Abstract
Objective:
Conventional antidepressants take two weeks before their therapeutic action begins. Recent studies have reported on the rapid antidepressant effect of ketamine when given as an intravenous (I.V.) infusion. Little is known about its intramuscular (I.M.) use in depression. Hence this study was conducted to compare the safety, tolerability and efficacy of I.M. versus. I.V. ketamine in Major Depression (ICD-10).
Materials and Methods:
It was a randomized open label parallel group study in a tertiary care teaching hospital. Study sample consisted of 27 subjects having major depression divided randomly into three groups of nine subjects each. Ketamine administered to each group in the dose of 0.5 mg/kg as an I.V. infusion, as 0.5 mg/kg I.M. or 0.25 mg/kg I.M. respectively. Depression rated on the Hamilton Depression Rating Scale (HAM-D) before the injection, two hours later, the next day, and after three days. Data analyzed using the Statistical Package for Social Sciences (SPSS).
Results:
Mean age of the sample was 36.81 years (SD 11.815). Two hours after the injection, HAM-D fell by 58.86%, 60.29% & 57.36% in each group respectively. The improvement was sustained for next three days. Adverse effects noticed were rare, of mild nature and transient, lasting less than an hour.
Conclusions:
Intramuscular ketamine in the dose of 0.25 mg/kg is as effective and safe as 0.5 mg/kg given either I.M. or I.V., substantially alleviating depressive symptoms within a few hours and sustained for 3 days.
Keywords: Acute antidepressant effects, antidepressant drugs, depression, intramuscular ketamine, intravenous ketamine, suicidal risk
INTRODUCTION
Ketamine is widely used as an anesthetic drug with wide therapeutic index. It also has an addiction potential and is classified in UK as class C drug. It has half-life of just 17 min and its urinary excretion occurs in just 2 h. When administered parenterally, it is totally eliminated from the body in 24 h.[1] Many animal studies using different animal models of depression have shown that N-methyl d-aspartate (NMDA) receptor antagonists, including ketamine, have antidepressant effects.[2] Ketamine and narcotic combinations are being used successfully for the last 20 years in many terminally ill patients in an intensive care unit to alleviate their anxiety, fear of death, severe depression, and suicidal ideation.[3] A few case reports and small randomized clinical trials using intravenous (IV) infusions of ketamine have shown significant, rapid, but short-lived antidepressant benefits.[4,5,6]
In what was the first ever reported randomized clinical trial (RCT), seven patients with major depression were treated with IV infusion of ketamine 0.5 mg/kg diluted in normal saline. It was a double-blind, crossover study, and showed significant, rapid, but short-lived antidepressant benefits.[7] In the next RCT, 18 patients with DSM-IV non-psychotic treatment-refractory major depressive disorder (MDD) were randomized to IV ketamine versus placebo infusion in a double-blind, crossover design study. Ketamine in the dose of 0.5 mg/kg, contained in 50 ml of normal saline was delivered across 40 min as an infusion. It was repeated after 1 week. One day later, 71% responded and 29% remitted. Effect sizes after 24 h and at 1 week were 1.5 and 0.7, respectively. (Placebo response rate was 0% at 1 day and 1 week.) Only 35% maintained response for >1 week. Adverse effects of ketamine included confusion, dizziness, perceptual disturbances, euphoria, and increased blood pressure. These did not last beyond 1-2 h after the infusion.[8]
In another study, 24 h after a single infusion of ketamine, there was significant reduction in Montgomery Asberg Depression Rating Scale (MADRS) suicide item scores in 23 patients with treatment-resistant depression. MADRS suicide item attenuation was sustained for 12 days by thrice-weekly ketamine infusion in an additional sample of nine patients. The benefits were likely a function of the sharp antidepressant effect observed with the drug.[9] In yet another open-label study, 33 patients with treatment-resistant MDD were treated with ketamine (0.5 mg/kg) infusion. From 40 min to 4 h later, there was significant reduction in the scale for suicidal ideation ratings, as well as MADRS and Hamilton Depression Rating Scale (HAM-D) suicide item scores. The benefits were likely a function of the sharp antidepressant effect observed with the drug.[10]
A recent study from India on 22 subjects with treatment-resistant depression reported similar rapid, albeit short-lived antidepressant effect of ketamine IV infusion.[11]
However, the studies reported so far have used IV route to deliver ketamine. Little is known about the potential role of intramuscular (IM) ketamine in the treatment of major depression. In the first ever case report of IM use of ketamine, it was given to a patient with metastatic ovarian cancer in the dose of 1 mg/kg as a course of six treatments with a remission of her depressive symptoms.[12] In another report, IM ketamine in the dose of 0.5 mg/kg was seen to have alleviated severe depression, especially the suicidal ideation, in two cases within a few hours.[13]
Even the oral ketamine has not been systematically studied yet. Only two cases have been reported in which a single oral dose of ketamine provided rapid and moderately sustained symptom relief for both depression and anxiety and no adverse effects were noted.[14]
While IV route is cumbersome, relatively expensive, and requires close monitoring during 40-min infusion, IM route may eliminate these disadvantages. To this purpose, we proposed to study the rapid antidepressant effects and safety of IM versus IV ketamine in major depression in sub-anesthetic doses.
