Abstract
Presbyopia is a physiologic inevitability that causes gradual loss of accommodation during the fifth decade of life. The correction of presbyopia and the restoration of accommodation are considered the final frontier of refractive surgery. Different approaches on the cornea, the crystalline lens and the sclera are being pursued to achieve surgical correction of this disability. There are however, a number of limitations and considerations that have prevented widespread acceptance of surgical correction for presbyopia. The quality of vision, optical and visual distortions, regression of effect, complications such as corneal ectasia and haze, anisometropia after monovision correction, impaired distance vision and the invasive nature of the currently techniques have limited the utilization of presbyopia surgery. The purpose of this paper is to provide an update of current procedures available for presbyopia correction and their limitations.
Keywords: Presbyopia, Surgical Correction, Treatment
INTRODUCTION
Presbyopia is a physiologic inevitability that causes the gradual loss of accommodation. The accommodative ability of the eye starts to decline usually after the age of 40-45 years. In 2005, the estimated global impact of presbyopia was 1.04 billion people, with over the half of not having adequate near-vision correction, and 410 million people listed as visually impaired (94% in developing countries).1 The quality of life is affected by presbyopia, that is associated with substantial negative effects in the US population.2
The pathophysiology of presbyopia still remains poorly understood. According to a theory proposed by Helmholtz, accommodation occurs as a result of the elastic properties of the lens and possibly the vitreous that allows the lens to expand and increase its power when zonular tension is relieved during ciliary muscle contraction.3 As the lens changes with age, the ability to expand and increase refractive power is lost. Helmholtz's theory of sclerosis of the crystalline lens as the cause of presbyopia has been challenged in 1992 by Schachar.4 Schachar suggests that the longitudinal muscle fibers of the ciliary muscle contract during accommodation, placing more tension on the equatorial zonules, while relaxing the anterior and posterior zonules.4 This force distribution causes an increase in the equatorial diameter of the lens, decreasing the peripheral volume while increasing the central volume. As the central volume increases, so does the power of the lens. Under this theory, presbyopia occurs because of the increasing equatorial diameter of the aging lens. Once the lens diameter reaches a critical size, usually during the fifth decade of life, the resting tension on the zonules is significantly reduced.5
Non-invasive methods of correcting presbyopia have been used for many years. While bifocal or multifocal progressive addition lenses, monofocal or bifocal contact lenses can provide satisfactory distance and near vision to presbyopes without the potential risks of a surgical procedure, they cannot restore or substitute the true process of accommodation of a younger individual. Several different methods have been used to correct presbyopia and restore the accommodation. However successful, repeatable correction still remains a challenge. These procedures are applied on the cornea, the crystalline lens or in the sclera. The purpose of this paper is to review the currently available procedures for the surgical correction of presbyopia and to analyze their advantages and disadvantages.
Current surgical procedures to treat presbyopia
In the last decades there have been several attempts to correct presbyopia in order to eliminate the dependence on reading glasses. The number of different techniques and the variety of approaches arises from the partial effectiveness of most methods to restore true accommodation. The anatomical site of the procedure differs, depending on the method utilized [Table 1]. Presbyopia correction can be achieved with Excimer or Femtosecond laser ablation on cornea (LASIK, PRK, Presbylasik, Supracor, Intracor, etc.), unilaterally as a monovision procedure or bilaterally. Another recent corneal approach is the insertion of inlays.6 Conductive keratoplasty was popular procedure for a brief period of time, however, it has seen limited use due to high regression rate in comparison to other techniques.7,8 Lens extraction with implantation of multifocal, monofocal (monovision) or accommodative intraocular lenses is another method for correction of presbyopia.9,10 Anterior ciliary sclerotomy with different expansion plug or band implantation has been proposed to correct presbyopia.
Table 1.
Current surgical treatment of presbyopia
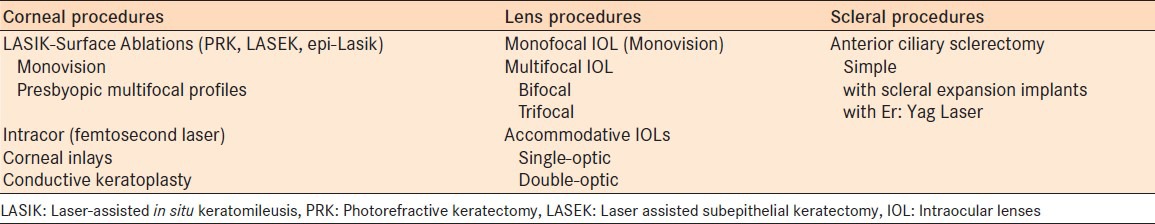
Corneal procedures
Monovision LASIK and surface ablations (PRK, LASEK, Epi-lasik)
Monovision has been used to compensate for presbyopia by optically correcting one eye for distance vision and the other eye for near vision.11 This strategy induces anisometropia with a consequent reduction in binocular acuity and stereopsis.12 Success rates for monovision refractive laser correction has been reported to be high (72 to 92%).12,13,14,15 Factors related to better outcomes are associated with anisometropia of less than 2.50 diopters (D), good distance correction of the dominant eye, stereoacuity reduction of less than 50’ of arc, distance esophoric shift of less than 0.6 prism diopters and highly motivated patient who can adapt to monovision.14,16,17,18
The amount of target refraction in monovision remains controversial. Some authors suggest correcting up to –2.50 D, whereas others suggest not exceeding –2.00 D.19,20 Patient age, occupation, needs and lifestyle plays a role in the decision of the amount of target correction in monovision. Patient selection is very important for the outcomes and high patient satisfaction of any monovision procedure. The surgeon has to spend sufficient time to explain and discuss the advantages and disadvantages of monovision and to be able to understand if the patient is a good candidate for this procedure, preoperatively. The patient has to be informed that the refractive status of the eye may change in the near future, due to changes in the lens and has to be aware that the effects of a corneal refractive procedure are not permanent. A complete ophthalmologic examination is important to exempt eyes with corneal or other abnormalities. As in all refractive surgery procedures, complications may occur. LASIK and surface ablation procedures are reasonably safe, effective and predictable in the treatment of presbyopic patients.21,22
