Abstract
CXCXL13, a B-cell chemokine, has been proposed as a biomarker in a variety of conditions, some of which can mimic multiple sclerosis (MS) and can have very high levels. In this case-control study, CSF CXCL13 was elevated in MS, neuromyelitis optica (NMO), and other inflammatory neurological controls compared to noninflammatory controls. Levels did not differentiate disease groups. For all subjects taken together, CSF CXCL13 correlated with CSF WBC, oligoclonal band numbers, CSF protein, EDSS, and neurofilament levels. In subgroup analyses, CSF CXCL13 correlated with CSF WBC in NMO and IgG index in MS. Additionally, serum CXCL13 was elevated in NMO.
Keywords: Multiple sclerosis, neuromyelitis optica, CXCL13, neuroinflammation, neurofilament, myelin basic protein, biomarker
INTRODUCTION
Neuroinflammatory diseases can mimic each other and be difficult to differentiate. Diagnostic tests are often not available or can lack specificity and/or sensitivity. Elevated levels of CXCL13 have been reported in the CSF of MS patients1,2 and even higher levels in NMO.3 Additionally, very high CSF levels of CXCL13 have been found in MS mimics, such as CNS lymphoma and neuroborreliosis; having been proposed as a specific biomarker in the latter disease.4 We sought to evaluate if levels of CSF CXCL13 could discriminate neuroinflammatory conditions. Another goal was to determine if CXCL13 in serum could help differentiate these conditions, as some neuroinflammatory conditions are not confined to the CNS.
CXCL13 is chemotactic for B cells, which are thought to be critical in the pathogenesis of MS and NMO. A role for B cells in MS is suggested by the efficacy of B cell depletion in Phase II studies.5,6 CXCL13 is produced in ectopic lymphoid follicles, which are associated with worse outcomes in MS7 and higher levels may help predict who will develop MS amongst those with clinically isolated demyelinating syndromes.2 The humoral immune system is implicated in NMO by presence of NMO-IgG and response to rituximab.8
METHODS
Setting and patient selection
CSF and serum samples collected concurrently within 2 months of symptom onset were obtained from our tissue repository. Nine NMO subjects meeting revised criteria9 and 9 MS patients meeting 2005 revised McDonald criteria were matched by gender and age (+/− 5 years). Diagnosis and Expanded Disability Status Scale (EDSS) score for disability were determined from records by consensus of AHC and EA. Other inflammatory (OIC, n=10) and noninflammatory (NIC, n = 9) neurological disease controls were selected, matching for gender and age (Table 1). A tenth OIC CSF sample was included to represent viral meningitis, a disease with reported high CXCL13 levels, although serum was unavailable. Two MS, three NMO, and two OIC subjects had received corticosteroids prior to collection. Subsequent analyses showed no significant differences between these subjects and those not on steroids. The local Institutional Review Board approved this study. Informed consent was obtained from each subject at collection.
Table 1.
Patient demographics.
| MS | NMO | OIC | NIC | |
|---|---|---|---|---|
| Number | 9 | 9 | 10a | 9 |
| Age (mean) | 36.0 | 37.9 | 40 | 39.3 |
| % Female | 88.9 | 88.9 | 60 | 77.8 |
| % Caucasian | 56.0 | 33.3 | 70 | 88.9 |
| % African American | 44.0 | 66.7 | 30 | 11.1 |
| Diagnoses | MS | NMO (6/9 were NMO-IgG +) | 4 - neurosarcoid, 3 - idiopathic TMa, 2 - primary CNS lymphoma, 1 - viral meningitisb |
4 - headache, 3 - paresthesias, 1 - seizure, 1 – cranial nerve III paresis |
| EDSS baseline, median (range) | 3.0 (2.0, 8.0) | 7.5(3.0, 8.5) | Not applicable | Not applicable |
| EDSS changec, median (range) | −1.0 (0, −2.0) | −1.0 (+3.0, −4.0) | Not applicable | Not applicable |
| CSF WBC, median (range) | 3 (0, 21) | 24 (0, 878) | 10 (0, 950) | 0 (0, 4) |
| CSF protein, median (range) | 24 (9, 39) | 81 (27, 637) | 72 (17, 189) | 28 (17, 49) |
| IgG index, median (range) | 0.82 (0.61, 1.44) | 0.72 (0.56, 1.00) | 0.54 (0.48,0.072) | 0.53 (0.48, 0.57) |
| CSF OCB median (range) | 8 (1, 11) | 3.5 (0, 6) | 0.5 (0, 8) | 0 |
NMO, neuromyelitis optica; OIC, other inflammatory neurological conditions; NIC, noninflammatory controls; NMO-IgG, NMO autoantibody to Aquaporin 4; TM, transverse myelitis; CNS, central nervous system; EDSS, Expanded Disability Status Scale; WBC, white blood cell; IgG, Immunogloblulin G; OCB, oligoclonal bands.
