Abstract
Background:
The aim of the present study was to evaluate the effect of risperidone in patients afflicted by autistic disorder especially with regards to its three core symptoms, including “relating to others”, “communication skills”, and “stereotyped behaviors” based on Childhood Autism Rating Scale (CARS).
Materials and Methods:
An 8-week open-label study of risperidone for treatment of autistic disorder in children 4-17 years old was designed. Risperidone dose titration was as follow: 0.02 mg/kg/day at the first week, 0.04 mg/kg/day at the second week, and 0.06 mg/kg/day at the third week and thereafter. The outcome measures were scores obtained by CARS, Aberrant Behavior Checklist (ABC), and Clinical Global Impression-Improvement (CGI-I) scale.
Results:
Fifteen patients completed this study. After 8 weeks, CARS total score decreased significantly, (P=0.001). At the end of the study, social interactions and verbal communication skills of the patients were significantly improved (P<0.001, P=0.03, respectively). However, stereotypic behaviors did not show any significant change in this study. Increase in appetite and somnolence were the most reported side effects.
Conclusion:
This study suggests that risperidone may be an effective treatment for the management of core symptoms of autistic disorder.
Keywords: Autistic disorder, childhood autism rating scale, core symptoms, risperidone
INTRODUCTION
Autistic disorder, a subtype of pervasive developmental disorders (PDDs), as defined in Diagnostic and Statistical Manual of Mental Disorders, fourth Edition, Text Revision (DSM-IV-TR), is a chronic mental disorder. The core symptoms of autism include impairments in social interactions, verbal and nonverbal communication skills, and stereotyped actions and tendencies.[1] Moreover, these patients may show other symptoms like irritability, anger outbursts, self-injurious behaviors, and inattention. Currently, risperidone and aripiprazole are the only two drugs approved by USA Food and Drug Administration (FDA) for the management of patients with autism. These drugs are approved primarily for the treatment of irritability related to autism.[2] Risperidone was the first drug approved by FDA for this disorder. Several studies have investigated the efficacy of risperidone in the management of autism; however, their results have been inconclusive especially in the domain of the core symptoms of this disorder. Some of these studies have reported a modest or even no effect of risperidone in the improvement of social interactions as well as in the verbal and nonverbal communication skills.[3,4,5,6] Several instruments have been used for measuring outcome in studies on patients with PDDs. Children's Psychiatric Rating Scale (CPRS), Clinical Global Impression-Improvement (CGI-I) scale, Children's Global Assessment Scale (C-GAS), Aberrant behavior checklist (ABC), Ritvo-Freeman Real Life Rating Scale, Nisonger Child Behavior Rating Form (N-CBRF), and Childhood Autism Rating Scale (CARS) are among such instruments.[3,4,6,7,8,9] ABC is one of the scales that has been extensively used for evaluation of drug therapy in patients with PDD. CARS is another scale that has been utilized to measure response in these patients, especially in those with autism.[10] This scale has been validated and is a reliable scale that assesses severity of the autistic symptoms and, therefore, is used to detect the degree of improvement.[8,11,12,13] However, it should be noted that changes in the subscales of CARS in addition to its total score after drug therapy have been analyzed in only a limited number of studies.[8,9,10,14,15,16] Risperidone has been used in the treatment of many children and adolescents with autism; however, its effectiveness on the core symptoms of autism has not been widely studied. The authors of this study have aimed to evaluate the efficacy of this drug in patients afflicted by autism, especially with regards to the three core symptoms, including relating to others, communication skills, and stereotyped behaviors, as measure by CARS (predominantly based on changes in its subscales and total score).
MATERIALS AND METHODS
This 8-week, open-label study was performed in 15 patients with established diagnosis of autistic disorder, made by a child and adolescent psychiatrist based on the DSM-IV-TR criteria. Included patients were between 4 years and 17 years of age, had not received a diagnosis of autism prior to this study and, therefore, never received a medication for this disorder. Patients with a history of epilepsy with no seizure attacks for at least one month before the initiation of the study were not excluded from the study.
Exclusion criteria included any neurologic disorder (except for epilepsy as explained above), substance abuse, history of neuroleptic malignant syndrome, history of severe allergic reactions to risperidone and pregnancy.
This study was approved by ethics committee of Tehran University of Medical Sciences. Subject's guardians have been given a written informed consent form and patient anonymity has been preserved.
