Abstract
Recessive Stargardt's macular degeneration is a blinding disease of children caused by mutations in the ABCA4 (ABCR) gene. Mice with a knockout mutation in abcr accumulate toxic lipofuscin pigments in ocular tissues, similar to affected humans. The major fluorophore of lipofuscin is the bis-retinoid, N-retinylidene-N-retinylethanolamine (A2E). In the current study, we sought to define the effect of increasing light on A2E accumulation. We crossed the abcr–/– mutation onto an albino background. The retinoid profiles in albino mice indicated higher retinal illuminance than in pigmented mice exposed to similar ambient light. Unexpectedly, A2E levels were not higher in the albino mice. Also, A2E levels in abcr–/– mice reared under cyclic light at 30, 120, or 1,700 lux were similar. Thus, increased retinal illuminance was not correlated with higher A2E. A2E has been shown to undergo light-dependent oxidation to yield a series of A2E epoxides or oxiranes. These oxiranes react with DNA in vitro, suggesting a potential mechanism for A2E cytotoxicity. We analyzed ocular tissues from abcr–/– mice for A2E oxiranes by mass spectrometry. Unlike A2E, the oxiranes were more abundant in albino vs. pigmented abcr–/– mice, and in abcr–/– mice exposed to increasing ambient light. These observations suggest that both the biosynthesis of A2E and its conversion to oxiranes are accelerated by light. Finally, we showed that the formation of A2E oxiranes is strongly suppressed by treating the abcr–/– mice with Accutane (isotretinoin), an inhibitor of rhodopsin regeneration.
Recessive Stargardt's disease is an inherited form of macular degeneration that causes progressive loss of central vision (1). The pathologic features of Stargardt's include accumulation of fluorescent lipofuscin pigments in cells of the retinal pigment epithelium (RPE) and degeneration of photoreceptors (2, 3). The RPE plays an important support role in maintaining photoreceptor viability (4). Photoreceptor degeneration in Stargardt's disease appears to result from loss of this support function as toxic lipofuscin pigments accumulate in cells of the RPE (5, 6). The gene affected in Stargardt's, ABCA4 (ABCR), encodes a retina-specific transporter protein in the rims of rod and cone outer segment discs (7–9). To study the function of ABCR and the molecular etiology of Stargardt's, we previously generated mice with a knockout mutation in the abcr gene (5). The phenotype in these mice includes elevated all-trans-retinaldehyde (atRAL) after light exposure, constitutively elevated phosphatidylethanolamine, and accumulation of lipofuscin pigments in cells of the RPE (5, 10, 11). These animals also manifest very slow photoreceptor degeneration (11). Based on biochemical analysis of abcr–/– mice (5, 10, 11) and the results of in vitro biochemical studies (12, 13), ABCR appears to function as a flippase for N-retinylidene-phosphatidylethanolamine (N-ret-PE), the Schiff-base conjugate of phosphatidylethanolamine and atRAL. According to this model, ABCR transfers N-ret-PE from the intradiscal to cytoplasmic leaflet of outer-segment disk membranes. Accordingly, ABCR may increase availability of substrate to all-trans-retinol dehydrogenase (Fig. 1A) and thereby accelerate removal of atRAL from rod outer segments (5).
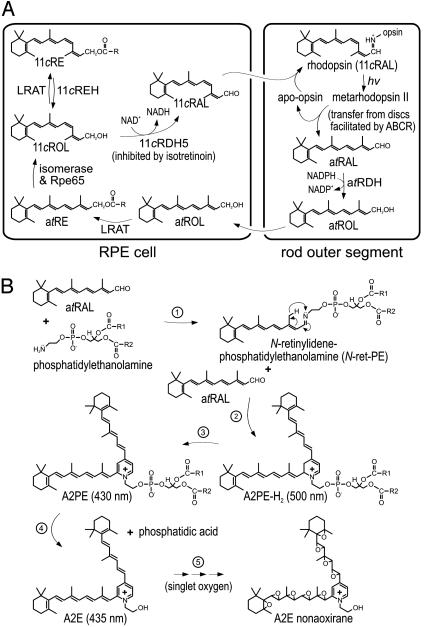
Fig. 1.
Retinoid pathways in the retina and RPE. (A) Visual cycle mediating rhodopsin regeneration. Absorption of a photon (hv) by a rhodopsin molecule in a rod outer segment disk induces photoisomerization of the 11cRAL chromophore, yielding activated metarhodopsin II. After several seconds, metarhodopsin II decays to yield apo-rhodopsin and free atRAL. ABCR functions to accelerate removal of atRAL from the interior of outer segment discs to the cytoplasmic space by flipping N-ret-PE (5). The atRAL is subsequently reduced to atROL or vitamin A by all-trans-retinol dehydrogenase. The atROL is released from the outer segment and taken up by an adjacent RPE cell where it is esterified by lecithin retinol acyl transferase (LRAT) to form an atRE. Chemical isomerization is effected by an isomerase that uses atREs as a substrate, in conjunction with Rpe65 (46). The resulting 11cROL is oxidized by 11cRDH to form 11cRAL chromophore. 11cRDH is inhibited by isotretinoin with a Ki of ≈0.1 μM (40, 47). 11cROL may also serve as a substrate for LRAT to form 11-cis-retinyl esters. The final step is recombination of 11cRAL with aporhodopsin in the outer segment to form a new molecule of light-sensitive rhodopsin. (B) Synthesis of A2E. After light exposure, newly released atRAL condenses reversibly with phosphatidylethanolamine to N-ret-PE (step 1). Rarely, a second molecule of atRAL will condense with N-ret-PE to form A2PE-H2 (step 2). The wavelength of maximal absorption (λmax) for A2PE-H2 is 500 nm. Within the acidic and oxidizing environment of RPE phagolysosomes, A2PE-H2 is oxidized to A2PE (λmax = 430 nm) (step 3). Hydrolysis of the phosphate ester yields A2E (λmax = 435 nm) and phosphatidic acid (step 4) (10). Double bonds along the polyene chains of A2E may react with singlet oxygen to form a series of one to nine (shown) oxiranes (step 5).
