Summary:
Gene regulation during cell cycle progression is an intricately choreographed process, ensuring accurate DNA replication and division. However, the translational landscape of gene expression underlying cell cycle progression remains largely unknown. Here, employing genome-wide ribosome profiling we uncover widespread translational regulation of hundreds of mRNAs serving as an unexpected mechanism for gene regulation underlying cell cycle progression. A striking example is the S-phase translational regulation of RICTOR, which is associated with cell cycle-dependent activation of mTORC2 signaling and accurate cell cycle progression. We further identified unappreciated coordination in translational control of mRNAs within molecular complexes dedicated to cell cycle progression, lipid metabolism, and genome integrity. This includes the majority of mRNAs comprising the cohesin and condensin complexes, controlling genome organization, which are coordinately translationally controlled in specific cell cycle phases via their 5′ UTRs. Our findings illuminate the prevalence and dynamic nature of translational regulation underlying the mammalian cell cycle.
Introduction
During cell division, exquisite temporal control of protein expression in distinct phases of the cell cycle underlies fundamental checkpoints that ensure accurate completion of chromosome duplication and segregation of a daughter cell. A central paradigm that has emerged is that rapid, dynamic, and fine-tuned reprogramming of gene expression occurs during specific phases of the cell cycle. For example, a large number of mRNAs, including those involved in promoting cell cycle progression, are transcriptionally activated in a cell cycle phase-dependent manner(Cho et al., 2001; Whitfield et al., 2002). In addition, degradation of many cell cycle checkpoint proteins, primarily through the ubiquitin proteasome pathway, at specific times during the cell cycle is required for progression to subsequent phases (Peters, 2006). Systems level mass spectrometry approaches are also beginning to elucidate targets of the ubiquitin-proteasome pathway, as well as cell cycle-specific patterns of post-translational modifications on a large number of proteins (Kim et al., 2011; Merbl et al., 2013). While these studies have provided great insight into the highly coordinated gene expression program of the cell cycle, a key step in modulating protein levels has remained undefined: the regulation of mRNA translation.
To date, the study of translational control during the mammalian cell cycle has generally focused on global reductions in protein synthesis during mitosis, monitored by a decrease in amino acid incorporation into proteins(Fan and Penman, 1970; Konrad, 1963). Conversely, a relatively modest number of mRNAs have been identified as actively translated during mitosis(Qin and Sarnow, 2004). Other studies have primarily investigated regulation of single mRNAs such as the translational regulation of Cyclin E, which is required for progression into S-phase(Lai et al., 2010). While limited in scope, these studies highlight both the importance of translational regulation during cell cycle progression and underscore the need for an unbiased, genome-wide analysis of the translational landscape during the mammalian cell cycle.
Here, we have employed ribosome profiling(Ingolia et al., 2009) to uncover widespread translational regulation during cell cycle progression. We defined remarkable, and previously unknown translational control of key cell cycle genes. Importantly, among these mRNAs, we uncovered translational regulation of RICTOR, which correlates with the cell cycle phase-specific signaling of the mTOR kinase pathway and suggests an important role in cell cycle progression. Moreover, we identify surprising, coordinate regulation in translational control of functionally related sets of mRNAs, suggesting a regulatory mechanism that dictates the coordinate expression and activity of key molecular machinery in the cell. Among these translationally controlled networks are mRNAs required for metabolism, nuclear transport, and DNA repair required to ensure genome fidelity. Together, this work highlights both the prevalence and dynamic nature of translational regulation during cell cycle progression. It further suggests a multifaceted mechanism for differential regulation of transcript-specific translational control in the execution of distinct steps of the cell cycle.
