Abstract
Superparamagnetic iron oxide nanoparticles (SPIONs), have played an important role in the promotion of image contrast in magnetic resonance imaging modality. The objective of present study is describing SPIONs conjugated with C595 monoclonal antibody (mAb) against MUC1-expressing ovarian cancer (OVCAR3) cell. Magnetic resonance imaging parameters of the prepared nanoconjugate was investigated in vitro: characterization, cell toxicity, flow cytometry, Prussian blue staining, and cellular uptake as well as biodistribution and magnetic resonance signal intensities under in vivo conditions were also investigated. Magnetic resonance imaging and biodistribution results showed good tumor accumulation and detection, no cytotoxicity, and potential selective as anti-ovarian cancer. In conclusion, based on the findings SPIONs-C595 nanosized-probe is potentially, a selective ovarian molecular imaging tool. Further subsequent in vivo studies and clinical trials are warranted.
Keywords: Flow cytometry, magnetic nanoparticles, magnetic resonance imaging, OVCAR3
Introduction
Superparamagnetic iron oxide nanoparticles (SPIONs), have played an important role in the promotion of image contrast in magnetic resonance imaging modality. Magnetic resonance imaging (MRI) can be combined with specific MR molecular imaging agents to improve the specificity and sensitivity of cancer imaging. Tumor cell targeting by use of biomarker target specific imaging probes is a promising strategy for molecular imaging.[1,2,3] Using of nanoparticles for molecular imaging is among the most important clinical breakthrough of the past decade.
Monoclonal antibodies (mAbs) are one of the potential selective cancer MR carriers of pharmaceuticals. One of the targets is ovarian specific membrane antigen, MUC-1, a high molecular weight trans-membrane glycoprotein antigen.[4,5,6] Additionally, tumor marker antigent mucin-1 (MUC1) is a proposed molecular target for a novel imaging for cancer. C595 mAb is an immunoglobulin (Ig)G3, a monoclonal antibody against human MUC1.[7] Several studies has been showed that C595 is a useful antibody either alone or in combination with other therapeutic methods to treat the human cancer.[5,7,8]
In particular, superparamagnetic iron oxide nanoparticles (SPIONs), conjugated with mAb enhance contrast in MRI. The use of antibody-conjugated MRI contrast agents to specifically target cancer cells has been demonstrated previously for several cancers.[9,10,11]
Over the past decade, significant advances have been made in the development and application of MRI and its role may shift from a problem-solving to a central management tool, possibly fulfilling a broad range of tasks from characterization, staging and even early detection of ovarian cancer.[12,13]
Owing to the fact that many types of ovary cancer cells express high levels of (MUC1) on their cell surface,[14,15] the imaging strategy is to use SPIONs, attached to an antibody that binds to the MUC1, to specifically enhance the contrast of MUC1-expressing ovarian cancer cells. Also, the use of monoclonal antibody C595, conjugated to a radioisotope, has been used for gamma camera imaging of MUC1-expressing cancers.[16,17] Hence, the production and evaluation of magnetic nanoprobe (SPIONs-C595) and its application as an MRI contrast agent for targeted molecular imaging of MUC1-expressing ovarian cancer cells was investigated.
Materials and Methods
All chemical materials were prepared as described in previous published paper by authors.[11] C595 monoclonal antibody was obtained from Professor Barry J. Allen (University of New South Wales, Kogarah, NSW 2217, Australia). Ovarian cancer cell line, OVCAR3, was purchased from National Cell Bank of Iran (Pasture Institute, Tehran, Iran).
The nanoprobe was synthesized as shown in [Figure 1], following a three-step process as described previously.[18] Briefly, in the first step, the sulfo-SMCC cross-linker was first added to nanomag®-D-SPIO particles with CLD-NH2 surface to introduce maleimide groups. The next step was the modification of mAb primary amines with 2-iminothiolane to introduce sulfhydryl groups into the antibody. The sulfhydryl groups of antibody react with the maleimide groups at the surface of sulfo-SMCC activated SPIONs.
Figure 1.