The major fluorophore of lipofuscin is the bis-retinoid pyridinium salt, N-retinylidene-N-retinylethanolamine (A2E) (14, 15). Significant accumulation of A2E is seen in the RPE of abcr–/– mice (5, 10) and humans with Stargardt's disease (2, 3, 10). A2E forms by sequential condensation of atRAL with phosphatidylethanolamine (Fig. 1B) (10, 16). Given that atRAL is both the product of photoisomerization (Fig. 1 A) and the first reactant in A2E biogenesis (Fig. 1B), A2E formation should depend on the level of ambient light. By 11 mo, A2E levels were ≈20-fold higher in RPE from abcr–/– compared to wild-type mice raised under normal vivarium lighting (5). However, A2E could be suppressed to wild-type levels when abcr–/– mice were raised in total darkness (10). A2E accumulation was similarly suppressed when abcr–/– mice, raised under normal lighting conditions, were treated with Accutane (isotretinoin) (17). This drug slows rhodopsin regeneration by inhibiting 11-cis-retinol dehydrogenase in RPE cells (Fig. 1 A) (18). These observations suggest that light restriction and treatment with isotretinoin may slow A2E accumulation in Stargardt's patients.
A2E has several potential cytotoxic effects on RPE cells, including destabilization of membranes (19–21), release of proapoptotic proteins from mitochondria (22, 23), sensitization of cells to blue-light damage (24–26), impaired lysosomal acidification (27), and impaired degradation of phospholipids from phagocytosed outer segments (28). Irradiation of A2E with blue (430-nm) light resulted in a series of oxirane products containing up to nine epoxide rings, formed by addition of singlet oxygen to double bonds along the polyene chains (29). These strained three-membered rings are susceptible to nucleophilic attack and hence are highly reactive. A2E oxiranes were shown to induce DNA fragmentation by forming adducts with purines and pyrimidines in cultured ARPE-19 cells (30, 31). This appears to represent an important mechanism of A2E cytotoxicity.
In previous studies, we measured A2E accumulation in abcr–/– mice exposed to 12-h cyclic light at 30 lux (5, 10, 17). The light intensity in a typical office environment, however, is at least 10-fold higher. Given the observed light dependence of A2E formation (10), we expected increased A2E accumulation in abcr–/– mice exposed to higher ambient light. In the current work, we examined the effects of increasing retinal illuminance on the formation of A2E and A2E oxiranes.
Materials and Methods
Mice. For analysis of visual retinoids, we used pigmented 129/SV and albino BALB/c strains on abcr+/+ (wild-type) or abcr–/– mutant backgrounds. For analysis of A2E, dihydro-N-retinylidene-N-retinyl-phosphatidylethanolamine (A2PE-H2), and A2E oxiranes, we used 129×B6 hybrids on both wild-type and abcr–/– backgrounds. Mice exposed to light at 30 lux were maintained under 12-h cyclic light at this intensity from birth. Mice exposed to 120 or 1,700 lux were raised under cyclic light at 30 lux for 2 mo, then transferred to cyclic light at the higher level for up to 5 additional months. For the isotretinoin treatment study, 129/SV abcr–/– mice were administered isotretinoin (Sigma) daily by i.p. injections at 20 mg/kg of body weight in 25 μl of DMSO for 2 mo. The treatment was started in 2-mo-old mice kept under 12-h cyclic light at 30 lux. Fluorescent lights were used in all studies, and light intensities were measured inside the mouse cages. We sequenced DNA from all mice studied to confirm homozygosity for the wild-type (L450) allele of rpe65, because the common L450M mutation in this gene affects the kinetics of rhodopsin regeneration (32, 33). Mice were anesthetized with ketamine (200 mg/kg) plus xylazine (10 mg/kg) administered i.p. before death by cervical dislocation.
Tissue Preparation and Extraction. After death, eyes were removed and hemisected. For analysis of 11-cis-retinyl esters, all-trans-retinyl esters (atREs), 11-cis-retinol (11cROL), all-trans-retinol (atROL), 11-cis-retinaldehyde (11cRAL), and atRAL, two mouse eyecups containing retinas plus RPE were dissected and homogenized in PBS, pH 7.2, containing 200 mM NH2OH. We added 1.0 ml of ethanol to all samples, extracted twice by addition of 4.0 ml of hexane, and centrifuged at 1,000 × g for 2 min. For analysis of A2PE-H2, and A2E, eyecups were dissected and homogenized in PBS. Samples were homogenized further by adding 4.0 ml of chloroform/methanol (2:1, vol/vol), extracted by addition of 4.0 ml of chloroform and 3.0 ml of dH2O, and centrifuged at 1,000 × g for 10 min. The resulting phospholipid extracts were dried under a stream of argon and the residues dissolved in 100 μl of hexane. For liquid chromatography–MS (LC-MS), dried A2E and A2E-oxirane-containing samples were dissolved in 50 μl of methanol, and 10 μl of 1-palmitoyl-2-hydroxy-sn-glycero-phosphocholine (lysophosphatidylcholine) (Avanti Polar Lipids) at 20 mg/ml in methanol was added as an internal standard for quantitation. All manipulations were done on ice under dim red light (Kodak Wratten 1A).