Presbyopic excimer laser ablation
Attempts to create multifocal profiles to correct presbyopia has been made since 1992 using Excimer laser.23 The introduction of LASIK in refractive surgery offered a more effective and controllable technique for the creation of multifocal, bifocal or other profiles.24 In central presbyopic laser profiles, the near distance is corrected with the central zone which is hyperpositive, whereas the peripheral zone of ablation is preserved for far vision.25 Alio et al. reported reduced contrast sensitivity at higher spatial frequencies, night halos and loss of two lines of distance best spectacle corrected visual acuity (BSCVA) with this technique.25 Other authors proposed a multifocal ablation profile using the peripheral zone for near vision.26,27,28,29 Telandro reported that binocular unaided near visual acuity was J3 or better in all eyes and J1 or better in 35 and 41% eyes, of the hyperopic and myopic group, respectively.26 Uy and Go concluded that the induced spherical aberration improves the depth of focus in patients with presbyopia.28 Danasoury et al. reported that 54% of hyperopes and 48% of myopes were satisfied or very satisfied with their postoperative near UCVA.29 An aspheric ablation profile to improve near vision in presbyopic patients with hyperopia was reported recently by Jackson et al. According to authors, contrast sensitivity reduction was clinically insignificant and negative spherical aberration was highly correlated with postoperative improvement of distance corrected near visual acuity (DCNVA).30 Other profiles were suggested by Vinciguerra et al. that involved the use of a mask consisting of a mobile diaphragm formed by two blunt blades to ablate a 10 to 17 micron deep semilunar-shaped zone immediately below the pupillary center, steepening the corneal curvature in that area in order to create bifocality.31 Alio et al. reviewed three different approaches that have been used for corneal multifocality: transitional multifocality, central presbyLasik (center for near) and peripheral presbyLasik (peripheral cornea for near).32 The authors concluded that although central presbyLasik creates a bifocal cornea, the other techniques increase the depth of focus based on the ablation of the peripheral cornea. Transitional multifocality creates intentionally an increase in coma aberration. Based on the published results, both central and peripheral presbyLasik resulted in adequate spectacle independence simultaneously for far and for near. A neuroadaptation process is necessary for peripheral presbyopic LASIK. Transitional techniques have a very limited use and very few outcomes were reported. Alarcon et al. evaluated two multifocal corneal models and an aspheric model designed to correct presbyopia by corneal photoablation.33 The design of each model was optimized to achieve the best visual quality possible for both near and distance vision.33 Additionally, they evaluated the effect of miosis and pupil decentration on visual quality.33 According to the authors, the corrected model with the central zone for near vision provides better results because it requires less ablated corneal surface area, permits higher addition values, presents more stable visual quality with pupil-size variations and lower high-order aberrations.33 Very recently, a new proprietary ablation pattern (Supracor, Bausch and Lomb/Technolas, Munich, Germany) was applied using a profile that steepens the center of the cornea to create hyperprolate shape resulting about 2 D near addition with controlled higher order aberrations (HOA).34 Ninety-six percent of the patients were satisfied with this procedure at 6 months.34
Different presbyopic laser ablation profiles currently offer an alternative treatment for presbyopia. Many of these techniques are still under evaluation today. Long-term scientific evidence is necessary to assess their role toward spectacle or contact lens independence for near.
Presbyopic femtosecond laser ablation (Intracor)
The utilization of femtosecond laser technology in ophthalmology, introduced new techniques in the field of refractive surgery. Femtosecond laser pulses applied in a concentric ring fashion inside the corneal stroma were able to induce changes in the corneal shape without cutting a flap. Ruiz et al. performed and published for the first time the Intracor procedure using a Technolas Femtosecond Laser (Bausch and Lomb Technolas, Munich, Germany).35 In this proprietary procedure, the pattern of laser delivery is entirely intrastromal, without impacting either the endothelium, Descemet's membrane, Bowman's layer, or epithelium at any point throughout the operation, creating a central steepening of the anterior corneal surface. Such a procedure has several potential advantages: No epithelial disruption, no pain and inflammation related to the absence of epithelium and quick recovery. Early results of this procedure yielded a significant and stable gain of uncorrected near visual acuity (UNVA) and corneal steepening, without a significant loss of endothelial cells or corneal thinning up to 18 months postoperatively. No significant regression of visual acuity or further corneal steepening occurred during the follow-up period.36,37 Intracor has also some disadvantages: It can lead to a reduction of mesopic contrast sensitivity and an increase of glare sensitivity according to the study conducted by Fitting et al.38 The authors suggested that possible consequences on night driving ability should be discussed with the patients prior to treatment. Very recently, a case with keratectasia after intracor combined with Supracor LASIK enhancement was reported in an eye without risk factors for keratectasia.39 This paper raised concerns on the mechanical stability of the cornea after the Intracor procedure, if combined with other corneal refractive surgery. Further studies with larger number of eyes are required to assess the safety, efficacy and long-term stability of this new procedure.
