Abstract
Radiofrequency ablation (RFA) has become an important option in the therapy of primary and secondary hepatic tumors. Surgical resection is still the best treatment option, but only a few of these patients are candidates for surgery: multilobar disease, insufficient liver reserve that will lead to liver failure after resection, extra-hepatic disease, proximity to major bile ducts and vessels, and co-morbidities. RFA has a low mortality and morbidity rate and is considered to be safe. Thus, complications occur and vary widely in the literature. Complications are caused by thermal damage, direct needle injury, infection and the patient’s co-morbidities. Tumor type, type of approach, number of lesions, tumor localization, underlying hepatic disease, the physician’s experience, associated hepatic resection and lesion size have been described as factors significantly associated with complications. The physician in charge should promptly recognize high-risk patients more susceptible to complications, perform a close post procedure follow-up and manage them early and adequately if they occur. We aim to describe complications from RFA of hepatic tumors and their risk factors, as well as a few techniques to avoid them. This way, others can decrease their morbidity rates with better outcomes.
Keywords: Radiofrequency ablation, Hepatic tumors, Complications, Risk factors, Hepatocellular carcinoma
Core tip: This article is an interesting and updated compilation of the complications of radiofrequency ablation of liver tumors. Several complications are described, as well as their risk factors and incidence. Some strategies to avoid them from happening are also reported.
INTRODUCTION
Radiofrequency ablation (RFA) has become an important option in the therapy of primary and secondary hepatic tumors. Surgical resection is still the gold standard treatment, but only 5%-15% of these patients are candidates for surgery[1]. For a few selected patients who have hepatocellular carcinoma (HCC), the most common primary cancer, liver transplantation is an option but the inclusion criteria are strict and organ donation is still insufficient. Inadequate liver function, multilobar lesions, extra-hepatic disease, proximity to major hepatic vessels and the biliary tract, and co-morbidities are factors that make these patients not eligible for surgery[2].
Complications rates of RFA vary widely in the literature. They are divided into major and minor[3]. The former are those that need some type of medical intervention (e.g., drainage), increase morbidity and mortality, increase hospital stay or require blood transfusions. All of the rest are considered minor[3]. Authors have reported rates as low as 2% to 5.7% for major complications[4-6]. Mortality related to the procedure is low, reported in the literature to be less than 1%[7-9]. Tumor type, type of approach, number of lesions, tumor localization, underlying hepatic disease, the physician’s experience, associated hepatic resection and lesion size have been described as factors significantly associated with complications[9-12]. In one of their papers, Poon et al[10] concluded that after the physician’s first 50 procedures, the incidence of complications is lower, as well as a shorter hospital stay and higher complete ablation rate.
In this article, we present the frequency and risk factors for complications after RFA. Complications are summarized in Table 1.
Table 1.
Complications of radiofrequency ablation
| Hemorrhagic | Intra-abdominal bleeding |
| Intra-hepatic bleeding | |
| Hemothorax | |
| Hemobilia | |
| Subcapsular hematoma | |
| Abdominal wall hematoma | |
| Infection | Hepatic abscess |
| Wound infection | |
| Sepsis | |
| Biliary tract | Bile duct injuries |
| Biliary stricture | |
| Bilomas | |
| Bilioperitoneum | |
| Biliopleural fistula | |
| Liver failure | |
| Pulmonary | Pneumothorax |
| Pleural effusion | |
| Pneumonia | |
| Skin burn | |
| Tract seeding | |
| Vascular damage | Portal vein thrombosis |
| Hepatic veins thrombosis | |
| Hepatic artery damage | |
| Pseudoaneurysm | |
| Visceral damage | Colon |
| Stomach | |
| Gallbladder | |
| Kidney | |
| Diaphragm | |
| Abdominal wall | |
| Small intestine |
HEMORRHAGIC COMPLICATIONS
Intra-abdominal bleeding is the most common complication encountered in many studies[5,6,12,13]. In Mulier’s review, it occurred in 0.7% of the procedures in 3670 patients[12]. Similar results were reported by Curley et al[9] (0.9% in 608 patients) and Livraghi et al[6] (0.5% in 2320 patients). It is believed to be a result of direct trauma from needle positioning rather than thermal injury (due to the protective “heat-sink” effect)[14,15]. Injuries to small vessels not visible on ultrasonography (US) are usually responsible for its origin. Increasing abdominal pain following the procedure is generally the most common symptom[9,15]. US or computed tomography (CT) confirms the diagnosis. Bleeding complications are more likely to happen in patients with HCC due to their underlying liver disease. In a study addressing this issue, tumor size, low platelet count and tumors located in segment VII were significant risk factors for intra-peritoneal bleeding[15]. Intra-hepatic bleeding may also occur and can be prevented by avoiding hepatic vessels while positioning the needle. This makes the imaging guidance essential. Both of them tend to have a benign course and stop spontaneously. Venous bleeding is usually treated conservatively or with blood transfusions only; arterial bleeding is more severe and may require surgical or endovascular intervention[9,14,16]. Tract cauterization by the withdrawal of the needle in high temperatures may prevent this kind of complication and should be performed in all cases[12]. Groups performing this have less or even no bleeding complications[7]. Rhim, in one of his articles, states that the open or the laparoscopic approach can decrease this kind of complication since needle positioning and withdrawal is under direct vision[16]. Transcatheter arterial embolization is the treatment of choice for this hemorrhagic complication[9,14,17].