MATERIALS AND METHODS
Study design
This was a randomized, open-label, parallel-group study.
Intervention
Inj. ketamine 0.5 mg/kg IV or 0.5 mg/kg IM or 0.25 mg/kg IM
Methodology
This study was conducted to assess the safety, tolerability, and efficacy of IM versus IV ketamine in major depression. It was carried out in a tertiary care teaching hospital of South India. Institutional Ethics Committee (IEC) approved the study protocol. Informed consent was obtained from all the study participants. The study was conducted from March 2011 to April 2012, and all the outpatients and inpatients who met the selection criteria for major depression (ICD-10) were enrolled. The study sample consisted of 27 male and female subjects of age 18 or above, who met ICD-10 criteria for diagnosis of major depression (F32.2) as per an assessment by the physician — investigator, scoring at least 16 on the Hamilton Depression Rating Scale (HAM-D-17).[15] Subjects included fresh cases, those not responding to ongoing antidepressant medication, subjects for whom electroconvulsive therapy (ECT) was indicated for their severe depression, and depressed subjects who were suicidal where urgent relief of symptoms was indicated. Patients were excluded from the study if they had any contraindication to the administration of ketamine, especially present diagnosis or antecedents of clinically relevant cardiovascular disorders such as stroke, heart attack, etc., those with present or past diagnosis of glaucoma, intracranial hypertension, current diagnosis of schizophrenia or other psychotic disorder (including psychotic disorder due to general medical condition, substance-induced psychotic disorder, or mania) as defined in the ICD-10, and patients with any known hypersensitivity to ketamine.
Pulse rate, blood pressure (BP), and body weight were recorded at baseline. The subjects were allotted to one of the three groups through block-randomization table which determined the route and dose of administration of ketamine. Accordingly, each subject was then administered ketamine either as an IV infusion in dose of 0.5 mg/kg body weight diluted in 100 ml of normal saline and given over 40 min (group 1) or as an IM injection in the dose of either 0.5 mg/kg (group 2) or 0.25 mg/kg (group 3). The subject was allowed to rest on the bed for the next 4 h and engaged in conversation. BP was measured after every 1 h. At the end of the second hour, severity of depression on HAM-D-17 was recorded. The subject was allowed to go home after 4 h of observation. Depression was rated on HAM-D-17 on the following day and later again after 3days. Adverse events emerging at any point of the study were recorded. They were allowed to continue their ongoing antidepressant medication. End points of the study were the percentage of responders, partial responders, and non-responders, in addition to the safety.
Patients were graded as non-responders if the reduction in HAM-D-17 scale was <25% from baseline, partial responders if the reduction was between 26 and 49% from baseline, and responders if there was ≥50% reduction in the scale from baseline.
Remission was defined as a reduction in HAM-D-17 scale from baseline to <7 and relapse defined as the return of fully symptomatic state from remission.
Data management and statistical analysis
Data were entered into Excel spreadsheet 2007. Data were analyzed by using the Statistical Package for Social Sciences (SPSS). Randomization list was prepared using GraphPad StatMate. Between-groups analysis was performed using Kruskal — Wallis test. In all cases, P < 0.05 was considered statistically significant. Negative sign indicates decrease and vice versa.
RESULTS
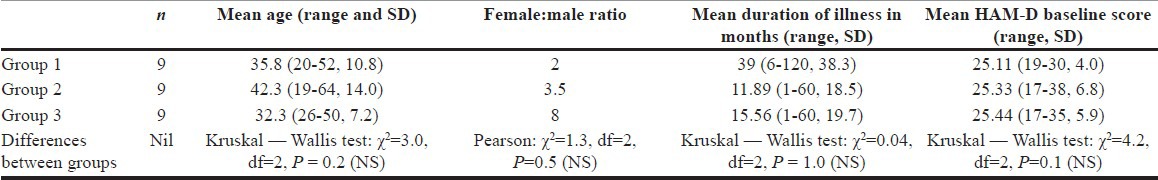
The present study enrolled 27 subjects in all. Mean age of the subjects was 36.81 years (SD 11.815, range 19-64), while the male to female ratio was 2:7 showing a female preponderance. The mean duration of illness when the subjects entered this study was 22.14 months (SD 28.73, range 1-120). The sample of 27 subjects was divided into three groups of 9 each, who received ketamine in different routes and doses. The three groups were similar with no significant differences between their mean age, mean duration of illness, or the severity of depression as measured on the HAM-D [Table 1].
