Abstract
Background:
Junctional ectopic tachycardia (JET) often occurs in the early postoperative period following surgery for congenital heart diseases and may lead to hemodynamic compromise. Its exact etiology is unknown, however, longer cardiopulmonary bypass (CPB) time, aortic cross clamp (ACC) time, catecholamines, and hypomagnesemia are known risk factors. JET is associated with increased postoperative morbidity and mortality.
Materials and Methods:
A prospective cohort study of 194 consecutive children who underwent open heart surgery on CPB over 1 year period, patients was divided into three groups; JET, non-JET arrhythmia, and no arrhythmia groups. Information on patient's demographics (sex, age, and body weight), type of surgical interventions, duration of CPB and ACC, the use of inotropic support, duration of intensive care unit (ICU) stay, and response to different therapeutic methods were collected.
Results:
JET was documented in 53 patients (27%) most commonly following tetralogy of Fallot (TOF) repair and was associated with longer CPB and ACC times (118 and 77 min, respectively) as compared to non-JET arrhythmia (93.9 and 55.3 min, respectively) and no arrhythmia groups (94.9 and 54.8 min, respectively). Patients with JET required more inotropic support and longer ICU stay as compared to other groups. Amiodarone was safe and effective in treatment of JET. Atrial electrocardiogram (ECG) and Lewis lead ECG were helpful tools in JET diagnosis. The mortality was 11.5% in JET patients.
Conclusions:
Incidence of JET was 27% possibly due to the large number of Fallot repair and Senning operation. Longer CPB and ACC times are risk factors for JET.
Keywords: JET, open heart surgery, postoperative arrhythmia
INTRODUCTION
Postoperative arrhythmias occur frequently in the early postoperative period following cardiac surgery in children. Junctional ectopic tachycardia (JET) is one of the more commonly encountered arrhythmia with an overall incidence estimated between 3.6 and 10.8% with two case series reporting incidence as high as 12 and 27%, respectively.[1,2,3,4,5,6,7,8]
JET is caused by abnormal automaticity of the atrioventricular (AV) node. Autopsy reports of children with fatal JET have shown hemorrhagic tracks invading the AV bundle originating from sutures close to conduction system.[9,10] JET is a narrow QRS tachycardia usually with AV dissociation and slower atrial than ventricular rate, it can also be associated with 1:1 retrograde ventriculoatrial conduction.[4,11]
Although a self-limiting tachycardia, JET can cause serious hemodynamic instability in the immediate postoperative period due to loss of AV synchrony and consequent loss of atrial contributions to cardiac output in the heart already compromised following surgery and CPB.[4,11,12]
Several risk factors have been associated with development of JET that include operations involving right ventricular outflow tract (RVOT) excision, young age, longer cardiopulmonary bypass (CBP) time and aortic cross clamp (ACC) time, use of catecholamines, and low serum magnesium level.[1,4,7]
There are several approaches to the management of JET comprising physiological alternation, drugs, external cardiac pacing, and extracorporeal support.[12,13] Patients who develop JET have increased morbidity as indicated by longer duration of mechanical ventilation and increased intensive care unit (ICU) stay and increased risk of mortality.[1,3,4,6,7]
The aims of this study were to determine the incidence of JET, identify patient, and procedure-related risk factors for development of JET, response of JET to different therapeutic modalities and impact of JET on postoperative morbidity.
MATERIALS AND METHODS
Patient population
All pediatric patients (1st month of life till 14-years-old) who underwent cardiac surgery with CBP at Cairo University Children Hospital between July 2010 and July 2011 were enrolled in our study. Patients who had preoperative arrhythmias were excluded.
Arrhythmia diagnosis
JET was defined as heart rate greater than 170 beats per min with the baseline QRS complex, AV dissociation with ventricular rate faster than the atrial rate or retrograde conduction with retrograde p-wave,[14] as detected by examining the telemetry using speed 50 m/s and 12-lead electrocardiogram (ECG) [Figures 1, 2 and 3].
Figure 1.
