–7. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 2.Magori K, Bajwa WI, Bowden S, Drake JM. Decelerating spread of west nile virus by percolation in a heterogeneous urban landscape. PLoS Comput Biol. 2011;7:e1002104. doi: 10.1371/journal.pcbi.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappole JH, Derrickson SR, Hubálek Z. Migratory birds and spread of west nile virus in the Western Hemisphere. Emerg Infect Dis. 2000;6:319–28. doi: 10.3201/eid0604.000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasci RS, White DJ, Stirling H, Oliver JA, Daniels TJ, Falco RC, et al. West nile virus isolates from mosquitoes in New York and New Jersey, 1999. Emerg Infect Dis. 2001;7:626–30. doi: 10.3201/eid0704.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindsey NP, Staples JE, Lehman JA, Fischer M. Centers for Disease Control and Prevention (CDC). Surveillance for human west nile virus disease- United States, 1999-2008. MMWR Surveill Summ. 2010;59:1–17. [PubMed] [Google Scholar]
- 6.Murray KO, Mertens E, Despres P. West nile virus and its emergence in the United States of America. Vet Res. 2010;41:67. doi: 10.1051/vetres/2010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen LR, Hayes EB. West nile virus in the Americas. Med Clin North Am. 2008;92:1307, ix–22. doi: 10.1016/j.mcna.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Malkinson M, Weisman Y, Pokamonski S, King R, Deubel V. Intercontinental transmission of west nile virus by migrating white storks. Emerg Infect Dis. 2001;7:540. doi: 10.3201/eid0707.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilpatrick AM, Daszak P, Jones MJ, Marra PP, Kramer LD. Host heterogeneity dominates west nile virus transmission. Proc Biol Sci. 2006;273:2327–33. doi: 10.1098/rspb.2006.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Public Health Agency of Canada. West Nile virus national surveillance report: Summary of 2002-2007 WNV seasons. [Accessed September 2010]. http://www.phac-aspc.gc.ca/wnv-vwn/mon-hmnsurv-archive-eng.php#a2002_07 .
- 11.Gubler DJ. The continuing spread of west nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039–46. doi: 10.1086/521911. [DOI] [PubMed] [Google Scholar]
- 12.Estrada-Franco JG, Navarro-Lopez R, Beasley DW, Coffey L, Carrara AS, Travassos da Rosa A, et al. West nile virus in Mexico: Evidence of widespread circulation since July 2002. Emerg Infect Dis. 2003;9:1604–7. doi: 10.3201/eid0912.030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Salas I, Contreras-Cordero JF, Blitvich BJ, González-Rojas JI, Cavazos-Alvarez A, Marlenee NL, et al. Serologic evidence of west nile virus infection in birds, Tamaulipas State, México. Vector Borne Zoonotic Dis. 2003;3:209–13. doi: 10.1089/153036603322662192. [DOI] [PubMed] [Google Scholar]
- 14.Elizondo-Quiroga D, Davis CT, Fernandez-Salas I, Escobar-Lopez R, Velasco Olmos D, Soto Gastalum LC, et al. West nile virus isolation in human and mosquitoes, Mexico. Emerg Infect Dis. 2005;11:1449–52. doi: 10.3201/eid1109.050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rios-Ibarra C, Blitvich BJ, Farfan-Ale J, Ramos-Jimenez J, Muro-Escobedo S, Martínez-Rodriguez HR, et al. Fatal human case of west nile disease, Mexico, 2009. Emerg Infect Dis. 2010;16:741–3. doi: 10.3201/eid1604.091614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulloa A, Ferguson HH, Méndez-Sánchez JD, Danis-Lozano R, Casas-Martínez M, Bond JG, et al. West nile virus activity in mosquitoes and domestic animals in Chiapas, México. Vector Borne Zoonotic Dis. 2009;9:555–60. doi: 10.1089/vbz.2008.0087. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) West nile virus activity - United States, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:497–501. [PubMed] [Google Scholar]