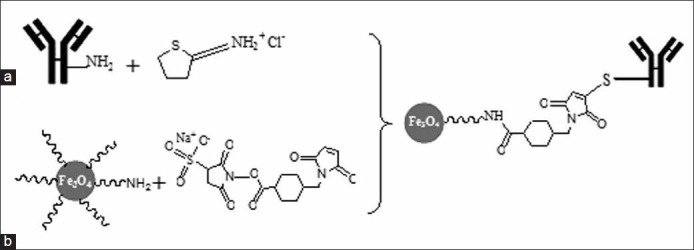
The scheme of superparamagnetic iron oxide nanoparticle (SPIONs) conjugation to the C595 monoclonal antibody (mAb): (a) Functionalization of SPIO-CLD-NH2 with sulfo-SMCC and (b) conjugation of thiolated antibody to maleimide functionalized SPIONs
In second step, SPIONs were modified with sulfo-SMCC to introduce maleimide groups. To remove unreacted sulfo-SMCC, the solution was washed with phosphate buffered saline-ethylenediaminetetraacetic acid (PBS-EDTA) buffer with size exclusion columns. In the last step, conjugation of mAb C595 with SPIONs was achieved by addition of the maleimide particle suspension to the SH-labeled mAb. Finally, the mAb labeled SPIONs were purified by magnetic columns in a high gradient magnetic field system.
Characterization
Transmission electron microscopy (TEM) (Tecnai 10, FEI Company, USA) operating at 80 kV was used to measure accurately the size distribution of particles. The samples for electron microscopy were prepared by deposition of a droplet of the nanoparticle solution onto a carbon-coated film supported on a copper grid and allowed to dry. The hydrodynamic particle size and the width of the particle size distribution (polydispersity index) of nanoparticles was obtained via photon correlation spectroscopy (PCS) using a Malvern Nano Series ZS, provided with a He/Ne laser of 633 nm wavelength.
To study the magnetic properties of synthesized nanoprobe, the nuclear magnetic resonance dispersion (NMRD) profiles (the longitudinal relaxivity, r1, as a function of the magnetic field) were recorded with a field cycling relaxometer (Spinmaster FFC2000, Stelar, Italy). Additional measurements of relaxation rate (R1, 2) were performed at 20 and 60 MHz and 310 K on Bruker Minispec system (Bruker, Karlsruhe, Germany) using an inversion recovery pulse sequence and the Carr-Purcell-Meiboom-Gill pulse sequence, respectively. The results of both measurements were published previously.[11]
The binding of mAb molecules to SPIONs and the amount of immobilized antibody were confirmed and determined by the Bradford assay method as well as the measurements of the hydrodynamic size. The iron concentration of samples was determined by the measurement of the longitudinal relaxation rate R1 at 20 MHz after digestion by microwave oven. Briefiy, the samples were mineralized by microwave digestion (Milestone MLS-1200, Sorisole, Italy) and the R1 value of the resulting solutions was recorded at 0.47 T and 37°C which also reported before.[11]
In vitro cytotoxicity
OVCAR3 cell line was grown in Roswell Park Memorial Institute (RPMI-1640) medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin followed by addition of 10 mg/ml insulin. The cells were cultured in 250 ml flasks, at 37°C in a humidified atmosphere with 5% CO2 to allow adherence of the cells.
The cytotoxic effects of Nanomag-D-SPIO particles and the corresponding C595 mAb conjugated nanoparticles (SPIONs-C595) in vitro against cell lines were examined by using the 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) assay which described in previous published study.[18] All experiments were performed in triplicate and cell survival was determined as a percentage of viable cells in comparison with controls.
Flow cytometry
Flow cytometry was used to detect and quantitatively analyze cell-surface expression of MUC1 on the cell surface.[19] In brief, cells were detached by tripsin and washed with PBS containing 0.1% FBS, and a 106 cell per tube of each cell were transferred in fluorescence activated cell sorting (FACS) tubes. The cells were re-suspended in 90 ml of washing buffer and were preblocked with human Fc receptors blocking (human) reagent (Miltenyi) for 10 min at room temperature in the dark. After blocking, primary C595 anti MUC1 antibody (1/150 dilution) was added to each cell tube (one tube of each cell line as a control), incubated for 30 min in the dark at room temperature, and then washed 3 × 5 min using a washing buffer.
After washing, the cells were re-suspended and incubated in goat anti-mouse fluorescein isothiocyanate (FITC) mAb for an additional 30 min at room temperature in the dark. Cells were then washed, resuspended in 0.5 ml of PBS, and analyzed immediately using a CyAN-ADP flow cytometer (Beckman Coulter).