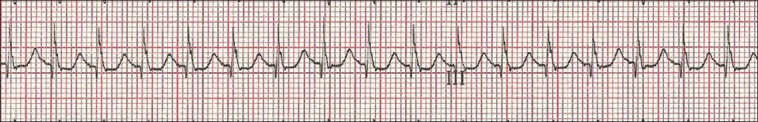
Electrocardiogram (ECG) showing junctional ectopic tachycardia (JET). The P waves could not be detected by usual ECG
Figure 2.
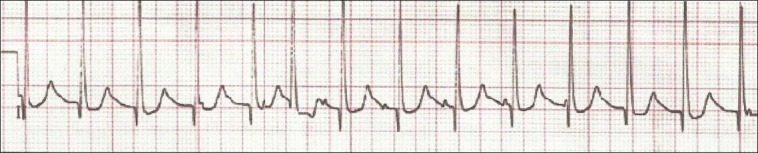
ECG of patient with JET using Lewis lead showing the P waves dissociated from QRS with atrial rate less than the ventricular rate
Figure 3.
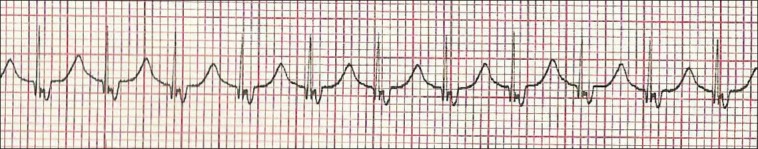
ECG using Lewis lead showing retrograde P wave with 1:1 ventriculoatrial conduction
Patients with wide QRS tachycardia especially following tetralogy of Fallot (TOF) repair due to right bundle branch block (RBBB), the diagnosis of JET was confirmed either by atrial ECG using atrial wires or esophageal lead if available or using Lewis lead arrangement to augment the P waves.
The Lewis lead configuration can help to detect atrial activity and its relationship to ventricular activity [Figures 2 and 3]. This technique was described by Sir Thomas Lewis (1881-1945) in his book (Clinical Electrocardiography), the right arm electrode applied to the right of the sternum at the second intercostal space and the left arm electrode applied to the fourth intercostal space. After recording, this tracing was interpreted in lead I.[15,16,17,18]
Risk factors
In order to determine the risk factors for development of JET, impact of JET on postoperative morbidity, the patients were divided into three groups: JET, non-JET arrhythmia, and patients with no arrhythmia in the early postoperative periods (1-30 days postoperative).
Information on patients demographics (sex, age, and body weight), type of surgical interventions, duration of CPB and ACC, serum electrolytes at tachyarrhythmia onset, the use of inotropic support, duration of ICU stay, and response to different therapeutic methods were collected.
Therapeutic interventions
Our staged protocol for JET therapy was as follow:
Step 1: Avoid hyperthermia; optimize electrolytes, sedation, and magnesium sulfate bolus of 30 mg/kg.
Step 2: Controlled hypothermia (target core temperature 35-36°C).
Step 3: Decrease the catecholamine dose if possible.
Step 4: Amiodarone was used as the first line anti-arrhythmic drug for all patients with hemodynamically compromising JET, bolus dose of 5 mg/kg over 2 h followed by continuous infusion of 10-20 mg/kg/24 h.
Pacing therapy was used using temporary atrial wires if available or esophageal pacing leads for overdrive or sequential pacing in selected cases.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) Excel for Windows (Microsoft, USA) and Graphpad software (Graphpad Software, San Diego, USA) were used for data statistics. The possible statistical significance of differences between sets of data was tested using one-way analysis of variance (ANOVA) test for unpaired data as well as Chi-square test. A significant difference was assumed for values of P < 0.05.