Had no subsequent neurological events, were NMO-IgG negative, had negative brain MRIs, and who did not meet criteria for MS or NMO.
Concurrent serum and CSF samples were studied from nine OIC patients. CSF only was available from the one patient with viral meningitis.
A negative number indicates an improvement in EDSS.
Sample processing
CSF and serum samples were centrifuged ×1250g and stored at −80°C. Routine CSF analyses were performed by Barnes-Jewish Hospital laboratory. OCB and NMO-IgG were performed at Mayo (Rochester, MN).
ELISA
CXCL13 in serum and CSF was measured by human CXCL13 ELISA (Quantikine; R&D Systems, Minneapolis, MN). CSF MBP and NF were determined by human MBP ELISA kit and human phosphorylated NF heavy chain ELISA kit, respectively (Beckman Coulter; Brea, CA; and BioVendor; Modrice, Czech Republic).
Statistical analysis
Patient groups were compared with non-parametric Kruskal-Wallis rank test and post-hoc Mann-Whitney U test. Correlation analyses utilized Spearman coefficient (rs) by rank. PASW Statistics (Armonk, NY) was used.
RESULTS
Patient demographics
Serum and CSF samples from 37 subjects with MS, NMO, OIC, or NIC, matched for age and gender, were studied (Table 1).
CXCL13 levels in CSF and serum
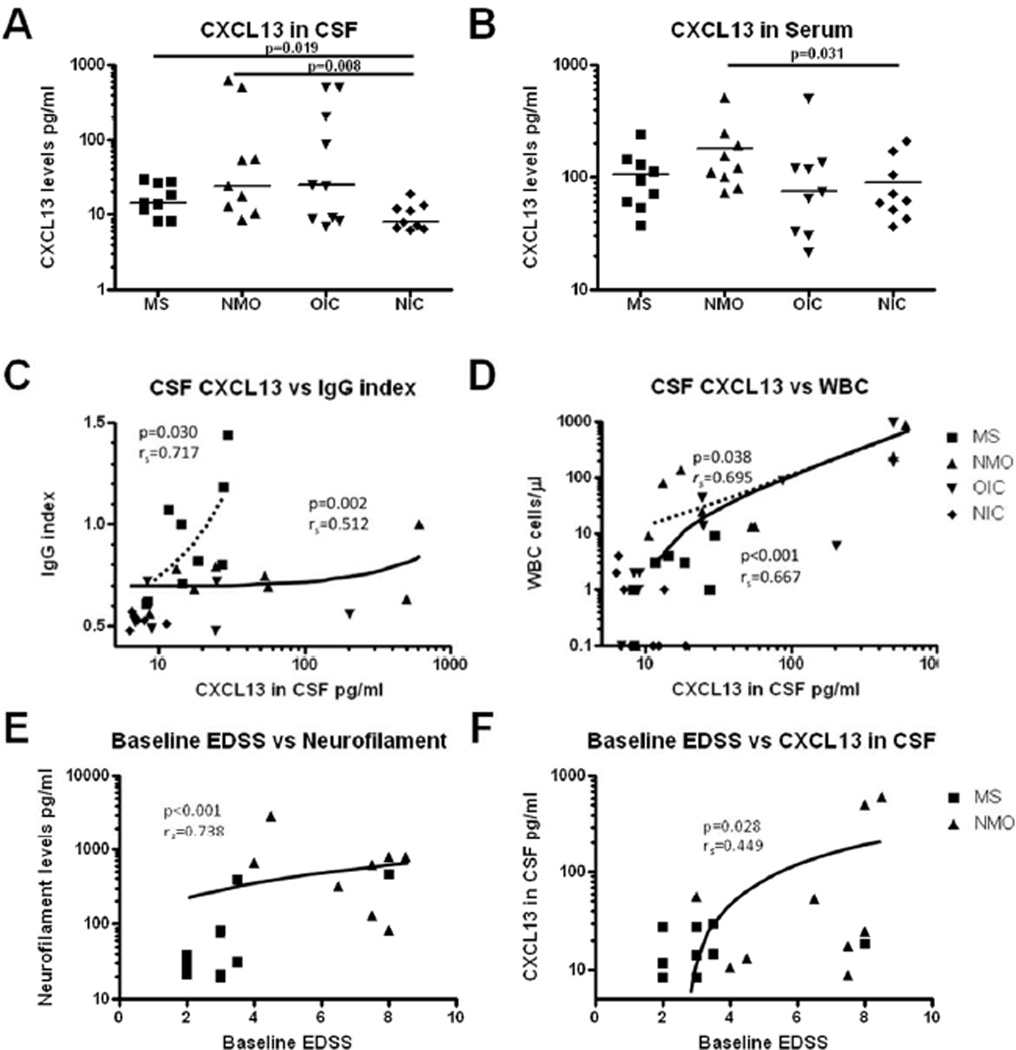
CSF CXCL13 levels were higher in subjects with MS (median 14.5pg/ml[range 8.2–29.5]) than NIC (7.9pg/ml[6.3–18.9](p=0.019)(Figure 1A). NMO CSF levels spanned a wide range (24.3pg/ml [8.7–614.6]) and were higher than NIC levels (p=0.008). CSF CXCL13 levels did not statistically differ between NMO and MS (p=0.258). Two NMO samples had very high CSF CXCL13 levels >500 pg/ml (Figure 1A), in the range seen in CNS lymphoma and viral meningitis.