Table 1.
Sample descriptive statistics
Even though the study was initially designed to follow-up the subjects weekly for a month following ketamine injection, most of them were irregular at the follow-ups after the 4th day review due to traveling constraints and did not turn up in numbers enough for any meaningful statistical analysis. Hence, the study analysis was limited to their follow-up on day 4 of the injection.
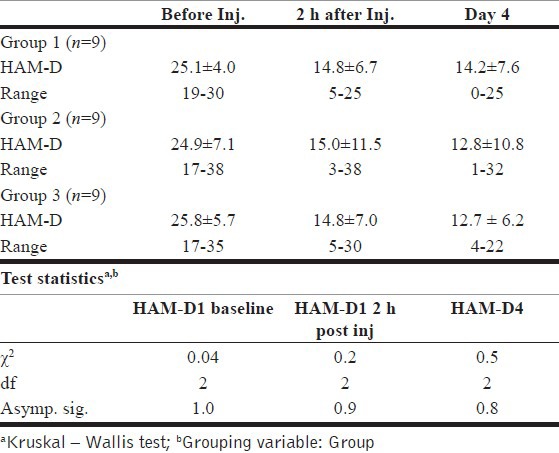
This study has shown that ketamine injection in small sub-anesthetic doses given either IV or IM results in a significant reduction of depressive symptoms in patients with major depression at 2 h, which is sustained till the 4th day after the injection [Table 2].
Table 2.
Test statisticsa

HAM-D1 pre inj, HAM-D score just before giving injection ketamine; HAM-D1_2h, HAM-D score at 2 h after administering the injection ketamine; HAM-D4, HAM-D score on the 4th day after the injection ketamine.
Kruskal — Wallis test revealed that there was no significant difference in the baseline HAM-D scores between the three groups. The test also showed that there was no significant difference between the three groups in their HAM-D scores, either at 2 h or on the 4th day after the injection [Table 3].
Table 3.
Response of depressive symptoms on HAM-D scores to various routes and doses of parenteral ketamine
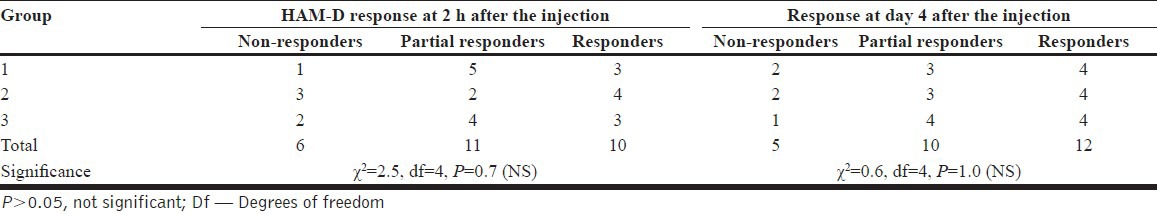
Also, there was no significant difference between the three study groups in the efficacy (as to the non-responders, partial responders, and the responders) [Table 4].
Table 4.
Number of subjects responded to Inj. ketamine (n=9 in each group)
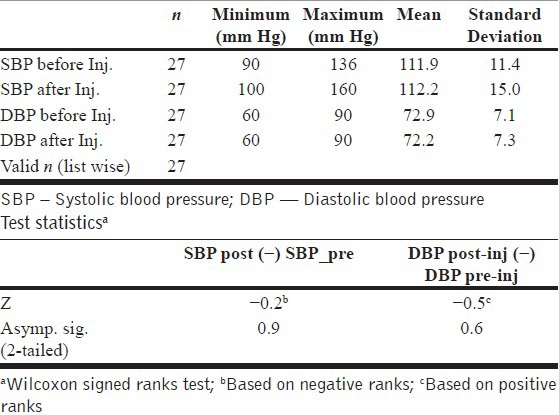
Ketamine caused no significant changes in the systolic or diastolic BP in each of the three study groups [Table 5].
Table 5.
