Abstract
The rules of the genetic code are established by aminoacylations of transfer RNAs by aminoacyl tRNA synthetases. New codon assignments, and the introduction of new kinds of amino acids, are blocked by vigorous tRNA-dependent editing reactions occurring at hydrolytic sites embedded within specialized domains in the synthetases. For some synthetases, these domains were present at the time of the last common ancestor and were fixed in evolution through all three of the kingdoms of life. Significantly, a well characterized domain for editing found in bacterial and eukaryotic threonyl– and all alanyl–tRNA synthetases is missing from archaebacterial threonine enzymes. Here we show that the archaebacterial Methanosarcina mazei ThrRS efficiently misactivates serine, but does not fuse serine to tRNA. Consistent with this observation, the enzyme cleared serine that was linked to threonine-specific tRNAs. M. mazei and most other archaebacterial ThrRSs have a domain, N2A, fused to the N terminus and not found in bacterial or eukaryotic orthologs. Mutations at conserved residues in this domain led to an inability to clear threonine-specific tRNA mischarged with serine. Thus, these results demonstrate a domain for editing that is distinct from all others, is restricted to just one branch of the tree of life, and was most likely added to archaebacterial ThrRSs after the eukaryote/archaebacteria split.
Aminoacyl tRNA synthetases are divided into two classes of 10 enzymes each, based on the active-site architecture shared by all members of the same class (1–4). This structural classification is based on the design of the ATP-binding and amino acid activation domain, which also interacts with the 3′ end of tRNA. For both class I and class II enzymes, the class-defining domain is considered the ancient, historical tRNA synthetase and appeared at or before the time of the last common ancestor. Surrounding and permeating these central domains are insertions and N- or C-terminal fusions of additional motifs, many of which were added later in evolution. These motifs provide tRNA-binding determinants and, in some cases, functions extraneous to aminoacylation, such as transcriptional and translational control (5, 6), RNA splicing (7, 8), and cytokine signaling (9–13).
In addition to these motifs, many synthetases have acquired a second active site that is designed to clear errors of aminoacylation (14–19). Two widely distributed designs for these active sites for editing have been described, one for class I enzymes (17, 20–24) and another for class II enzymes (25–27). In many instances, such as class I isoleucyl–, leucyl–, and valyl–tRNA synthetases, the domain for editing is conserved through evolution and is believed to have been present at the time of the split of the tree of life into three branches (28). In contrast, the universally conserved (in all three kingdoms) domain for editing in the class II alanyl–tRNA synthetase, although present in bacterial and eukaryote threonyl–tRNA synthetases, is missing in archaebacterial threonyl–tRNA synthetases (Fig. 1A) (25, 27).
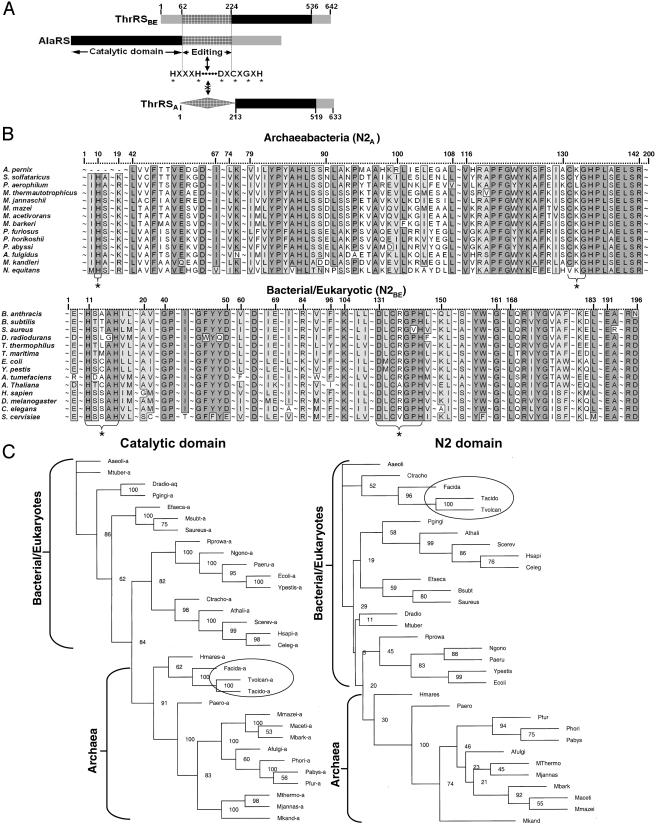
Fig. 1.