RESULTS
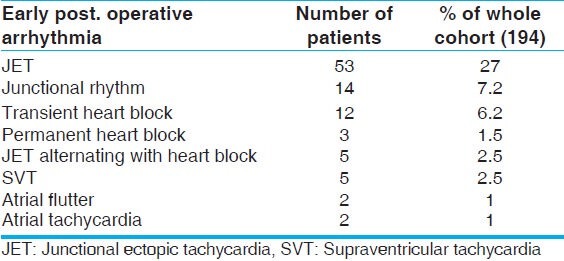
Males represent 54% while females 46% of study population. Their ages ranged from 0.5 to 144 months with mean of 29 ( ±27) months. Their weights ranged from 3 to 33 kg with mean of 10.5 kg ( ±5 kg). JET was documented in 53 out of 194 patients who represented an overall incidence of 27%. Other types of early postoperative arrhythmias that were documented in our patients are shown in Table 1. The diagnosis of JET was clear using the conventional ECG in 44 patients (83.1%), atrial leads confirmed the diagnosis in five patients (9.4%) and Lewis lead confirmed the diagnosis in four patients (7.5%). Atrial leads were not available in all patients, as it is not routinely done in our institute.
Table 1.
Different types of early postoperative arrhythmias in our patients
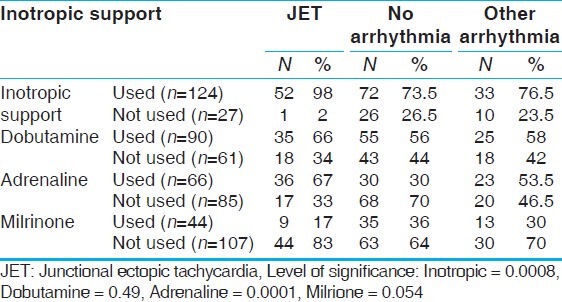
In our study, JET was noted most commonly following TOF repair (52%, n = 24), Senning operation (50%, n = 10), and AV canal repair (42.8%, n = 6). In order to define the risk factors that probably increase the incidence of JET in the early postoperative period, we did a comparison between patients with JET and those with no arrhythmia, and those with postoperative arrhythmias other than JET, [Table 2] using the following variables; age, weight, CBP time, and ACC time. It was found that a longer bypass time and ischemic time are significant prognostic factors for incidence of JET. It was also noted that patients with JET had longer length of ICU stay as compared to other arrhythmias and non-arrhythmia groups. Inotropic support was used more often in JET (98%, n = 53) patients as compared to non-JET group (76.5%, n = 33) and no arrhythmia group (73.5%, n = 72). There was a statistically significant correlation between the use of adrenaline and dobutamine and increased incidence of JET and on the other hand, use of milrinone was not associated with increased incidence of JET [Table 3]. JET successfully resolved in 86.5% (n = 47) of patients with 57% (n = 30) responding to conventional measures (cooling, magnesium sulfate, sedation and minimizing catecholamines) and the remaining 43% (n = 23) were controlled by intravenous (IV) amiodarone infusion in addition to conventional measures. Amiodarone was used in hemodynamically significant JET not responding to the initial steps in management, 23 patients received amiodarone, only two patients experienced hypotension that managed by boluses of fluid. The standard protocol for amiodarone administration in our study was to give a bolus loading dose of 5 mg/kg IV infusion over 2 h, followed by a continuous infusion of 10-20 mg/kg/day until desired effect of a sinus rhythm or slower junctional rate with improved hemodynamics was achieved. Before return to sinus rhythm patients were either in accelerated junctional rhythm or in intermittent sinus rhythm with accelerated junctional rhythm. When a slower junctional rhythm was obtained without improvement in hemodynamics, sequential pacing was used using temporary epicardial atrial wires if available or by the use of an esophageal lead.
Table 2.
JET versus no arrhythmia group
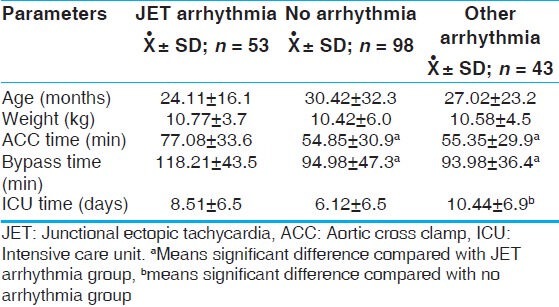
Table 3.