Figure 1. CXCL13 levels in neuroinflammatory conditions.
CXCL13 levels in A) CSF and B) serum in subjects with multiple sclerosis (MS), neuromyelitis optica (NMO), other inflammatory neurological conditions (OIC), and noninflammatory controls (NIC). CXCL13 correlates with C) IgG index and D) white blood cell count (WBC) across patient groups (solid lines) although correlations are still seen for IgG index in MS subjects (C) and WBC for NMO subjects (D)(dashed lines represent subgroup correlations of interest). Disability, as assessed using the expanded disability status scale (EDSS), correlated with E) CSF neurofilament and F) CSF CXCL13. Note that logarithmic scales were used to better demonstrate values.
Serum CXCL13 levels were higher in samples from NMO subjects (120.7pg/ml[72.2–516.1]) than in NIC (61.2pg/ml[36.4–211.9])(p=0.031), with a trend versus MS subjects (94.2pg/ml[37.4–239.6])(p=0.060)(Figure 1B). Serum CXCL13 was not significantly higher (p=0.471) in MS than in NIC (Figure 1B) samples. CSF and serum levels of CXCL13 were independent of NMO-IgG status.
CXCL13 correlation with other biomarkers
As a surrogate for axonal damage, CSF NF heavy chain levels were measured. NMO subjects (609.2pg/ml[77.6–2918.6]) had higher levels than NIC (44.6pg/ml[24.9–381.1])(p=0.001) or MS (31.2pg/ml [19.5–466.9])(p=0.006) (eFigure 1A). MS subjects did not differ versus NIC (p=0.666). As a biomarker of demyelination, CSF MBP was measured. MBP was higher in NMO samples (17.79ng/ml[0.74–140.52]) than in NIC (1.14 ng/ml [0.62–2.35])(p=0.006), and a trend versus MS (1.83 pg/ml[0.30–84.08])(p=0.063) (eFigure 1B). For all subgroups combined, CSF NF correlated with CXCL13 (p=0.013,rs=0.384) (eFigure 2B) and with MBP (p=0.043,rs=0.318) (eFigure 2C). CSF CXCL13 did not correlate with MBP (eFigure 2D).
For all subgroups combined, CSF CXCL13 levels correlated strongly with CSF WBC (p<0.001rs=0.667)(Figure 1D) and moderately with CSF protein (p=0.001rs=0.487)(eFigure 1C). Within disease groups, CSF WBC correlated strongly with CXCL13 in NMO samples (p=0.038,rs=0.695), but not in MS (p=0.295)(Figure 1D). For subjects with OCB number and IgG index data (9 MS, 8 NMO, 6 NIC, and 6 OIC), IgG index correlated moderately with CXCL13 (p=0.002,rs=0.512)(Figure 1C). This correlation was driven by MS subjects (p=0.030,rs =0.717), and not found in NMO (p=0.385). Overall, CSF OCB number correlated with CXCL13 (p<0.001,rs=0.619) (eFigure 1D), and with IgG index (p <0.001, rs=0.725).
Biomarker correlation with clinical measures
EDSS was lower in MS (median 3.0[2.0–8.0]) than NMO (7.5[3.0–8.5])(p=0.007). EDSS in both MS and NMO correlated with NF (p<0.001,rs=0.738) (Figure 1E), CSF protein (p=0.003,rs=0.663), CSF WBC (p=0.005,rs =0.630)(eFigure 1E–F), and CSF CXCL13 levels (p=0.028,rs=0.449)(Figure 1F).
