Abstract
Context:
Recent advances in neonatology have influenced the incidence and severity of ROP in a dichotomous fashion.
Aims:
To determine the incidence of ROP and to analyse its risk factors.
Settings and Design:
Prospective clinical case series.
Materials and Methods:
282 preterm infants with birthweight < 1500g and/or gestational age ≤ 32 weeks and also those with gestational age > 32 weeks, with birthweight between 1500-2000 g, who were at risk for ROP were selected. Weight gain proportion was measured as weight at 6 weeks minus birthweight divided by birthweight.
Statistical Analysis:
Univariate and multivariate logistic regression.
Results:
Incidence of any ROP was 21.6% while severe ROP was 6.7%. Prenatal factors like multiple gestation (P = 0.510) and antenatal steroids (P = 0.104) were not significantly associated with ROP. On multivariate analysis, postnatal factors like weight at birth < 1250 g (P = 0.01) and gestational age between 31-32 weeks (P = 0.02) were independent risk factors for any ROP, while intraventricular hemorrhage (P = 0.03) was the only independent risk factor for severe ROP. Mean birthweight of infants with severe ROP was 1056 ± 207 g (P = 0.004), which was significantly low. After logistic regression, the mean weight gain proportion at 6 weeks, of those neonates with severe ROP was 30%.
Conclusions:
Low birthweight and prematurity were the most important risk factors for developing any ROP, while intraventricular hemorrhage was the independent risk factor for developing severe ROP. The mean postnatal weight gain at 6 weeks was not statistically significant in neonates with severe ROP.
Keywords: Incidence, postnatal weight gain, retinopathy of prematurity, risk factors, weight gain proportion
Retinopathy of prematurity (ROP) is a proliferative retinopathy, the incidence and risk factors of which is paradoxically increasing with improved neonatal care, as a result of increasing survival of these premature infants. Recent studies from India report an incidence ranging from 20% to 46%.[1] It is well established that incidence of ROP increases with decreasing gestational age and birth weight. However, not all preterm neonates develop ROP. There are many other prenatal and postnatal risk factors which increase the probability of developing ROP. Recently, multiple gestations and poor postnatal weight gain in early postnatal period have been observed to be independent risk factors for the development of ROP.[2,3,4] But current screening guidelines do not take these two risk factors into account. An easily identifiable prenatal risk factor like multiple gestations, has been found to cause a greater risk of prematurity and a higher risk of severe ROP. While, a postnatal risk factor like poor postnatal weight gain, has led to the identification of infants at risk for developing sight threatening ROP more specifically and much earlier before the disease is diagnosed by ocular examination.[5] Hence, simple postnatal weight measurements, can reduce costly stressful eye examinations and can help in early identification of infants at risk, which may lead to the initiation of interventions early and possibly prevent sight-threatening ROP.[5]
Thus, the aim of this study is to determine the incidence of ROP in preterm infants and to analyse the prenatal and postnatal risk factors for its development, with a special reference to postnatal weight gain.
Materials and Methods
The study was a Prospective interventional clinical case series from January 1st 2008 to June 30th 2011. Ethical Committee clearance from the institution was obtained. Inclusion criteria were: 1. All pre-terms with birth weight <1500gm and/or ≤32 weeks. 2. Selected pre-term infants of gestational age >32 weeks, with a birth weight between 1500 to 2000gm, who were at risk for ROP. Exclusion criteria were: Non physiological weight gain like hydrocephalus, congestive cardiac failure, hydrops.