Inotropic support for JET group versus no arrhythmia group
Sinus rhythm attained in 47 patients (88.5%), six patients died (11.5%) of whom JET was the direct cause of mortality in one patient and a contributing factor in others.
DISCUSSION
JET is a common tachyarrhythmia in the early postoperative period after pediatric cardiac surgery, the cause of JET is thought to be a result of a combination of factors, including underlying heart disease, type of surgical procedure, hemodynamic instability, and electrolyte imbalance, specially hypomagnesemia. The pathological mechanism is believed to be a result of direct trauma to the AV node and bundle of His, although JET occurs in patients in whom there is no operation near the AV node (e.g., transplantation and extracardiac Fontan operation).[11,19,20,21,22,23,24,25]
The purpose of our study was to analyze the incidence, risk factors and outcome of JET in an unselected population (congenital heart defects operated using CBP) to provide broader information about JET in the postoperative cardiac ICU after repair of congenital heart disease.
In our cohort, we found that the overall incidence of JET in children undergoing cardiac surgery with CBP was 27%, which is a higher incidence as compared to other studies that reported incidence of 10-15%.[1,2,6] This higher incidence of JET may be related to the large number of cases with TOF, many of these cases were done in older age than usual. Since the outcome of arterial switch operation is not very good in our institution, we were forced to do large number of Senning operations, which might play a role in this high incidence. The higher incidence of JET in our study highlights the importance of analysis of the possible risk factors in a trial to avoid the preventable causes and treat the curable ones.
In our study, JET was observed most commonly following repair of TOF (52% n = 24), Senning operation (50%, n = 10), and common AV canal (CAVC) canal repair (42.8%, n = 6). Previous studies reported high incidence of JET following TOF repair.[7,8,11]
Several studies have implicated various clinical, surgical, and therapeutic associations with the genesis of JET. Our study, as does others, suggests that the cause of postoperative JET still remains largely unknown in any individual patient. For example, several patients did not have intracardiac surgical procedures and still experienced JET (for example, cavopulmonary anastomosis or the Glenn shunt). Injury to the conduction system was the proposed mechanism either by resection or excision of muscle bundles or relief of the RVOT though the right atrium commonly associated with a repair of tetralogy of Fallot.[11]
In our study, patients experienced JET in the immediate postoperative period (range 1-24 h post bypass; median, 6.8 h). JET patients had a longer bypass and ACC times (118 and 77 min, respectively) as compared to non-JET arrhythmia (93.9 and 55.3 min) and no arrhythmia groups (94.9 and 54.8 min), greater use of inotropic support and this in consistent with the previous reports.[1,2,4,5,6] Longer CPB is probably a result of more difficult surgical correction and larger surgical trauma.
Although a self-limiting arrhythmia, JET can lead to a marked hemodynamic instability in the early postoperative period due to impaired ventricular filling, loss of AV synchrony, and atrial contribution to cardiac output which is essential in patients with diastolic dysfunction of right ventricle (RV) as those following TOF repair. The duration of JET in our study was ranging between 6 and 168 h with mean of 33.8 h, JET rate was ranging between 170 and 220, hemodynamic instability was observed in 31 out of 53 patients with JET (60%).
The diagnosis of JET usually is based on electrocardiographic evidence of a narrow complex tachycardia, heart rate ranging from 170 to 260 beats/min and regular with AV dissociation.[5,26] The QRS is usually a narrow complex and is wide in cases associated with RBBB, P waves may be hidden, dissociated, or retrograde. This was confirmed in all our patients. When there is retrograde conduction, adenosine administration can rule out the diagnosis of AV reciprocating tachycardia, because of tachycardia cycle lengthening without termination of the arrhythmia. Diagnosis should be suspected in those anatomical substrates in which JET is common, that is, TOF, RVOT reconstruction, and AV septal defect (AVSD). Atrial ECG is usually confirmatory in difficult cases. In comparing atrial ECG with surface ECG, AV dissociation can be clearly demonstrated.[27] We performed atrial ECG in five patients and Lewis lead arrangement in four patients to visualize the P wave clearly. Although not used regularly in clinical practice, we would like to promote the use of the Lewis lead configuration in those situations in which differentiation between wide QRS tachycardia is difficult.