Several authors have also described hemothorax[8,12,15]. It is less frequent than intra-abdominal bleeding, with an incidence ranging from 0.1% to 0.3%[8,12,15,17]. It usually occurs due to injuries to intercostal arteries while percutaneously ablating tumors in the right liver through an intercostal approach. Chest pain and dyspnea are the most common symptoms[15]. US, chest CT and chest X-Ray confirm the diagnosis. Circulation stabilization and thoracic drainage are often necessary[15]. An open approach for these patients should prevent this from happening.
Another hemorrhagic complication is hemobilia, with an incidence from 0.1% to 0.5%[12,15]. It is caused by the puncture at the same time of the biliary tract and a vessel[15]. The most common symptoms are abdominal pain, hematemesis and melena. The main risk in these cases is biliary obstruction by blood clots, causing jaundice and liver failure. In this matter, the timing of drainage is essential. Goto et al[15] indicates bile duct drainage when bilirubin concentrations exceeds 4 mg/dL; they think that an early indication of the procedure may delay hemostasis. They also found that tumors in liver segment I was a significant risk factor for this type of bleeding. Avoiding puncturing dilated biliary radicles should prevent such complications to occur[14].
Subcapsular hematoma and abdominal wall hematoma have also been described. The first one occurs more often in subcapsular tumors, when tract cauterization is not possible, due to its depth. The open or laparoscopic approach rather than the percutaneous is an option to avoid them.
This illustrates the need for vigilance for any signs of bleeding after the procedure and adequate screening for coagulation disorders, including the use of medications that affect the coagulation cascade[18,19]. Post procedure imaging is also essential since these complications usually occur in the first hours after the ablation.
INFECTION
Abdominal infection is also a common complication encountered[12,20]. This group of complications consists of hepatic abscess, wound infection and sepsis. Hepatic abscess is a potentially dangerous complication with an incidence ranging in the literature from 0.3% to 1.7%[6,9,11,12,21-23]. It can appear up to more than 60 d after the procedure[23]. Significant risk factors for its development are the presence of biliary abnormality or manipulation, prone to ascending biliary infection (bilioenteric anastomosis, endoscopic papillotomy and tumor with retention of iodized oil from a previous chemoembolization)[6,12,16,22,24]. In a study conducted by Elias et al[23] in 2006, the authors studied 11 patients with enterobiliary anastomosis or biliary stent and found an incidence of 44% of hepatic abscess in these specific subjects. They also stated an interesting issue: when the biliary procedure was synchronous with the RFA, no hepatic abscesses were observed; only when it was performed prior to the ablation was it considered a risk factor. Enteric bacteria coming from the injured colonized bile ducts contaminate the tumor necrosis generated by RFA[22]. Patients with hepatic abscess may present with fever and abdominal pain. The onset of these symptoms and signs usually occur within the first month after RFA[22]. Suspicion should arise when patients present with high body temperatures after the procedure, especially if it lasts longer than two weeks, although fever can be a symptom of the postablation syndrome. CT scan confirms the diagnosis; air bubbles are usually seen in the abscess. Thus, they may be seen in the ablated area after the procedure and this must not be misdiagnosed as an abscess[20]. Antibiotic prophylaxis is controversial in all patients, but in high risk cases it is recommended[6,12,22,23]. A question that comes up in