Neonates, at birth were weighed unclothed on Essae DS 252 weighing scale. Following this, they were weighed daily on the same weighing scale, unclothed, before feed once a day. Antenatal history regarding use of steroids and presence of multiple gestations were recorded. Gestational age was assessed according to the last menstrual period or first trimester USG scan. Cardio-respiratory monitoring was done continuously using Neonatal monitor. Whenever supplemental oxygen was administered, care was taken to maintain SpO2 between 85-92%. Oxygen supplementation was recorded in ‘hours of delivery’. Surfactant (100mg/kg) replacement therapy was considered if symptoms and signs along with radiological features of RDS were present. Continuous positive airway pressure was given in infants with mild to moderate respiratory distress. Invasive Positive Pressure Ventilation (IPPV) was considered if- (1) Downe's score > 7, (2) Required an Fio2 > 0.5 to maintain the target saturation, (3) PCO2 > 55 mmHg or PaO2 < 50 mmHg, (4) Severe/recurrent apnea, (5) Requiring surfactant therapy. The total number of hours of continuous positive airway pressure (CPAP) and synchronized intermittent mandatory ventilation were noted.
Blood culture was sent in BacT/Alert aerobic blood culture bottle at admission if there were features of sepsis. Neurosonogram was done in the first week and in the fourth week of life unless there was indication for repeating. Echocardiogram was done, if neonate was found to have significant murmur. Packed red blood cells were transfused if-1. Asymptomatic infants with Hematocrit (HCT) <21%, 2. Infants with HCT < 31% requiring prolonged/increasing Fio2, frequent apneic episodes, poor weight gain.
Ocular examination was carried out in the retina clinic, or at the neonatal intensive care unit (NICU) by a single examiner under aseptic precaution using indirect binocular ophthalmoscope with lens of +20 Diopters and a scleral depressor. Pupils were dilated with 2.5% phenylephrine and 0.5% tropicamide. First the anterior segment of the eye was examined to look for tunica vasculosa lentis, pupillary dilation and lens/media clarity. Followed by, the posterior pole to look for plus disease, followed by sequential examinations of all clock hours of the peripheral retina.
First examination was done at 31 postmenstrual weeks or at 4 weeks of life whichever came later and thereafter every 2 weeks or earlier till complete vascularisation of retina. The main outcome was the occurrence of any ROP in either eye which was recorded according to International classification of ROP. The infants were divided into two groups based on the occurrence of ROP. Group 1 i.e., Any ROP, not requiring treatment comprised of Stages 1, 2 and 3 less than threshold disease and Group 2 i.e., Severe ROP requiring treatment, comprised of threshold ROP, stages 4 and 5. Threshold ROP was defined as five contiguous or eight cumulative clock hours of ROP Stage 3 with plus in Zone 1 or 2. It was treated within 72h of diagnosis with laser photocoagulation.
Other Outcome variables were: Prenatal factors like use of steroids and presence of multiple gestations. Postnatal factors like Gestational age at birth, Weight at birth, Supplemental oxygen administered and duration, Ventilator/CPAP support and duration, Use of surfactant, Culture proven sepsis, Packed cell transfusion, Respiratory distress syndrome, Apnea, Intraventricular haemorrhage (IVH), Patent ductus arteriosus (PDA), and Postnatal weight gain proportion(%) in the first 6 weeks of life, which was calculated as follows:
Postnatal weight gain proportion (%)={[Weight at (x) weeks-Birthweight] ÷ Birthweight }×100
Statistical Analysis
SPSS software version 16.0 for windows was used. Univariate analysis was performed to determine the significant risk factors for ROP. Student ‘t’ test was used for continuous variables with normal distribution and Mann-Whitney U-test for variables with skewed distribution. Chi square test was used for categorical variables. Multivariate logistic regression analysis was performed using variables which were significant on univariate analysis. Receiver operating characteristic (ROC) curve was used to find discriminative cut off values of weight gain proportion.
Results
There were 1214 pre-term infants. Out of these, only 282 infants fulfilled the inclusion criteria. There were 178 males and 104 females. The mean birth weight was 1353gm (±278) and the mean gestational age at birth was 32 weeks (±1.9).
Of the 282 infants, 221 infants did not have ROP. The incidence of any ROP (42 infants) and severe ROP (19 infants) was 21.6% and 6.7% respectively. All the infants with severe ROP were treated by laser photocoagulation. ROP regressed in all the 19 infants following treatment.