Medication schedule
Risperidone was started at 0.02 mg/kg/day for one week, followed by 0.04 mg/kg/day at the second week and ultimately 0.06 mg/kg/day at the third week and afterwards. The maximum allowed dose of risperidone was 3 mg/day. In case of severe side effect, the dose was decreased back to the previous tolerable amount and then gradually increased to the targeted, tolerable amount.
Baseline assessment and outcome measures
ABC, CARS, and Clinical Global Impression-Severity scale (CGI-S) were utilized to assess patients at baseline. Patients were assessed by ABC and CGI-I at week 4 and by ABC, CARS, and CGI-I at week 8.
ABC: This checklist includes 58 items divided into five subscales; each item may be scored 0-3. ABC is an instrument used extensively in previous studies for evaluation of treatment response in PDDs including autistic disorder.[17]
CARS: This questionnaire contains 15 items each scored 1-4 that collectively adds up to a total score of 15-60.[7] As mentioned above, CARS is used for the diagnosis and evaluation of symptoms in patients with autism.[8]
CGI: This scale has three categories for evaluation of illness severity (CGI-S), evaluation of improvement relative to the previous situation (CGI-I), and evaluation of the treatment efficacy (efficacy index). CGI-S is scored as following: 0 (not assessed), 1 (Normal, not at all ill), 2 (Borderline mentally ill), 3 (Mildly ill), 4 (Moderately ill), 5 (Markedly ill), 6 (Severely ill), 7 (Among the most extremely ill patients). Similarly, CGI-I is also scored as following: 0 (Not assessed), 1 (Very much improved), 2 (Much improved), 3 (Minimally improved), 4 (No change), 5 (Minimally worse), 6 (Much worse), and 7 (Very much worse).[18]
Monitoring of side effects
Patients were asked about any potential side effects since they started using the study drug at weeks 2, 4 and 8 of the trial.
Statistical analysis
One-way repeated measure analysis of variance (ANOVA) was used for analyzing the ABC data; Paired samples t-test was used for CARS data analysis. P≤0.05 were considered for statistical significance.
RESULTS
Participants include five females and 10 males between 4 years and 17 years old (mean±SD: 6.5±3.5 years). Based on the CGI-S scale, six patients were noted to be markedly ill (40%), six patients severely ill (40%), and three patients (20%) moderately ill. The total score of CARS showed that use of risperidone resulted in a significant decrease of the symptoms intensity (mean±SD: From 42.85±4.6 at baseline to 35.2±5.6 at week 8) (t=4.6, P=0.001). The CARS items decreased significantly after 8 weeks of treatment included “relating to people”, “emotional response”, “body use”, “object use”, “adaptation to change”, “visual response”, “listening response”, “verbal communication”, “activity level”, and “general impression” (t=6.98, P<0.001; t=4.46, P=0.001; t=2.7, P=0.01; t=2.75, P=0.01; t=4.78, P<0.001; t=2.44, P=0.02; t=3.38, P=0.004; t=2.38, P=0.03; t=4.75, P<0.001; t=6.61, P<0.001, respectively). Table 1 and Figure 1 show scores of CARS items before treatment and after 8 weeks of treatment.
Table 1.
Scores of CARS items at baseline and after 8 weeks of treatment
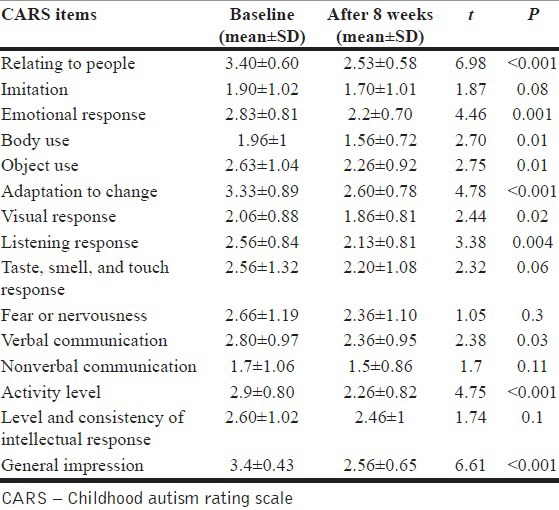
Figure 1.