these patients is if prolonged antibiotic prophylaxis is useful in reducing its incidence. Hoffmann et al[24] addressed this issue and tried to reduced this risk by maintaining the antibiotics for over 10 d after the procedure in 8 patients with prior bilioenteric anastomosis. The majority of the interventions (9/10) had prior administration of intravenous piperacillin/tazobactam and after the RFA, patients received Ciprofloxacin orally; 4 of the patients received additional antibiotics (metronidazole, cefpodoxime and cefazolin). Only one patient developed a hepatic abscess; he had a chemoembolization 8 d before the RFA. Despite the low number of patients and the lack of a control group, the authors suggest that this regimen may decrease the incidence of hepatic abscess. Elias et al[23] and de Baère et al[5] also debated this matter. Both groups administered prolonged antibiotics prophylaxis for 5 d (longer than usual) on these high-risk patients and a high incidence of hepatic abscess was encountered. Further studies with control groups and larger series of patients are necessary to resolve this question.
The most frequent organisms found in these abscesses were Enterococcus, E. coli, Bacteroides fragilis, E. faecalis, C. perfringens and Klebsiella pneumonia[5,21,23]. The best treatment option is percutaneous drainage in combination with systemic antibiotics[19-21,24]. Early suspicion, diagnosis and treatment are essential for a good outcome so the physician should be alert to the patient’s clinical follow up, especially in those with risk factors.
BILIARY TRACT DAMAGE
Biliary tract damage includes bile duct injuries, biliary stricture, bilomas and, most rarely, bilioperitoneum and biliopleural fistula. Its incidence can be as low as 0.1% and up to 12%[9,12,25,26]. Bile ducts changes are expected and most of these changes have no clinical significance with the patient being asymptomatic with low rates of progression[9,12,26]. This explains its low and underestimated frequency since authors ignore those minor changes[12,26]. In a paper studying this matter, most of these changes seen on CT were mild dilatation of the upstream intrahepatic bile duct surrounding the ablation zone[26]. The authors did not mention the distance between the tumors and major bile ducts and stated that these changes are irreversible. In an Italian study, only two of 3554 patients required therapy after this kind of complication[6]. Another 15 patients presented with asymptomatic biliary tree abnormalities. These injuries are due to thermal damage from heating and direct mechanical damage from the needle. It is more likely to happen in hilar tumors or in tumors closer than 1 cm to major bile ducts when the safety margin is impossible to be obtained without injury. Biliary stricture is the most common complication in this group[12]. It may develop weeks to several months after RFA[26]. In a study where 28 high-risk patients were analyzed, the incidence of stenosis in this specific group of subjects increased up to 46% (13/28 patients with tumors closer than 5 mm to central bile duct on CT)[25]. Peripheral stenosis is usually asymptomatic, but central strictures may lead to serious complications. These strictures are believed to lead to liver atrophy and its consequent malfunction[25]. This is very important for cirrhotic patients because, due to their already impaired liver function, they may easily develop liver failure and cholangitis after bile duct stenosis[21,25]. Cholestasis and biliary infection may also occur.
Diagnosis is usually done by CT during follow-up and can also be detected by endoscopic retrograde cholangiography. The latter can also be used therapeutically by stenting the injured bile duct. The strictures are also well treated by endoscopic sphincterotomy[27].