Effect of ketamine on blood pressure
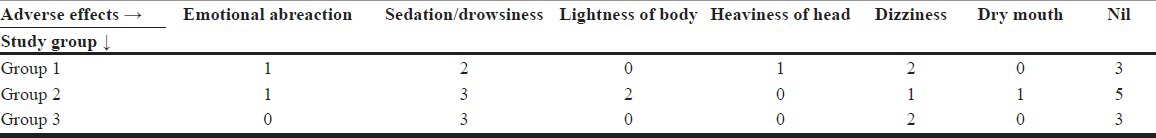
Adverse effects such as sedation and dizziness were reported by some subjects, but they subsided within an hour of the infusion/injection. One patient with IV infusion and another with IM 0.5 mg/kg had manifested mild emotional abreaction which subsided within an hour of the injection. Some subjects had reported more than one of the adverse effects mentioned in Table 6. None of them manifested any perceptual disturbances or psychotic symptoms. The subjects who were outpatients were well enough to travel back to their homes after the 4-h observation period.
Table 6.
Incidence of adverse effects after injection ketamine
DISCUSSION
In the present study, parenteral ketamine has resulted in a rapid and significant improvement in the depressive symptoms. Two hours after the injection of ketamine, groups 1, 2, and 3 showed a reduction in the HAM-D score of 58.86%, 60.29%, and 57.36%, respectively. The said improvement was sustained after 1 day, as well as for 3 days after the injection. Differences in the antidepressant efficacy between various routes (IV or IM) or the doses (0.5 mg/kg or 0.25 mg/kg) were not statistically significant. Adverse effects noticed were of mild nature and transient lasting less than an hour. Thus, IM ketamine in small doses of 0.25 mg/kg is as effective and safe as when given in the dose of 0.5 mg/kg either in IM or IV route.
During the course of the study, two of the subjects attempted suicide. Both were of high intentionality and high lethality, but survived the attempts. First one was a spinster, a dental student aged 20 years who was suffering from a recurrent depressive disorder of 5 years duration and had already attempted suicide four times before entry into this study. She also had significant financial as well as psychosocial stressors. She was a non-responder to ketamine injection given IV in this study. She had therefore received ECT (six treatments) in addition to antidepressant medication, but still did not respond, and finally after 50 days of the injection, she attempted suicide. The second case was a 64-year-old married doctor who had a comorbid chronic generalized anxiety disorder ever since his youth. He received one injection of ketamine in the dose of 0.5 mg/kg IM as a part of this study. He too was a non-responder to ketamine injection and attempted suicide 10 days after the injection. In both of these cases, suicidality cannot be attributed to ketamine injection. It is also evident that not all patients would respond to ketamine as is the case with conventional antidepressant drugs or ECT. We had 6 subjects who were non-responders out of 27 in this study, which amounts to 22.22%. Thus, the exact cause of depressive illness at the molecular level is still elusive.
As ketamine's pharmacokinetics assures us of its excellent safety profile, much is being speculated in the recent literature on its mode of action in alleviating depression.
Glutamate is a major “excitatory” neurotransmitter that helps activate brain cells. Ketamine directly blocks NMDA receptors (NMDARs) and effectively increases the levels of glutamate in the brain. Because other studies have found that ketamine affects additional brain molecules, however, it may be that this drug triggers downstream biological changes that have antidepressant effects.[16] A series of studies completed over the past 12 years suggest that a novel class of drugs that directly target the glutamatergic neurotransmitter system may produce rapid and robust antidepressant effects in patients who had previously not responded to the standard monoaminergic antidepressant medications. Several ongoing clinical trials in this area have been amply described in a recent review.[17] Ketamine is reported to reduce depressive symptoms by activating the mammalian target of rapamycin (mTOR), which results in an increase in synapse-related proteins.[18] In an in vivo study, ketamine was reported to induce distinct oscillatory dynamics not only in pyramidal cells but also in at least seven different types of cornu ammonis (CA1) interneurons including putative basket cells, chandelier cells, bistratified cells, and O-LM cells. These emergent unique oscillatory dynamics may very well reflect the intrinsic temporal relationships within the CA1 circuit.[19] Ketamine has also been described as a chiral drug that has a greater affinity for the phencyclidine binding site on the NMDAR. However, chronic administration of ketamine and imipramine did not modify the brain-derived neurotropic factor (BDNF) protein levels in the rat hippocampus.[20] Ketamine and other NMDAR antagonists have been shown to produce fast-acting behavioral antidepressant-like effects in mouse models, and these effects have been observed to depend on the rapid synthesis of BDNF.[21] But in human beings, ketamine's rapid initial antidepressant effects were found not to be mediated by BDNF.[22] Thus, the exact mechanism of ketamine's antidepressant action remains yet to be established.