(A) Depictions of organization of editing domains in AlaRS and ThrRS. Hatched rectangle indicates canonical bacterial/eukaryotic (N2BE) class II editing domain with conserved sequence motifs given below. Noncanonical archaeal domain (N2A, hatched diamond) is appended to the N terminus, with a sequence different from bacterial and eukaryotic orthologs but nonetheless highly conserved among archaebacteria. (B) Representative alignments of bacterial/eukaryotic (N2BE) and archaebacterial (N2A) domains prepared by clustalw and illustrated by bioedit. Positions with >90% identity are shaded in light gray, whereas positions with 100% identity are in dark gray. Universally conserved N2BE sequence motifs and potentially analogous N2A motifs are bracketed below and marked (*). Extensive regions with <90% identity are deleted from the alignment and replaced by (∼). (C) Phylogenetic relationship of catalytic domain of ThrRS and the N2 domain of ThrRS. Archaeal organisms possessing N2BE are circled.
In this work, we set out to understand how an archaebacterial enzyme achieved an accuracy of aminoacylation that could compensate for the absence of the editing domain found in all of its bacterial and eukaryotic orthologs. However, most strikingly, the archaebacterial threonine-specific enzymes have a previously undescribed domain (see below) that is appended to the N terminus. Because domains idiosyncratically appended to the N or C termini of some tRNA synthetases are known to have functions other than editing (such as RNA or protein binding, as in the multisynthetase complex; refs. 29 and 30), and because other class I and class II enzymes (such as AlaRS) have strictly conserved editing domains through evolution, we imagined that archaebacterial threonyl–tRNA synthetases could either function without an editing function or had acquired a domain for editing that was specific to archaebacteria.
Materials and Methods
Construction of Plasmids. Using a procedure previously detailed, construction of genes for tRNAs for in vitro transcription was accomplished by annealing four overlapping oligonucleotides encoding the nucleotide sequences of the tRNAs (31). The annealed oligonucleotide cassettes were ligated into PstN1/BamHI-digested plasmid pUC18 (32). The coding sequences within the plasmids were confirmed by DNA sequencing. The plasmids for expression of wild-type Escherichia coli ThrRS and ΔN2-ThrRS were constructed through PCR amplification of the gene for ThrRS, by using colony PCR with oligonucleotides containing attB1 and attB2 sites for Gateway cloning by recombination (Invitrogen). After generation of entry clones into plasmid pDONR-201 (Invitrogen), a second recombination reaction was performed into a Gateway-converted version of pET28a (Novagen), producing an N-terminal His tag and plasmids pH8GW-ecThrRS and pH8GW-ecΔN2-ThrRS.
To clone the Methanosarcina mazei ThrRS, an entry clone was generated by PCR with oligonucleotides containing attB1 and attB2 sites plus NdeI and XhoI sites, respectively. M. mazei genomic DNA was obtained from American Type Culture Collection. QuikChange mutagenesis (Stratagene) was performed to eliminate an internal NdeI site, and the entry clone was partially digested and ligated into the NdeI and XhoI sites of pET21b (Novagen), yielding plasmid pET21b-mmThrRSH6. To eliminate the C-terminal His-tag embedded in pET21b, QuikChange mutagenesis was used to generate a stop codon before the His tag and thereby yield plasmid pET21b-mmThrRS. DNA sequencing was used to verify the coding sequence of M. mazei ThrRS in plasmid pET21b-mmThrRS. Spreading of all E. coli transformants was accomplished by the Copacabana method (33).
RNA Preparation. Plasmids (see above) were linearized with BstN1 and transcribed with T7 RNA polymerase as described (34). The transcript was resuspended in 50 mM Hepes (pH 7.5) and folded as previously detailed by heat denaturation and slow cooling to room temperature (27). [3H]Ser–tRNAThr was prepared by mischarging unfractionated E. coli or bovine tRNA (Roche Diagnostics and Novagen, respectively) (5 μg/μl) and [3H]Ser (16 μM), in charging buffer at 37°C with ecΔN2-ThrRS (5 μM). Reactions were incubated for 30 min, extracted twice with phenol (pH 4.7), ethanol-precipitated, resuspended in 2 mM MgCl2, and stored at –80°C until use.