HPLC and LC-MS. Retinoids were analyzed by normal-phase HPLC on a silica column (Zorbax Rx-SIL 4.6 × 250 mm, 5 μm, Agilent, Palo Alto, CA) by using gradient elution (0.2–10% dioxane in hexane) at 2.0 ml/min in an Agilent model 1100 liquid chromatograph equipped with a UV photodiode-array detector. The eluted peaks were identified by UV spectral analysis and by coelution with authentic retinoid standards. Retinoids (atROL, atRAL, and atRP) were purchased from Sigma or synthesized from 11cRAL (11cROL and 11cRP) according to published methods (34). Oxime standards (syn- and anti-isomers) were prepared by reacting 11cRAL or atRAL with hydroxylamine (NH2OH) according to published procedures (35, 36). Quantitation of retinoids was done by comparing the sample-peak areas to calibration curves established with the retinoid standards using published molar extinction coefficients (37, 38).
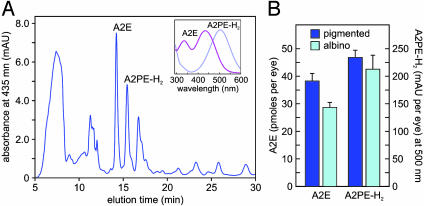
Samples of A2E and A2PE-H2 were analyzed by normal-phase HPLC on a silica column (Microsorb 5 μm Si, 250 × 4.6 mm, Sigma) by using the mobile phase: hexane/2-propanol/ethanol/25 mM potassium phosphate/acetic acid (485:376:100:50:0.275 vol/vol). Column and solvent temperatures were maintained at 30°C. Chromatographic conditions and quantitation of A2E and A2PE-H2 were as described (5, 10). A2PE, another precursor of A2E (Fig. 1B), eluted between 11 and 12 min (Fig. 3A) under these conditions but was insufficiently resolved to permit quantitation.
Fig. 3.
A2E and A2PE-H2 in abcr–/– eyes. (A) Representative chromatogram of a phospholipid extract from a pigmented abcr–/– eyecup showing absorbance at 435 nm. (Inset) UV spectra acquired from the peaks labeled A2E and A2PE-H2. Note the λmax of A2E at 435 and A2PE-H2 at 500 nm. (B) Quantitation of A2E (pmol per eye) and A2PE-H2 (milli-absorbance unit per eye at 500 nm) in eyecups from 3-mo-old pigmented (dark blue bars) and albino (cyan bars) abcr–/– mice raised under cyclic light at 30 lux. Error bars show standard deviations (n = 4).
For LC-MS analysis, A2E and A2E-oxirane-containing samples were analyzed by reverse-phase chromatography on a C18-column (Zorbax 300 SB-C18; 5 μm, 2.1 × 150 mm, Agilent) by using gradient elution [0–100% chloroform in methanol/water (95:5 vol/vol); 50 μl/min flow rate, 30°C column temperature] in an Agilent model 1100 capillary chromatograph coupled to an LCQ Deca XP-Plus ion-trap mass spectrometer (ThermoFinnigan, San Jose, CA). MS conditions were: electrospray ionization at 5.0 kV; 80-μA spray current; 40-unit sheath gas flow; +15.0-V capillary voltage; 55.0-V tube lens offset; 200°C capillary temperature; and 40% normalized collision energy. A2E and the A2E oxiranes were quantitated by comparing the ion intensities at their respective m/zs to the ion intensity of the lysophosphatidylcholine internal standard (m/z = 496). No ions with a m/z corresponding to this internal standard were present in the tissue extracts. This approach is valid for relative ion-intensity quantitation of A2E or A2E oxiranes within an experiment. It does not permit comparison of quantitative values between figures, nor is it suitable for comparing the level of A2E to the A2E oxiranes because of their different ionization efficiencies.
A2E and Epoxides. A2E was synthesized and purified following published methods (16). A2E epoxides were generated by oxidation of A2E with meta-chloroperoxybenzoic acid (MCPBA, Sigma) (A2E/MCPBA, 1:10 molar ratio) according to published methods (29). The solution in chloroform was mixed for 48 h on a mutator. Samples were dried under argon, resuspended in methanol, and directly infused into the MS system at 50.0 μl/min. Conditions for MS were otherwise as described above.