- 18.Komar O, Robbins MB, Contreras GG, Benz BW, Klenk K, Blitvich BJ, et al. West nile virus survey of birds and mosquitoes in the Dominican Republic. Vector Borne Zoonotic Dis. 2005;5:120–6. doi: 10.1089/vbz.2005.5.120. [DOI] [PubMed] [Google Scholar]
- 19.Quirin R, Salas M, Zientara S, Zeller H, Labie J, Murri S, et al. West nile virus, Guadeloupe. Emerg Infect Dis. 2004;10:706–8. doi: 10.3201/eid1004.030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komar O, Robbins MB, Klenk K, Blitvich BJ, Marlenee NL, Burkhalter KL, et al. West nile virus transmission in resident birds, Dominican Republic. Emerg Infect Dis. 2003;9:1299–302. doi: 10.3201/eid0910.030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupuis AP, 2nd, Marra PP, Kramer LD. Serologic evidence of west nile virus transmission, Jamaica, West Indies. Emerg Infect Dis. 2003;9:860–3. doi: 10.3201/eid0907.030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pupo M, Guzmán MG, Fernández R, Llop A, Dickinson FO, Pérez D, et al. West nile virus infection in humans and horses, Cuba. Emerg Infect Dis. 2006;12:1022–4. doi: 10.3201/eid1206.051235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz L, Cardenas VM, Abarca M, Rodriguez T, Reyna RF, Serpas MV, et al. Short report: Serological evidence of west nile virus activity in El Salvador. Am J Trop Med Hyg. 2005;72:612–5. [PubMed] [Google Scholar]
- 24.EID Weekly Updates: Emerging and Reemerging Infectious Diseases, Region of the Americas, Vol. 2, No. 11-18. 2004 Mar [Google Scholar]
- 25.Dupuis AP, 2nd, Marra PP, Reitsma R, Jones MJ, Louie KL, Kramer LD. Serologic evidence for west nile virus transmission in Puerto Rico and Cuba. Am J Trop Med Hyg. 2005;73:474–6. [PubMed] [Google Scholar]
- 26.Barrera R, Hunsperger E, Muñoz-Jordán JL, Amador M, Diaz A, Smith J, et al. First isolation of west nile virus in the Caribbean. Am J Trop Med Hyg. 2008;78:666–8. [PubMed] [Google Scholar]
- 27.Hunsperger EA, McElroy KL, Bessoff K, Colón C, Barrera R, Muñoz-Jordán JL. West nile virus from blood donors, vertebrates, and mosquitoes, Puerto Rico, 2007. Emerg Infect Dis. 2009;15:1298–300. doi: 10.3201/eid1508.090333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beatty ME, Hunsperger E, Long E, Schürch J, Jain S, Colindres R, et al. Mosquitoborne infections after Hurricane Jeanne, Haiti, 2004. Emerg Infect Dis. 2007;13:308–10. doi: 10.3201/eid1302.061132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales-Betoulle ME, Morales H, Blitvich BJ, Powers AM, Davis EA, Klein R, et al. West nile virus in horses, Guatemala. Emerg Infect Dis. 2006;12:1038–9. doi: 10.3201/eid1206.051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattar S, Edwards E, Laguado J, González M, Alvarez J, Komar N. West nile virus antibodies in Colombian horses. Emerg Infect Dis. 2005;11:1497–8. doi: 10.3201/eid1109.050426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hobson-Peters J, Arévalo C, Cheah WY, Blitvich BJ, Tan CS, Sandis A, et al. Detection of antibodies to west nile virus in horses, Costa Rica, 2004. Vector Borne Zoonotic Dis. 2011;11:1081–4. doi: 10.1089/vbz.2010.0198. [DOI] [PubMed] [Google Scholar]