Protein Expression and Purification. E. coli wild-type and E. coli ΔN2-ThrRS were prepared from expression of its gene encoded by plasmids pH8GW-ecThrRS or pH8GW-ecΔN2-ThrRS in BL21-CodonPlus(DE3)-RIL cells (Stratagene). Overnight cultures were diluted 100-fold, grown (37°C) to mid-log phase, and induced with 400 μM isopropyl β-d-thiogalactoside (IPTG) for 3–5 h at 37°C. Cells were harvested by centrifugation, lysed by sonication, and purified by nickel-nitrilotriacetic (Ni-NTA) chromatography. For M. mazei ThrRS, cells were treated as detailed above but lysed in 50 mM K/PO4, pH 7.4/100 mM NaCl/1.2 M NH4SO4/20 mM 2-mercaptoethanol/1 mM DTT. The lysate was centrifuged 30 min at 110,000 × g, diluted 4-times in the above buffer and loaded onto a self-packed phenyl-Sepharose column (10 ml). A gradient of 1.2 M to 0.1 M NH4SO4 was applied, and fractions exhibiting threonine-dependent ATP–PPi exchange were pooled and concentrated with Centriprep-30 (Millipore). The concentrate was diluted eight times with MonoQ buffer A (25 mM Tris, pH 7.5/100 mM NaCl/20 mM 2-mercaptoethanol/1 mM DTT) and loaded onto a MonoQ 5/5 (Amersham Pharmacia). A gradient of 100–275 mM NaCl was applied, and active fractions were pooled, concentrated with Centriprep-30, and stored at –20°C in 50% glycerol. Concentrations of enzymes were determined by active-site titration (35).
Aminoacylation and Deacylation Assays. Aminoacylation assays were performed at 37°C as described (36) in charging buffer (50 mM Hepes, pH 7.5/20 mM KCl/4 mM DTT/10 mM MgCl2). For deacylation assays, ThrRS (40–176 nM) was added to [3H]Ser–tRNA (2 μg/μl) in charging buffer at 23°C and the reaction was stopped at several time intervals by addition to filter paper saturated in 5% trichloroacetic acid (31).
Sequence Information and Generation of the Alignments. All protein sequences were obtained from the NCBI protein database (www.ncbi.nlm.nih.gov). The complete sequences of all analyzed proteins were directly aligned by using clustalw (37). The alignments generated by clustalw were edited to remove gaps, and used for the phylogenetic studies. bioedit was used to display sequence alignments for figures.
Phylogenetic Calculations. Maximum parsimony (MP) and neighbor joining (NJ) analyses were performed with the package phylip 3.57c (38). The numbers and lengths of MP minimal trees were estimated from 1,000 replicate random heuristic searches, and confidence limits of branch points were estimated from 1,000 bootstrap replications. The NJ phylogeny was based on pairwise distances using the programs neighbor and protdist. The Dayhoff 120 matrix was used in the protdist program to estimate the expected amino acid replacements per position. The programs seqboot and consense were used to estimate the confidence limits of branching points.
Results
A Domain in Archaebacterial ThrRS. The N-terminal sequence seen in the bacterial and eukaryotic ThrRSs (referred to as “N2BE domain”) is remarkably different in archaebacteria (Fig. 1 A). Secondary structure predictions of the archaebacterial sequence (N2A) suggested an α+β structure, as seen in the orthologous bacterial and eukaryotic enzymes, but with no obvious sequence similarity to the N2BE domain (Fig. 1B). Also, the conserved (in N2BE) HXXXH and D/EXCXGXH motifs are absent in the archaebacterial N2A domain. (Most archaeal ThrRSs have a CXG tripeptide, but its significance is unclear because of the shortness of the sequence.) Certain mutations of amino acids within these motifs of N2BE abolished editing activities of ThrRS and AlaRS (25, 27). psi-blast iterations to convergence with sequences of the bacterial/eukaryotic N2BE and of the archaeal N2A domains failed to produce alignments with other sequences encompassing either the bacterial/eukaryotic or archaeal group, respectively. Interestingly, the genomes of Sulfolobus solfataricus and Aeropyrum pernix encode sequences similar to N2A that exist independent of the catalytic domain of ThrRS. Moreover, the N2A domain is not fused to the body of ThrRS in these two organisms. A further peculiarity is noted in that some archaeal organisms possess N2BE in place of N2A (Fig. 1C and Table 1).