Conductive keratoplasty
Conductive keratoplasty (CK) is a noninvasive, in-office procedure for the correction of hyperopia, hyperopic astigmatism, and management of presbyopia. It is based on radiofrequency energy delivered through a fine needle tip that is inserted into the peripheral corneal stroma in a ring pattern. A series of spots (8-32) are placed in up to three rings of 6-, 7-, 8-mm optical zones in the corneal periphery. The shrinkage of collagen between the spots creates a band of tightening, which results in steepening of the central cornea. The United States Food and Drug Administration approved this procedure about a decade ago for the temporary correction of mild to moderate spherical hyperopia in people over 40 years old.40 It is applied as a monovision procedure in the non-dominant eye of presbyopic individuals. The advantages of CK include that it is a minimally invasive, in-office and relatively cost-effective procedure. However, it has significant contraindications in eyes with corneal disease and dry eye syndrome. Various studies evaluated the safety and efficacy of CK.41,42 Although satisfactory NUCVA was reported initially, significant regression of refractive and keratometric effects of CK has been observed over short-, mid- and long-term follow up period,7,8,43,44 limiting the usage of this procedure.
Corneal inlays
Intracorneal implantation of a lens is not a novel idea. Jose Barraquer developed and experimented with the first prototype in 1949.45 He soon abandoned this idea because of the corneal tissue's aggressive response to the flint glass material. The discovery of hydrogel and other more biocompatible materials revived the concept of corneal inlays, two decades later. The new materials were transparent and permeable to nutrients and promised to be well-tolerated by the corneal tissue. They had, however, many complications: Thinning and melting of the overlying stroma, corneal opacification, decentration and haze that led to explantation of many of these devices.46,47,48,49
Keates et al (1995) reported their results of using the small-diameter corneal inlay to create a bifocal cornea to correct presbyopia.50 According to the authors, uncorrected near vision had improved from J4 to better than J2 in four of the five eyes implanted at 12 months postoperatively. The newest generation of corneal inlays is made of materials with enhanced biocompatibility.51,52 Advances in technology, such as femtosecond lasers, facilitate the easier, more reliable creation of stromal pockets, offer better estimation of implantation depth and improve centration of corneal inlays.
Currently, there are three different designs of presbyopic corneal inlays
Small aperture inlay (Kamra, Acufocus Inc, Irvine, CA, USA) that increases the depth of field using the pin-hole effect to restore near and intermediate visual acuity without significantly affecting distance vision. The AcuFocus Kamra corneal inlay is a 5μ-microperforated artificial aperture (3.8 mm outer diameter; 1.6 mm inner diameter) made of polyvinylidene fluoride, a material reported to be highly biocompatible in vitro.53 It is implanted unilaterally in the non-dominant eye. The inlay received the Conformité Européenne (CE) mark for use in the European Union in 2005. The initial reports show an improvement in all tested reading performance parameters in emmetropic presbyopic patients, as the result of an increased depth of field.53,54 The Kamra inlay implantation can be combined with LASIK improving near vision with a minimal effect on distance vision, resulting in high patient satisfaction and less dependence on reading glasses according to a recent paper by Tomita et al.55
Space-occupying inlays that create a hyperprolate cornea (Raindrop, Revision Optics, Lake Forest, CA, USA). The Raindrop Near Vision inlay is made of hydrogel, is 32 μ thick and has a diameter of 2 mm. In the first published paper in a peer-reviewed journal, Garza et al. concluded that the hydrogel corneal inlay improved uncorrected near and intermediate visual acuity in 20 patients with emmetropic presbyopia, with high patient satisfaction and little effect on distance visual acuity at 1 year postoperatively.56 One dissatisfied patient requested the explantation of the inlay.56 Although the Raindrop Near Vision Inlay was implanted unilaterally in this study,56 it can be implanted in bilaterally.
Refractive annular addition lenticules that work as bifocal optical inlays separating distance and near focal points (Flexivue Microlens, Presbia, Irvine, CA, USA). The Presbia Flexivue Microlens is made of a hydrophilic polymer, has a diameter of 3 mm and its edge thickness is approximately 15 μm57 [Figure 1]. The central 1.6 mm zone of the inlay is optically neutral. The Flexivue Microlens has a 0.5 mm hole in the center for allowing adequate nutritional flow in the cornea. Limnopoulou et al. reported uncorrected near visual acuity of 20/32 or better in 75% of operated eyes, whereas mean uncorrected distance visual acuity (UDVA) decreased statistically significantly from 0.06 logMAR (20/20) preoperatively to 0.38 logMAR (20/50) postoperatively.58 Mean binocular UDVA was not significantly altered.58 Overall, higher order aberrations increased and contrast sensitivity decreased in the operated eye.58 No tissue alterations were found using corneal confocal microscopy.58
Figure 1.
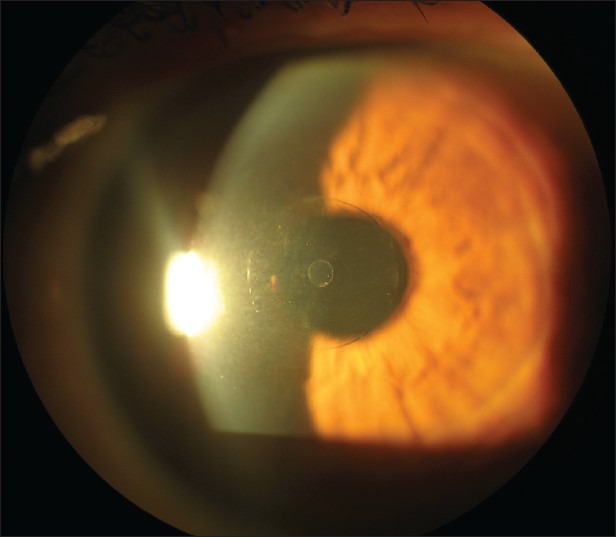
Flexivue Corneal Inlay, (photo courtesy of Prof. I. Pallikaris, University of Crete, Greece)
A great advantage of the corneal inlays is their potential reversibility. Although the initial papers show encouraging results, further studies and longer follow up are needed for the clinical assessment of the inlays, which are considered investigational devices and are currently not available in the USA.