Results
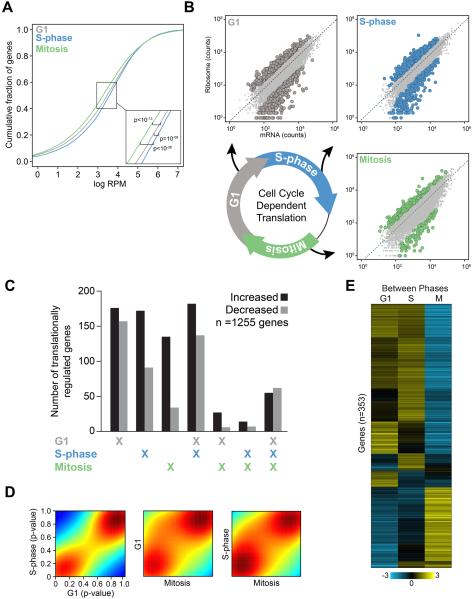
To understand the extent and impact of translational regulation during cell cycle progression, we employed ribosome profiling to identify individual mRNAs exhibiting unexpected levels of ribosome association during each phase of the cell cycle. After synchronizing human Hela cells in G1, S-phase, or mitosis, libraries for total mRNA and ribosome-protected RNA fragments were prepared, sequenced, and analyzed using a sophisticated statistical framework (see Methods) (Figure S1A)(Olshen et al., 2013). We initially characterized global levels of ribosome occupancy in the expressed transcriptome of each phase of the cell cycle. This data revealed a significant decrease in the overall level of ribosome-bound mRNAs during mitosis compared to either G1 or S-phase, which is consistent with a decline in cap-dependent translation during mitosis (Figure 1A)(Fan and Penman, 1970; Konrad, 1963). Beyond these global changes in translation, our principal aim was to understand the extent of gene-specific translational regulation during cell cycle progression. We therefore developed a computational framework for determining the statistical significance of relative ribosome occupancy of mRNAs within individual phases of the cell cycle. This analysis quantifies higher or lower levels of ribosome occupancy than predicted from transcript abundance (Figure 1B, highlighted points; see Methods). This approach enabled us to quantify the landscape of mRNA translation during a given cell cycle phase, or to directly assess differential translational regulation between any two phases of the cell cycle.
Figure 1. Systematic and multifaceted translational control of gene expression during the mammalian cell cycle.
(A) The cumulative fraction of ribosome-bound mRNA on all expressed transcripts in each phase of the cell cycle is shown as a function of increasing ribosome-bound mRNA. X-axis represents the scaled fraction of total ribosome-bound reads and the Y-axis represents the fraction of expressed transcripts. (B) Representative scatter plots illustrate ribosome occupancy as a function of mRNA abundance (measured as sequencing read counts). The dashed line represents the expected level of ribosome occupancy given mRNA abundance (see Supplemental Experimental Procedures). mRNAs with statistically significant translational regulation are those with greater or less than the expected levels of ribosome occupancy given their mRNA expression (FDR < 1%; G1 is grey, S-phase is blue, and Mitosis is green). (C) The total number of genes with significantly increased (black) or decreased (grey) ribosome occupancy are shown, including those unique to a given phase or shared between multiple phases of the cell cycle. (D) Density plots of the nominal p-value of ribosome occupancy for each gene between any two phases of the cell cycle (colors designate the density of genes in a given region where red and blue are the most and least dense respectively). (E) Unsupervised hierarchical clustering of mRNA translation across the phases of the cell cycle using the 353 differentially translationally regulated transcripts between any two phases in direct comparisons. Genes and cell cycle phases are clustered based on the level of normalized ribosome occupancy (mean-centered translational efficiency by gene, scales and colors indicate the direction and magnitude of mRNA translation S is S phase, M is Mitosis). See also Figure S1, Tables S1-2.
Strikingly, we observed extensive and dynamic translational control of individual mRNAs during different phases of the cell cycle (Figure 1B, Table S1). In total, we identified 1255 mRNAs, representing 12% of the expressed transcripts in these cells, that exhibit higher or lower than the expected levels of ribosome occupancy in any cell cycle phase [false discovery rate (FDR) < 1%]. We next sought to determine how the translation of these 1255 mRNAs varies among cell cycle phases. Transcript-specific translational regulation was most prevalent in G1 and S-phases. (Figure 1C). Indeed, genome-wide there was far greater similarity in the level of ribosome occupancy in mRNAs between G1 and S-phase than existed when comparing either of these to mitosis (Figure 1D). Importantly, these differences in transcript-specific translational control during the cell cycle are independent from global changes in the levels of protein synthesis that occur during specific cell cycle phases as we measured the levels of ribosome association for specific mRNAs relative to the background level of ribosome association during each individual phase. This is especially relevant during mitosis, when global protein synthesis levels are lower compared to G1 or S-phase (Figure 1A)(Fan and Penman, 1970; Konrad, 1963). Additionally, we also identified core groups of mRNAs that exhibit increased or decreased translation in all phases of the cell cycle, suggesting they may share a mechanism to maintain high or low levels of translation throughout the cell cycle (Figure 1C). Moreover, the specific patterns obtained by hierarchical clustering of the 1255 translationally regulated mRNAs were not observed when comparing corresponding transcript expression levels, indicating that translational regulation is indeed a distinct regulatory system, uncoupled from transcription, controlling gene expression during the cell cycle (Figure S1B).
