Abstract
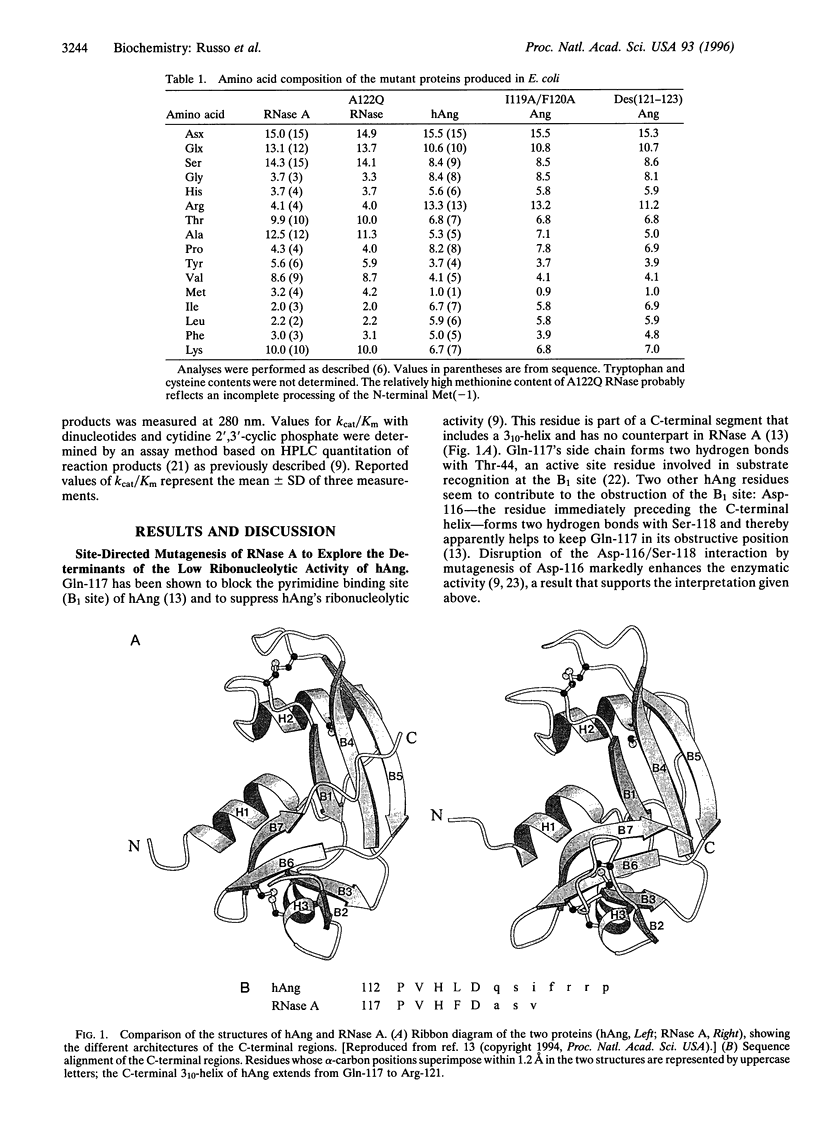
The ribonucleolytic activity of angiogenin (Ang) is essential to Ang's capacity to induce blood vessel formation. Previous x-ray diffraction and mutagenesis results have shown that the active site of the human protein is obstructed by Gln-117 and imply that the C-terminal region of Ang must undergo a conformational rearrangement to allow substrate binding and catalysis. As a first step toward structural characterization of this conformational change, additional site-directed mutagenesis and kinetic analysis have been used to examine the intramolecular interactions that stabilize the inactive conformation of the protein. Two residues of this region, Ile-119 and Phe-120, are found to make hydrophobic interactions with the remainder of the protein and thereby help to keep Gln-117 in its obstructive position. Furthermore, the suppression of activity by the intramolecular interactions of Ile-119 and Phe-120 is counterbalanced by an effect of the adjacent residues, Arg-121, Arg-122, and Pro-123 which do not appear to form contacts with the rest of the protein structure. They contribute to enzymatic activity, probably by constituting a peripheral subsite for binding polymeric substrates. The results reveal the nature of the conformational change in human Ang and assign a key role to the C-terminal region both in this process and, presumably, in the regulation of human Ang function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya K. R., Shapiro R., Allen S. C., Riordan J. F., Vallee B. L. Crystal structure of human angiogenin reveals the structural basis for its functional divergence from ribonuclease. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2915–2919. doi: 10.1073/pnas.91.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya K. R., Shapiro R., Riordan J. F., Vallee B. L. Crystal structure of bovine angiogenin at 1.5-A resolution. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2949–2953. doi: 10.1073/pnas.92.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beintema J. J., Schüller C., Irie M., Carsana A. Molecular evolution of the ribonuclease superfamily. Prog Biophys Mol Biol. 1988;51(3):165–192. doi: 10.1016/0079-6107(88)90001-6. [DOI] [PubMed] [Google Scholar]
- Bond M. D., Strydom D. J., Vallee B. L. Characterization and sequencing of rabbit, pig and mouse angiogenins: discernment of functionally important residues and regions. Biochim Biophys Acta. 1993 Mar 5;1162(1-2):177–186. doi: 10.1016/0167-4838(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Curran T. P., Shapiro R., Riordan J. F. Alteration of the enzymatic specificity of human angiogenin by site-directed mutagenesis. Biochemistry. 1993 Mar 9;32(9):2307–2313. doi: 10.1021/bi00060a023. [DOI] [PubMed] [Google Scholar]