Table 1. Archaeal organisms grouped by sequence of N2 domain.
| Archaeal (N2A) | Bacterial/eukaryotic (N2BE) | Other |
|---|---|---|
| M. mazei | Halobacterium sp NRC-7 | Sulfolobus tokodaii* |
| Methanosarcina acetivorans | Thermoplasma acidophilum | |
| M. barkeri | Ferroplasma acidarmanus | |
| Pyrococcus furiosus | Thermoplasma volcanium | |
| Pyrococcus horikoshii | ||
| Pyrococcus abyssi | ||
| Archaeoglobus fulgidus | ||
| Methanocaldococcus jannaschii | ||
| Methanopyrus kandleri | ||
| Methanothermobacter thermautotrophicus | ||
| Pyrobaculum aerophilum | ||
| Nanoarchaeum equitans | ||
| S. solfataricus† | ||
| A. pernix† |
S. tokodaii has a reported sequence that resembles neither N2BE nor N2A.
A sequence from S. solfataricus and A. pernix resembling N2A exists as a separate gene and is not fused to a full-length ThrRS.
Selection of Archaeal Candidate, Purification, and Assay for Amino Acid Misactivation. Because assays could be compromised by high temperatures required by archaebacterial thermophiles and hyperthermophiles, we chose to investigate ThrRS from M. mazei, which grows at 30–40°C (39). The gene for the enzyme was cloned, and the protein was expressed in E. coli and subsequently purified. Initial assays sought to determine whether M. mazei ThrRS was able to misactivate serine at levels similar to those observed with E. coli ThrRS. [One possibility for obviating the necessity for editing by archaebacterial ThrRSs would be if these particular enzymes gained an enhancement (such as active site mutations) that allowed for more effective discrimination of serine versus threonine. Although sequence alignments did not reveal any striking differences within the active site regions of bacterial, eukaryote, and archaebacterial ThrRS, the possibility of a subtle combination of mutations affecting the active site conformation could not be excluded.] M. mazei ThrRS was challenged with serine in the standard amino acid-dependent ATP–PPi exchange assay shown below.
 |
[1] |
The assay was performed at a concentration of serine (82 mM) that is the same as the reported Km for misactivation of serine by E. coli ThrRS (26). Robust amino acid activation was observed in the standard amino acid-dependent ATP–PPi exchange assay, which monitors production of the aminoacyl adenylate (Ser–AMP) (Fig. 2A). This result suggested that the site for adenylate synthesis was not sufficiently specific to obviate the need for an editing activity.
Fig. 2.
(A) Amino acid activation assay (ATP–PPi exchange) with serine (82 mM) or a no amino acid control. Either E. coli or M. mazei ThrRS (50 nM) was assayed for serine-dependent PPi exchange at pH 7.5, 37°C. (B) Aminoacylation of total bovine (2.7 mg/ml) or E. coli tRNA (10 mg/ml) and 40 nM ThrRS at pH 7.5, 37°C. (C) Mischarging of bovine tRNA with serine. Wild-type E. coli or M. mazei ThrRS, or E. coli ΔN2ThrRS (900 nM) were added to bovine tRNA and assayed as in B.
Aminoacylation of Bovine tRNAThr by M. mazei ThrRS. To test the ability of ThrRS to edit mischarged substrates, a suitable model tRNA was required. We sought to obtain a substrate by in vitro transcription of the gene for M. mazei tRNAThr. Transcripts of the three M. mazei ThrRS isoacceptors were prepared in vitro. All were tested and failed to be significantly aminoacylated by M. mazei ThrRS. In contrast, E. coli ThrRS efficiently charged these three substrates (data not shown), thus establishing that the transcripts were viable. We also attempted to aminoacylate naturally modified tRNA isolated from E. coli with M. mazei ThrRS, but no aminoacylation was observed (Fig. 2B). However, when bovine tRNA was used, aminoacylation with M. mazei ThrRS was seen (Fig. 2B). (Fortuitously, the inability of M. mazei ThrRS to aminoacylate E. coli tRNA allowed us to exclude the possibility of endogenous E. coli ThrRS contaminating the M. mazei ThrRS expressed in E. coli.) Therefore, we used the bovine tRNA as a model substrate for further studies.