Our current treatment strategy includes the active avoidance of hyperthermia, hypothermia, optimal sedation and pain control, optimizing the electrolytes, magnesium sulfate IV bolus of 30 mg/kg, minimizing exogenous catecholamines if possible, and the use of amiodarone. Atrial or sequential pacing to optimize hemodynamics in selected patients.
JET successfully resolved in 86.5% (n = 47) of patients, with 57% (n = 30) responding to conventional measures (cooling, magnesium sulfate, sedation, and minimizing catecholamines) and the remaining 43% (n = 23) were controlled by IV amiodarone infusion in addition to conventional measures. IV amiodarone was found to be safe and effective; only two patients develop hypotension during amiodarone loading dose administration that was managed by boluses of fluids.
The rationale for amiodarone use differs in previous studies for treatment of JET.[1,2,3,11,28] Some institutions, like ours, use amiodarone as a primary treatment,[7] because of the good response to intravenous amiodarone in patients who failed to respond to conventional therapies or were hemodynamically unstable. It has also been recommended as an early first-line treatment for JET.[6,10,29,30]
If the ventricular rate is not adequately controlled or AV synchrony not achieved by means of pacing strategies, JET may increase further postoperative problems (i.e., low cardiac output and hypotension). A recent study noted that the cardiac ICU stay increased from 4.5 to 8.8 days when comparing when comparing those without and with postoperative JET; respectively.[4] In our study, there was a statistically significant correlation between JET and increased length of ICU stays (mean of 8.5 days) as compared to non-arrhythmias group (6.5 days). This can be explained by the treatment protocol for hemodynamically unstable JET patients that included sedation, muscle paralysis, and ventilator therapy. The patients could not be transferred to regular ward until JET has subsided. Both longer ventilator time and longer ICU stay increase other possible complications, that is, infections, and also increase the cost of treatment. In other studies, JET has been associated with worse outcome and increased death rate compared to non-JET patients,[1,6,10] in our cohort, six (11.5%) patients out of 53 patients with JET died, JET was a direct cause of mortality in one patient only and a contributing factor in the other five patients.
CONCLUSIONS
In conclusion, of 194 patients who underwent cardiac surgical procedures in our institution, 53 (27%) experienced JET. Longer CPB and ACC times, use of catecholamines were risk factors for JET. Twenty-three patients (43%) required amiodarone infusion. JET was associated with increased postoperative morbidity and mortality.
We strongly recommend placement of temporary atrial wires for surgical procedure with high likelihood of JET as TOF repair, Senning operation and AVSD repair, atrial wires are useful tool for diagnosis, and pacing therapy of JET. We recommend the use of Lewis lead ECG placement in case of JET with wide QRS as a simple way to clearly visualize the P waves in the event atrial wires are unavailable.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Andreasen JB, Johnsen SP, Ravn HB. Junctional ectopic tachycardia after surgery for congenital heart disease in children. Intensive Care Med. 2008;34:895–902. doi: 10.1007/s00134-007-0987-2. [DOI] [PubMed] [Google Scholar]