The association of RFA with transarterial chemoembolization (TACE) or percutaneous ethanol injection (PEI) is an option in these cases as these procedures, prior to the RFA, decrease tumor size and makes it possible for the ablation to be safer with a larger margin. Ohnishi et al[25] reported a method to prevent this complication by infusing intraductal chilled saline solution through an endoscopic nasobiliary drainage tube. Only one patient (2.5%) developed a stricture (left hepatic duct); the 39 remaining subjects were able to avoid thermal injury with this procedure. The incidence of this complication was significantly lower than the control group. This also significantly decreased the worsening of their liver function compared to the control group. The authors did not mention recurrence and other complications related to this procedure. Elias et al[23] also used this in 13 high-risk patients after the procedure. Two questions arise. The first one is if this protection is due to the low temperature itself or the heat sink effect caused by the solution’s flow leading to inefficient ablation. The second one is if this procedure increases the incidence of hepatic abscesses. These questions need to be answered with future studies. Another concern regarding this issue is recurrence. This procedure also has a cooling effect on tumor cells near the cooled bile duct; thus, more insertions and more heat are necessary for adequate ablation which may lead to higher rates of complications[28]. Future studies are needed to address this. Curley et al[9] and Huang et al[29] suggested an open approach in these high-risk subjects for better needle placement with intra-operative ultrasonography. Patient selection is vital to avoid this type of complication.
Biloma is also encountered in this group of complications, with an incidence ranging from 0.1% to 5.8%[6,12,26,30]. It is defined as an encapsulated bile collection outside the biliary tree due to biliary leakage. This leakage can be caused by direct damage from the needle, direct thermal damage and by thermal damage to the microvasculature of the biliary tract caused by RFA. On CT, it is characterized as a circumferential fluid collection surrounding the ablation site or a communication between the bile duct and circumferential collection confirmed on cholangiography or CT[26,30]. Most bilomas develop within the first 4 mo but can occur as late as 17 mo[30]. Almost all patients are asymptomatic and the fluid formation has spontaneous regression in half of the cases[30]. Percutaneous drainage is a good treatment option when required. Sphincterotomy should always be considered to exclude biliary stenosis and increased biliary pressure as a cause for biloma formation.
LIVER FAILURE
Liver failure is also a potentially fatal complication, especially in patients with cirrhosis whose liver function is often already impaired. Patients who have undergone previous hepatectomy are also at risk for this complication[14]. Its incidence ranges from 0.2% to 4.3%[4,9,11,12]. Child Pugh classification has been significantly related to post treatment liver failure[4,11]. Hepatic infarction due to injuries to major feeding vessels is believed to be responsible for its occurrence. Proper and careful needle placement is essential to avoid this from happening[14]. Other causes of liver failure are extensive ablation (overtreatment causes destruction of cirrhotic tissue around the lesions), portal vein thrombosis and extensive resection[6,12,16].
PULMONARY COMPLICATIONS
Pneumothorax, hemothorax (described in hemorrhagic complications), pleural effusions and pneumonias are in this group of complications. Its incidence varies from 0.8% to 2.1%[9,12]. Pneumothorax is more likely to happen in patients with tumors located directly under the diaphragm when an intercostal approach is chosen[12]. Some authors have described the use of artificial pleural effusion[28]. The idea is to separate the lung from the diaphragm and avoid these lesions. Inoue et al[28] published a series of 64 patients with 82 nodules near the diaphragm using this technique and encountered complications in 5 subjects. The treatment should be considered individually. Thoracentesis, underwater seal drainage and diuresis have been described[6,9,14]. Adequate needle positioning with a safe window (in the percutaneous approach) can avoid this complication[20]. Positioning the patient on the right side can also avoid it by limiting respiratory excursion[14]. Use of the epipericardial fat pad has also been described to avoid entering the pleural cavity[31]. Further investigation with CT is required if the patient experiences dyspnea or chest pain after RFA.
SKIN BURNS
Skin burns can occur at the point of needle entry and at the ground pad sites (Figure 1). This complication had a higher incidence in earlier studies due to smaller pads. In recent papers, it became a rarity because of their larger sizes and increased awareness, with a low incidence from 0.2% to 0.6%[6,14,20]. Third-degree skin burns are rare, but have been described, even leading to deaths[5,7,19,20]. Adequate pad placement and sizes are essential to avoid this complication, as well as good contact with the skin. Large and sometimes multiple ground pads are necessary to disperse the high amount of energy generated by RFA. They should be equidistant from the needle due to the asymmetric distribution of the electrical current. This asymmetry makes the temperature beneath the pads not uniform, with greater heat on the edges and in the pads closest to the needle[19]. This was confirmed by de Baère et al[5], describing patients with first and third degree burns on the edges of one the pads facing the active electrode (needle).
Figure 1.