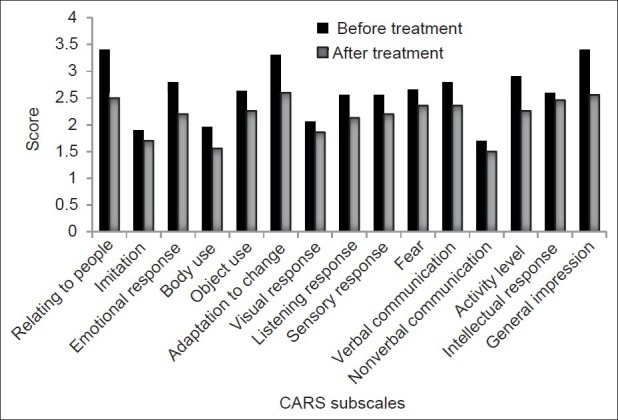
CARS item changes after 8 weeks of treatment
Based on the ABC score, mean irritability score decreased significantly after 4 and 8 weeks of treatment; interestingly, the difference between the fourth and eighth week scores was significant as well (F=71.3, P<0.001). Decrements in the mean of the two subscales of “lethargy/social withdrawal” and “hyperactivity” were significant after 4 and 8 weeks of treatment; besides the difference between the fourth and eighth week was also significant for the above subscales (lethargy/social withdrawal subscale: F=21.3, P<0.001) (attention deficit/hyperactivity subscale: F= 41.8, P<0.001).
The subscale of inappropriate speech showed a significant decrease after 4 weeks (F=5.6, P=0.03) but the difference between fourth and eighth weeks was not considered to be significant. Risperidone did not exert any significant effect on stereotypy subscale (F=3.6, P=0.6). Results of ABC subscales are presented in Table 2 and Figure 2.
Table 2.
Scores of the ABC subscales at baseline and after 4 and 8 weeks of treatment
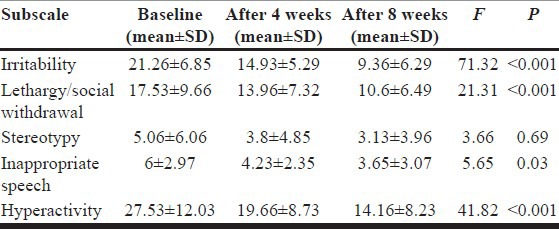
Figure 2.
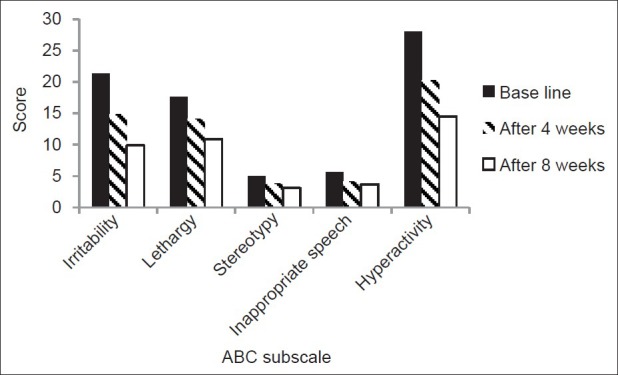
ABC subscales changes after 4 and 8 weeks of treatment
Results of the CGI-I scale 4 and 8 weeks after the initiation of the study were as follow. After 4 weeks: Three patients (20%) were reported with very much improvement, nine patients (60%) with much improvement, two patients (13.3%) with minimal improvement, and one patient (6.7%) with no change. After 8 weeks: Four patients (26.7%) were very much improved, seven patients (46.7%) much improved, and three patients (20%) minimally improved while one patient (6.6%) had no change.
Side effects
No patient reported any serious side effect during this study. Increase in appetite (without any significant weight gain) was the most reported side effect (in eight patients, 53.3%). Other reported side effects included somnolence (in five patients, 33.3%), fever (in four patients, 26.6%), indifference to self-defense (in three patients, 20%), and enuresis (in two patients, 13.3%).
DISCUSSION
Our study indicated that risperidone is an effective treatment for core symptoms of autism. The present trial noted a significant improvement in social interactions after 4 weeks based on the changes in “lethargy/social withdrawal” subscale of ABC and after 8 weeks based on the changes in ABC and “relating to people” item of CARS.