Lens procedures
In the last decades cataract surgery has evolved to a refractive procedure, in which the ophthalmic surgeon attempts to correct all or most of the refractive errors of the patient, including presbyopia, in a single operation. The growing interest for the treatment of presbyopia among individuals without cataract has led surgeons to perform operations that correct reading disability by exchanging the crystalline lens with intraocular lenses (IOL) designed for this purpose. Currently, there are different approaches in IOL optic design to compensate for the loss of accommodation. One approach is to provide the visual system with two simultaneous images, either monocularly using multifocal IOLs or binocularly through monovision.59 In monovision, one eye is optimized for distance vision and the other eye for near, as described in the corneal procedures. Another option is to utilize accommodative IOLs that use the ciliary muscle contraction to change the refractive state of the eye by shifting the IOL position.60
The multifocality of the latest generation IOLs is based on refractive and diffractive technology. Excellent clinical outcomes have been reported with different IOLs.61,62,63 Patient selection is very crucial in order to avoid patient dissatisfaction and secondary procedures for IOL exchange. Until recently, most multifocal IOLs could provide satisfactory vision for far and either near or intermediate distance [Figure 2]. They were actually bifocal lenses. The most recent multifocal IOLs with improved optics have enhanced intermediate distance, giving the patient a full range of vision.64,65 Multifocal IOLs reduce contrast sensitivity and cause more glare and halos in comparison to monofocal IOLs.66 In some cases, these optical phenomena can be disturbing and a secondary intervention and IOL explantation might be required.67 A study by Mamalis et al. on IOLs requiring explantation, the second most frequently explanted IOL was the multifocal hydrophobic acrylic IOL (23%). The most common reason for explantation or secondary intervention was glare/optical aberrations (68%), followed by incorrect IOL power (21%). Precise biometry for IOL calculation and correction of astigmatism is crucial for a good outcome after multifocal IOL implantation. Corneal astigmatism reduced through-focus image quality and depth of focus with all IOLs evaluated in a study by Zheleznyak et al.68 However, the multifocal IOLs had the most severe decline in depth of focus. In eyes with astigmatism of 0.75 D and above, a toric multifocal IOL provides better quality of vision in all distances. In cases where a multifocal IOL is not indicated, monovision can been used to provide near, intermediate, and distance vision and is one of the most common methods used in cataract patients to correct presbyopia.59
Figure 2.
Diffractive Apodized Multifocal IOL
Current accommodative IOL designs have either single or double lens systems that are based on the “focus shift” principle. Theoretically, the contraction of the ciliary muscle moves the optic anteriorly, thereby increasing the dioptric power of the eye. Menapace et al. found that axial shift and thus true accommodative effect was small or even absent, and also very variable, making an individual prediction impracticable.60 In a study by Klaproth et al., comparing different accommodative IOLs based on the principle of using ciliary muscle contraction for moving the IOL or changing its thickness and/or surface radii during accommodation in order to change the ocular refractive power, the authors concluded that a proof of principle of such lenses under physiological, non-pharmacologically stimulated conditions is still lacking.69 In a recent paper by Zamora-Alejo et al., no significant signs of accommodation were found with a single-optic accommodative IOL.70 The accommodative IOL showed some benefit for intermediate visual function compared to monofocal IOLs with both groups wearing full correction for distance. Alio et al. compared the visual and ocular optical performance in eyes with a single-optic or a dual-optic accommodating intraocular lens.71 They found that eyes with the dual-optic IOL had significantly better ocular optical quality. Further studies are necessary to provide evidence of the efficacy of single- or double-optic accommodative lenses.
Scleral procedures
Anterior ciliary sclerotomy is based on Schachar's theory4 and involves making radial incisions in the sclera overlying the ciliary muscle. According to this theory, radial sclerotomies allow expansion of the sclera overlying the ciliary body, increasing the space between the lens equator and the ciliary body.72 This may place more resting tension on the equatorial zonules, allowing for increased tension to develop during ciliary muscle contraction. The procedure is hypothesized to restore accommodative amplitude in presbyopic subjects. In 2002, in a prospective controlled study of 9 eyes, Hamilton et al. reported that anterior ciliary sclerotomy does not restore accommodation in presbyopic eyes and can cause significant complications such as perforation of the anterior chamber and mild postoperative anterior segment ischemia manifested by sectoral iris akinesis.72 Fukasaku and Marron suggested the placement of silicone plugs in the incisions to prevent scleral healing, yielding a mean accommodative amplitude gain of 1.5 D at 12 months.73 T-shaped collagen implants were used in a study by Malyougin et al. to enhance the outcome, but the authors reported that the effect was temporary and diminished with time.74 Another report by Ito et al. raised concerns on ocular integrity after Er: Yag laser scleral incisions.75 Recently, a new type of scleral expansion implant, the Presview (PSI, Refocus-Group, Dallas, Texas, USA) is being evaluated as a treatment for presbyopia, in an FDA monitored investigational device exemption (IDE) clinical trial currently underway in the USA76. During the initial phase of the study, it was found that the implants also appeared to have an IOP lowering effect on the normotensive presbyopic emmetropes, in whom they were implanted. Although not clinically significant in this group of patients without glaucoma, a statistically significant reduction in IOP of approximately 20% was found in patients with baseline IOP of higher than 16 mmHg. Anterior ciliary sclerotomy or any other scleral surgical technique has not been shown to be an effective treatment for the correction of presbyopia. Better controlled studies are needed for the evaluation and the possibility of utilization of this technique in the future, based on scientific evidence.