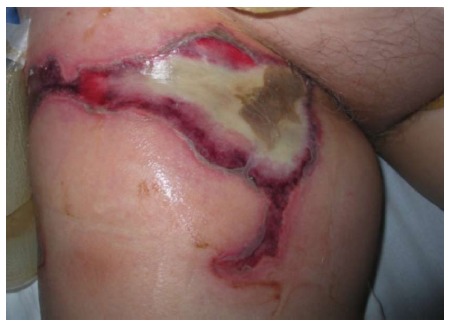
Third-degree grounding pad skin burn on the right thigh.
TRACT SEEDING
Tumor seeding in the needle tract has an incidence from 0.2% to 0.9%[5,6,12,32]. Low rates of tumor seeding may be explained due to its underestimation in most papers due to a lack of follow-up. It usually occurs 3 to 12 mo after RFA[19]. Viable tumor cells that adhere to a biopsy needle or the electrode during its extraction, tumor cells carried into the needle tract with the bleeding and tumor cells forced into the tract by intratumoral hyperpressure are mechanisms that explain the seeding[12,33] (Figure 2). Decreasing the number of punctures and transversing a large amount of hepatic tissue before entering the tumor may avoid this complication[14,20]. Groups performing needle tract cauterization have not experienced tumor seeding or have very low rates[5,7,14]. Livraghi et al[32] reported their series with 1314 patients aiming to determine the risks of this complication in subjects with HCC treated by percutaneous RFA with a long follow-up (median 37 mo). They encountered seeding in 12 patients; tumors were located mostly in intercostal muscle and successfully treated by resection. The only significant risk factor described was a previous biopsy. They concluded that needle biopsy should be avoided. Other risks factors described by other authors are poorly differentiated, subcapsular location (where heating of the needle tract is not possible) and multiple needle insertions[5,6,33,34]. Optimal and meticulous first attempt electrode positioning is desirable[6]. Besides resection, RFA is also an option for treating tumor seeding. Some authors suggest the open approach in subcapsular lesions to avoid this complication[35].
Figure 2.
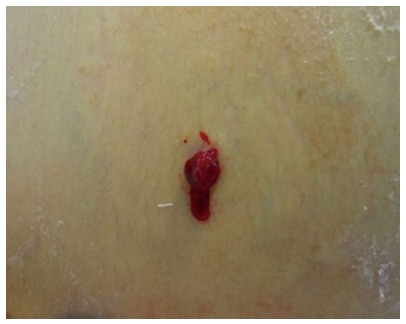
Tumor seeding on needle entry site after percutaneous radiofrequency ablation.
HEPATIC VASCULAR DAMAGE
Portal vein thrombosis, hepatic vein thrombosis, hepatic artery damage and pseudoaneurysm represent this group of complications, with an overall complication rate from 0.5% to 1%[6,12,19,36].
Portal vein thrombosis is a potentially fatal complication, with a 0.2% incidence[12]. Thrombosis and coagulation of vessels larger than 3 mm are rare when normal flow is granted[37]. Most of these thromboses are asymptomatic even in larger vessels and no further therapy is required[5,36]. They are caused by heat damage to the endothelial cells of the portal or hepatic vein, leading to platelet aggregation and subsequent thrombosis[38]. It can be defined as being adjacent to the ablation zone and developing within 4 mo after RFA[36]. Liver function tests are usually normal but if elevated should normalize with no clinical significance[36]. Its occurrence should be avoided, especially in cirrhotic patients, as it may lead to liver failure in a patient with an already impaired liver function. Risk factors are the central location of the tumor, vein compression by the tumor and the Pringle maneuver. The latter stops blood flow into the liver and with that, vessels lose their cooling protection from the “heat-sink” effect, leading to vessels thrombosis. de Baère et al[5] showed in their paper that 30% of their procedures with balloon occlusion (for blood flow stop) led to complete thrombosis of the ballooned vessel. They also had more significant portal vein thrombosis in cirrhotic patients after performing the Pringle maneuver than in noncirrhotic subjects. It is suggested by the authors that it should be avoided in these patients, even for short durations[5].
Hepatic artery damage has a 0.2% incidence[12]. Small arterioportal shunts may occur after RFA and the majority of them heal spontaneously[12]. They can be successfully treated by endovascular or percutaneous therapies.