Results
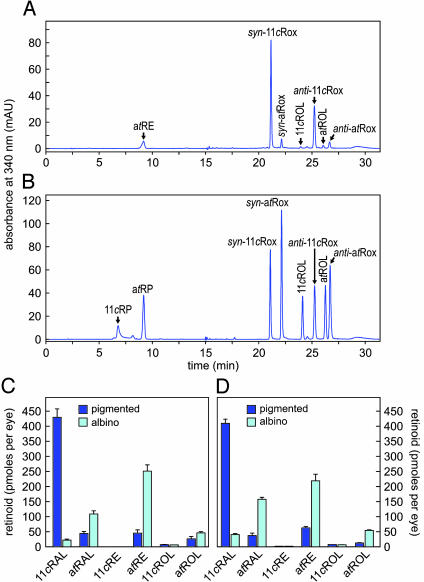
Visual Retinoids and A2E in Light-Adapted Albino and Pigmented Mice. Albino mice lack melanin pigments due to mutations in the gene for tyrosinase. In ocular tissues, melanin pigments function to prevent extrapupillary light from entering the eye and to absorb scattered light. At similar ambient light intensities, 11cRAL should be lower and atRAL higher in albino vs. pigmented mice, due to increased photobleaching in the albino mice. To confirm this prediction, we light-adapted 2- to 3-mo-old wild-type albino (BALB/c) and pigmented (129/SV) mice for 3 h at 30 lux then measured levels of visual retinoids in retina and RPE by HPLC. Fig. 2 shows a representative chromatogram of retinoids from a wild-type pigmented mouse (Fig. 2 A) compared to a chromatogram of retinoid standards (Fig. 2B). 11cRAL was ≈20-fold lower and atRAL 2.5-fold higher in albino vs. pigmented mice (Fig. 2C). The albino mice also showed higher levels of atROL and atRE compared to pigmented mice, consistent with a higher rates of photoisomerization in the albino mice.
Fig. 2.
Visual retinoids in pigmented and albino mice. (A) Representative HPLC chromatogram of a retinoid extract from the eyecup of a 2-mo-old pigmented wild-type mouse showing absorbance at 340 nm. Labeled peaks corresponding to atREs, atROL, and the syn- and anti-oximes of 11cRAL (11cRox) and atRAL (atRox) were confirmed by spectral analysis. (B) Chromatogram of authentic retinoid standards at 340-nm absorbance. (C) Quantitation of retinoids from eyecups of 2- to 3-mo-old wild-type pigmented (dark blue bars) and albino (cyan bars) mice, light adapted at 30 lux. Values for the indicated retinoids are shown in pmol per eye. (D) Quantitation of retinoids from eyecups of 2- to 3-mo-old pigmented and albino abcr–/– mice light adapted at 30 lux. Error bars show standard deviations (n = 4).
The abcr–/– knockout mutation was originally generated in pigmented 129/SV mice (5). We moved the abcr–/– mutation onto an albino background by repeatedly crossing abcr–/+ heterozygotes with BALB/c mice until all offspring were albino. Intercrossing yielded albino abcr–/– homozygotes. We light adapted 2- to 3-mo-old pigmented and albino abcr–/– mice at 30 lux for 3 h and measured levels of visual retinoids by HPLC. Similar to wild-type mice (Fig. 2C), 11cRAL was much lower, whereas atRAL, atRE, and atROL were higher in abcr–/– albino vs. pigmented mice (Fig. 2D). These data show higher retinal illuminance in albino vs. pigmented abcr–/– mice exposed to similar ambient light.
Next, we measured levels of A2E and its precursor, A2PE-H2 (10), in 3-mo-old pigmented and albino abcr–/– mice raised under cyclic light at 30 lux. Unexpectedly, A2E was lower and A2PE-H2 unchanged in the albino mice (Fig. 3B) despite the higher levels of atRAL (Fig. 2D).
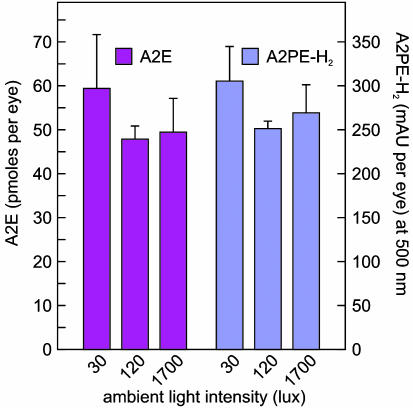
Accumulation of A2E Does Not Increase with Ambient Light. To explore further the effects of retinal illuminance on A2E and A2PE-H2 accumulation, we exposed 2-mo-old pigmented abcr–/– mice to cyclic light at different intensities for 3 mo. We analyzed eyecup extracts from these mice at 5 mo for lipofuscin fluorophores by HPLC. We observed no significant difference in A2E levels with exposure to 30, 120, or 1,700 lux (Fig. 4). Levels of A2PE-H2 were similarly unaffected by ambient light (Fig. 4). These data corroborate our observations in albino mice but appear inconsistent with the established mechanism of A2E biogenesis (10, 16) shown in Fig. 1B.
Fig. 4.
Effects of light on A2E and A2PE-H2. Quantitation of A2E (magenta bars) in pmol per eye and A2PE-H2 (blue bars) in milli-absorbance unit per eye from 5-mo-old pigmented abcr–/– mice raised under cyclic light at 30, 120, or 1,700 lux. Error bars show standard deviations (n = 3).