DISCUSSION
CXCL13 may play a role in MS and NMO pathogenesis by recruiting inflammatory cells into the CNS. Although the sample numbers were low, we found sufficient overlap in CSF CXCL13 levels between groups to believe that CXCL13 will not distinguish the disease groups studied. Some NMO CSF samples contained CXCL13 concentrations in the very high range reported in CNS lymphoma and viral meningitis. Additionally, some MS and NMO samples had low levels in the range of noninflammatory controls. CSF CXCL13 correlated with inflammation (WBC count, total protein, IgG index, and OCB number) for all samples taken together, but in specific disease subgroups CSF CXCL13 correlated with only some markers. As in prior reports,1,2 CSF CXCL13 in MS samples correlated with IgG index. We did not find an association with WBC. In samples from NMO subjects, who often have elevated WBC but not IgG index, CSF CXCL13 correlated with CSF WBC but not IgG index. In the present study CSF CXCL13 levels in MS and NMO correlated with disability, which will need to be explored further.
NMO-IgG is often present at higher titers in serum than CSF,10 suggesting that the autoimmune process in NMO is not CNS restricted. Our finding that NMO patients have elevated serum CXCL13 levels, not described previously, also supports a systemic pathophysiology. Serum and CSF levels of CXCL13 were not correlated (eFigure 1A), suggesting separate sources or differential degradation in the CNS and periphery.
Supplementary Material
Acknowledgments
Funding
This project was supported by the Washington University Institute of Clinical and Translational Sciences from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH) [grant number UL1 TR000448, CO6 RR020092, and K23NS052430-01A1]; and the Barnes-Jewish Hospital Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS, NIH, or the Barnes-Jewish Hospital Foundation.
Support
Dr. Alvarez has received honoraria from Teva Neurosciences. Salary and training support is provided by the Sylvia Lawry Physician Clinical Fellowship from the National MS Society and Gateway chapter (FP 1772-A-1) and Predoctoral Training Program at Washington University (TR000448).
Dr. Piccio is a recipient of the Harry Weaver Neuroscience Award from the National MS Society (JF 2144A2/1) and funded by the Fondazione Italiana Sclerosi Multipla (FISM) (2009/R/33).
Dr. Klawiter has received speaking honoraria from Bayer Healthcare, Genzyme Corporation, and Teva Neuroscience. Salary and training support is provided by an American Academy of Neurology
Foundation Clinical Research Training Fellowship and the National Institutes of Health (K23 NS078044-01 and UL1RR024992).
Dr. Parks has received speaking honoraria, and consulting fees from Biogen-Idec, EMD Serono, Novartis and Teva Neuroscience.
Dr. Naismith has received speaking honoraria for speaking and consulting for Acorda, Bayer, Biogen-Idec, EMD Serono, and Teva Neurosciences, and research support through Acorda.
Dr. Cross has received consulting honoraria from GlaxoSmithKline, Questcor, Teva Neurosciences, Biogen-Idec, Hoffman-La Roche, Novartis, and Sanofi-Aventis. AHC serves on advisory boards for the National MS Society and the NIH. AHC was supported in part by the Manny and Rosalyn Rosenthal-Dr. John L. Trotter Chair in Neuroimmunology.
Footnotes
Mr. Mikesell has nothing to disclose.
REFERENCES
- 1.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129:200–211. doi: 10.1093/brain/awh680. [DOI] [PubMed] [Google Scholar]
- 2.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17:335–343. doi: 10.1177/1352458510389102. [DOI] [PubMed] [Google Scholar]
- 3.Zhong X, Wang H, Dai Y, et al. Cerebrospinal fluid levels of CXCL13 are elevated in neuromyelitis optica. Journal of neuroimmunology. 2011;240–241:104–108. doi: 10.1016/j.jneuroim.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt C, Plate A, Angele B, et al. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology. 2011;76:1051–1058. doi: 10.1212/WNL.0b013e318211c39a. [DOI] [PubMed] [Google Scholar]
- 5.Takano R, Misu T, Takahashi T, Sato S, Fujihara K, Itoyama Y. Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology. 2010;75:208–216. doi: 10.1212/WNL.0b013e3181e2414b. [DOI] [PubMed] [Google Scholar]
- 6.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England journal of medicine. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 7.Naismith RT, Piccio L, Lyons JA, et al. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74:1860–1867. doi: 10.1212/WNL.0b013e3181e24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Archives of neurology. 2008;65:1443–1448. doi: 10.1001/archneur.65.11.noc80069. [DOI] [PubMed] [Google Scholar]
- 9.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain : a journal of neurology. 2007;130:1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.