CONCLUSION
At present, the ophthalmic surgeon has several options for the correction of presbyopia in individuals who wish to decrease their dependence on reading glasses. Improvements in technology have advanced surgical options, offering a variety of approaches. A unique and ideal solution is not yet available. Among the procedures described in this article, monovision (LASIK or pseudophakic) and multifocal IOL insertion are the most widely used methods. Patient selection is very important for a good outcome. Age, occupation, lifestyle, the neuroadaptive ability of the patient, and the condition of the eye are important issues for the selection of the most appropriate surgical procedure. The surgeon should decide which option is the best treatment for each patient. During preoperative assessment, it is imperative to advise the patients of realistic expectations after a refractive procedure. They should be well informed of the limitations and the compromises in the quality of vision after surgery. The restoration of accommodation, which is considered the final frontier in refractive surgery, still remains a challenge.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Holden BA, Fricke TR, Ho SM, Wong R, Schlenther G, Cronjé S, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol. 2008;126:1731–9. doi: 10.1001/archopht.126.12.1731. [DOI] [PubMed] [Google Scholar]
- 2.McDonnell PJ, Lee P, Spritzer K, Lindblad AS, Hays RD. Associations of presbyopia with vision-targeted health-related quality of life. Arch Ophthalmol. 2003;121:1577–81. doi: 10.1001/archopht.121.11.1577. [DOI] [PubMed] [Google Scholar]
- 3.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–72. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 4.Schachar RA. Cause and treatment of presbyopia with a method for increasing the amplitude of accommodation. Ann Ophthalmol. 1992;24:445–7. 452. [PubMed] [Google Scholar]
- 5.Toricelli A, Junior JB, Santiago M, Bechara S. Surgical Management of Presbyopia. Clin Ophthalmol. 2012;6:1459–66. doi: 10.2147/OPTH.S35533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seyeddain O, Hohensinn M, Riha W, Nix G, Rückl T, Grabner G, et al. Small-aperture corneal inlay for the correction of presbyopia: 3-year follow-up. J Cataract Refract Surg. 2011;38:35–45. doi: 10.1016/j.jcrs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Ayoubi MG, Leccisotti A, Goodall EA, McGilligan VE, Moore TC. Femtosecond laser in situ keratomileusis versus conductive keratoplasty to obtain monovision in patients with emmetropic presbyopia. J Cataract Refract Surg. 2010;36:997–1002. doi: 10.1016/j.jcrs.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Moshirfar M, Anderson E, Hsu M, Armenia JM, Mifflin MD. Comparing the rate of regression after conductive keratoplasty with or without prior laser-assisted in situ keratomileusis or photorefractive keratectomy. Middle East Afr J Ophthalmol. 2012;19:377–81. doi: 10.4103/0974-9233.102743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobi PC, Dietlein TS, Lüke C, Jacobi FK. Multifocal intraocular lens implantation in prepresbyopic patients with unilateral cataract. Ophthalmology. 2002;109:680–6. doi: 10.1016/s0161-6420(01)01029-6. [DOI] [PubMed] [Google Scholar]
- 10.Bellucci R. Multifocal intraocular lenses. Curr Opin Ophthalmol. 2005;16:33–7. doi: 10.1097/00055735-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Farid M, Steinert RF. Patient selection for monovision laser refractive surgery. Curr Opin Ophthalmol. 2009;20:251–4. doi: 10.1097/ICU.0b013e32832a0cdb. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Arora I, Azar DT. Success of monovision in presbyopes: Review of the literature and potential applications to refractive surgery. Surv Ophthalmol. 1996;40:491–9. doi: 10.1016/s0039-6257(96)82015-7. [DOI] [PubMed] [Google Scholar]
- 13.Wright KW, Guemes A, Kapadia MS, Wilson SE. Binocular function and patient satisfaction after monovision induced by myopic photorefractive keratectomy. J Cataract Refract Surg. 1999;25:177–82. doi: 10.1016/s0886-3350(99)80123-0. [DOI] [PubMed] [Google Scholar]
- 14.Reilly CD, Lee WB, Alvarenga L, Caspar J, Garcia-Ferrer F, Mannis MJ. Surgical monovision and monovision reversal in LASIK. Cornea. 2006;25:136–8. doi: 10.1097/01.ico.0000178722.19317.7b. [DOI] [PubMed] [Google Scholar]
- 15.Braun EH, Lee J, Steinert RF. Monovision in LASIK. Ophthalmology. 2008;115:1196–202. doi: 10.1016/j.ophtha.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Westin E, Wick B, Harrist RB. Factors influencing success of monovision contact lens fitting: Survey of contact lens diplomates. Optometry. 2000;71:757–63. [PubMed] [Google Scholar]