Cellular SPIONs uptake studies
To measure the iron uptake, human ovarian cancer, OVCAR3, cell were detached and washed three times with PBS and approximately 4 × 106 cell per tube of cells were suspended in 15 ml tube and incubated with culture medium containing Nanomag-D-SPIO or SPIONs-C595 at Fe concentrations of 2 mM (one tube control) for 2 h at room temperature with gentle shaking.
After incubation, cells were washed with PBS three times and mineralized in 0.5 ml of 5 M HCl for 3 h in a water bath at 80°C. The iron concentrations of the samples were measured by relaxometry measurements at 20 MHz after digestion of samples by microwave oven. This was achieved by mineralization of sample in acidic conditions (0.2 ml sample, 0.6 ml HNO3, and 0.3 ml H2O2) by microwave oven (Milestone MLS-1200, Sorisole, Italy). The millimolar iron concentration was determined from the longitudinal relaxivity (R1) of samples, the same as procedure described in the previous work.[11]
Also, the potential of nanoprobes as MRI agent was investigated in vitro using 1.5 T MRI system by use of spin-echo pulse sequence as follow: TE= 30 ms, TR= 2,500 ms, slice thickness = 3 mm, and matrix size = 256 × 256. The data from region of interest (ROI) drawn to consistently measure mean signal intensity at the identical position within each phantom vial.
Prussian blue staining
The procedure of Prussian blue staining was described in the previous publication.[11] Briefly, OVCAR3 cells were detached and washed three times with PBS and about 106 cells per tube of cells were suspended in 15 ml tube and incubated with culture medium containing SPIONs-C595 at Fe concentrations of 2 mM (one tube control) for 1 h at room temperature.
After incubation, the cells were washed three times with PBS to remove excess nanoparticles. Then, cells were fixed on 22 × 22 mm square glass coverslips with 4% glutaraldehyde, washed and stained using specific iron Prussian blue method to observe nanoparticles accumulation. Accumulation of iron oxide nanoparticles were showed in cells as dark blue grains under microscope light using a Nikon Eclipse TS100 microscope (Nikon Corp., Tokyo, Japan).
Animals
The animal studies were performed with 15 nude mice, 6-8-week-old with a mean weight of 20 g. Mice were randomly divided into three groups of five. Each group was housed per cage in humidity and temperature controlled, isolated animal in house. All mice were fed sterilized standard mouse chow and water ad libitum.
The studied cell line (specific ovarian cancer xenograft tumors OVCAR3) was grown in tissue culture (2.5 × 106 cells, 120 ml) and injected subcutaneously into both flanks of nude mice. Three weeks after tumor implantation, when the tumor diameter was about 2 mm (mean weight of tumors was approximately 100 mg), all mice received the amount of 0.5 mg Fe/kg (130 mg Fe) intravenously (i.v.) injection of the both nanoprobe MRI agents (SPIONs-C595 and Nanomag-D-SPIO). All nanoparticle agents were diluted in physiological saline to a final concentration as injected in bolus doses.
In vivo magnetic resonance imaging
Contrast in MRI depends on the T1 and T2 relaxation times. SPIONs affect T2 and work as negative contrast agents, hence decreases signal intensity. In other words, a higher intracellular concentration of SPIONs may results in the reduction of T2 relaxation time. The mice were anesthetized using a nose cone that delivered isoflurane and oxygen mixture and imaged using 1.5 T MRI scanner (Signa, GE Medical System, Milwaukee, WI, USA) and a standard circular polarizable head coil (Clinical MR solutions, Brookfield, WI, USA). All images were obtained using the T2-weighted imaging method by multi spin-echo pulse sequence technique, with TE values of 12, 24, 36, and 48 ms, TR= 3,000 ms, 3 mm slice thickness, 2.5 × 2.5 cm field of view, and matrix size of 256 × 256. MR image signal intensity was measured using the signal intensity of ROI (region of interest) at different time (0, 1, 5, 10, 15, and 20 h) after injection of both synthesized and commercially available nanosized probe agents using the Dicom Works version 1.3.5 (Dicom-Works, Lyon, France) by use of the following equation:
(S = S0e−TE/T2)
where S is the signal intensity after administration of nanosized agent and S0 is the signal intensity when no any nanosized agent is applied.
Biodistribution studies
To investigate the biodistribution of nanosized probe MRI contrast agents (SPIONs-C595, Nanomag-D-SPIO, and control) in tumor and other organs 24 h after injection all mice were sacrificed and critical organs (including tumor, bladder, liver, spleen, and kidney) of each three group were removed and their iron content was determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES), after doing acid digestion procedure.[10] The percentage of iron concentration (mg) per gram of organ was obtained as biodistribution of the conjugate in the studied organs.