In addition to defining the relative level of translation for specific genes within each cell cycle phase, we also identified a large number of mRNAs that undergo significant changes in translation between phases of the cell cycle. We identified 353 mRNAs with a statistically significant change in translation between any two phases of the cell cycle (FDR<5%) (Figure 1E, Table S2). Among these, 112 mRNAs were translationally regulated exclusively between specific phases of the cell cycle. These mRNAs comprise a number of important cell cycle regulatory genes, including CLASP2 and KNTC1, which are involved in establishing the mitotic spindle checkpoint. Overall, these data demonstrate a previously unrecognized level of systematic and multi-faceted translational control of gene expression during progression through the mammalian cell cycle.
We next sought to understand the regulatory logic underlying general changes in translational regulation observed among specific phases of the cell cycle. To determine certain parameters of cell cycle-dependent translational regulation, we examined specific structural characteristics of 5′ untranslated regions (UTRs) including their length, %G+C content, and minimum free energy (MFE). In the G1 phase of the cell cycle, there is a significant association between ribosome occupancy and the length of 5′ UTRs such that mRNAs with shorter UTRs had high levels of ribosome occupancy. In both G1 and S-phase, there is an inverse relationship between the G+C content of 5′ UTRs and ribosome occupancy levels. Finally, mRNAs with higher ribosome occupancy in all phases of the cell cycle studied were associated with higher predicted MFE, revealing that their 5′UTRs possess less complex secondary structures (Figure S1C). Together, these findings suggest that several of the defining features of 5′UTRs may contribute to the translational efficiency of the mammalian genome within specific phases of the cell cycle. However, these very general characteristics of the 5′UTR do not account for all the patterns of translational regulation observed here, therefore there are likely to be additional important determinants of transcript-specific translational control (see below).
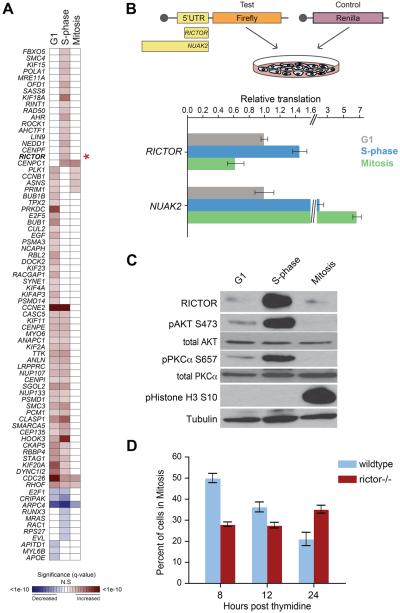
The translational dynamics of key cell cycle regulators is still poorly understood. We therefore assessed the pattern of statistically significant translational regulation among bone fide cell cycle regulatory genes during each phase of the cell cycle (Figure 2A)(Subramanian et al., 2005). This analysis uncovered extensive translational regulation among these genes, highlighting the prevalence of translational control during cell cycle progression. For example, we observed high levels of ribosome occupancy in G1 and S-phase for CCNE2, a cyclin known to be translationally regulated. On the contrary, our data revealed low levels of ribosome occupancy of E2F1, also during G1 and S-phase, which is consistent with cell cycle-dependent miRNA-mediated regulation of E2F1 (Pulikkan et al., 2010). Furthermore, this analysis identified translational regulation among several cell cycle mediators including PLK1 and BUB1, two mitotic checkpoint kinases. Notably, RICTOR, the defining subunit of mTOR Complex 2 (mTORC2) (Laplante and Sabatini, 2012), displayed a significant change in the level of ribosome occupancy, with almost a 3 fold increase from G1 to S-phase and greater than 13 fold decrease from S-phase to mitosis. Our findings further show an accumulation of RICTOR protein, but not other components of mTORC2, that mirrors this pattern of ribosome occupancy (Figure 2A,C, S2B), which suggests that RICTOR protein accumulation is, at least in part, mediated by translational control. To further investigate the mechanisms responsible for cell cycle-dependent translational control of RICTOR, we assessed the activity of its 5′UTR. Strikingly, the 5′ UTR of the RICTOR mRNA is sufficient to direct increased protein expression during S-phase, which then decreases upon entry into mitosis, a pattern consistent with the cell-cycle dependent ribosome occupancy we observed (Figure 2A, B, S2A). We compared the translation directed by the RICTOR 5′UTR to the 5′UTR of an mRNA that exhibits a distinct pattern of cell cycle phase specific translation, NUAK2 (Figure 2B). In our ribosome profiling experiments, NUAK2 showed a significant increase in ribosome occupancy during mitosis compared to either G1 or S-phase (q-values = 9.5e-4 and 1.3e-3 respectively, Table S2). RICTOR and NUAK2 5′UTRs promote unique patterns of translation in the luciferase reporter assay, suggesting that translational regulation of these mRNAs is specific and the patterns of translation we observe are not due to global changes in protein synthesis (Figure 2B).