Mischarging with E. coli ΔN2–ThrRS but not with Wild-Type E. coli or M. mazei ThrRS. Although serine is misactived by M. mazei ThrRS at a rate comparable to that of its E. coli ortholog, and although M. mazei ThrRS charged bovine tRNA with threonine, M. mazei ThrRS did little or no acylation of bovine tRNA with serine (Fig. 2C). This result suggests that M. mazei ThrRS has an editing activity that clears the misactivated serine. Similarly, wild-type E. coli ThrRS charged bovine tRNA with threonine, but not with serine. In contrast, ΔN2BE E. coli ThrRS, which lacks the editing domain of the E. coli protein, misacylated bovine tRNA with serine. To further investigate the possibility that the N2A domain of M. mazei ThrRS encoded an activity for deacylation of Ser–tRNAThr, we created a construct, designated ΔN2AThrRS, which lacked the appended N2A domain. However, the solubility of this protein was so low that we were unable to obtain amounts sufficient for investigation.
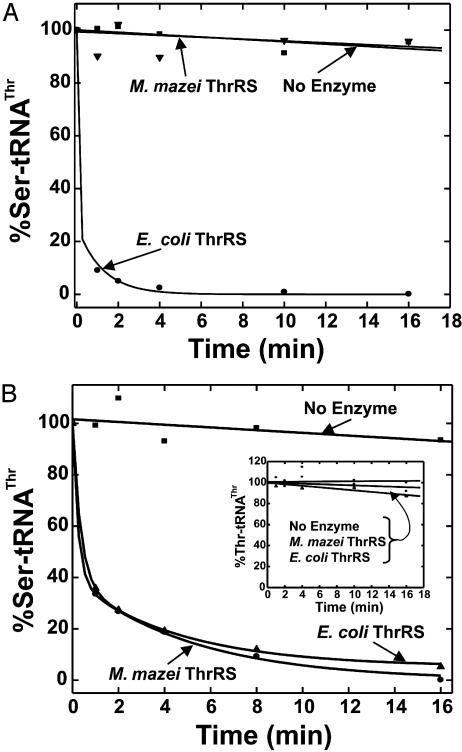
Deacylation of Ser–tRNAThr. Because it is not known whether E. coli ThrRS can execute tRNA-dependent hydrolysis of Ser–AMP (pretransfer editing), we sought directly to examine the deacylation of Ser–tRNAThr (posttransfer editing). To pursue the question of whether M. mazei ThrRS could deacylate Ser–tRNAThr, a source of mischarged E. coli tRNAThr was required. Because M. mazei ThrRS did not charge E. coli tRNAThr, we imagined it would be inactive on E. coli Ser–tRNAThr. We used ΔN2BE E. coli ThrRS to prepare E. coli Ser–tRNAThr. The mischarged substrate was subjected to enzymatic deacylation by either wild-type E. coli or M. mazei ThrRS. M. mazei ThrRS did not deacylate mischarged E. coli tRNA. In contrast, wild-type E. coli ThrRS rapidly cleared the misacylated species (Fig. 3A). Thus, the archaeal protein had the same lack of activity on E. coli tRNAThr in editing that it had in aminoacylation.
Fig. 3.
Posttransfer editing activities of E. coli or M. mazei ThrRS (40 nM) at pH 7.5, 23°C. The clearance of Ser–tRNAThr is plotted as a function of time. Deacylation of Ser–tRNAThr from E. coli (A) or Ser–tRNAThr from M. mazei (B). (Inset) Deacylation of Thr–tRNAThr.
In contrast, M. mazei ThrRS efficiently deacylated bovine Ser–tRNAThr, as did E. coli ThrRS (Fig. 3B). Thus, the same specificity observed in the aminoacylation reaction is seen in deacylation. To investigate whether M. mazei ThrRS is amino acid-specific for deacylation, bovine Thr–tRNAThr was also tested. No significant deacylation of bovine tRNA aminoacylated with the cognate amino acid was detected (Fig. 3B Inset).
Mutations in M. mazei ThrRS That Result in a Loss of Editing. To further explore the origin of the editing activity seen in M. mazei ThrRS, mutations were placed in the N2A domain. Because no blocks of residues are conserved between the N2A and N2BE domains, alignments of sequences of all of the N2A domains (Fig. 1B) were used to select highly conserved residues for mutation. Because C125–R135 in N2A of M. mazei ThrRS has a conserved cysteine, and because a conserved cysteine is important for editing by the class II AlaRS (27), we replaced C125–R135 with AAASAAASAAA to give M. mazei ThrRSA125–135. (This particular 11-aa change was chosen as a broad, generic replacement.) M. mazei ThrRSA125–135 misactivates serine as efficiently, as did the wild-type enzyme (Fig. 4A Inset). Thus, the C125–R135 substitution in N2A did not affect the active site for adenylate synthesis and its capacity for misactivation. Despite a 5-fold loss in aminoacylation activity (Fig. 4A), no deacylation of bovine Ser–tRNAThr by M. mazei ThrRSA125–135 was observed even at 5-fold higher protein concentrations (176 nM) that compensated for the diminution in the rate of aminoacylation (Fig. 4B). These data further corroborated the idea that the N2A domain is required for editing by archaebacterial ThrRS.