Several double-blind, placebo-controlled trials have found that risperidone may not be effective in improvement of social interactions.[3,4,19,20] However, McDougle et al., noted that social interactions improved 8 weeks after initiation of risperidone based on the subscale II Ritvo-Freeman Real life Rating Scale. This improvement was not found to be significant (P=0.07), however, this P value may show a trend toward significance. If a more sensitive instrument had been used, the results may have been shown to be significant.[4]
Along those lines, there are reports regarding the effectiveness of risperidone in improving social interactions.[21] In an open-label study in 24 PDDs patients, treatment with risperidone resulted in significant reduction in “withdrawal subscale” of Children's Psychiatry Rating Scale (CPRS) and “relating to people” item of CARS after 16 weeks.[8] Another open-label study of risperidone in 11 PDDs patients showed significant improvement in “relating to people” item of CARS after 6 months of treatment.[9] A double-blind, placebo-controlled, randomized clinical trial (RCT) of risperidone in 79 PDDs patients showed significant improvement in “Lethargy/social withdrawal” subscale of ABC after 8 weeks.[6] Another double-blind RCT in 80 patients with autism showed significant improvement in “lethargy/social withdrawal” subscale of ABC after 8 weeks of risperidone treatment.[22]
Speech disturbances in our trial were also improved significantly after 4 weeks based on “inappropriate speech” subscale of ABC and after 8 weeks based on “verbal communication” item of CARS. These findings were similar to the results of some studies of risperidone in PDDs patients[3,6] with some exceptions observed in some other trials in which no significant improvement in language and verbal skills were noted after administration of risperidone.[4,9,19,22]
In our study, risperidone showed a significant improvement in “body use” (including stereotyped behaviors) after 8 weeks of treatment based on CARS; however, no significant improvement was noted in the stereotyped behavior after 4 and 8 weeks of treatment based on ABC. The difference seen in the results obtained by using these two different scales may have been due to the broader evaluation of symptoms by “body use” item of CARS that includes balanced and stereotyped movements as well as self-injurious behaviors. Even though several studies have noted insignificant effects of risperidone on stereotypic behaviors of patients with autistic disorder,[5,23] several other studies have shown the effectiveness of risperidone in improving these behaviors.[4,5,6,19,23] For instance, in a study on 101 patients with autistic disorder, risperidone did not significantly decrease “stereotypy” subscale of ABC.[3]
There are several limitations in our study as follows. Our study was performed on a small number of patients, based on an open-label design and used only three instruments for its outcome measures. Autism is considered as one of the hard-to-manage psychiatric disorders with tremendous socioeconomic burden and enormous health costs. As such, even though reisperidone has been proven to be an effective and safe medication for this group of patients, especially for treatment of core symptoms, however, there seems to be a much further need for newer medications or new application of some of the current medications in order to achieve the best possible outcomes across all domains of impairment in autism.
ACKNOWLEDGMENT
This study was a part of Dr. Nikvarz's thesis toward graduation from the residency program in clinical pharmacy and was supported by Tehran University of Medical Sciences (TUMS) (Grant number: 91-01-33-16991).
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Doyle CA, Mc Dougle CJ. Pharmacologic treatments for the behavioral symptoms associated with autism spectrum disorders across the lifespan. Dialogues Clin Neurosci. 2012;14:263–79. doi: 10.31887/DCNS.2012.14.3/cdoyle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Cur Treat Options Neurol. 2010;12:529–38. doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]
- 3.McCracken AT, Mc-Gough J, Shah B, Cronin P, Hong D. Risperidone in children with autism and serious behavioral problems. N Engl J Med. 2002;347:314–21. doi: 10.1056/NEJMoa013171. [DOI] [PubMed] [Google Scholar]
- 4.McDougle CJ, Scahill L, Aman MG, McCracken JT, Tierney E, Davies M, et al. Risperidone for the core symptom domains of autism: Results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry. 2005;162:1142–48. doi: 10.1176/appi.ajp.162.6.1142. [DOI] [PubMed] [Google Scholar]