Results and Discussion
Characterization
The particle size distribution of SPIONs before and after mAb conjugation was obtained by PCS [Figure 2]. The hydrodynamic particle diameters were obtained to be 19.46 ± 0.80 and 27.22 ± 1.22 nm for Nanomag-D-SPIO and SPIONs-C595, respectively. Figure 3a and b show TEM images for the spherical shaped plain and mAb conjugated SPIONs, respectively. The average particle size calculated from TEM was 10-20 nm for two studied nanoparticle agents.
Figure 2.
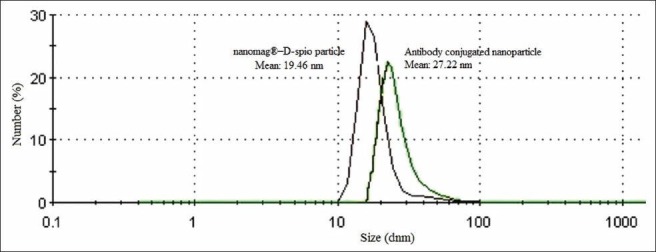
Nanoparticle size before and after conjugation
Figure 3.
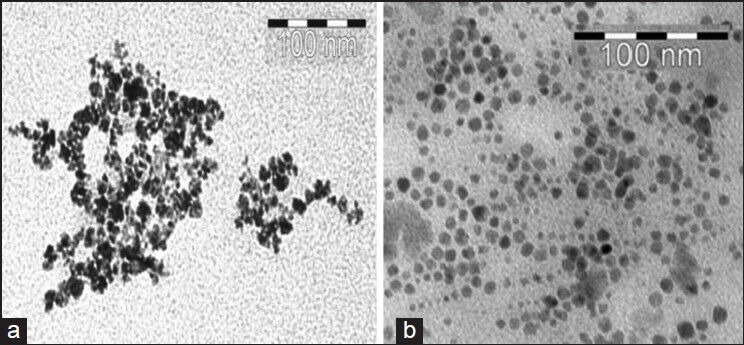
TEM images for: (a) Nanomag-D-SPIO and (b) SPIONs-C595; antibody binding causes a significant reduction of particle agglomeration. The average particle size of particles estimated from TEM images was about 10-20 nm
In vitro cytotoxicity
The in vitro cytotoxic effect of Nanomag-D-SPIO and the synthesized nanoprobe was assessed using the standard MTT assay, using ovarian cancer OVCAR3 cell line. As indicated in Figure 4, the results after different incubation times with different iron concentrations for cell line showed more than 80% cell viability in relation to the control. Moreover, because of their selective accumulation at cancer cells expressing the MUC1 cell receptor, only cells retaining the nanoparticles will be safe and nontoxic. These results are in good agreement with the results of previous published work,[18] which also showed a high affinity of SPIONs-C595 for OVCAR3 cell line and was a reason why this cell line is used for further investigation in this study.
Figure 4.
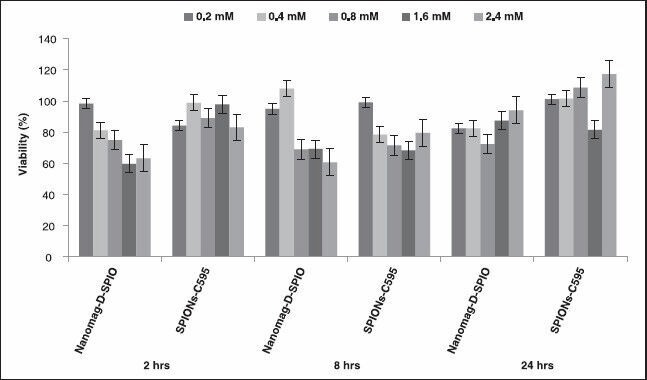
In vitro cytotoxicity of Nanomag-D-SPIO and SPIONs-C595 in OVCAR3 cell line by the 3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide (MTT) assay. The cells were incubated with Nanomag-D-SPIO or SPIONs-C595 at equivalent Fe concentrations ranging from 0.2 to 2.4 mM for 2, 8, and 24 h
Flow cytometry
Flow cytometric analysis was performed to confirm the availability and quantitative analysis of desired ovarian cancer cell surface antigen (MUC1). Immuno-fiuorescence staining of OVCAR3 cell line showed that OVCAR3 cell express high levels of MUC1 on their cell surface (88.6 ± 4.6%), as indicated in Figure 5. These results are in good agreement with previously published study.[18]
Figure 5.