Figure 2. Phase-dependent translational control of key cell cycle regulators including RICTOR and mTOR signaling.
(A) A heat map of the significance of translational regulation among cell cycle progression genes (asterisk: RICTOR). Shading represents significance level of increased or decreased translational regulation (red and blue, as indicated). (B) A diagram describing the 5′ UTR luciferase reporter assay (top panel). 5′ UTRs cloned into the firefly reporter are scaled to length. Capped mRNAs are transfected into synchronized cells prior to assaying reporter levels. Levels of translation directed by RICTOR or NUAK2 5′UTRs are shown: Y-axis is reporter value relative to cells in G1 (bottom panel). (C) Western blots showing RICTOR protein levels and phosphorylation of mTORC2 targets (AKT-S473, PKCα-S657). pHistone H3 is a mitosis marker. Tubulin is a loading control. (D) Mitotic progression of thymidine-synchronized MEFs: Y-axis is percent mitotic cells. Bars represent the mean +/- SD. See also Figure S2.
These data suggest that regulation of RICTOR mRNA translation and its accumulation at the protein level during S-phase could modulate the activity of mTOR during the cell cycle. To test this hypothesis, we assessed phosphorylation levels of mTORC2 targets AKT (S473) and PKCα (S657) in lysates from synchronized cells (Figure 2C). We identified a strong correlation between the levels of RICTOR protein and the phosphorylation of these two mTORC2 targets during the S-phase of the cell cycle. Moreover, the phosphorylation of AKT during S-phase is dependent on RICTOR, since it does not occur in Rictor-/- mouse embryonic fibroblasts (MEFs) (Figure S2C). Upstream signaling pathways, such as PI3-kinase/PDPK1, can activate AKT by phosphorylating T308(Alessi et al., 1996; Alessi et al., 1997). The fact that phosphorylation of AKT at T308 does not change during the cell cycle, further suggests that the RICTOR dependent phosphorylation of AKT at S473 during S phase is independent from the induction of upstream PI3-kinase signaling (Figure S2B). Importantly, we also observe an increase in the phosphorylation of p27, a downstream target of AKT, specifically during S-phase (Figure S2B). Phosphorylation of p27 by AKT inhibits its activity, thereby promoting cell cycle progression(Shin et al., 2002). These findings suggest that the accumulation of RICTOR protein levels in S-phase may have an important function in cell cycle progression. Rictor-/- MEFs have been previously shown to possess a proliferation defect (Shiota et al., 2006). We therefore determined when the proliferation defect in Rictor-/- MEFs manifests during the cell cycle relative to the increase in translation of Rictor observed in S-phase. Rictor-/- MEFs exhibit a defect in progression through mitosis as evidenced by a lag in the accumulation of diploid cells after synchronization in S-phase (Figure 2D), associated with a marked decrease in S-phase cells compared to wildtype (p-value = 0.0006, Student’s t-test; Figure S2D). Together, our findings suggest that translational regulation, at least in part, leads to an increase in RICTOR protein that underlies cell cycle-dependent mTORC2 activity and is associated with S-phase to mitosis progression of the mammalian cell cycle program.
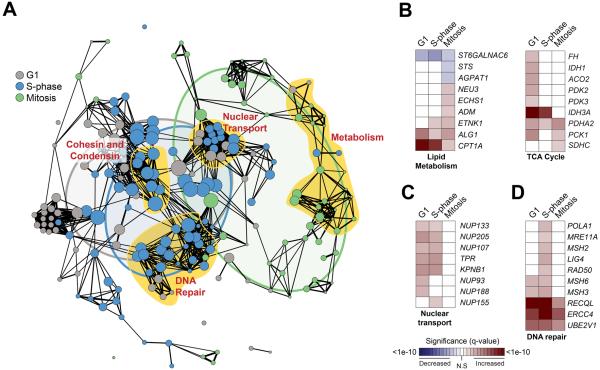
An outstanding question is whether functionally related groups of genes may be translationally co-regulated as a means to simultaneously control the expression of important mRNA networks. Strikingly, we identified numerous clusters of functionally related genes among translationally co-regulated mRNAs (Table S3). These include genes central to the control of metabolism, nuclear transport, and DNA repair (Figure 3A, Table S3). Among the metabolism genes identified, there was a particular enrichment of genes involved in lipid metabolism and the TCA cycle (Figure 3B). Furthermore, we identified a significant enrichment of translationally regulated mRNAs, primarily during G1 and S-phase, involved in nuclear-cytoplasmic transport including a large number of core scaffolding components of the Nuclear Pore Complex (NPC) (Figure 3C). In fact, over 20% of NPC components are translationally regulated during cell cycle progression, primarily during interphase when the number of NPCs increases dramatically (Antonin et al., 2008). Furthermore, translationally regulated genes are components of multiple DNA repair pathways, including mismatch repair and double strand break repair pathways (Figure 3D). This suggests that translational control may be a key contributor in regulating the response to diverse types of DNA damage that arise during cell cycle progression.