- Curran T. P., Shapiro R., Riordan J. F. Alteration of the enzymatic specificity of human angiogenin by site-directed mutagenesis. Biochemistry. 1993 Mar 9;32(9):2307–2313. doi: 10.1021/bi00060a023. [DOI] [PubMed] [Google Scholar]
- D'Alessio G. Oligomer evolution in action? Nat Struct Biol. 1995 Jan;2(1):11–13. doi: 10.1038/nsb0195-11. [DOI] [PubMed] [Google Scholar]
- Di Donato A., Cafaro V., D'Alessio G. Ribonuclease A can be transformed into a dimeric ribonuclease with antitumor activity. J Biol Chem. 1994 Jul 1;269(26):17394–17396. [PubMed] [Google Scholar]
- Di Donato A., Cafaro V., de Nigris M., Rizzo M., D'Alessio G. The determinants of the dimeric structure of seminal ribonuclease are located in its N-terminal region. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1440–1445. doi: 10.1006/bbrc.1993.1986. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Strydom D. J., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry. 1985 Sep 24;24(20):5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- Hallahan T. W., Shapiro R., Vallee B. L. Dual site model for the organogenic activity of angiogenin. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2222–2226. doi: 10.1073/pnas.88.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. A covalent angiogenin/ribonuclease hybrid with a fourth disulfide bond generated by regional mutagenesis. Biochemistry. 1989 Feb 21;28(4):1875–1884. doi: 10.1021/bi00430a067. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Vallee B. L. Mutagenesis of aspartic acid-116 enhances the ribonucleolytic activity and angiogenic potency of angiogenin. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7139–7143. doi: 10.1073/pnas.85.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. V., Vallee B. L. Neovascularisation of the meniscus with angiogenin. An experimental study in rabbits. J Bone Joint Surg Br. 1991 Jul;73(4):587–590. doi: 10.1302/0301-620X.73B4.1712788. [DOI] [PubMed] [Google Scholar]
- Moenner M., Gusse M., Hatzi E., Badet J. The widespread expression of angiogenin in different human cells suggests a biological function not only related to angiogenesis. Eur J Biochem. 1994 Dec 1;226(2):483–490. doi: 10.1111/j.1432-1033.1994.tb20073.x. [DOI] [PubMed] [Google Scholar]
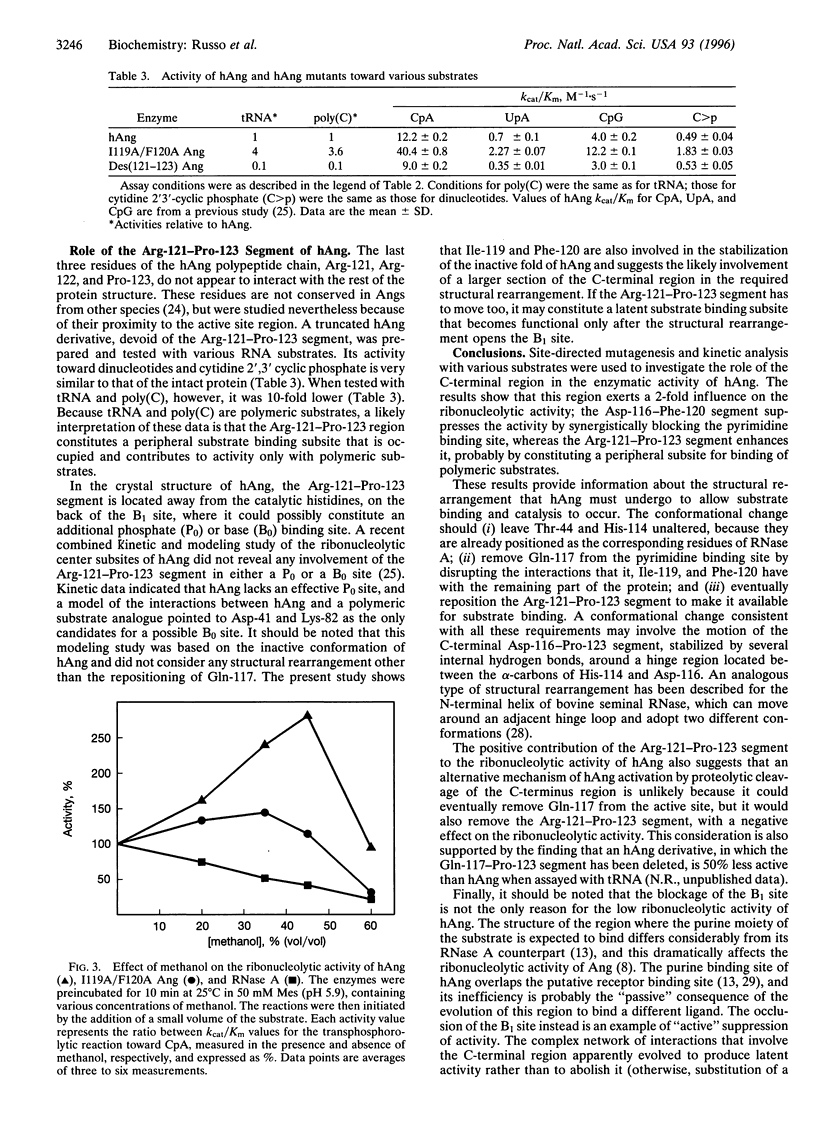