- 17.Sippel KC, Jain S, Azar DT. Monovision achieved with excimer laser refractive surgery. Int Ophthalmol Clin. 2001;41:91–101. doi: 10.1097/00004397-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg DB. Comparison of myopes and hyperopes after laser in situ keratomileusis monovision. J Cataract Refract Surg. 2003;29:1695–701. doi: 10.1016/s0886-3350(03)00462-0. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg DB. Laser in situ keratomileusis monovision. J Cataract Refract Surg. 2001;27:1449–55. doi: 10.1016/s0886-3350(01)01001-x. [DOI] [PubMed] [Google Scholar]
- 20.Cox CA, Krueger RR. Monovision with laser vision correction. Ophthalmol Clin North Am. 2006;19:71–5. doi: 10.1016/j.ohc.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Jain S, Ou R, Azar DT. Monovision outcomes in presbyopic individuals after refractive surgery. Ophthalmology. 2001;108:1430–3. doi: 10.1016/s0161-6420(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 22.Ghanem RC, de la Cruz J, Tobaigy FM, Ang LP, Azar DT. LASIK in the presbyopic age group: Safety, efficacy, and predictability in 40- to 69-year-old patients. Ophthalmology. 2007;114:1303–10. doi: 10.1016/j.ophtha.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Moreira H, Garbus JJ, Fasano A, Lee M, Clapham TN, McDonnell PJ. Multifocal corneal topographic changes with excimer laser photorefractive keratectomy. Arch Ophthalmol. 1992;110:994–9. doi: 10.1001/archopht.1992.01080190100036. [DOI] [PubMed] [Google Scholar]
- 24.Bauerberg JM. Centered vs. inferior off-center ablation to correct hyperopia and presbyopia. J Refract Surg. 1999;15:66–9. doi: 10.3928/1081-597X-19990101-13. [DOI] [PubMed] [Google Scholar]
- 25.Alió JL, Chaubard JJ, Caliz A, Sala E, Patel S. Correction of presbyopia by technovision central multifocal LASIK (presbyLASIK) J Refract Surg. 2006;22:453–60. doi: 10.3928/1081-597X-20060501-06. [DOI] [PubMed] [Google Scholar]
- 26.Telandro A. Pseudo-accommodative cornea: A new concept for correction of presbyopia. J Refract Surg. 2004;20(Suppl 5):S714–7. doi: 10.3928/1081-597X-20040903-17. [DOI] [PubMed] [Google Scholar]
- 27.Pinelli R, Ortiz D, Simonetto A, Bacchi C, Sala E, Alió JL. Correction of presbyopia in hyperopia with a center-distance, paracentral-near technique using the Technolas 217z platform. J Refract Surg. 2008;24:494–500. doi: 10.3928/1081597X-20080501-07. [DOI] [PubMed] [Google Scholar]
- 28.Uy E, Go R. Pseudoaccommodative cornea treatment using the NIDEK EC-5000 CXIII excimer laser in myopic and hyperopic presbyopes. J Refract Surg. 2009;25(Suppl 1):S148–55. doi: 10.3928/1081597X-20090115-13. [DOI] [PubMed] [Google Scholar]
- 29.El Danasoury AM, Gamaly TO, Hantera M. Multizone LASIK with peripheral near zone for correction of presbyopia in myopic and hyperopic eyes: 1-year results. J Refract Surg. 2009;25:296–305. doi: 10.3928/1081597X-20090301-10. [DOI] [PubMed] [Google Scholar]
- 30.Jackson WB, Tuan KM, Mintsioulis G. Aspheric wavefront-guided LASIK to treat hyperopic presbyopia: 12-month results with the VISX platform. J Refract Surg. 2011;27:519–29. doi: 10.3928/1081597X-20101110-02. [DOI] [PubMed] [Google Scholar]
- 31.Vinciguerra P, Nizzola GM, Bailo G, Nizzola F, Ascari A, Epstein D. Excimer laser photorefractive keratectomy for presbyopia: 24-month follow-up in three eyes. J Refract Surg. 1998;14:31–7. doi: 10.3928/1081-597X-19980101-08. [DOI] [PubMed] [Google Scholar]
- 32.Alió JL, Amparo F, Ortiz D, Moreno L. Corneal multifocality with excimer laser for presbyopia correction. Curr Opin Ophthalmol. 2009;20:264–71. doi: 10.1097/icu.0b013e32832a7ded. [DOI] [PubMed] [Google Scholar]
- 33.Ryan A, O’Keefe M. Corneal approach to hyperopic presbyopia treatment: Six-month outcomes of a new multifocal excimer laser in situ keratomileusis procedure. J Cataract Refract Surg. 2013;39:1226–33. doi: 10.1016/j.jcrs.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Alarcón A, Anera RG, del Barco LJ, Jiménez JR. Designing multifocal corneal models to correct presbyopia by laser ablation. J Biomed Opt. 2012;17:018001. doi: 10.1117/1.JBO.17.1.018001. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz LA, Cepeda LM, Fuentes VC. Intrastromal correction of presbyopia using a femtosecond laser system. J Refract Surg. 2009;25(10):847–854. doi: 10.3928/1081597X-20090917-05. [DOI] [PubMed] [Google Scholar]
- 36.Holzer MP, Mannsfeld A, Ehmer A, Auffarth GU. Early outcomes of INTRACOR femtosecond laser treatment for presbyopia. J Refract Surg. 2009;25:855–61. doi: 10.3928/1081597X-20090917-06. [DOI] [PubMed] [Google Scholar]
- 37.Menassa N, Fitting A, Auffarth GU, Holzer MP. Visual outcomes and corneal changes after intrastromal femtosecond laser correction of presbyopia. J Cataract Refract Surg. 2012;38:765–73. doi: 10.1016/j.jcrs.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 38.Fitting A, Menassa N, Auffarth GU, Holzer MP. Effect of intrastromal correction of presbyopia with femtosecond laser (INTRACOR) on mesopic contrast sensitivity. Ophthalmologe. 2012;109:1001–7. doi: 10.1007/s00347-012-2624-x. [DOI] [PubMed] [Google Scholar]