Figure 3. Translational co-regulation of large molecular complexes during cell cycle progression.
(A) Among translationally regulated genes during the cell cycle, a network of statistically significant functional enrichments (nodes, nominal p-value < 0.05; radius scaled to the number of genes) and their relatedness (edges, spearman correlation ρ ≥ 0.3) indicate a highly interconnected set of modules of molecular function. Groups of nodes closely related by function are highlighted in yellow and labeled. A Venn diagram overlay represents the relative overlap of enriched molecular function between cell cycle phases (G1 is grey, S-phase is blue, and Mitosis is green). Heatmaps highlight the significance of translational regulation of genes that define representative functional categories including lipid metabolism and TCA cycle (B), nuclear transport (C), and DNA repair (D). Shading represents significance level of increased or decreased translational regulation (red and blue, as indicated). See also Table S3.
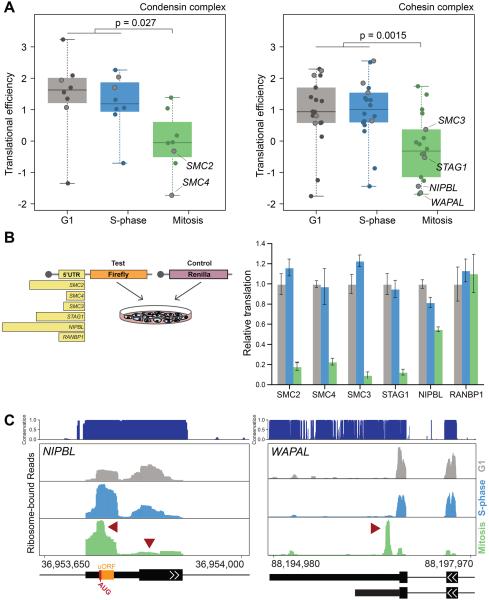
Given this striking pattern of translational regulation among genes involved in DNA repair, we sought to identify cell cycle phase-specific translational regulation of mRNAs required for genome fidelity. Strikingly, the majority of genes comprising the cohesin and condensin complexes are translated in a cell cycle phase-specific manner (Figure 4A). mRNAs from components of both the condensin and cohesin complexes exhibit relatively high levels of ribosome occupancy during G1 and S-phase that decrease in mitosis (Figure 4A). As these complexes are loaded onto DNA during S phase or G2 in order to prepare chromosomes for segregation during mitosis(Hirano, 2012; Wood et al., 2010), the ribosome occupancy we observe is consistent with the requirement for the condensin and cohesin complexes to be produced prior to mitosis. We determined that the molecular basis for these translational patterns in gene expression is, at least in part, through 5′UTR mediated regulation. For example, the 5′ UTRs of the core components of the condensin complex, SMC2 and SMC4, direct similar patterns of translational activation during G1 and S-phase that decreases in mitosis and mirrors the cell cycle ribosome occupancy profile. Likewise, the 5′ UTRs of SMC3, STAG1, and NIPBL, components of the cohesin complex, direct translation that is high during G1 and S-phase, and decreased during mitosis (Figure 4B). On the contrary the 5′ UTR from RANBP1, a control mRNA whose pattern of ribosome occupancy is distinct from components of the condensin and cohesin complexes and is functionally unrelated, does not (Figure 4B).
Figure 4. Condensin and cohesion complex components are coordinately translationally regulated during the cell cycle at the level of their 5′UTR.
(A) The translational efficiency of components of the condensin (left) and cohesin (right) complexes during each cell cycle phase are indicated (specific genes mentioned elsewhere are outlined for clarity). (B) A diagram of the luciferase reporter assay with 5′UTRs scaled to length (left). Levels of translation directed by specific 5′ UTRs are indicated: Y-axis is reporter value relative to cells in G1 (right). Bars represent mean +/- SD from 6 replicates. (C) The level of bound ribosome in the 5′ UTR of NIPBL (left) and the 3′ UTR of WAPAL (right), with peaks of interest denoted by red arrows (evolutionary conservation is indicated; G1 is grey, S-phase is blue, and Mitosis is green). Representative gene features are indicated: uORF is designated by an orange box, narrow or wide black bars represent UTRs and coding exons respectively, black lines indicate introns, and arrows indicate the coding strand. Numbers represent absolute genomic positions. See also Figure S3.