- 32.Thompson NN, Auguste AJ, Coombs D, Blitvich BJ, Carrington CV, da Rosa AP, et al. Serological evidence of Flaviviruses and alphaviruses in livestock and wildlife in Trinidad. Vector Borne Zoonotic Dis. 2012;12:969–78. doi: 10.1089/vbz.2012.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bosch I, Herrera F, Navarro JC, Lentino M, Dupuis A, Maffei J, et al. West nile virus, Venezuela. Emerg Infect Dis. 2007;13:651–3. doi: 10.3201/eid1304.061383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monge Maillo B, López-Vélez R, Norman F, de Ory F, Sanchez-Seco MP, Giovanni Fedele C. Importation of west nile virus infection from Nicaragua to Spain. Emerg Infect Dis. 2008;14:1171–3. doi: 10.3201/eid1407.071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pauvolid-Corrêa A, Morales MA, Levis S, Figueiredo LT, Couto-Lima D, Campos Z, et al. Neutralising antibodies for west nile virus in horses from Brazilian Pantanal. Mem Inst Oswaldo Cruz. 2011;106:467–74. doi: 10.1590/s0074-02762011000400014. [DOI] [PubMed] [Google Scholar]
- 36.Melandri V, Guimarães AÉ, Komar N, Nogueira ML, Mondini A, Fernandez-Sesma A, et al. Serological detection of west nile virus in horses and chicken from Pantanal, Brazil. Mem Inst Oswaldo Cruz. 2012;107:1073–5. doi: 10.1590/s0074-02762012000800020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soares CN, Castro MJ, Peralta JM, Freitas MR, Puccioni-Sohler M. Is west nile virus a potential cause of central nervous system infection in Brazil? Arq Neuropsiquiatr. 2010;68:761–3. doi: 10.1590/s0004-282x2010000500016. [DOI] [PubMed] [Google Scholar]
- 38.Morales MA, Barrandeguy M, Fabbri C, Garcia JB, Vissani A, Trono K, et al. West nile virus isolation from equines in Argentina, 2006. Emerg Infect Dis. 2006;12:1559–61. doi: 10.3201/eid1210.060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adrián Diaz L, Komar N, Visintin A, Dantur Juri MJ, Stein M, Lobo Allende R, et al. West nile virus in birds, Argentina. Emerg Infect Dis. 2008;14:689–91. doi: 10.3201/eid1404.071257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gould LH, Fikrig E. West nile virus: A growing concern? J Clin Invest. 2004;113:1102–7. doi: 10.1172/JCI21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rappole JH, Compton BW, Leimgruber P, Robertson J, King DI, Renner SC. Modeling movement of west nile virus in the Western hemisphere. Vector Borne Zoonotic Dis. 2006;6:128–39. doi: 10.1089/vbz.2006.6.128. [DOI] [PubMed] [Google Scholar]
- 42.Center for Disease Control and Prevention. CDC-West Nile Virus avian mortality database from 1999-present. [Accessed June 2011]. http://www.cdc.gov/ncidod/dvbid/westnile/birdspecies.htm .
- 43.US Department of Agriculture, A. a. P. H. I. S. o. A. Veterinary servicces, center for epidemiology and animal health, national surveillance unit. Summary of West Nile Virus Cases in the United States. 2010;2012. 2011 [Google Scholar]
- 44.Deardorff E, Estrada-Franco J, Brault AC, Navarro-Lopez R, Campomanes-Cortes A, Paz-Ramirez P, et al. Introductions of west nile virus strains to Mexico. Emerg Infect Dis. 2006;12:314–8. doi: 10.3201/eid1202.050871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed KD, Meece JK, Henkel JS, Shukla SK. Birds, migration and emerging zoonoses: West nile virus, lyme disease, influenza A and enteropathogens. Clin Med Res. 2003;1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farfán-Ale JA, Blitvich BJ, Loroño-Pino MA, Marlenee NL, Rosado-Paredes EP, García-Rejón JE, et al. Longitudinal studies of west nile virus infection in avians, Yucatán State, México. Vector Borne Zoonotic Dis. 2004;4:3–14. doi: 10.1089/153036604773082942. [DOI] [PubMed] [Google Scholar]