- 39.Taneri S, Oehler S. Keratectasia after treating presbyopia with INTRACOR followed by SUPRACOR enhancement. J Refract Surg. 2013;29:573–6. doi: 10.3928/1081597X-20130620-02. [DOI] [PubMed] [Google Scholar]
- 40.McDonald MB, Hersh PS, Manche EE, Maloney RK, Davidorf J, Sabry M. Conductive keratoplasty for the correction of low to moderate hyperopia: US clinical trial 1-year results on 355 eyes. Ophthalmology. 2002;109:1978–89. doi: 10.1016/s0161-6420(02)01255-1. [DOI] [PubMed] [Google Scholar]
- 41.Rojas MC, Manche EE. Comparison of videokeratographic functional optical zones in conductive keratoplasty and laser in situ keratomileusis for hyperopia. J Refract Surg. 2003;19:333–7. doi: 10.3928/1081-597X-20030501-10. [DOI] [PubMed] [Google Scholar]
- 42.McDonald MB, Durrie D, Asbell P, Maloney R, Nichamin L. Treatment of presbyopia with conductive keratoplasty: Six-month results of the 1-year United States FDA clinical trial. Cornea. 2004;23:661–8. doi: 10.1097/01.ico.0000126321.13143.a0. [DOI] [PubMed] [Google Scholar]
- 43.Esquenazi S, He J, Kim DB, Bazan NG, Bui V, Bazan HE. Wound-healing response and refractive regression after conductive keratoplasty. J Cataract Refract Surg. 2006;32:480–6. doi: 10.1016/j.jcrs.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 44.Ehrlich JS, Manche EE. Regression of effect over long-term follow-up of conductive keratoplasty to correct mild to moderate hyperopia. J Cataract Refract Surg. 2009;35:1591–6. doi: 10.1016/j.jcrs.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Barraquer JI. Queratoplatica refractiva. Estudios e informaciones Oftalmologicas. 1949;2:10. [Google Scholar]
- 46.Choyce P. The present status of intracameral and intracorneal implants. Can J Ophthalmol. 1968;3:295–311. [PubMed] [Google Scholar]
- 47.Lane SL, Lindstrom RL, Cameron JD, Thomas RH, Mindrup EA, Waring GO, 3rd, et al. Polysulfone corneal lenses. J Cataract Refract Surg. 1986;12:50–60. doi: 10.1016/s0886-3350(86)80057-8. [DOI] [PubMed] [Google Scholar]
- 48.Klyce SD, Dingeldein S, Bonanno J, McDonald M, Kaufman H. Hydrogel implants: evaluation of first human trial. Invest Ophthalmol Vis Sci Suppl. 1988;29:393. [Google Scholar]
- 49.Keates RH, Martines E, Tennen DG, Reich C. Small-diameter corneal inlay in presbyopic or pseudophakic patients. J Cataract Refract Surg. 1995;21:519–21. doi: 10.1016/s0886-3350(13)80209-x. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney DF, Vannas A, Hughes TC, Evans MD, McLean KM, Xie RZ, et al. Synthetic corneal inlays. Clin Exp Optom. 2008;91:56–66. doi: 10.1111/j.1444-0938.2007.00198.x. [DOI] [PubMed] [Google Scholar]
- 51.Laroche G, Marois Y, Guidoin R, King MW, Martin L, How T, et al. Polyvinylidene fluoride (PVDF) as a biomaterial: From polymeric raw material to monofilament vascular suture. J Biomed Mater Res. 1995;29:1525–36. doi: 10.1002/jbm.820291209. [DOI] [PubMed] [Google Scholar]
- 52.Waring GO, 4th, Klyce SD. Corneal inlays for the treatment of presbyopia. Int Ophthalmol Clin. 2011;51:51–62. doi: 10.1097/IIO.0b013e31820f2071. [DOI] [PubMed] [Google Scholar]
- 53.Yilmaz OF, Bayraktar S, Agca A, Yilmaz B, McDonald MB, van de Pol C. Intracorneal inlay for the surgical correction of presbyopia. J Cataract Refract Surg. 2008;34:1921–7. doi: 10.1016/j.jcrs.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 54.Dexl AK, Seyeddain O, Riha W, Hohensinn M, Hitzl W, Grabner G. Reading performance after implantation of a small-aperture corneal inlay for the surgical correction of presbyopia: Two-year follow-up. J Cataract Refract Surg. 2011;37:525–31. doi: 10.1016/j.jcrs.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 55.Tomita M, Kanamori T, Waring GO, 4th, Nakamura T, Yukawa S. Small-aperture corneal inlay implantation to treat presbyopia after laser in situ keratomileusis. J Cataract Refract Surg. 2013;39:898–905. doi: 10.1016/j.jcrs.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 56.Garza EB, Gomez S, Chayet A, Dishler J. One-year safety and efficacy results of a hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg. 2013;29:166–72. doi: 10.3928/1081597X-20130129-01. [DOI] [PubMed] [Google Scholar]
- 57.Bouzoukis DI, Kymionis GD, Limnopoulou AN, Kounis GA, Pallikaris IG. Femtosecond laser-assisted corneal pocket creation using a mask for inlay implantation. J Refract Surg. 2011;27:818–20. doi: 10.3928/1081597X-20110706-01. [DOI] [PubMed] [Google Scholar]
- 58.Limnopoulou AN, Bouzoukis DI, Kymionis GD, Panagopoulou SI, Plainis S, Pallikaris AI, et al. Visual outcomes and safety of a refractive corneal inlay for presbyopia using femtosecond laser. J Refract Surg. 2013;29:12–8. doi: 10.3928/1081597X-20121210-01. [DOI] [PubMed] [Google Scholar]