We further identified unique cell cycle-dependent patterns of ribosome occupancy within specific regions of transcripts belonging to the cohesin complex. For example, the density of bound ribosomes on the NIPBL mRNA, a member of the cohesin complex, shifts from the primary initiation codon of the coding region to an upstream open reading frame (uORF) 59 nucleotides upstream of the primary initiation codon in a highly evolutionarily conserved region of the 5′UTR as cells progress through the cell cycle into mitosis (Figure 4C, left, Figure S3A). uORFs often act to decrease the translation of primary open reading frames, which is consistent with the decrease in translation observed from the NIPBL 5′UTR in the luciferase reporter assay (Figure 4B). In the case of WAPAL, a gene that promotes dissociation of cohesin from sister chromatids during mitosis(Gandhi et al., 2006; Kueng et al., 2006), we identified an unexpected peak of ribosome density downstream of the translation termination codon in the 3′ UTR that is only present during mitosis (Figure 4C, right). This region of the WAPAL 3′UTR is highly conserved and contains two additional in-frame stop codons, suggesting that WAPAL could produce a C-terminally extended protein product during mitosis (Figure 4C, right, Figure S3B). Together, these data suggest that components of the condensin and cohesin complexes utilize multiple modes of translational regulation to coordinate their expression during the cell cycle. Furthermore, translational regulation may help to facilitate the assembly or modulate the activity of large protein complexes by ensuring individual components of these complexes are coordinately expressed.
Discussion
Our work delineates the unexpected magnitude and dynamic nature of translational regulation during the mammalian cell cycle. We have presented a comprehensive network of inter-related and coordinately translationally regulated mRNAs underlying this fundamental biological process. These data suggest that translational control is a particularly well-suited mechanism for fine-tuning gene expression during dynamic processes such as cell cycle progression. For example, we uncovered unexpected translational regulation of a key component of the mTOR pathway, a key regulator of cell growth. RICTOR becomes translationally induced specifically upon transitioning into S-phase of the cell cycle. Although we cannot exclude that other mechanisms, such as control of protein stability, may also cooperate in modulating RICTOR protein abundance during the cell cycle, our findings show that accumulation of RICTOR during S-phase modulates mTORC2 signaling to promote the phosphorylation of specific downstream targets including AKT and PKCα. Phosphorylation of both AKT and PKCα by mTORC2 during S-phase is consistent with their roles in promoting cell growth and proliferation, and reveals how this process may be regulated at the level of translational control. Moreover, we did not observe overt translational regulation of other mTOR complex components, highlighting the specificity in RICTOR 5′UTR translational activation in controlling mTOR signaling during cell cycle progression. Elucidating the precise mechanisms governing translational regulation of RICTOR will be an important area of future research that may play an important role in mTORC2 signaling during cell cycle progression.
One surprising finding from our data is the translational co-regulation of the molecular machinery responsible for maintaining genome integrity. A number of translationally regulated genes are involved in sensing multiple types of DNA damage, such as base pair mismatches and double strand breaks, that are specifically translationally activated during S-phase. Notably, multiple orthogonal DNA repair pathways are controlled by translation, suggesting a critical regulatory mechanism that maintains the fidelity of the genome, adding a robust level of protection in safeguarding the genome. This may also be true for protein complexes that are responsible for organizing the higher order structure of chromosomes, which also show co-regulated patterns of translational control. The primary roles of the cohesin and condensin complexes are to package the genome to ensure faithful segregation of chromosomes during cell division(Hirano, 2012; Wood et al., 2010). The mRNAs comprising the majority of these two complexes exhibit high levels of translation during both G1 and S-phase that may ensure sufficient, stoichiometric amounts of the proteins required to package chromosomes prior to their segregation.
Interestingly, cohesins can promote transcription, and disruption of the cohesin complex impairs ribosomal RNA transcription, thus leading to defects in protein synthesis(Bose et al., 2012). Most notably, mutations in cohesin genes characterize an entire class of human disorders termed cohesinopathies. Cohesinopathies manifest as developmental disorders with characteristic limb defects, including oligodactyly, and neurodevelopmental delay. These features overlap with the phenotypic spectrum of ribosomopathies where ribosome components are found mutated, suggesting a possible relationship between these two fundamental biological processes. Moreover, it is notable that a mutation in an uORF in the 5′ UTR of NIPBL, leads to a decrease in NIPBL translation and is associated with a cohesinopathy known as Cornelia de Lange Syndrome, suggesting that alterations in translational regulation may underlie this human disease(Borck et al., 2006). This mutation disrupts an uORF in the 5′UTR of NIPBL, which could be responsible for the observed decrease in translation. This finding is consistent with our studies showing a critical role for the 5′UTR in regulating the translation of mRNAs belonging to the cohesin complex. These results also suggest a potential feedback mechanism between the cohesin complex and the translation machinery that may be of great importance to the etiology of cohesinopathies.