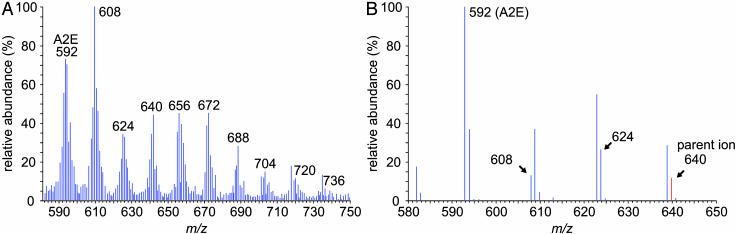
Light-Dependent Formation of A2E Oxiranes. A2E oxiranes form by reaction of singlet oxygen with A2E in the presence of blue light (29). A possible explanation for the unchanged or lower levels of A2E with increased retinal illuminance is light-dependent conversion of A2E to oxiranes. Addition of epoxides interrupts the resonating polyene chains of A2E, greatly altering its UV-spectral and chromatographic properties. Oxiranes of A2E therefore cannot be identified by routine LC. They are detectable, however, by MS (29). To identify oxiranes of A2E in abcr–/– eyes, we first synthesized A2E in vitro from atRAL and ethanolamine (16). We oxidized the A2E with meta-chloroperoxybenzoic acid (29) and analyzed the resulting oxirane mixture by LC-MS. A representative spectrum is shown in Fig. 5A. The monoisotopic mass of A2E is 592.45. Addition of n oxygens to A2E increases the mass of the resulting oxirane by n × 16. All nine potential oxiranes were formed by MCPBA oxidation of A2E (Fig. 5A).
Fig. 5.
Identification of A2E oxiranes generated in vitro and in vivo by MS. (A) Full-scan MS in the range 580–750 m/z of oxiranes after chemical oxidation of in vitro synthesized A2E. Note A2E at 592 and the family of ions that increase by 16 mass units to nonaoxirane at 736. (B) Product-ion spectrum showing fragmentation of a 640-m/z parent ion in a phospholipid extract of 7-mo-old abcr–/– eyecups. Note the formation of bisoxirane (624), monooxirane (608), and A2E (592).
We analyzed phospholipid extracts from eyecups of 7-moold abcr–/– mice by LC-MS. The resulting spectra included ions with m/z values corresponding to A2E and the nine oxiranes. To confirm the presence of A2E oxiranes in these extracts, we did tandem MS on each candidate oxirane ion. Collision-induced fragmentation of the oxiranes yielded daughter ions corresponding to the series of lower oxiranes and A2E. A representative product-ion spectrum is shown for the 640-m/z oxirane (Fig. 5B). Here, formation of the 624 and 608 oxiranes and A2E during collision-induced fragmentation confirms our identification of A2E oxiranes in the eyecup phospholipid extracts.
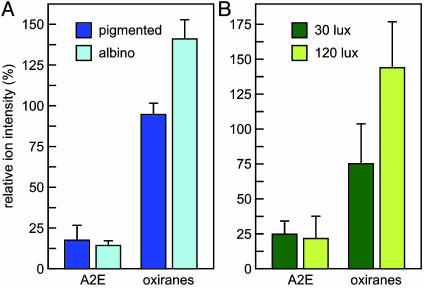
Next, we measured A2E and oxiranes in eyecup extracts from 7-mo-old albino and pigmented abcr–/– mice raised under 30-lux cyclic illumination. To compare the levels in pigmented versus albino mice of A2E and A2E oxiranes, we divided the intensity of each parent ion by that of lysophosphatidylcholine, which was added to the extracts as an internal standard. To simplify comparison, we combined the relative ion intensities of the nine oxiranes observed in each sample. Although A2E levels were similar, total oxiranes were 1.5-fold higher in eyecup extracts from albino compared to pigmented mice (Fig. 6A). The similar levels of A2E observed in this experiment seem inconsistent with the data obtained by HPLC analysis (Fig. 3B). This may be due to differences in the molecular species measured by the two techniques. For example, the geometric isomers of A2E have the same molecular mass but exhibit different chromatographic properties. All isomers of A2E are detected by MS, whereas only those that Colette with bis-all-trans-A2E are detected by HPLC. Therefore, the lower level of A2E in albino eyecups observed by UV-detection may indicate a greater abundance of A2E isomers that were not quantified in Fig. 3B.
Fig. 6.
A2E and oxiranes in abcr–/– mice at different retinal illuminance. (A) Quantitation of A2E and total oxiranes in eyecups from 7-mo-old abcr–/– pigmented (dark blue bars) and albino (cyan bars) mice raised under cyclic light at 30 lux determined by LC-MS (n = 4). (B) Quantitation of A2E and total oxiranes in 7-mo-old abcr–/– pigmented mice raised under cyclic light at 30 (dark green bars) or 120 lux (light green bars). Values are expressed as ion intensities relative to a lysophosphatidylcholine internal standard. Error bars show standard deviations (n = 3). Note the similar levels of A2E and elevated oxiranes with increased illuminance in both experiments.
We also measured A2E and oxirane levels in 7-mo-old pigmented abcr–/– mice raised under cyclic light at 30 or 120 lux. Here again, A2E levels were similar under the two lighting conditions (Fig. 6B). Total oxiranes, however, were 1.9-fold higher in eyecups from mice reared under 120 vs. 30 lux. The results in Fig. 6 suggest that A2E is converted in vivo to oxiranes in a light-dependent manner.
We did similar studies on pigmented and albino wild-type mice. A2E levels in wild-type mice were at least 10-fold lower than in abcr–/– mice, as previously described (5, 10). Oxirane levels tended to increase with retinal illuminance in wild-type mice (not shown). The levels of these oxiranes were not sufficiently above the detection threshold to permit quantitation.