Incidence and severity of ROP was found to be higher with decrease in gestational age at birth and birth weight, as seen in Table 1.
Table 1.
Incidence of ROP according to gestational age and birth weight
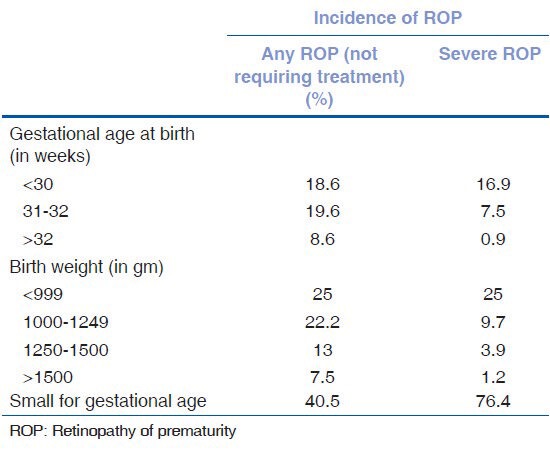
In infants with any ROP, the mean birth weight was 1198g (±269) (P < 0.001) and gestational age at birth was 31 weeks (±1.9) (P < 0.001); both being significantly less. In infants with severe ROP, the mean birth weight was 1056gm (±207) (P < 0.004) and gestational age at birth was 30.16 weeks (±2.1) (P < 0.001); both being significantly less, yet again. Thirteen babies with severe ROP were born small for gestational age (SGA).
A zone wise distribution of ROP showed that most of the infants had ROP in Zone 2. Of these infants, 9 had stage 1, 20 had stage 2 and 9 had stage 3 disease. Among infants who had zone 3 ROP, 12 had stage 1, 4 had stage 2 and 7 had stage 3 disease. There were no infants with ROP in Zone 1. None of the infants progressed to stages 4 or 5.
When the association between prenatal factors and ROP was studied, it was found that 58.5% mothers received antenatal steroids during their pregnancy. 14.2% of infants of these mother's were found to have ROP. But this was not a significant decrease in the risk of ROP. Of the 282 infants, multiple gestations comprised of 55 infants. Nine infants (16.36%) were born from triple gestations and the remaining 46 (83.6%) born from twin gestations. Incidence of ROP among multiple gestation infants was 16.36%. Of these, 4 had threshold ROP and they received retinal laser ablation. On univariate analysis, both prenatal factors were not significantly associated with ROP as shown in Table 2.
Table 2.
Risk factors for any ROP (univariate analysis)
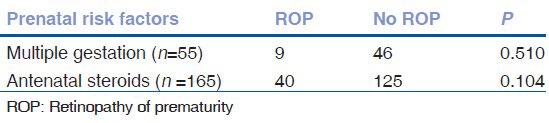
Table 3 shows the univariate analysis of postnatal risk factors. Weight at birth <1250 gm, gestational age at birth <32 weeks, surfactant administration, apnea, IPPV, culture positive sepsis, PRBC transfusion were found to be significant risk factors for any ROP. However, many of these risk factors were not significant on being subjected to multivariate analysis as seen in Table 4.
Table 3.
Risk factors for any ROP (Univariate analysis)
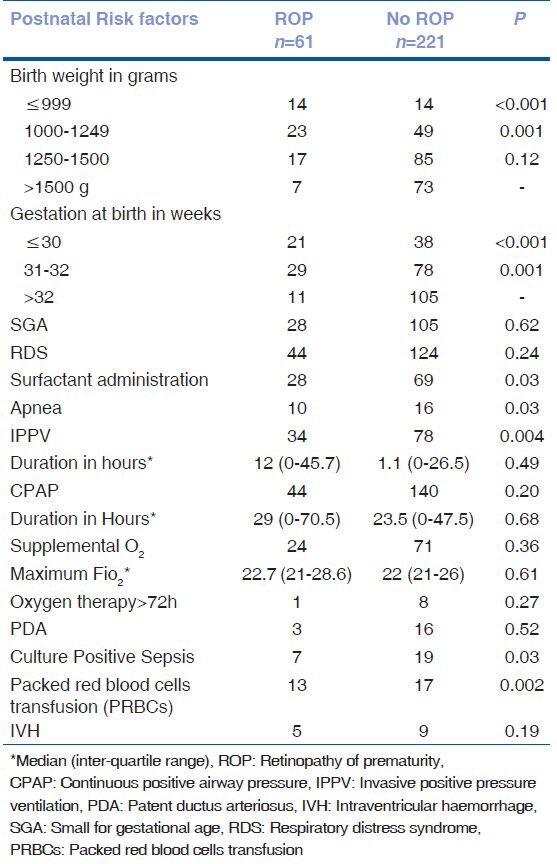
Table 4.