Fig. 4.
Enzymatic activity of mutant M. mazei ThrRS. (A) Aminoacylation of total bovine (2.7 mg/ml) tRNA and 40–176 nM of either wild-type or M. mazei ThrRSA125–135 at pH 7.5, 37°C. (Inset) Serine-dependent ATP–PPi exchange activity of wild-type or M. mazei ThrRSA125–135 as described in Fig. 2. (B) Posttransfer editing activities of wild-type M. mazei ThrRS (40 nM) or 176 nM M. mazei ThrRSA125–135 at pH 7.5, 23°C.
Discussion
We cannot rule out the possibility that the archaebacterial N2A domain is a structural, if not a sequence, homolog of the N2BE domain. Examples of domains related by structure but not sequence include (among others) the oligonucleotide/oligosaccharide binding folds (OB folds) such as the EMAP II-like domain of human TyrRS (40, 41) and Trbp111 (42), and catalytic regions of mandelate racemase, enolase, and muconate lactonizing enzyme (43, 44) and of bacterial and eukaryote nucleotide phosphorylases (45, 46). Nonetheless, the sequence of the N2A domain is clearly segregated from the bacterial and eukaryote N2BE domain. Thus, regardless of the structure of the N2A domain and its potential relatedness to N2BE, the unique sequence of the archaebacteria-specific N2A domain suggests that it was added to ThrRS after the eukaryote/archaebacterial split.
The N2A domain is missing from S. solfataricus and A. pernix ThrRSs. However, in these organisms, the genome encodes a free-standing N2A. In earlier work, the CP1 editing domain imbedded in class I tRNA synthetases was shown to act as a free-standing, editing-proficient protein, when dissected out of specific synthetases (14). For example, CP1IleRS clears Val–tRNAIle, and CP1ValRS clears Thr–tRNAVal (14). Similarly, the insertion (INS) domain of ProRS, when produced as a free-standing protein, clears Ala–tRNAPro (47). Recent work of Söll and coworkers (48) identified free-standing domains in Methanosarcina barkeri and Clostridium sticklandii that clear Gly/Ser-tRNAAla and Ala–tRNAPro, respectively. Thus, the N2A domains found in S. solfataricus and A. pernix most likely are responsible for clearing Ser–tRNAThr.
A few sequences within the archaebacterial kingdom appear, by sequence alignment, to be distinctly N2BE (Table 1). These sequences phylogenetically group independently from the N2A sequences (Fig. 1C), even though the catalytic domains group in a kingdom-specific way. We surmise that, in these instances, horizontal gene transfer events fused the N2BE domain to the catalytic portions of the respective synthetase.
At this point in our analysis, we cannot rule out the possibility that M. mazei ThrRS acts in both pre- (49, 50) and posttransfer editing (16), as is the case for class 1 IleRS, which is the most extensively investigated synthetase with respect to the editing reaction. In pretransfer editing, the misactivated amino acid is translocated in cis from the active site to the center for editing, a distance of ≈25 Å in IleRS (51, 52). The adenylate is then hydrolyzed before reaction with the 3′ end of tRNA. In this work, we only demonstrate the posttransfer clearance of Ser–tRNAThr when the mischarged tRNA is added to ThrRS. Whether posttransfer editing can occur in cis, as it does for class I IleRS, also remains to be determined. Mechanistic and structural details aside, the N2A domain of ThrRS is an archaebacteria-specific domain for editing that has not been seen in any other class I or class II enzyme.
Acknowledgments
We thank Professor Ya-Ming Hou for helpful comments on this work. This work was supported by National Institutes of Health Grants GM 15539 and 23562 and a National Foundation for Cancer Research fellowship. K.B. was a National Institutes of Health postdoctoral fellow (2001–2004).