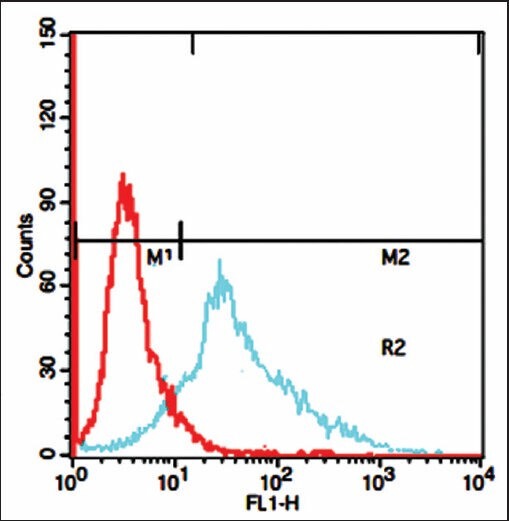
Graphs of flow cytometry test for OVCAR3 cell line
Cellular SPIONs uptake
The millimolar iron concentration was determined from the longitudinal relaxivity (R1) of samples as indicated in Figure 6. This figure shows calibration curve of iron concentration versus relaxation rate at 20 MHz. Various blue-staining particles were found in the cells labeled with the molecular probe at different iron concentrations, and the density of particles was positively related to the iron concentration.
Figure 6.
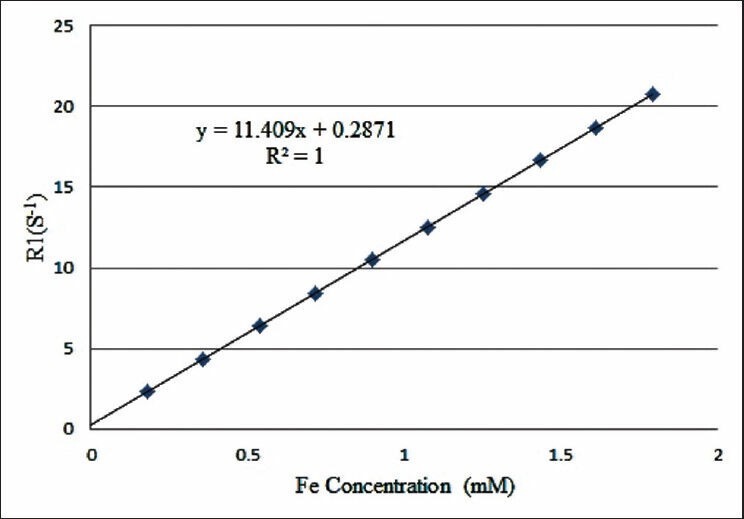
Calibration curve of iron concentration versus relaxation rate at 20 MHz
The capability of the synthesized nanoprobe as a specific MRI agent was shown in Figure 7. This figure demonstrated that the nanoprobe functionalized C595 mAb reducing 95% MR image signal intensity in OVCAR3 compared with nonspecific agent of nanomag-D-SPIO.
Figure 7.
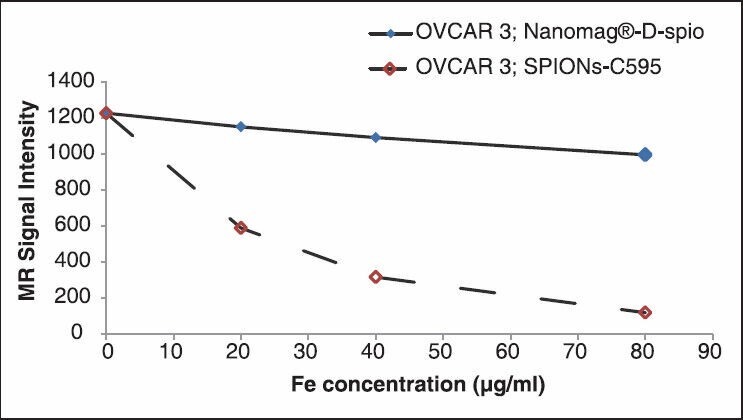
Magnetic resonance (MR) image signal intensity of both C595- SPIONs and Nanomag-D-SPIO at different Fe concentration
Prussian blue staining
The qualitative information on the cell surface antigen expression as well as specificity and cellular uptake of SPIONs-C595 and Nanomag-D-SPIO to the cells were done by Prussian blue staining test. Blue areas as shown in Figure 8, illustrated the targeting effect and SPIONs uptake of functionalized particles (SPIONs-C595) on the cellular uptake behavior. As can be seen from this figure, there was no blue color appearance in the cells incubated with nonfunctionalized particles.