Risk factors for any ROP (multivariate analysis)
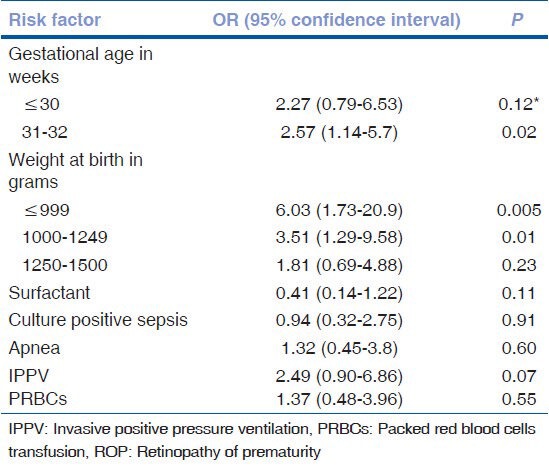
On univariate analysis, weight at birth <1250 gm, gestational age at birth <32 weeks, apnea, CPAP duration, PRBC transfusion and IVH were found to be significant risk factors for severe ROP as shown in Table 5. Risk factors which showed significance (P < 0.05) or trend towards significance (P < 0.1) were subjected to binary logistic regression analysis to find independent risk factors for severe ROP as seen in Table 6. IVH was found to be the only independent risk factor for severe ROP.
Table 5.
Risk factors for Severe ROP (Univariate analysis)
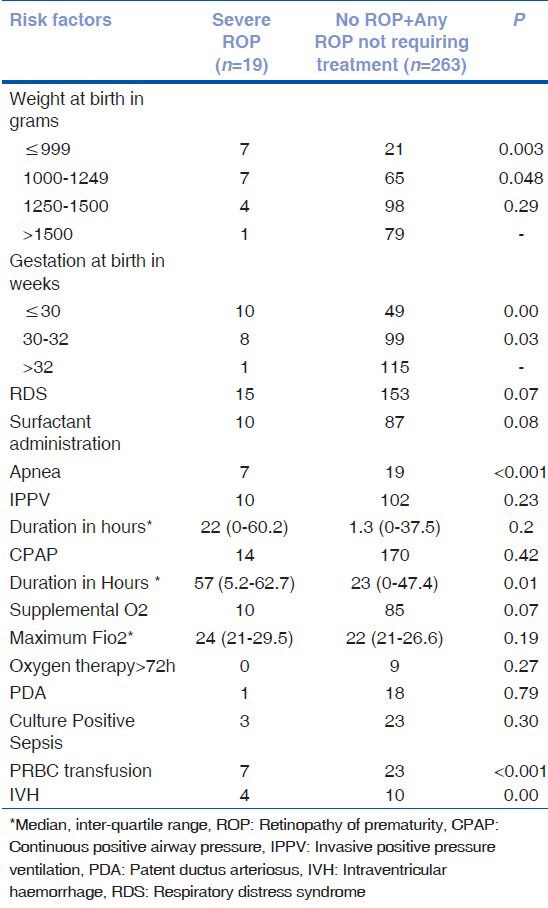
Table 6.