- 5.Posey DJ, Erickson CA, McDougle CJ. Developing drugs for core social and communication impairment in autism. Child Adolesc Psychiatr Clin N Am. 2008;17:787–99. doi: 10.1016/j.chc.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea S, Turgay A, Carroll A, Schulz M, Orlik H, Smith I, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics. 2004;114:e634–41. doi: 10.1542/peds.2003-0264-F. [DOI] [PubMed] [Google Scholar]
- 7.Chez MG, Buchanan TM, Becker M, Kessler J, Aimonovitch MC, Mrazek SR. Donepezil hydrochloride: A double-blind study in autistic children. J Pediatr Neurol. 2003;1:83–8. [Google Scholar]
- 8.Masi G, Cosenza A, Mucci M, Brovedani A. Open trial of risperidone in 24 young children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry. 2001;40:1206–14. doi: 10.1097/00004583-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Zuddas A, Martino AD, Muglia P, Cianchetti C. Long-term risperidone for pervasive developmental disorder: Efficacy, tolerability, and discontinuation. J Child Adolesc Psychopharmacol. 2000;10:79–90. doi: 10.1089/cap.2000.10.79. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraj R, Singhi P, Malhi P. Risperidone in chidren with autism: Randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21:450–5. doi: 10.1177/08830738060210060801. [DOI] [PubMed] [Google Scholar]
- 11.Rellini E, Tortolani D, Trillo S, Carbone S, Montecchi F. Childhood Autism Rating Scale (CARS) and Autism Behavior Checklist (ABC) correspondence and conflicts with DSM-IV criteria in diagnosis of autism. J Autism Dev Disord. 2004;34:703–8. doi: 10.1007/s10803-004-5290-2. [DOI] [PubMed] [Google Scholar]
- 12.Perry A, Condillac RA, Freeman NL, Dunn-Geier J, Belair J. Multi-site study of the Childhood Autism Rating Scale (CARS) in five clinical groups of young children. J Autism Dev Disord. 2005;35:625–34. doi: 10.1007/s10803-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 13.Masi G, Cosenza A, Millepiedi S, Muratori F, Pari C, Salvadori F. Aripiprazole monotherapy in children and young adolescents with pervasive developmental disorders, A retrospective study. CNS drugs. 2009;23:511–21. doi: 10.2165/00023210-200923060-00005. [DOI] [PubMed] [Google Scholar]
- 14.Masi G, Cosenza A, Mucci M, De Vito G. Risperidone monotherapy in preschool children with pervasive developmental disorders. J Child Neurol. 2001;16:395–400. doi: 10.1177/088307380101600602. [DOI] [PubMed] [Google Scholar]
- 15.Luby J, Mrakotsky C, Stalets MM, Belden A, Heffelfinger A, Williams M. Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006;16:575–87. doi: 10.1089/cap.2006.16.575. [DOI] [PubMed] [Google Scholar]
- 16.Diler RS, Firat S, Avci A. An open label trial of risperidone in children with autism. Curr Ther Res. 2002;63:91–102. [Google Scholar]
- 17.Karabekiroglu K, Aman MG. Validity of the Aberrant Behavior Checklist in a clinical sample of toddlers. Child Psychiatry Hum Dev. 2009;40:99–110. doi: 10.1007/s10578-008-0108-7. [DOI] [PubMed] [Google Scholar]
- 18.Kadouri A, Corruble E, Falissard B. The improved Clinical Global Impression Scale (iCGI): Development and validation in depression. BMC Psychiatry. 2007;7:1–7. doi: 10.1186/1471-244X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDougle CJ, Holmes JP, Carlson DC, Pelton GH, Cohen DJ, Price LH. A double-blind placebo controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Arch Gen Psychiatry. 1998;55:633–41. doi: 10.1001/archpsyc.55.7.633. [DOI] [PubMed] [Google Scholar]
- 20.Kent JM, Kushner S, Ning X, Karcher K, Ness S, Aman M, et al. Risperidone dosing in children and adolescents with autistic disorder: A double-blind, placebo-controlled study. J Autism Dev Disord. 2012 doi: 10.1007/s10803-012-1723-5. [DOI] [PubMed] [Google Scholar]
- 21.Williams SK, Scahill L, Vitiello B, Aman MG, Arnold E, McDougle CJ, et al. Risperidone and adaptive behavior in children with autism. J Am Acad Child Adolesc Psychiatry. 2006;45:431–39. doi: 10.1097/01.chi.0000196423.80717.32. [DOI] [PubMed] [Google Scholar]
- 22.Pandina GJ, Bossie CA, Youssef E, Zhu Y, Dunbar F. Risperidone improves behavioral symptoms in children with autism in a randomized, double-blind, placebo-controlled trial. J Autism Dev Disord. 2007;37:367–73. doi: 10.1007/s10803-006-0234-7. [DOI] [PubMed] [Google Scholar]
- 23.McPheeters ML, Warren Z, Sathe N, Bruzek JL, Krishnaswami S, Jerome RN. A systematic review of medical treatments for children with Autism Spectrum Disorders. Pediatrics. 2011;127:e1312–21. doi: 10.1542/peds.2011-0427. [DOI] [PubMed] [Google Scholar]