Together, our studies shed light on the unexpected dynamics of translational control in the regulation of gene expression during fundamental cellular programs, such as the mammalian cell cycle. The magnitude of this translational regulation, involving hundreds of mRNAs, suggests that yet unknown regulatory mechanisms and transcript-specific translational regulators may endow remarkable specificity to the post-transcriptional gene expression program that is fundamental for accurate replication and cell division.
Experimental Procedures
Tissue culture and cell cycle analysis.
Hela cells and MEFs were cultured under normal growth conditions. Synchronization in specific cell cycle phases was achieved by release from thymidine block (G1 and S-phase) and nocodazole treatment (mitosis) as previously described (Jackman and O’Connor, 2001). Cell cycle analysis was performed on cells fixed in 80% ethanol prior to RNase digestion and staining with 40μg/ml propidium iodide or with the Click-iT EdU Flow Cytometry Assay (Life Technologies).
Library preparation and sequencing
Next generation sequencing libraries were prepared as described previously(Hsieh et al., 2012). Briefly, synchronized cells were treated with cycloheximide, lysed, and split into pools for isolating total mRNA and ribosome bound mRNA. Ribosome-protected mRNA fragments were isolated by centrifugation. Total mRNA was alkaline fragmented and size selected. Both samples were processed for small RNA library sequencing. Libraries from two biological replicates per cell cycle phase were sequenced on an Illumina HiSeq 2000.
Alignment and analysis of ribosome profiling data
Sequencing reads were processed and aligned to the human genome using standard procedures (see Supplemental Experimental Procedures). Translational regulation was inferred using an errors-in-variables regression model. This analytical model estimates p-values in each replicate to represent lower or higher than expected ribosome-given-mRNA counts. We similarly developed methods for combining p-values among replicates within cell cycle phases and for testing differential translational regulation, all of which is described in greater detail in the Supplemental Experimental Procedures.
5′ UTR characterizations
Genes were classified as having either a higher, lower, or expected level of ribosome occupancy given their mRNA levels within each phase of the cell cycle as described in Supplemental Experimental Procedures. The length and %G+C content of the 5′ UTR of each expressed gene was computed from Gencode version 11, while the predicted minimum free energy was computed with the Vienna suite; RNAfold version 1.8.4(Gruber et al., 2008). The significance of each 5′UTR feature was determined with Wilcoxon rank sum test statistics.
Luciferase reporter assays
5′UTRs from Hela cDNA were cloned upstream of firefly luciferase in pGL3-T7. RNAs were in vitro transcribed using the T7 Megascript kit (Life Technologies). Purified RNA was capped using a vaccinia RNA capping system (New England Biolabs). Luciferase reporter RNA was transfected at a ratio of 20:1 with a control Renilla reporter RNA using the TransIT mRNA transfection kit (Mirus Bio). Luciferase levels were read after a 4-hour incubation using the Dual Luciferase Assay (Promega). Firefly luciferase signal was normalized to Renilla signal for each sample, averaged, and the signal relative to G1 was calculated.
Western blotting
Proteins were analyzed by standard western blotting protocols. Antibodies used were: RICTOR, AKT phospho-S473, total AKT, AKT phospho-T308, total PKCα, PROTOR-2 (Cell Signaling), PKCα phospho-S657 (Santa Cruz), PROTOR-1 (GeneTex), SIN1 (Bethyl Laboratories) phospho-p27 (R&D Systems), total p27 (BD Biosciences), Histone H3 phospho-S10 (Millipore), α-Tubulin (Sigma-Aldrich).
Accession Numbers
Sequencing data were deposited in the NCBI Short Read Archive: accession number SRA099816.
Supplementary Material
Highlights.