- 2.Batra AS, Chun DS, Johnson TR, Maldonado EM, Kashyap BA, Maiers J, et al. A prospective analysis of the incidence and risk factors associated with junctional ectopic tachycardia following surgery for congenital heart disease. Pediatr Cardiol. 2006;27:51–5. doi: 10.1007/s00246-005-0992-6. [DOI] [PubMed] [Google Scholar]
- 3.Delaney JW, Moltedo JM, Dziura JD, Kopf GS, Snyder CS. Early postoperative arrhythmias after pediatric cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:1296–300. doi: 10.1016/j.jtcvs.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Dodge-Khatami A, Miller OI, Anderson RH, Gil-Jaurena JM, Goldman AP, De Leval MR. Impact of junctional ectopic tachycardia on postoperative morbidity following repair of congenital heart defects. Eur J Cardiothorac Surg. 2002;21:255–9. doi: 10.1016/s1010-7940(01)01089-2. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman TM, Bush DM, Wernovsky G, Cohen MI, Wieand TS, Gaynor JW, et al. Postoperative junctional ectopic tachycardia in children: Incidence, risk factors, and treatment. Ann Thorac Surg. 2002;74:1607–11. doi: 10.1016/s0003-4975(02)04014-6. [DOI] [PubMed] [Google Scholar]
- 6.Mildh L, Hiippala A, Rautiainen P, Pettila V, Sairanen H, Happonen JM. Junctional ectopic tachycardia after surgery for congenital heart disease: incidence, risk factors and outcome. Eur J Cardiothorac Surg. 2011;39:75–80. doi: 10.1016/j.ejcts.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Rekawek J, Kansy A, Miszczak-Knecht M, Manowska M, Bieganowska K, Brzezinska-Paszke M, et al. Risk factors for cardiac arrhythmias in children with congenital heart disease after surgical intervention in the early postoperative period. J Thorac Cardiovasc Surg. 2007;133:900–4. doi: 10.1016/j.jtcvs.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Yildirim SV, Tokel K, Saygili B, Varan B. The incidence and risk factors of arrhythmias in the early period after cardiac surgery in pediatric patients. Turk J Pediatr. 2008;50:549–53. [PubMed] [Google Scholar]
- 9.Till JA, Ho SY, Rowland E. Histopathological findings in three children with his bundle tachycardia occurring subsequent to cardiac surgery. Eur Heart J. 1992;13:709–12. doi: 10.1093/oxfordjournals.eurheartj.a060239. [DOI] [PubMed] [Google Scholar]
- 10.Haas NA, Plumpton K, Justo R, Jalali H, Pohlner P. Postoperative junctional ectopic tachycardia (JET) Z Kardiol. 2004;93:371–80. doi: 10.1007/s00392-004-0067-3. [DOI] [PubMed] [Google Scholar]
- 11.Dodge-Khatami A, Miller OI, Anderson RH, Goldman AP, Gil-Jaurena JM, Elliott MJ, et al. Surgical substrates of postoperative junctional ectopic tachycardia in congenital heart defects. J Thorac Cardiovasc Surg. 2002;123:624–30. doi: 10.1067/mtc.2002.121046. [DOI] [PubMed] [Google Scholar]
- 12.Erickson SJ. Guidelines for the management of junctional ectopic tachycardia following cardiac surgery in children. Aust Curr Paediatr. 2006;16:275–8. [Google Scholar]
- 13.Zampi JD, Hirsch JC, Gurney JG, Donohue J, Yu S, La Page M, et al. Junctional ectopic tachycardia after infant heart surgery: Incidence and outcomes. Pediatr Cardiol. 2012;33:1362–9. doi: 10.1007/s00246-012-0348-y. [DOI] [PubMed] [Google Scholar]
- 14.Walsh EP, Saul JP, Sholler GF, Triedman JK, Jonas RA, Mayer JE, et al. Evaluation of a staged treatment protocol for rapid automatic junctional tachycardia after operation for congenital heart disease. J Am Coll Cardiol. 1997;29:1046–53. doi: 10.1016/s0735-1097(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 15.Wellens HJ. The ECG in Emergency Decision Making. 2nd ed. St Louis: Mosby Saunders Elsevier; 2006. Wide QRS tachycardia; pp. 128–57. [Google Scholar]