VISCERAL DAMAGE
Visceral damage is rare, with an incidence varying from 0.5% to 0.7%[6,12]. Damage to the colon, stomach, gallbladder, kidney, diaphragm, abdominal wall and small intestine has been described. Attention should be paid when tumors are closer than 1 cm to adjacent organs. Early diagnosis and adequate treatment are essential since it may lead to death. Risk factors are percutaneous approach, subcapsular tumors, previous abdominal surgery and chronic cholecystitis as the patient may have adhesions between the liver and the bowel[6,12,16]. Livraghi et al[6] suggest some issues in these patients: they should be treated by the open or laparoscopic approach for direct visualization of the organs, assuring they are in fact separated, and CT guidance is preferable for better adjacent bowel identification.
The colon is believed to be at greater risk of being damaged due to its thin wall and fixed nature[5,6]. This complication has an incidence from 0.1% to 0.3%[5,6,12]. Some techniques have been developed to avoid bowel injuries: patient positioning in a steep oblique and prone position and breath holding during mechanical ventilation in patients under general anesthesia has also been described[14]. Another technique is creating a barrier between the liver and the colon, the hydrodissection. The use of 5% dextrose and saline solutions has been reported[14,28,39]. The former is preferred due to its properties since it does not conduct electricity and hence provides a thermal barrier around the organ[39]. Song et al[39] and Inoue et al[28] used artificial ascites and had no gastrointestinal injuries. The stomach and small bowel are less injured because adhesions along the gastrohepatic ligament are rare as the gastric wall is very thick and the small bowel has great mobility and peristalsis[6,19]. One should keep in mind that the onset of the symptoms of perforation is delayed; therefore, treatment is also usually delayed and the patient presents with a severe clinical status, eventually leading to death. A high level of suspicion is essential and close follow-up is important in these subjects.
Ribeiro et al[7] in their series routinely performed open cholecystectomy prior to RFA in tumors near the gallbladder, with the intention to avoid cholecystitis and incomplete ablation. Minimal wall thickening is expected on imaging after RFA, usually with no clinical significance. This probably happens due to the capacity of the fluid inside the gallbladder to dissipate the heat[16].
Injury to the diaphragm occurs in 0.1% of the cases[6,12]. It frequently results in severe shoulder pain[14]. Usually, RFA causes thickening of the muscle but perforation and hernia have been described[40]. Artificial ascites can also be used to decrease it.
CONCLUSION
Complication rates of RFA are low, making it a safe and feasible procedure. Every component of the treatment should be thoroughly analyzed. Proper patient selection is essential; subjects with exclusion criteria may lead to higher complication rates. Type of approach is also vital; depending on tumor location, one type may lead to a higher complication rate than another. This also fits for imaging guidance, where some tumors locations are better visualized by a specific method over another. The physician’s experience is very important as well. Identification of high-risk subjects (with close follow-up), early diagnosis of known complications and a high level of suspicion are acquired with time and may lead to better outcomes and reduced risk of complications.