- Russo N., Acharya K. R., Vallee B. L., Shapiro R. A combined kinetic and modeling study of the catalytic center subsites of human angiogenin. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):804–808. doi: 10.1073/pnas.93.2.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N., Shapiro R., Acharya K. R., Riordan J. F., Vallee B. L. Role of glutamine-117 in the ribonucleolytic activity of human angiogenin. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2920–2924. doi: 10.1073/pnas.91.8.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak S. M., Vallee B. L. Base cleavage specificity of angiogenin with Saccharomyces cerevisiae and Escherichia coli 5S RNAs. Biochemistry. 1988 Apr 5;27(7):2288–2294. doi: 10.1021/bi00407a007. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fett J. W., Strydom D. J., Vallee B. L. Isolation and characterization of a human colon carcinoma-secreted enzyme with pancreatic ribonuclease-like activity. Biochemistry. 1986 Nov 18;25(23):7255–7264. doi: 10.1021/bi00371a002. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Fox E. A., Riordan J. F. Role of lysines in human angiogenin: chemical modification and site-directed mutagenesis. Biochemistry. 1989 Feb 21;28(4):1726–1732. doi: 10.1021/bi00430a045. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Riordan J. F., Vallee B. L. Characteristic ribonucleolytic activity of human angiogenin. Biochemistry. 1986 Jun 17;25(12):3527–3532. doi: 10.1021/bi00360a008. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Strydom D. J., Olson K. A., Vallee B. L. Isolation of angiogenin from normal human plasma. Biochemistry. 1987 Aug 11;26(16):5141–5146. doi: 10.1021/bi00390a037. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Identification of functional arginines in human angiogenin by site-directed mutagenesis. Biochemistry. 1992 Dec 15;31(49):12477–12485. doi: 10.1021/bi00164a026. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Vallee B. L. Site-directed mutagenesis of histidine-13 and histidine-114 of human angiogenin. Alanine derivatives inhibit angiogenin-induced angiogenesis. Biochemistry. 1989 Sep 5;28(18):7401–7408. doi: 10.1021/bi00444a038. [DOI] [PubMed] [Google Scholar]
- Shapiro R., Weremowicz S., Riordan J. F., Vallee B. L. Ribonucleolytic activity of angiogenin: essential histidine, lysine, and arginine residues. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8783–8787. doi: 10.1073/pnas.84.24.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strydom D. J., Fett J. W., Lobb R. R., Alderman E. M., Bethune J. L., Riordan J. F., Vallee B. L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985 Sep 24;24(20):5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Wlodawer A., Svensson L. A., Sjölin L., Gilliland G. L. Structure of phosphate-free ribonuclease A refined at 1.26 A. Biochemistry. 1988 Apr 19;27(8):2705–2717. doi: 10.1021/bi00408a010. [DOI] [PubMed] [Google Scholar]