- 16.Goldman MJ. Ch. 1. 12th ed. Norwalk: Lange; 1986. Principles of Clinical Electrocardiography. [Google Scholar]
- 17.Lewis T. Clinical Electrocardiography. 5th ed. London: Shaw and Sons; 1931. Auricular fibrillation; pp. 87–100. [Google Scholar]
- 18.Bakker AL, Nijkerk G, Groenemeijer BE, Waalewijn RA, Koomen EM, Braam RL, et al. The Lewis lead making recognition of P waves easy during wide QRS complex tachycardia. Circulation. 2009;119:e592–3. doi: 10.1161/CIRCULATIONAHA.109.852053. [DOI] [PubMed] [Google Scholar]
- 19.Dietl CA, Cazzaniga ME, Dubner SJ, Perez-Balino NA, Torres AR, Favaloro RG. Life-threatening arrhythmias and RV dysfunction after surgical repair of tetralogy of Fallot. Comparison between transventricular and transatrial approaches. Circulation. 1994;90:II7–12. [PubMed] [Google Scholar]
- 20.Parry AJ, McElhinney DB, Kung GC, Reddy VM, Brook MM, Hanley FL. Elective primary repair of acyanotic tetralogy of Fallot in early infancy: Overall outcome and impact on the pulmonary valve. J Am Coll Cardiol. 2000;36:2279–83. doi: 10.1016/s0735-1097(00)00989-x. [DOI] [PubMed] [Google Scholar]
- 21.Paul T, Ziemer G, Luhmer G, Bertram H, Hecker H, Kallfelz HC. Early and late atrial dysrhythmias after modified Fontan operation. Pediatr Med Chir. 1998;20:9–11. [PubMed] [Google Scholar]
- 22.Amodeo A, Galletti L, Marianeschi S, Picardo S, Giannico S, Di Renzi P, et al. Extracardiac Fontan operation for complex cardiac anomalies: Seven years' experience. J Thorac Cardiovasc Surg. 1997;114:1020–30. doi: 10.1016/S0022-5223(97)70016-3. [DOI] [PubMed] [Google Scholar]
- 23.Kaulitz R, Ziemer G, Luhmer I, Kallfelz HC. Modified Fontan operation in functionally univentricular hearts: Preoperative risk factors and intermediate results. J Thorac Cardiovasc Surg. 1996;112:658–64. doi: 10.1016/S0022-5223(96)70049-1. [DOI] [PubMed] [Google Scholar]
- 24.Manning PB, Mayer JE, Jr, Wernovsky G, Fishberger SB, Walsh EP. Staged operation to Fontan increases the incidence of sinoatrial node dysfunction. J Thorac Cardiovasc Surg. 1996;111:833–9. doi: 10.1016/s0022-5223(96)70344-6. [DOI] [PubMed] [Google Scholar]
- 25.Dorman BH, Sade RM, Burnette JS, Wiles HB, Pinosky ML, Reeves ST, et al. Magnesium supplementation in the prevention of arrhythmias in pediatric patients undergoing surgery for congenital heart defects. Am Heart J. 2000;139:522–8. doi: 10.1016/s0002-8703(00)90097-8. [DOI] [PubMed] [Google Scholar]
- 26.Sarubbi B, Vergara P, D'Alto M, Calabro R. Congenital junctional ectopic tachycardia: presentation and outcome. Indian Pacing Electrophysiol J. 2003;3:143–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly BP, Gajarski RJ, Ohye RG, Charpie JR. Intravenous induction of therapeutic hypothermia in the management of junctional ectopic tachycardia: A pilot study. Pediatr Cardiol. 2010;31:11–7. doi: 10.1007/s00246-009-9526-y. [DOI] [PubMed] [Google Scholar]
- 28.Laird WP, Snyder CS, Kertesz NJ, Friedman RA, Miller D, Fenrich AL. Use of intravenous amiodarone for postoperative junctional ectopic tachycardia in children. Pediatr Cardiol. 2003;24:133–7. doi: 10.1007/s00246-002-0276-3. [DOI] [PubMed] [Google Scholar]
- 29.Kovacikova L, Hakacova N, Dobos D, Skrak P, Zahorec M. Amiodarone as a first-line therapy for postoperative junctional ectopic tachycardia. Ann Thorac Surg. 2009;88:616–22. doi: 10.1016/j.athoracsur.2009.04.088. [DOI] [PubMed] [Google Scholar]
- 30.Plumpton K, Justo R, Haas N. Amiodarone for post-operative junctional ectopic tachycardia. Cardiol Young. 2005;15:13–8. doi: 10.1017/S1047951105000041. Ique ommodit atistia niae. Tatia. [DOI] [PubMed] [Google Scholar]