Footnotes
P- Reviewers: Chetty R, Ramani K S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Zhang DN
References
- 1.Chaib E, Ribeiro Jr. MAF, Saad WA. Epidemiologia e Fatores de Risco. In: Carcinoma Hepatocelular: dos fatores de risco ao tratamento., editor. São Paulo: Editora Atheneu; 2004. pp. 5–16. [Google Scholar]
- 2.McGrane S, McSweeney SE, Maher MM. Which patients will benefit from percutaneous radiofrequency ablation of colorectal liver metastases? Critically appraised topic. Abdom Imaging. 2008;33:48–53. doi: 10.1007/s00261-007-9313-2. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2005;16:765–778. doi: 10.1097/01.RVI.0000170858.46668.65. [DOI] [PubMed] [Google Scholar]
- 4.Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008;23:e445–e450. doi: 10.1111/j.1440-1746.2007.05078.x. [DOI] [PubMed] [Google Scholar]
- 5.de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, Gamal El Din M, Letoublon C, Elias D. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 6.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro MA, Rodrigues JJ, Habr-Gama A, Chaib E, D’Ipolitto G, Fonseca AZ, Saad WA, Saad WA. Radiofrequency ablation of primary and metastatic liver tumors--4 years experience. Hepatogastroenterology. 2007;54:1170–1175. [PubMed] [Google Scholar]
- 8.Kasugai H, Osaki Y, Oka H, Kudo M, Seki T. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72 Suppl 1:72–75. doi: 10.1159/000111710. [DOI] [PubMed] [Google Scholar]
- 9.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, Wong J. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004;239:441–449. doi: 10.1097/01.sla.0000118565.21298.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong WT, Zhang WW, Qiu YD, Zhou T, Qiu JL, Zhang W, Ding YT. Major complications after radiofrequency ablation for liver tumors: analysis of 255 patients. World J Gastroenterol. 2009;15:2651–2656. doi: 10.3748/wjg.15.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 13.Rhim H, Lim HK, Kim YS, Choi D, Lee WJ. Radiofrequency ablation of hepatic tumors: lessons learned from 3000 procedures. J Gastroenterol Hepatol. 2008;23:1492–1500. doi: 10.1111/j.1440-1746.2008.05550.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendiratta-Lala M, Brook OR, Midkiff BD, Brennan DD, Thornton E, Faintuch S, Sheiman RG, Goldberg SN. Quality initiatives: strategies for anticipating and reducing complications and treatment failures in hepatic radiofrequency ablation. Radiographics. 2010;30:1107–1122. doi: 10.1148/rg.304095202. [DOI] [PubMed] [Google Scholar]
- 15.Goto E, Tateishi R, Shiina S, Masuzaki R, Enooku K, Sato T, Ohki T, Kondo Y, Goto T, Yoshida H, et al. Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol. 2010;44:374–380. doi: 10.1097/MCG.0b013e3181b7ed76. [DOI] [PubMed] [Google Scholar]
- 16.Rhim H. Complications of radiofrequency ablation in hepatocellular carcinoma. Abdom Imaging. 2005;30:409–418. doi: 10.1007/s00261-004-0255-7. [DOI] [PubMed] [Google Scholar]
- 17.Koda M, Murawaki Y, Hirooka Y, Kitamoto M, Ono M, Sakaeda H, Joko K, Sato S, Tamaki K, Yamasaki T, et al. Complications of radiofrequency ablation for hepatocellular carcinoma 283 346 treated nodules in 13 in a multicenter study: An analysis of 16 patients. Hepatol Res. 2012;42:1058–1064. doi: 10.1111/j.1872-034X.2012.01025.x. [DOI] [PubMed] [Google Scholar]
- 18.Nemcek AA. Complications of radiofrequency ablation of neoplasms. Semin Intervent Radiol. 2006;23:177–187. doi: 10.1055/s-2006-941448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhim H, Dodd GD, Chintapalli KN, Wood BJ, Dupuy DE, Hvizda JL, Sewell PE, Goldberg SN. Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics. 2004;24:41–52. doi: 10.1148/rg.241025144. [DOI] [PubMed] [Google Scholar]
- 20.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, Lee WJ, Lim HK, Nam GJ, Han SS, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134; discussion 134-136. doi: 10.1148/rg.231025054. [DOI] [PubMed] [Google Scholar]
- 21.Shibata T, Yamamoto Y, Yamamoto N, Maetani Y, Shibata T, Ikai I, Terajima H, Hatano E, Kubo T, Itoh K, et al. Cholangitis and liver abscess after percutaneous ablation therapy for liver tumors: incidence and risk factors. J Vasc Interv Radiol. 2003;14:1535–1542. doi: 10.1097/01.rvi.0000099532.29957.4f. [DOI] [PubMed] [Google Scholar]