Figure 8.
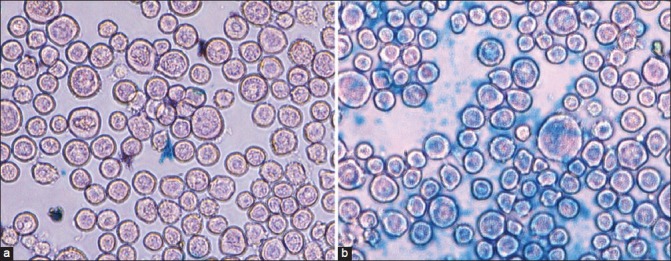
Prussian blue staining images (objective magnification: ×40) for OVCAR3 cells after 1 h incubation with (a) Nanomag-D-SPIO and (b) SPIONs-C595 nanoprobe. SPIONs-labeled OVCAR3 shows blue spots located inside cells, suggesting presence of iron oxide particles
MR signal intensity
MR images of studied animals before nanoprobe injection and after injection of both agents was was shown in Figure 9. For in vivo MRI, distinct changes of signal intensities and T2 values of ovarian cancers were detected after the injection of SPIONs-C595 compared to Nanomag-D-SPIO [Figure 10]. As shown in Figure 10, the reduction of determined signal intensity were 56 and 10% for SPIONs-C595 and Nanomag-D-SPIO, respectively.
Figure 9.
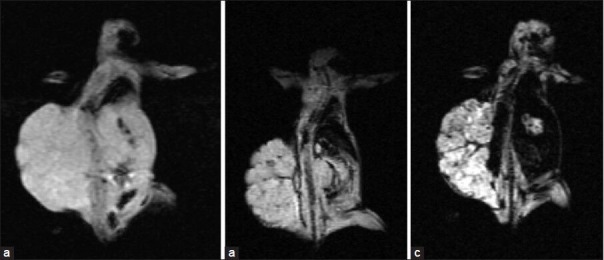
MR image of mice: (a) Before injection of agents, (b) after injection of Nanomag-D-SPIO, and (c) after injection of C595-SPIONs
Figure 10.
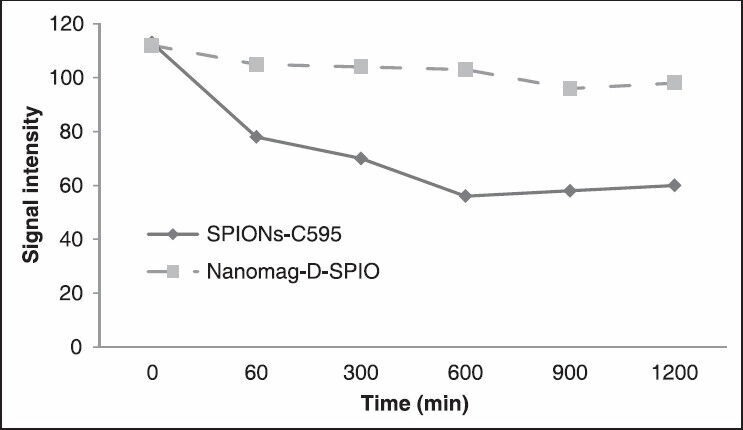
MR image signal intensity of tissues from some region of interest (ROI) at different time after injection of two agents
Biodistribution
The percentage of injected dose per gram of each organ was measured by ICP-AES test showed that the most (90%) of injected dose was found in tumor. Liver was the second organ which attained SPIONs (50%) after 24 h post injection and its clearance is so fast in other organs. The results suggested that SPIONs-C595 as characterized in this study might be potentially used as nanoparticle contrast agent in MRI for ovarian cancer detection. The conjugated nanoparticles’ affinity to OVCAR3 was higher than that of nonconjugated nanoparticles. Both MRI and ICP-AES results show significant preferential uptake of the SPIONs-C595 nanoparticles by human ovarian carcinoma cells (OVCAR3) as compared to the other studied organs [Figure 11]. In vivo results also showed that tumor uptake of SPIONs-C595 was about two times higher than the other organs. The biodistribution of nanosized agents in mice showed dramatic uptake in reticuloendothelial system after 24 h.