References
- 1.Webster, T. A., Tsai, H., Kula, M., Mackie, G. A. & Schimmel, P. (1984) Science 226, 1315–1317. [DOI] [PubMed] [Google Scholar]
- 2.Ludmerer, S. W. & Schimmel, P. (1987) J. Biol. Chem. 262, 10801–10806. [PubMed] [Google Scholar]
- 3.Cusack, S., Berthet-Colominas, C., Hartlein, M., Nassar, N. & Leberman, R. (1990) Nature 347, 249–255. [DOI] [PubMed] [Google Scholar]
- 4.Eriani, G., Delarue, M., Poch, O., Gangloff, J. & Moras, D. (1990) Nature 347, 203–206. [DOI] [PubMed] [Google Scholar]
- 5.Moine, H., Romby, P., Springer, M., Grunberg Manago, M., Ebel, J. P., Ehresmann, B. & Ehresmann, C. (1990) J. Mol. Biol. 216, 299–310. [DOI] [PubMed] [Google Scholar]
- 6.Torres Larios, A., Dock Bregeon, A. C., Romby, P., Rees, B., Sankaranarayanan, R., Caillet, J., Springer, M., Ehresmann, C., Ehresmann, B. & Moras, D. (2002) Nat. Struct. Biol. 9, 343–347. [DOI] [PubMed] [Google Scholar]
- 7.Rho, S. B. & Martinis, S. A. (2000) RNA 6, 1882–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho, S. B., Lincecum, T. L. & Martinis, S. A. (2002) EMBO J. 21, 6874–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakasugi, K. & Schimmel, P. (1999) Science 284, 147–151. [DOI] [PubMed] [Google Scholar]
- 10.Wakasugi, K. & Schimmel, P. (1999) J. Biol. Chem. 274, 23155–23159. [DOI] [PubMed] [Google Scholar]
- 11.Wakasugi, K., Slike, B. M., Hood, J., Ewalt, K. L., Cheresh, D. A. & Schimmel, P. (2002) J. Biol. Chem. 277, 20124–20126. [DOI] [PubMed] [Google Scholar]
- 12.Wakasugi, K., Slike, B. M., Hood, J., Otani, A., Ewalt, K. L., Friedlander, M., Cheresh, D. A. & Schimmel, P. (2002) Proc. Natl. Acad. Sci. USA 99, 173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otani, A., Slike, B. M., Dorrell, M. I., Hood, J., Kinder, K., Ewalt, K. L., Cheresh, D., Schimmel, P. & Friedlander, M. (2002) Proc. Natl. Acad. Sci. USA 99, 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin, L., Hale, S. P. & Schimmel, P. (1996) Nature 384, 33–34. [DOI] [PubMed] [Google Scholar]
- 15.Fersht, A. (1985) Enzyme Structure and Mechanism (Freeman, New York), pp. 347–368.
- 16.Eldred, E. W. & Schimmel, P. R. (1972) J. Biol. Chem. 247, 2961–2964. [PubMed] [Google Scholar]
- 17.Schmidt, E. & Schimmel, P. (1995) Biochemistry 34, 11204–11210. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt, E. & Schimmel, P. (1994) Science 264, 265–267. [DOI] [PubMed] [Google Scholar]
- 19.Schreier, A. A. & Schimmel, P. R. (1972) Biochemistry 11, 1582–1589. [DOI] [PubMed] [Google Scholar]
- 20.Lin, L. & Schimmel, P. (1996) Biochemistry 35, 5596–5601. [DOI] [PubMed] [Google Scholar]
- 21.Nureki, O., Vassylyev, D. G., Tateno, M., Shimada, A., Nakama, T., Fukai, S., Konno, M., Hendrickson, T. L., Schimmel, P. & Yokoyama, S. (1998) Science 280, 578–582. [DOI] [PubMed] [Google Scholar]
- 22.Silvian, L. F., Wang, J. & Steitz, T. A. (1999) Science 285, 1074–1077. [PubMed] [Google Scholar]
- 23.Hale, S. P. & Schimmel, P. (1997) Tetrahedron 53, 11985–11994. [Google Scholar]
- 24.Starzyk, R. M., Webster, T. A. & Schimmel, P. (1987) Science 237, 1614–1618. [DOI] [PubMed] [Google Scholar]
- 25.Dock-Bregeon, A., Sankaranarayanan, R., Romby, P., Caillet, J., Springer, M., Rees, B., Francklyn, C. S., Ehresmann, C. & Moras, D. (2000) Cell 103, 877–884. [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan, R., Dock-Bregeon, A. C., Rees, B., Bovee, M., Caillet, J., Romby, P., Francklyn, C. S. & Moras, D. (2000) Nat. Struct. Biol. 7, 461–465. [DOI] [PubMed] [Google Scholar]