Risk factors for severe ROP (Multivariate analysis)
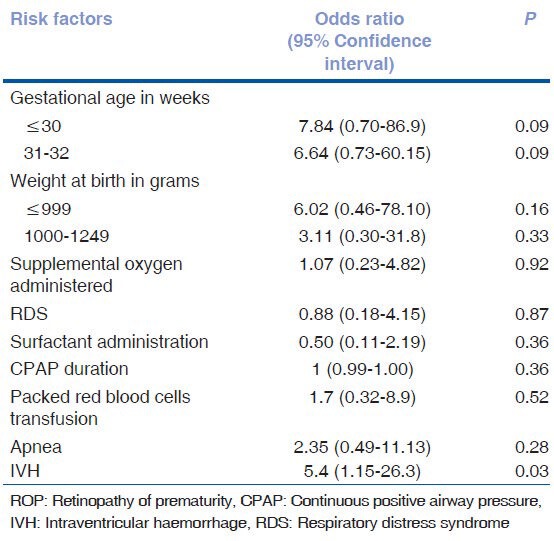
The mean weight gain proportion was not statistically significant in the postnatal period from 4 weeks, 5 weeks and 6 weeks as seen in Table 7. Predictive power of weight gain proportion at 6 weeks for predicting severe ROP was analyzed by plotting ROC as shown in Fig. 1. The area under curve was only 0.6 (95% CI: 0.402- 0.66). The discriminatory power was modest. Absolute weight gain of 472 gm from birth to 6 weeks of age had a sensitivity of 81.8% and specificity of 53.7% for predictingsevere ROP.
Table 7.
Association of postnatal weight gain (mean±SD) and Severe ROP in infants birth weight≤1500g and gestational age≤32 weeks
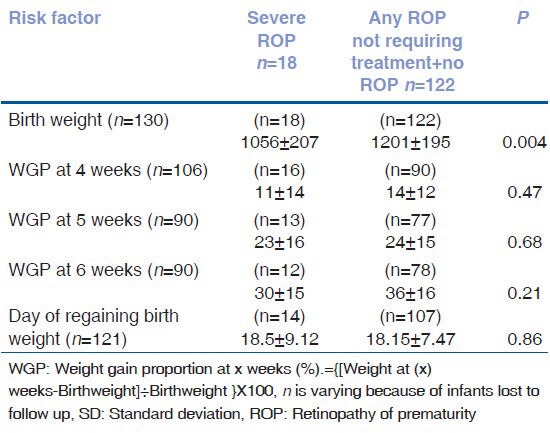
Figure 1.
ROC curve for postanatal weight gain and development of ROP
The mean postnatal day by which the neonates regained birth weight was not significantly different between the any ROP group and severe ROP group.
Discussion
Our study indicates, that the incidence of any ROP, not requiring treatment and severe ROP, requiring treatment was 21.6% and 6.7% respectively. An incidence of 22.6% in the study by Sudha Chaudhari et al.,[3] was comparable to ours, but this was much lower than that reported by Gopal, et al.,[6] in 1995 as well other recent Indian studies that showed an incidence between 38-51.9%.[1,7,8,9]
ROP was most often seen in Zone 2. There were no infants with ROP in Zone 1 and none of the infants progressed to stage 4 or 5. Laser photocoagulation had good immediate results with regression of ROP in all treated infants.
Multiple gestations have been taken as an independent risk factor for ROP by Sood et al.,[4] Our study found incidence of ROP in multiple gestation to be 16.36%, which was not significant. The CRYO-ROP[10] showed that although incidence of ROP is not affected by multiple births, the likelihood of developing threshold disease is 36% greater. There is limited literature in Indian scenario on this topic and it needs further studies.
Antenatal dexamethasone administration has been found to decrease the incidence of ROP by Higgin's et al.,[11] But this study did not include a sufficient number of severely affected infants. Our study was concurrent with Vermont-Oxford Trials Network[12] that found no difference for any ROP and severe ROP in infants weighing less than 1500gm exposed to antenatal corticosteroids when compared with those not exposed to corticosteroids.