Translational regulation is prevalent and dynamic during cell cycle progression
Distinct cellular functions are coordinately translated during the cell cycle
Cell cycle-dependent translational regulation controls key signaling pathways
Translational regulation of large protein complexes promotes genome fidelity
Acknowledgments
We thank Maria Barna and members of the Ruggero lab for critical insights during this work and D. Byrd, K. Tong, and C. Milentis for reading the manuscript. Mark Magnuson provided the rictor-/- MEFs. C.R.S. is supported by NIH/NRSA (F32CA162634). This work was supported by NIH R01CA140456 (D.R.), NIH R01CA154916 (D.R.) and NIH P30CA82103 (A.B.O.). B.S.T. is a Prostate Cancer Foundation Young Investigator. D.R. is a Leukemia & Lymphoma Society Scholar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Antonin W, Ellenberg J, Dultz E. Nuclear pore complex assembly through the cell cycle: regulation and membrane organization. FEBS Lett. 2008;582:2004–2016. doi: 10.1016/j.febslet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- Borck G, Zarhrate M, Cluzeau C, Bal E, Bonnefont JP, Munnich A, CormierDaire V, Colleaux L. Father-to-daughter transmission of Cornelia de Lange syndrome caused by a mutation in the 5′ untranslated region of the NIPBL Gene. Hum Mutat. 2006;27:731–735. doi: 10.1002/humu.20380. [DOI] [PubMed] [Google Scholar]
- Bose T, Lee KK, Lu S, Xu B, Harris B, Slaughter B, Unruh J, Garrett A, McDowell W, Box A, et al. Cohesin proteins promote ribosomal RNA production and protein translation in yeast and human cells. PLoS Genet. 2012;8:e1002749. doi: 10.1371/journal.pgen.1002749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RJ, Huang M, Campbell MJ, Dong H, Steinmetz L, Sapinoso L, Hampton G, Elledge SJ, Davis RW, Lockhart DJ. Transcriptional regulation and function during the human cell cycle. Nat Genet. 2001;27:48–54. doi: 10.1038/83751. [DOI] [PubMed] [Google Scholar]
- Fan H, Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during . J Mol Biol. 1970;50:655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Gillespie PJ, Hirano T. Human Wapl is a cohesin-binding protein that promotes sister-chromatid resolution in mitotic prophase. Curr Biol. 2006;16:2406–2417. doi: 10.1016/j.cub.2006.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber AR, Lorenz R, Bernhart SH, Neubock R, Hofacker IL. The Vienna RNA websuite. Nucleic Acids Res. 2008;36:W70–74. doi: 10.1093/nar/gkn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Condensins: universal organizers of chromosomes with diverse functions. Genes Dev. 2012;26:1659–1678. doi: 10.1101/gad.194746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485:55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman J, O’Connor PM. Methods for synchronizing cells at specific stages of the cell cycle. Curr Protoc Cell Biol. 2001 doi: 10.1002/0471143030.cb0803s00. Chapter 8, Unit 8 3. [DOI] [PubMed] [Google Scholar]
- Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, Sowa ME, Rad R, Rush J, Comb MJ, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad CG. Protein Synthesis and Rna Synthesis during Mitosis in Animal Cells. J Cell Biol. 1963;19:267–277. doi: 10.1083/jcb.19.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueng S, Hegemann B, Peters BH, Lipp JJ, Schleiffer A, Mechtler K, Peters JM. Wapl controls the dynamic association of cohesin with chromatin. Cell. 2006;127:955–967. doi: 10.1016/j.cell.2006.09.040. [DOI] [PubMed] [Google Scholar]
- Lai MC, Chang WC, Shieh SY, Tarn WY. DDX3 regulates cell growth through translational control of cyclin E1. Mol Cell Biol. 2010;30:5444–5453. doi: 10.1128/MCB.00560-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merbl Y, Refour P, Patel H, Springer M, Kirschner MW. Profiling of ubiquitin-like modifications reveals features of mitotic control. Cell. 2013;152:1160–1172. doi: 10.1016/j.cell.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshen AO, Hsieh AC, Stumpf CR, Olshen RA, Ruggero D, Taylor BS. Assessing gene-level translational control from ribosome profiling. Bioinformatics. 2013 doi: 10.1093/bioinformatics/btt533. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Pulikkan JA, Dengler V, Peramangalam PS, Peer Zada AA, Muller-Tidow C, Bohlander SK, Tenen DG, Behre G. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood. 2010;115:1768–1778. doi: 10.1182/blood-2009-08-240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Sarnow P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J Biol Chem. 2004;279:13721–13728. doi: 10.1074/jbc.M312854200. [DOI] [PubMed] [Google Scholar]
- Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield ML, Sherlock G, Saldanha AJ, Murray JI, Ball CA, Alexander KE, Matese JC, Perou CM, Hurt MM, Brown PO, et al. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AJ, Severson AF, Meyer BJ. Condensin and cohesin complexity: the expanding repertoire of functions. Nat Rev Genet. 2010;11:391–404. doi: 10.1038/nrg2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.