- 27.Beebe, K., Ribas de Pouplana, L. & Schimmel, P. (2003) EMBO J. 22, 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribas de Pouplana, L. & Schimmel, P. (2001) Cold Spring Harbor Symp. Quant. Biol. 66, 131–166. [DOI] [PubMed] [Google Scholar]
- 29.Mirande, M. (1991) Prog. Nucleic Acid Res. 40, 95–142. [DOI] [PubMed] [Google Scholar]
- 30.Norcum, M. T. & Dignam, J. D. (1999) J. Biol. Chem. 274, 12205–12208. [DOI] [PubMed] [Google Scholar]
- 31.Farrow, M. A., Nordin, B. E. & Schimmel, P. (1999) Biochemistry 38, 16898–16903. [DOI] [PubMed] [Google Scholar]
- 32.Yanisch-Perron, C., Viera, J. & Messing, J. (1985) Gene 33, 103–119. [DOI] [PubMed] [Google Scholar]
- 33.Worthington, M. T., Luo, R. Q. & Pelo, J. (2001) BioTechniques 30, 738–740, 742. [DOI] [PubMed] [Google Scholar]
- 34.Steer, B. A. & Schimmel, P. (1999) Biochemistry 38, 4965–4971. [DOI] [PubMed] [Google Scholar]
- 35.Fersht, A. R., Ashford, J. S., Bruton, C. J., Jakes, R., Koch, G. L. E. & Hartley, B. S. (1975) Biochemistry 14, 1–4. [DOI] [PubMed] [Google Scholar]
- 36.Buechter, D. D. & Schimmel, P. (1993) Biochemistry 32, 5267–5272. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994) Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felsenstein, J. (1988) Annu. Rev. Genet. 22, 521–565. [DOI] [PubMed] [Google Scholar]
- 39.Sowers, K. R. & Schreier, H. J. (1995) in Archaea: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), p. 446.
- 40.Yang, X. L., Skene, R. J., McRee, D. E. & Schimmel, P. (2002) Proc. Natl. Acad. Sci. USA 99, 15369–15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kleeman, T. A., Wei, D., Simpson, K. L. & First, E. A. (1997) J. Biol. Chem. 272, 14420–14425. [DOI] [PubMed] [Google Scholar]
- 42.Swairjo, M. A., Morales, A. J., Wang, C. C., Ortiz, A. R. & Schimmel, P. (2000) EMBO J. 19, 6287–6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasson, M. S., Schlichting, I., Moulai, J., Taylor, K., Barrett, W., Kenyon, G. L., Babbitt, P. C., Gerlt, J. A., Petsko, G. A. & Ringe, D. (1998) Proc. Natl. Acad. Sci. USA 95, 10396–10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neidhart, D. J., Kenyon, G. L., Gerlt, J. A. & Petsko, G. A. (1990) Nature 347, 692–694. [DOI] [PubMed] [Google Scholar]
- 45.Mao, C., Cook, W. J., Zhou, M., Koszalka, G. W., Krenitsky, T. A. & Ealick, S. E. (1997) Structure (London) 5, 1373–1383. [DOI] [PubMed] [Google Scholar]
- 46.Galperin, M. Y., Walker, D. R. & Koonin, E. V. (1998) Genome Res. 8, 779–790. [DOI] [PubMed] [Google Scholar]
- 47.Wong, F. C., Beuning, P. J., Silvers, C. & Musier Forsyth, K. (2003) J. Biol. Chem. 278, 52857–52864. [DOI] [PubMed] [Google Scholar]
- 48.Ahel, I., Korencic, D., Ibba, M. & Söll, D. (2003) Proc. Natl. Acad. Sci. USA 100, 15422–15427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fersht, A. R. (1977) Biochemistry 16, 1025–1030. [DOI] [PubMed] [Google Scholar]
- 50.Fersht, A. R. & Kaethner, M. M. (1976) Biochemistry 15, 3342–3346. [DOI] [PubMed] [Google Scholar]
- 51.Nomanbhoy, T. K., Hendrickson, T. L. & Schimmel, P. (1999) Mol. Cell 4, 519–528. [DOI] [PubMed] [Google Scholar]
- 52.Hendrickson, T. L., Nomanbhoy, T. K., de Crécy Lagard, V., Fukai, S., Nureki, O., Yokoyama, S. & Schimmel, P. (2002) Mol. Cell 9, 353–362. [DOI] [PubMed] [Google Scholar]