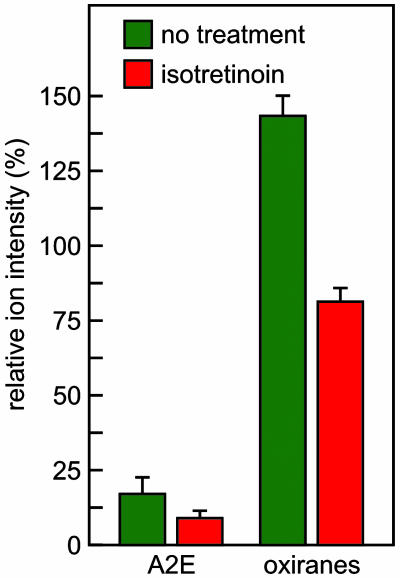
Isotretinoin Inhibits Oxirane Formation. A possible explanation for the unchanged A2E and higher oxirane levels with increasing retinal illuminance is that both the synthesis and oxidative conversion of A2E are accelerated to a similar degree by light. This explanation predicts significantly reduced oxirane levels with inhibited synthesis of A2E. To test this possibility, we treated pigmented abcr–/– mice with isotretinoin for 2 mo. Levels of A2E and oxiranes in the treated mice were 52% and 56%, respectively, of those in untreated age-matched controls (Fig. 7). Thus, by inhibiting synthesis of visual chromophore we significantly reduced formation of A2E and the A2E oxiranes.
Fig. 7.
Effect of treatment with isotretinoin on A2E and oxiranes in abcr–/– mice. Quantitation of A2E and total oxiranes in eyecups from pigmented 4-mo-old abcr–/– mice raised under cyclic light at 30 lux and treated (red bars) or not treated (green bars) with isotretinoin for 2 mo. Error bars show standard deviations (n = 4). Note the reduction of both A2E and oxiranes in the treated mice.
Discussion
We began this study to determine the effects of increased light on the production of A2E in abcr–/– mice. Two strategies were used to vary the incident light on retinas in vivo. The first was to move the abcr–/– mutation onto an albino background. At similar ambient lighting, the rate of rhodopsin photoisomerization should be higher in albino vs. pigmented mice, due to the absence of melanin in the iris, RPE, and choroid of albino mice. We tested this prediction by measuring the steady-state levels of visual retinoids in similarly light-adapted pigmented and albino mice. As expected, 11cRAL was lower and atRAL higher in albino compared to pigmented mice on both wild-type and abcr–/– backgrounds. These observations confirm higher retinal illuminance in albino mice.
Despite the higher levels of atRAL, A2E was lower and A2PE-H2 unchanged in 3-mo-old albino compared to pigmented abcr–/– mice (Fig. 3B). Similarly, A2E and A2PE-H2 were not significantly changed in 5-mo-old pigmented abcr–/– mice after prolonged exposure to light at 120 or 1,700 vs. 30 lux (Fig. 4). Previously, we showed strong light dependence of A2E production in abcr–/– mice raised under total darkness vs. cyclic light at 30 lux (10). Together, these results suggest that the light-dependent increase in A2E production plateaus between 30 and 120 lux ambient light. A2E was shown to form oxiranes in vitro by addition of singlet oxygen (29). Further, lipofuscin was shown to generate reactive oxygen species, including singlet oxygen, on exposure to blue light (39). Thus, the A2E-laden RPE in abcr–/– mice exposed to bright light should strongly promote formation of oxiranes. By MS analysis of RPE extracts from abcr–/– mice, we observed the presence of all predicted A2E oxiranes. These compounds have not been previously described in animal tissues. Unlike A2E, the levels of oxiranes varied directly with retinal illuminance (Fig. 6). The rate of A2E formation probably also varied with retinal illuminance. However, simultaneous increased oxidation of A2E may explain the similar levels seen in mice exposed to light at 30, 120, and 1,700 lux (Fig. 4). The dependence of oxirane formation on retinal illuminance is further evidence that Stargardt's disease patients may slow the progression of visual loss by limiting their exposure to light.
Isotretinoin inhibits 11cROL dehydrogenase (40, 41) (Fig. 1 A). Treatment of mice with isotretinoin results in lower levels of 11cRAL chromophore and arrested A2E formation (17). In the current study, both A2E and A2E oxiranes were reduced by nearly 50% in abcr–/– mice treated with isotretinoin (Fig. 7). These data are consistent with the previous observation that rpe65–/– mice lacking rhodopsin do not accumulate lipofuscin (42) and further support the model that A2E is an intermediate in oxirane formation. These oxiranes are likely to mediate the blue-light sensitivity conferred on RPE cells by A2E (31). The large reduction in A2E oxiranes observed in treated abcr–/– mice provides further evidence that isotretinoin may be useful to slow disease progression in humans with Stargardt's macular degeneration. Might inhibitors of 11cROL dehydrogenase be useful in the treatment of retinal or macular degenerations of other etiologies? Recently, mice with null mutations in the genes for monocyte chemoattractant protein (Ccl-2) and its cognate receptor (Ccr-2) were reported. Loss of Ccl-2 or Ccr-2 resulted in delayed clearance of immune complexes due to impaired infiltration of macrophages into various tissues (43, 44). Interestingly, ccl2–/– and ccr2–/– mice manifested many pathologic features of age-related macular degeneration including deposition of drusen beneath the basal RPE, thickening of Bruch's membrane, photoreceptor degeneration, choroidal neovascularization, and lipofuscin formation in RPE cells (45). Importantly, these mice also accumulated A2E in the RPE (45). A2E accumulation in animal models of age-related macular degeneration caused by defects in immune function suggests that A2E formation may be a common etiologic mechanism in macular degenerations of diverse cause, including those unrelated to ABCA4 mutations. If so, the above observations suggest that isotretinoin may be generally useful in the treatment of macular degeneration.