Whatever may be its mode of action, treatment with IM ketamine can thus be a very useful tool as a first aid to help patients who are depressed and suicidal, pending the onset of action of the newly started antidepressants or pending arrangements for ECT. It can be useful to treat depressed patients who are unwilling for ECT or where ECT is relatively contraindicated such as in brain tumors, recent myocardial infarction, etc. It thus paves way for an important breakthrough in the emergency management of severe depression. It can be useful for students who are severely depressed before their examinations, where ECT can interfere with their memory and, hence, is not preferred. It also can serve community psychiatry as a safe tool in the hands of primary care physicians (civilian or military) or mental health workers who can provide first aid relief to severely depressed suicidal patients under their care in the rural or remote areas where psychiatrists are not available, or to render them fit enough to travel to a psychiatric center. It is, however, recommended that patients be made to take bed rest for a couple of hours following the injection in view of possible sedation and other minor adverse effects in some of them.
Limitations
The sample size in this study was relatively small and it was not a double-blind controlled study. Due to ethical constraints, placebo control could not be included in this study. Besides, due to practical considerations, the study was limited to 3 days, whereas a longer duration could have shown the late effects of ketamine. Even though this study shows modest benefits from IM injection of ketamine in depression, it needs replication with double-blinded studies using larger samples and preferably placebo-controlled.
CONCLUSIONS
Ketamine, when given in the dose of 0.25 mg/kg IM, has been found to be as safe and effective as when given in the higher dose of 0.5 mg/kg either in IM or IV route. It brought about a rapid reduction of depressive symptoms within a few hours, as measured on the HAM-D. The improvement was sustained for the following 3 days. The few emergent adverse effects were mild and subsided within 1 h of the injection. Thus, it has an important role in the emergency management of severe depression, at least till a better alternative is discovered.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Winstock AR, Schifano F. Disorders relating to the use of ecstasy and other ‘party drugs’. In: Gelder MG, Andreasen NC, lopez-Ibor JJ Jr, Geddes JR, editors. New Oxford Textbook of Psychiatry. 2nd ed. Vol. 1. New York: Oxford University Press Inc; 2011. pp. 499–501. [Google Scholar]
- 2.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;28:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao TS, Andrade C. Innovative approaches to treatment-refractory depression: The ketamine story. Indian J Psychiatry. 2010;52:97–9. doi: 10.4103/0019-5545.64573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebrenz M, Borgeat A, Leisinger R, Stohler R. Intravenous Ketamine therapy in a patient with a treatment-resistant major depression. Swiss Med Wkly. 2007;137:234–6. doi: 10.4414/smw.2007.11852. [DOI] [PubMed] [Google Scholar]
- 5.Sivagnanam G. Chronotherapy in hypertension. J Pharmacol Pharmacother. 2012;3:209. [Google Scholar]
- 6.Thangathurai D, Mogos M. Ketamine alleviates fear, depression, and suicidal ideation in terminally ill patients. J Palliat Med. 2011;14:389. doi: 10.1089/jpm.2010.0499. [DOI] [PubMed] [Google Scholar]
- 7.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of Ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 8.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 9.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous Ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–6. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, et al. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakurta RG, Ray P, Kanji D, Das R, Bisui B, Singh OP. Rapid Antidepressant Response with Ketamine: Is it the Solution to Resistant Depression? Indian J Psychol Med. 2012;34:56–60. doi: 10.4103/0253-7176.96161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med. 2012;15:400–3. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- 13.Harihar C, Dasari P, Srinivas JS. Intramuscular ketamine in acute depression: A report on two cases. Indian J Psychiatry. 2013;55:186–8. doi: 10.4103/0019-5545.111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13:903–8. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Attention deficit hyperactivity disorder and sleep. Insomnia and other sleep problems may worsen symptoms of ADHD; treatment options exist. Harv Ment Health Lett. 2010;27:6–7. [PubMed] [Google Scholar]
- 17.Sanacora G. Ketamine-Induced Optimism: New Hope for the Development of Rapid-Acting Antidepressants. Psychiatric Times. 2012 Jul;29:1–2. [Google Scholar]
- 18.Murrough JW, Charney DS. Cracking the moody brain: Lifting the mood with ketamine. Nat Med. 2010;16:1384–5. doi: 10.1038/nm1210-1384. [DOI] [PubMed] [Google Scholar]
- 19.Roca CP, Lozano S, Arenas A, Sánchez A. Topological traps control flow on real networks: The case of coordination failures. PLoS One. 2010;5:e15210. doi: 10.1371/journal.pone.0015210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, et al. Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic Clin Pharmacol Toxicol. 2008;103:502–6. doi: 10.1111/j.1742-7843.2008.00210.x. [DOI] [PubMed] [Google Scholar]
- 21.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–6. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]