- 22.Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, Lim JH, Paik SW, Yoo BC, Choi MS, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860–1867. doi: 10.2214/ajr.184.6.01841860. [DOI] [PubMed] [Google Scholar]
- 23.Elias D, Di Pietroantonio D, Gachot B, Menegon P, Hakime A, De Baere T. Liver abscess after radiofrequency ablation of tumors in patients with a biliary tract procedure. Gastroenterol Clin Biol. 2006;30:823–827. doi: 10.1016/s0399-8320(06)73327-9. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann R, Rempp H, Schmidt D, Pereira PL, Claussen CD, Clasen S. Prolonged antibiotic prophylaxis in patients with bilioenteric anastomosis undergoing percutaneous radiofrequency ablation. J Vasc Interv Radiol. 2012;23:545–551. doi: 10.1016/j.jvir.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi T, Yasuda I, Nishigaki Y, Hayashi H, Otsuji K, Mukai T, Enya M, Omar S, Soehendra N, Tomita E, et al. Intraductal chilled saline perfusion to prevent bile duct injury during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e410–e415. doi: 10.1111/j.1440-1746.2007.05091.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Lim HK, Choi D, Lee WJ, Kim SH, Kim MJ, Lee SJ, Lim JH. Changes in bile ducts after radiofrequency ablation of hepatocellular carcinoma: frequency and clinical significance. AJR Am J Roentgenol. 2004;183:1611–1617. doi: 10.2214/ajr.183.6.01831611. [DOI] [PubMed] [Google Scholar]
- 27.Razafindratsira T, Isambert M, Evrard S. Complications of intraoperative radiofrequency ablation of liver metastases. HPB (Oxford) 2011;13:15–23. doi: 10.1111/j.1477-2574.2010.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, Minami Y, Chung H, Hayaishi S, Ueda T, Tatsumi C, Takita M, Kitai S, Hatanaka K, Ishikawa E, et al. Radiofrequency ablation for hepatocellular carcinoma: assistant techniques for difficult cases. Oncology. 2010;78 Suppl 1:94–101. doi: 10.1159/000315236. [DOI] [PubMed] [Google Scholar]
- 29.Huang JW, Hernandez-Alejandro R, Croome KP, Yan LN, Wu H, Chen ZY, Prasoon P, Zeng Y. Surgical vs percutaneous radiofrequency ablation for hepatocellular carcinoma in dangerous locations. World J Gastroenterol. 2011;17:123–129. doi: 10.3748/wjg.v17.i1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang IS, Rhim H, Kim SH, Kim YS, Choi D, Park Y, Lim HK. Biloma formation after radiofrequency ablation of hepatocellular carcinoma: incidence, imaging features, and clinical significance. AJR Am J Roentgenol. 2010;195:1131–1136. doi: 10.2214/AJR.09.3946. [DOI] [PubMed] [Google Scholar]
- 31.Brennan DD, Ganguli S, Brecher CW, Goldberg SN. Thinking outside the abdominal box: safe use of the epipericardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol. 2008;19:133–136. doi: 10.1016/j.jvir.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 32.Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856–858. doi: 10.1002/bjs.4986. [DOI] [PubMed] [Google Scholar]
- 33.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 34.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, Sherman M, Grant DR, Greig PD, Gallinger S. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16:485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 35.Latteri F, Sandonato L, Di Marco V, Parisi P, Cabibbo G, Lombardo G, Galia M, Midiri M, Latteri MA, Craxì A. Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis. 2008;40:684–689. doi: 10.1016/j.dld.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 36.Kim AY, Rhim H, Park M, Lee MW, Kim YS, Choi D, Lim HK. Venous thrombosis after radiofrequency ablation for hepatocellular carcinoma. AJR Am J Roentgenol. 2011;197:1474–1480. doi: 10.2214/AJR.11.6495. [DOI] [PubMed] [Google Scholar]
- 37.Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47–51. doi: 10.2214/ajr.178.1.1780047. [DOI] [PubMed] [Google Scholar]
- 38.Ng KK, Lam CM, Poon RT, Shek TW, Fan ST, Wong J. Delayed portal vein thrombosis after experimental radiofrequency ablation near the main portal vein. Br J Surg. 2004;91:632–639. doi: 10.1002/bjs.4500. [DOI] [PubMed] [Google Scholar]
- 39.Song I, Rhim H, Lim HK, Kim YS, Choi D. Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol. 2009;19:2630–2640. doi: 10.1007/s00330-009-1463-x. [DOI] [PubMed] [Google Scholar]
- 40.Koda M, Ueki M, Maeda N, Murawaki Y. Diaphragmatic perforation and hernia after hepatic radiofrequency ablation. AJR Am J Roentgenol. 2003;180:1561–1562. doi: 10.2214/ajr.180.6.1801561. [DOI] [PubMed] [Google Scholar]