Acknowledgments
R.A.R. was a postdoctoral fellow on the Jules Stein Vision Science Training Grant. G.H.T. is the Charles Kenneth Feldman and Jules and Doris Stein Research to Prevent Blindness Professor. This work was supported by grants from the National Eye Institute, the Foundation Fighting Blindness, the Ruth and Milton Steinbach Fund, and the Macula Vision Research Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RPE, retinal pigment epithelium; atRAL, all-trans-retinaldehyde; N-ret-PE, for N-retinylidene-phosphatidylethanolamine; A2E, N-retinylidene-N-retinylethanolamine; atRE, all-trans-retinyl esters; 11cROL, 11-cis-retinol; atROL, all-trans-retinol; 11cRAL, 11-cis-retinaldehyde; A2PE-H2, dihydro-N-retinylidene-N-retinyl-phosphatidylethanolamine; LC-MS, liquid chromatography–MS.
References
- 1.Lee, B. L. & Heckenlively, J. R. (1999) in Retina-Vitreous-Macula, eds. Guyer, D. R., Yannuzzi, L. A., Chang, S., Shields, J. A. & Green, W. R. (Saunders, Darien, IL), pp. 978–988.
- 2.Eagle, R. C., Jr., Lucier, A. C., Bernardino, V. B., Jr., & Yanoff, M. (1980) Ophthalmology 87, 1189–1200. [DOI] [PubMed] [Google Scholar]
- 3.Birnbach, C. D., Jarvelainen, M., Possin, D. E. & Milam, A. H. (1994) Ophthalmology 101, 1211–1219. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg, R. H. (1985) Doc. Ophthalmol. 60, 327–346. [DOI] [PubMed] [Google Scholar]
- 5.Weng, J., Mata, N. L., Azarian, S. M., Tzekov, R. T., Birch, D. G. & Travis, G. H. (1999) Cell 98, 13–23. [DOI] [PubMed] [Google Scholar]
- 6.Sun, H. & Nathans, J. (2001) J. Bioenerg. Biomembr. 33, 523–530. [DOI] [PubMed] [Google Scholar]
- 7.Allikmets, R., Shroyer, N. F., Singh, N., Seddon, J. M., Lewis, R. A., Bernstein, P. S., Peiffer, A., Zabriskie, N. A., Li, Y., Hutchinson, A., et al. (1997) Science 277, 1805–1807. [DOI] [PubMed] [Google Scholar]
- 8.Illing, M., Molday, L. L. & Molday, R. S. (1997) J. Biol. Chem. 272, 10303–10310. [DOI] [PubMed] [Google Scholar]
- 9.Azarian, S. M. & Travis, G. H. (1997) FEBS Lett. 409, 247–252. [DOI] [PubMed] [Google Scholar]
- 10.Mata, N. L., Weng, J. & Travis, G. H. (2000) Proc. Natl. Acad. Sci. USA 97, 7154–7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mata, N. L., Tzekov, R. T., Liu, X. R., Weng, J., Birch, D. G. & Travis, G. H. (2001) Invest. Ophthalmol. Visual Sci. 42, 1685–1690. [PubMed] [Google Scholar]
- 12.Sun, H., Molday, R. S. & Nathans, J. (1999) J. Biol. Chem. 274, 8269–8281. [DOI] [PubMed] [Google Scholar]
- 13.Ahn, J., Wong, J. T. & Molday, R. S. (2000) J. Biol. Chem. 275, 20399–20405. [DOI] [PubMed] [Google Scholar]
- 14.Sakai, N., Decatur, J., Nakanishi, K. & Eldred, G. E. (1996) J. Am. Chem. Soc. 118, 1559–1560. [Google Scholar]
- 15.Reinboth, J. J., Gautschi, K., Munz, K., Eldred, G. E. & Reme, C. E. (1997) Exp. Eye Res. 65, 639–643. [DOI] [PubMed] [Google Scholar]
- 16.Parish, C. A., Hashimoto, M., Nakanishi, K., Dillon, J. & Sparrow, J. (1998) Proc. Natl. Acad. Sci. USA 95, 14609–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radu, R. A., Mata, N. L., Nusinowitz, S., Liu, X., Sieving, P. A. & Travis, G. H. (2003) Proc. Natl. Acad. Sci. USA 100, 4742–4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sieving, P. A., Chaudhry, P., Kondo, M., Provenzano, M., Wu, D., Carlson, T. J., Bush, R. A. & Thompson, D. A. (2001) Proc. Natl. Acad. Sci. USA 98, 1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eldred, G. E. & Lasky, M. R. (1993) Nature 361, 724–726. [DOI] [PubMed] [Google Scholar]
- 20.Sparrow, J. R., Parish, C. A., Hashimoto, M. & Nakanishi, K. (1999) Invest. Ophthalmol. Visual Sci. 40, 2988–2995. [PubMed] [Google Scholar]
- 21.De, S. & Sakmar, T. P. (2002) J. Gen. Physiol. 120, 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suter, M., Reme, C., Grimm, C., Wenzel, A., Jaattela, M., Esser, P., Kociok, N., Leist, M. & Richter, C. (2000) J. Biol. Chem. 275, 39625–39630. [DOI] [PubMed] [Google Scholar]