Figure 11.
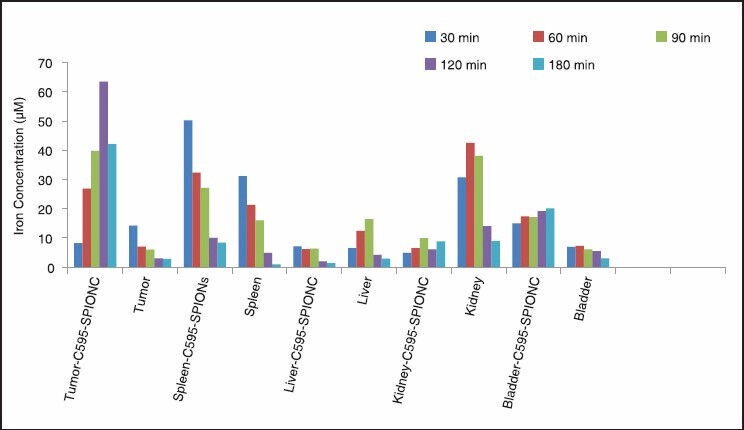
Biodistribution of iron uptakes in different studied organs 24 h after injection of nanoparticles
Conclusions
MRI is occupying an increasing niche in the clinical diagnostic workup of several cancers, including ovarian cancers. Although a role for MRI is yet to be established in this initial setting or in staging, some studies have shown that high sensitivity may be achieved with contrast agent-enhanced MRI for detection of recurrent disease. Currently, effective diagnostic and therapeutic schemes are lacking for treating ovarian cancer, and consequently ovarian cancer has a high mortality rate.[20,21]
The novel MRI nanosized probe used in this study was prepared with a mAb C595 conjugated to SPIONs to target the MUC1 receptor expressed by most of ovarian cancer cells.
Results of this study showed that, functionalization of SPIONs-C595 to the MUC1-expressing cells are achievable both in vitro and in vivo. The results of flow cytometry, Prussian blue staining, signal intensity, and biodistribution and measurement of iron uptake by cells showed high targeting and affinity of SPIONs-C595 to MUC1 positive ovarian cancer cells (OVCAR3). Findings of in vivo study demonstrated also high sensitivity of this agent as an MRI nanosized contrast agent for the detection of ovarian cancer cells. Further, subsequent clinical trials are warranted.
Acknowledgements
This work was financially supported by Isfahan University of Medical Sciences, Isfahan, Iran (Grant No. 189018).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, Murase K. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by marcrophages. Int J Nanomed. 2012;7:5415–21. doi: 10.2147/IJN.S33709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–7. [PubMed] [Google Scholar]
- 3.Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol. 1997;183:311–7. doi: 10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Shahbazi-Gahrouei D, Rizvi SM, Williams MA, Allen BJ. In-vitro studies of gadolinium-DTPA conjugated with monoclonal antibodies as cancer-specific magnetic resonance imaging contrast agents. Australas Phys Eng Sci Med. 2002;25:31–8. doi: 10.1007/BF03178372. [DOI] [PubMed] [Google Scholar]
- 5.Shahbazi-Gahrouei D. Novel MR imaging contrast agents for cancer detection. J Res Med Sci. 2009;14:141–7. [PMC free article] [PubMed] [Google Scholar]
- 6.von Mensdorff-Pouilly S, Gouretvich MM, Kenemans P, Verstraeten AA, van Kamp GJ, Kok A, et al. An enzyme-linked immunosorbent assay for the measurement of circulating antibodies to polymorphic epithelial mucin (MUC1) Tumor Biol. 1998;19:186–95. doi: 10.1159/000030006. [DOI] [PubMed] [Google Scholar]
- 7.Sagara M, Yonezawa S, Nagata K, Tezuka Y, Natsugoe S, Xing PX, McKenzie IF, Aikou T, Sato E. Expression of mucin-1 (MUC1) in esophageal squamous-cell carcinoma: Its relationship with prognosis. Int J Cancer. 1999;84:251–7. doi: 10.1002/(sici)1097-0215(19990621)84:3<251::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Shahbazi-Gahrouei D, Roufeh M, Tavakoli MB. Gadolinium-diethylenetriaminepenta-acetic acid conjugated with monoclonal antibody as new magnetic resonance imaging contrast agents for breast cancer (MCF-7) detection. Iran Biomed J. 2006;10:209–13. [Google Scholar]