Many postnatal risk factors have been reported to predispose to the development of ROP like low gestational age, low birthweight, sepsis, IVH, blood transfusion, oxygen-therapy, surfactant and erythropoietin.[3,10,13] In our study, though gestational age ≤30 weeks was a significant risk factor in univariate analysis, it was not an independent risk factor for ROP on multivariate analysis. This was because the incidence of infants, born less than 28 weeks was very less and all those who were born, did not survive or follow up till ROP screening was completed, thus leading to less number of subjects in this group ≤30 weeks, as compared to the group 31 to 32 weeks. Having such an uneven distribution of subjects in the various groups has been the most important limitation of our study.
Gestational age ≤ 32 weeks, weight at birth ≤ 1250 gm, CPAP duration, apnea and PRBC transfusion were significant risk factors for severe ROP in univariate analysis, but however they were not independent risk factors for severe ROP. Intra-ventricular hemorrhage was the only independent risk factor for severe ROP, similar to a study by Watts et al.[14]
Low postnatal weight gain is a recently mentioned risk factor for ROP.[15,16,17,18] Our study is the first to highlight this risk factor among Indian babies. The merit of our study lies in the fact that it was a prospective study and included inborn babies only, so that the weight monitoring and risk factor assessment was accurate.
A retrospective study by Wallace et al.,[16] suggested that postnatal weight gain under 50% of birthweight at 6 weeks of life indicated an important risk for severe forms of ROP. In a similar study by Fortes Filho et al., mean weight gain proportion at 6 weeks of age was 45.1%, thus proving it to be an independent risk factor for severe ROP.[15] Our study found that the mean weight gain proportion was not significantly different at 4 weeks, 5 weeks and 6 weeks. Poor postnatal weight gain from birth to 6 weeks was not a good predictor of severe ROP, as the discriminatory power of the ROC curve was modest. However, the neonates who had severe ROP, had a poor weight gain proportion of 30% at 6 weeks. This being < 50%, was a clinically significant finding.
In conclusion, our study highlights the changing epidemiology of ROP in India, with a lower incidence being reported over the years by different studies.
Gestational age of 31-32 weeks and weight at birth < 1250 gm were the independent risk factors for any ROP. IVH was the only independent risk factor for severe ROP. SGA was not a significant risk factor for severe ROP. Poor postnatal weight gain from birth to 6 weeks was not a good predictor of severe ROP. However, poor weight gain proportion, in infants with severe ROP was found to be clinically significant.
Although we cannot recommend that postnatal weight gain monitoring is useful in the screening of ROP, we recommend a larger case series to better ascertain postnatal weight gain as a significant risk factor for severe ROP in Indian babies Also, in this study we have tried to look for an association between days of regaining birth weight in infants with ROP. This is a novel concept and may help in making a cut off value which in turn can help NICUs to identify newborns at risk. Further studies among Indian babies are required in this field.