- 23.Sparrow, J. R. & Cai, B. (2001) Invest. Ophthalmol. Visual Sci. 42, 1356–1362. [PubMed] [Google Scholar]
- 24.Cubeddu, R., Taroni, P., Hu, D. N., Sakai, N., Nakanishi, K. & Roberts, J. E. (1999) Photochem. Photobiol. 70, 172–175. [PubMed] [Google Scholar]
- 25.Sparrow, J. R., Nakanishi, K. & Parish, C. A. (2000) Invest. Ophthalmol. Visual Sci. 41, 1981–1989. [PubMed] [Google Scholar]
- 26.Schutt, F., Davies, S., Kopitz, J., Holz, F. G. & Boulton, M. E. (2000) Invest. Ophthalmol. Visual Sci. 41, 2303–2308. [PubMed] [Google Scholar]
- 27.Holz, F. G., Schutt, F., Kopitz, J., Eldred, G. E., Kruse, F. E., Volcker, H. E. & Cantz, M. (1999) Invest. Ophthalmol. Visual Sci. 40, 737–743. [PubMed] [Google Scholar]
- 28.Finnemann, S. C., Leung, L. W. & Rodriguez-Boulan, E. (2002) Proc. Natl. Acad. Sci. USA 99, 3842–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Shabat, S., Itagaki, Y., Jockusch, S., Sparrow, J. R., Turro, N. J. & Nakanishi, K. (2002) Angew. Chem. Int. Ed. Engl. 41, 814–817. [DOI] [PubMed] [Google Scholar]
- 30.Sparrow, J. R., Vollmer-Snarr, H. R., Zhou, J., Jang, Y. P., Jockusch, S., Itagaki, Y. & Nakanishi, K. (2003) J. Biol. Chem. 278, 18207–18213. [DOI] [PubMed] [Google Scholar]
- 31.Sparrow, J. R., Zhou, J. & Cai, B. (2003) Invest. Ophthalmol. Visual Sci. 44, 2245–2251. [DOI] [PubMed] [Google Scholar]
- 32.Danciger, M., Matthes, M. T., Yasamura, D., Akhmedov, N. B., Rickabaugh, T., Gentleman, S., Redmond, T. M., La Vail, M. M. & Farber, D. B. (2000) Mamm. Genome 11, 422–427. [DOI] [PubMed] [Google Scholar]
- 33.Wenzel, A., Reme, C. E., Williams, T. P., Hafezi, F. & Grimm, C. (2001) J. Neurosci. 21, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bridges, C. D. & Alvarez, R. A. (1982) Methods Enzymol. 81, 463–485. [DOI] [PubMed] [Google Scholar]
- 35.Groenendijk, G. W., De Grip, W. J. & Daemen, F. J. (1980) Biochim. Biophys. Acta 617, 430–438. [DOI] [PubMed] [Google Scholar]
- 36.Ozaki, K., Terakita, A., Hara, R. & Hara, T. (1986) Vision Res. 26, 691–705. [DOI] [PubMed] [Google Scholar]
- 37.Garwin, G. G. & Saari, J. C. (2000) Methods Enzymol. 316, 313–324. [DOI] [PubMed] [Google Scholar]
- 38.Yang, M. & Fong, H. K. W. (2002) J. Biol. Chem. 277, 3318–3324. [DOI] [PubMed] [Google Scholar]
- 39.Rozanowska, M., Jarvis-Evans, J., Korytowski, W., Boulton, M. E., Burke, J. M. & Sarna, T. (1995) J. Biol. Chem. 270, 18825–18830. [DOI] [PubMed] [Google Scholar]
- 40.Law, W. C. & Rando, R. R. (1989) Biochem. Biophys. Res. Comm. 161, 825–829. [DOI] [PubMed] [Google Scholar]
- 41.Gamble, M. V., Shang, E., Zott, R. P., Mertz, J. R., Wolgemuth, D. J. & Blaner, W. S. (1999) J. Lipid Res. 40, 2279–2292. [PubMed] [Google Scholar]
- 42.Katz, M. L. & Redmond, T. M. (2001) Invest. Ophthalmol. Visual Sci. 42, 3023–3030. [PubMed] [Google Scholar]
- 43.Kuziel, W. A., Morgan, S. J., Dawson, T. C., Griffin, S., Smithies, O., Ley, K. & Maeda, N. (1997) Proc. Natl. Acad. Sci. USA 94, 12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu, B., Rutledge, B. J., Gu, L., Fiorillo, J., Lukacs, N. W., Kunkel, S. L., North, R., Gerard, C. & Rollins, B. J. (1998) J. Exp. Med. 187, 601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ambati, J., Anand, A., Fernandez, S., Sakurai, E., Lynn, B. C., Kuziel, W. A., Rollins, B. J. & Ambati, B. K. (2003) Nat. Med. 9, 1390–1397. [DOI] [PubMed] [Google Scholar]
- 46.Mata, N. L., Moghrabi, W. N., Lee, J. S., Bui, T. V., Radu, R. A., Horwitz, J. & Travis, G. H. (2004) J. Biol. Chem. 279, 635–643. [DOI] [PubMed] [Google Scholar]
- 47.Gamble, M. V., Mata, N. L., Tsin, A. T., Mertz, J. R. & Blaner, W. S. (2000) Biochim. Biophys. Acta 1476, 3–8. [DOI] [PubMed] [Google Scholar]