- 59.Zhang F, Sugar A, Jacobsen G, Collins M. Visual function and spectacle independence after cataract surgery: Bilateral diffractive multifocal intraocular lenses versus monovision pseudophakia. J Cataract Refract Surg. 2011;37:853–8. doi: 10.1016/j.jcrs.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 60.Menapace R, Findl O, Kriechbaum K, Leydolt-Koeppl C. Accommodating intraocular lenses: A critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol. 2007;245:473–89. doi: 10.1007/s00417-006-0391-6. [DOI] [PubMed] [Google Scholar]
- 61.Chiam PJ, Chan JH, Haider SI, Karia N, Kasaby H, Aggarwal RK. Functional vision with bilateral ReZoom and ReSTOR intraocular lenses 6 months after cataract surgery. J Cataract Refract Surg. 2007;33:2057–61. doi: 10.1016/j.jcrs.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 62.Cionni RJ, Chang DF, Donnenfeld ED, Lane SS, McCulley JP, Solomon KD. Clinical outcomes and functional visual performance: Comparison of the ReSTOR apodised diffractive intraocular lens to a monofocal control. Br J Ophthalmol. 2009;93:1215–9. doi: 10.1136/bjo.2008.146647. [DOI] [PubMed] [Google Scholar]
- 63.Santhiago MR, Wilson SE, Netto MV, Espíndola RF, Shah RA, Ghanem RC, et al. Visual performance of an apodized diffractive multifocal intraocular lens with+3.00-d addition: 1-year follow-up. J Refract Surg. 2011;27:899–906. doi: 10.3928/1081597X-20110816-01. [DOI] [PubMed] [Google Scholar]
- 64.Montés-Micó R, Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, Pons AM. In vitro optical quality differences between multifocal apodized diffractive intraocular lenses. J Cataract Refract Surg 013. 39:928–36. doi: 10.1016/j.jcrs.2012.12.038. [DOI] [PubMed] [Google Scholar]
- 65.Gatinel D, Houbrechts Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. J Cataract Refract Surg. 2013;39:1093–9. doi: 10.1016/j.jcrs.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 66.Gil MA, Varón C, Cardona G, Vega F, Buil JA. Comparison of far and near contrast sensitivity in patients symmetrically implanted with multifocal and monofocal IOLs. Eur J Ophthalmol. 2013;24:44–52. doi: 10.5301/ejo.5000335. [DOI] [PubMed] [Google Scholar]
- 67.Mamalis N, Brubaker J, Davis D, Espandar L, Werner L. Complications of foldable intraocular lenses requiring explantation or secondary intervention--2007 survey update. J Cataract Refract Surg. 2008;34:1584–91. doi: 10.1016/j.jcrs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 68.Zheleznyak L, Kim MJ, MacRae S, Yoon G. Impact of corneal aberrations on through-focus image quality of presbyopia-correcting intraocular lenses using an adaptive optics bench system. J Cataract Refract Surg. 2012;38:1724–33. doi: 10.1016/j.jcrs.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 69.Klaproth OK, Titke C, Baumeister M, Kohnen T. Accommodative intraocular lenses--principles of clinical evaluation and current Results. Klin Monbl Augenheilkd. 2011;228:666–75. doi: 10.1055/s-0031-1281673. [DOI] [PubMed] [Google Scholar]
- 70.Zamora-Alejo KV, Moore SP, Parker DG, Ullrich K, Esterman A, Goggin M. Objective accommodation measurement of the Crystalens HD compared to monofocal intraocular lenses. J Refract Surg. 2013;29:133–9. doi: 10.3928/1081597X-20130117-09. [DOI] [PubMed] [Google Scholar]
- 71.Alió JL, Plaza-Puche AB, Montalban R, Ortega P. Near visual outcomes with single-optic and dual-optic accommodating intraocular lenses. J Cataract Refract Surg. 2012;38:1568–75. doi: 10.1016/j.jcrs.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 72.Hamilton DR, Davidorf JM, Maloney RK. Anterior ciliary sclerotomy for treatment of presbyopia: A prospective controlled study. Ophthalmology. 2002;109:1970–6. doi: 10.1016/s0161-6420(02)01252-6. [DOI] [PubMed] [Google Scholar]
- 73.Fukasaku H, Marron JA. Anterior ciliary sclerotomy with silicone expansion plug implantation: effect on presbyopia and intraocular pressure. Int Ophthalmol Clin. 2001;41:133–41. doi: 10.1097/00004397-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 74.Malyugin B, Antonian S, Lohman BD. Anterior ciliary sclerotomy using collagen T-shaped implants for treatment of presbyopia. Ann Ophthalmol (Skokie) 2008;40:130–6. [PubMed] [Google Scholar]
- 75.Ito M, Asano-Kato N, Fukagawa K, Arai H, Toda I, Tsubota K. Ocular integrity after anterior ciliary sclerotomy and scleral ablation by the Er: YAG laser. J Refract Surg. 2005;21:77–81. doi: 10.3928/1081-597X-20050101-14. [DOI] [PubMed] [Google Scholar]
- 76.Soloway B, Rifkind A. Scleral spacing procedure and its indications in glaucoma treatment. Ophthalmol Times Eur [Internet] 2011. [Last cited 2011 Jun 1]. p. 7. Available from: http://www.oteurope.com/ophthalmologytimeseurope/ophthalmologytimeseurope/Glaucoma/Scleral-spacing-procedure-and-its-indications-in-g/ArticleStandard/Article/detail/728673 .