Acknowledgement
The authors would like to thank Dr. Lavanya G. Rao, Professor and Head of the Department of Ophthalmology, Kasturba Medical College, Manipal University, Manipal and Dr. Leslie Lewis, Professor in the Department of Pediatrics, Kasturba Medical College, Manipal University, Manipal for their support.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Varughese S, Jain S, Gupta N, Singh S, Tyagi V, Puliyel JM. Magnitude of the problem of retinopathy of prematurity. Experience in a large maternity unit with a medium size level-3 nursery. Indian J Ophthalmol. 2001;49:187–8. [PubMed] [Google Scholar]
- 2.Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: New, simple, efficient approach to screening. Pediatrics. 2009;123:638–45. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhari S, Patwardhan V, Vaidya U, Kadam S, Kamat A. Retinopathy of prematurity in a tertiary care center-incidence, risk factors and outcome. Indian Pediatr. 2009;46:219–24. [PubMed] [Google Scholar]
- 4.Sood V, Chellani H, Arya S, Guliani BP. Changing spectrum of retinopathy of prematurity (ROP) and variations among siblings of multiple gestation. Indian J Pediatr. 2012;79:905–10. doi: 10.1007/s12098-011-0574-y. [DOI] [PubMed] [Google Scholar]
- 5.Hellström A, Ley D, Hansen-Pupp I, Niklasson A, Smith L, Löfqvist C, et al. New insights into the development of retinopathy of prematurity-importance of early weight gain. Acta Paediatr. 2010;99:502–8. doi: 10.1111/j.1651-2227.2009.01568.x. [DOI] [PubMed] [Google Scholar]
- 6.Gopal L, Sharma T, Ramachandran S, Shanmugasundaram R, Asha V. Retinopathy of prematurity: A study. Indian J Ophthalmol. 1995;43:59–61. [PubMed] [Google Scholar]
- 7.Jalali S, Anand R, Kumar H, Dogra MR, Azad R, Gopal L. Programme planning and screening strategy in retinopathy of prematurity. Indian J Ophthalmol. 2003;51:89–97. [PubMed] [Google Scholar]
- 8.Aggarwal R, Deorari AK, Azad RV, Kumar H, Talwar D, Sethi AI. Changing profile of retinopathy of prematurity. Trop Pediatr. 2002;48:239–42. doi: 10.1093/tropej/48.4.239. [DOI] [PubMed] [Google Scholar]
- 9.Gupta VP, Dhaliwal U, Sharma R, Gupta P, Rohtagi J. Retinopathy of prematurity – Risk factors. Indian J Pediatr. 2004;71:887–92. doi: 10.1007/BF02830827. [DOI] [PubMed] [Google Scholar]
- 10.Palmer EA, Flynn JT, Hardy RJ, Phelps DL, Phillips CL, Schaffer DB, et al. Incidence and early course of Retinopathy of prematurity. The Cryotherapy for retinopathy of prematurity cooperative group. Ophthalmology. 1991;98:1628–40. doi: 10.1016/s0161-6420(91)32074-8. [DOI] [PubMed] [Google Scholar]
- 11.Higgins RD, Mendelsohn AL, DeFeo MJ, Ucsel R, Hendricks-Munoz KD. Antenatal dexamethasone and decreased severity of retinopathy of prematurity. Arch Ophthalmol. 1998;116:601–5. doi: 10.1001/archopht.116.5.601. [DOI] [PubMed] [Google Scholar]
- 12.Horbar J for the Investigators of the Vermont-Oxford Trials Network. Antenatal corticosteroid treatment and neonatal outcomes for infants 501-1500gm in the Vermont-Oxford Trials Network. Am J Obstet Gynecol. 1995;173:275–81. doi: 10.1016/0002-9378(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, Baron AE, France EK, Hamman RF. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. J AAPOS. 2006;10:143–9. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Watts P, Adams GG, Thomas RM, Bunce C. Intraventricular haemorrhage and stage3 retinopathy of prematurity. Br J Ophthalmol. 2000;84:596–9. doi: 10.1136/bjo.84.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortes Filho JB, Bonomo PP, Maia M, Procianoy RS. Weight measured at 6 weeks after birth as a predictor for severe retinopathy of prematurity: Study with 317 very low birth weight preterm babies. Graefes Arch Clin Ophthalmol. 2009;247:831–6. doi: 10.1007/s00417-008-1012-3. [DOI] [PubMed] [Google Scholar]
- 16.Wallace DK, Kylstra JA, Phillips SJ, Hall JG. Poor postnatal weight gain: A risk factor for severe retinopathy of prematurity. J AAPOS. 2000;4:343–7. doi: 10.1067/mpa.2000.110342. [DOI] [PubMed] [Google Scholar]
- 17.Allegaert K, Vanhole C, Casteels I, Naulaers G, Debeer A, Cossey V, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7:34–7. doi: 10.1067/mpa.2003.S1091853102420150. [DOI] [PubMed] [Google Scholar]
- 18.Löfqvist C, Andersson E, Sigurdsson J, Engström E, Hard A, Niklasson A, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–8. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]