- 9.Xu F, Lei D, Du X, Zhang C, Xie X, Yin D. Modification of MR molecular imaging probes with cysteine-terminated peptides and their potential for in vivo tumor detection. Contrast Media Mol Imaging. 2011;6:46–54. doi: 10.1002/cmmi.403. [DOI] [PubMed] [Google Scholar]
- 10.Shahbazi-Gahrouei D, Williams M, Rizvi S, Allen BJ. In vivo studies of Gd-DTPA-monoclonal antibody and Gd-porphyrins: Potential magnetic resonance imaging contrast agents for melanoma. J Magn Reson Imaging. 2001;14:169–74. doi: 10.1002/jmri.1168. [DOI] [PubMed] [Google Scholar]
- 11.Abdolahi M, Shahbazi-Gahrouei D, Laurent S, Sermeus C, Firozian F, Allend BJ, et al. Synthesis and in vitro evaluation of MR molecular imaging probes using J591 mAb-conjugated SPIONs for specific detection of prostate cancer. Contrast Media Mol Imaging. 2013;8:175–84. doi: 10.1002/cmmi.1514. [DOI] [PubMed] [Google Scholar]
- 12.Forstner R, Meissnitzer MW, Schlattau A, Spencer JA. MRI in ovarian cancer. Imaging Med. 2012;4:59–75. [Google Scholar]
- 13.Li W, Chu C, Cui Y, Zhang P, Zhu M. Diffusion-weighted MRI: A useful technique to discriminate benign versus malignant ovarian surface epithelial tumors with solid and cystic components. Abdom Imaging. 2012;37:897–903. doi: 10.1007/s00261-011-9814-x. [DOI] [PubMed] [Google Scholar]
- 14.Feng H, Ghazizadeh M, Konishi H, Araki T. Expression of MUC1 and MUC2 mucin gene products in human ovarian carcinomas. Jpn J Clin Oncol. 2002;32:525–9. doi: 10.1093/jjco/hyf111. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins AC, et al. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol Oncol. 2007;105:695–702. doi: 10.1016/j.ygyno.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Song EY, Qu CF, Rizvi SM, Raja C, Beretov J, Morgenstern A, et al. Bismuth-213 radioimmunotherapy with C595 anti-MUC1 monoclonal antibody in an ovarian cancer ascites model. Cancer Biol Ther. 2008;7:76–80. doi: 10.4161/cbt.7.1.5132. [DOI] [PubMed] [Google Scholar]
- 17.Hughes OD, Perkins AC, Frier M, Wastie ML, Denton G, Price MR, et al. Imaging for staging bladder cancer: A clinical study of intravenous 111indium-labelled anti-MUC1 mucin monoclonal antibody C595. BJU Int. 2001;87:39–46. doi: 10.1046/j.1464-410x.2001.00985.x. [DOI] [PubMed] [Google Scholar]
- 18.Shahbazi-Gahrouei D, Abdolahi M. A novel method for quantitative analysis of anti-MUC1 expressing ovarian cancer cell surface based on magnetic cell separation. J Med Sci. 2012;12:256–66. [Google Scholar]
- 19.Shahbazi-Gahrouei D, Abdolahi M, Zarkesh-Esfahani SH, Laurent S, Sermeus C, Gruettner C. Functionalized magnetic nanoparticles for the detection and quantitative analysis of cell surface antigen. Biomed Res Int. 2013;2013:349408. doi: 10.1155/2013/349408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranganathan R, Madanmohan S, Kesavan A, Baskar G, Krishnamoorthy YR, Santosham R, et al. Nanomedicine: Towards development of patient-friendly drug-delivery systems for oncological applications. Int J Nanomedicine. 2012;7:1043–60. doi: 10.2147/IJN.S25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao W, Luo J, Jain T, Riggs JW, Tseng HP, Henderson PT, et al. Biodistribution and pharmacokinetics of a telodendrimer micellar palitaxel nanoformulation in a mouse xenograft model of ovarian cancer. Int J Nanomedicine. 2012;7:1587–97. doi: 10.2147/IJN.S29306. [DOI] [PMC free article] [PubMed] [Google Scholar]