- 47.Farfán-Ale JA, Blitvich BJ, Marlenee NL, Loroño-Pino MA, Puerto-Manzano F, García-Rejón JE, et al. Antibodies to west nile virus in asymptomatic mammals, birds, and reptiles in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2006;74:908–14. [PubMed] [Google Scholar]
- 48.Blitvich BJ, Fernandez-Salas I, Contreras-Cordero JF, Marlenee NL, Gonzalez-Rojas JI, Komar N, et al. Serologic evidence of west nile virus infection in horses, Coahuila State, Mexico. Emerg Infect Dis. 2003;9:853–6. doi: 10.3201/eid0907.030166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez G. The first regional workshop for strengthening and training in surveillance, control, and prevention activities for WNV in Mexico. Hermosillo, Sonora. 2005 [Google Scholar]
- 50.EID Weekly Updates: Emerging and Reemerging Infectious Diseases, Region of the Americas, Vol. 1, No. 23-18. 2003 Dec [Google Scholar]
- 51.Osorio JE, Ciuoderis KA, Lopera JG, Piedrahita LD, Murphy D, Levasseur J, et al. Characterization of west nile viruses isolated from captive American Flamingoes (Phoenicopterus ruber) in Medellin, Colombia. Am J Trop Med Hyg. 2012;87:565–72. doi: 10.4269/ajtmh.2012.11-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tesh RB, Travassos da Rosa AP, Guzman H, Araujo TP, Xiao SY. Immunization with heterologous Flaviviruses protective against fatal west nile encephalitis. Emerg Infect Dis. 2002;8:245–51. doi: 10.3201/eid0803.010238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao SY, Guzman H, da Rosa AP, Zhu HB, Tesh RB. Alteration of clinical outcome and histopathology of yellow fever virus infection in a hamster model by previous infection with heterologous Flaviviruses. Am J Trop Med Hyg. 2003;68:695–703. [PubMed] [Google Scholar]
- 54.Komar N, Clark GG. West nile virus activity in Latin America and the Caribbean. Rev Panam Salud Publica. 2006;19:112–7. doi: 10.1590/s1020-49892006000200006. [DOI] [PubMed] [Google Scholar]
- 55.Papa A, Karabaxoglou D, Kansouzidou A. Acute west nile virus neuroinvasive infections: Cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol. 2011;83:1861–5. doi: 10.1002/jmv.22180. [DOI] [PubMed] [Google Scholar]
- 56.Fernández-Salas I, de Lourdes Garza-Rodríguez M, Beaty BJ, Jiménez JR, Rivas-Estilla AM. Presence of west nile virus in northeast Mexico. Salud Publica Mex. 2007;49:210–7. doi: 10.1590/s0036-36342007000300006. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez Mde L, Rodriguez DR, Blitvich BJ, López MA, Fernández-Salas I, Jimenez JR, et al. Serologic surveillance for west nile virus and other Flaviviruses in febrile patients, encephalitic patients, and asymptomatic blood donors in northern Mexico. Vector Borne Zoonotic Dis. 2010;10:151–7. doi: 10.1089/vbz.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nielsen CF, Reisen WK, Armijos MV, Maclachlan NJ, Scott TW. High subclinical west nile virus incidence among nonvaccinated horses in northern California associated with low vector abundance and infection. Am J Trop Med Hyg. 2008;78:45–52. [PubMed] [Google Scholar]
- 59.Farfan-Ale JA, Loroño-Pino MA, Garcia-Rejon JE, Hovav E, Powers AM, Lin M, et al. Detection of RNA from a novel west nile-like virus and high prevalence of an insect-specific Flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–9. [PMC free article] [PubMed] [Google Scholar]
- 60.Beasley DW, Davis CT, Estrada-Franco J, Navarro-Lopez R, Campomanes-Cortes A, Tesh RB, et al. Genome sequence and attenuating mutations in west nile virus isolate from Mexico. Emerg Infect Dis. 2004;10:2221–4. doi: 10.3201/eid1012.040647. [DOI] [PMC free article] [PubMed] [Google Scholar]


