Abstract
Background: Oral Submucous fibrosis (OSMF) is a common potentially malignant disease, which is characterized by generalized fibrosis of the oral soft tissues. It is due to disproportion in the collagen deposition and degradation. The excess deposition of collagen results in juxta epithelial fibro elastic changes and epithelial atrophy. Neoplastic changes in the epithelium usually precede the changes in the connective tissue. The purpose of the study was to evaluate the changes in birefringence of collagen using picrosirius stain and compare with haematoxylin and eosin stained section.
Materials & Methods: The study was conducted on 50 subjects, which included 45 patients diagnosed with different functional and histopathological grades of OSMF and 5 in control group. Picrosirius red stain was used to analyze collagen both histopathologically and qualitatively using polarizing microscopy. Chi-square test at p value <0.05 was used to analyze the results and the significance was tabulated.
Results: Collagen fibers showed mixed birefringence with a shift in polarizing colour from yellow to red-orange in lamina propria, around the muscle and blood vessels, which was correlating with the conventional H&E stain.
Conclusion: The results of present study show a significant change in birefringence of collagen between the various components of connective tissue. This change in birefringence colors and arrangement of collagen fibers might give an assumption of impending neoplastic change in OSMF.
How to cite the article: Velidandla S, Gaikwad P, Ealla KK, Bhorgonde KD, Hunsingi P, Kumar A. Histochemical analysis of polarizing colors of collagen using Picrosirius Red staining in oral submucous fibrosis. J Int Oral Health 2014;6(1):33-8.
Key Words: : Birefringence, collagen, oral submucous fibrosis, picrosirius red stain, polarizing colors
Introduction
Oral Submucous Fibrosis (OSMF) is a chronic disease of insidious onset and a prevailing potentially malignant disorder characterized by juxta-epithelial inflammatory reaction along with mucosal fibrosis. 1 It is a well recognized entity with frequency of malignant transformation reported in the range of 7-13%. 2 - 3 The precancerous nature was first mentioned by Paymaster 4 who observed the development of squamous cell carcinoma in one-third of his OSMF patients.
The condition is usually preceded by symptoms like burning sensation of the oral mucosa, ulceration and pain. The characteristic features that follow initial symptoms include reduced movement and depapillation of the tongue, blanching and leathery texture of oral mucosa and
progressive reduction of mouth opening. 5
Collagen forms the major portion of the connective tissue and provides it with a unique combination of flexibility and tensile strength, but is not very elastic. 6 The structural integrity and the tissue function is maintained by Collagen fibres. 7 The amount of collagen deposited and degraded is a well regulated process, not only to control the amount of collagen produced but also to control the fiber architecture. Collagen synthesis is determined by a variety of mediators, which include growth factors, hormones, cytokines and lymphokines, however TGF-β plays a pivotal role in the production of collagens and other matrix components. 8
OSMF is characterized by deposition of dense collagen in the connective tissue. Traditional stains such as Van-Gieson and trichrome were used to detect collagen fibres, however these stains failed to demonstrate thin fibres. The connective tissue integrity is maintained by both thick and thin fibres, emphasizing the importance to demonstrate them in order to evaluate any connective tissue changes. Picrosirius red a special stain was found to be promising to demonstrate both thin and thick fibres, which stains the thin fibres intensely and increases their birefringence. 9
In oral mucosa extracellular matrix of the lamina propria is a dense lattice network of collagen and elastin embedded in a ground substance of proteoglycans and glycoproteins. Type I collagen occupies the major portion of the connective tissue, while some amount of Type III is also present. In normal buccal mucosa the bulk of collagen is composed of Type I whereas Type III appeared around blood vessels, salivary glands, muscles and at the epithelialconnective tissue junction. 10
Picrosirius red special stain was used because the Sirius red, a strong cationic dye, stains collagen by reacting with its sulfonic acid and basic groups present in the collagen molecules. Collagen molecules, being rich in basic amino acids, strongly react with acidic dyes. Sirius red is an elongated dye molecule, which reacts with collagen and promotes the enhancement of its normal birefringence due to the fact that many dye molecule are aligned parallel with the long axis of the each collagen molecule. 11
An attempt has been made in the present study to compare the histopathological findings obtained using H&E stain with the polarizing colors of collagen in OSMF using picrosirius red stain, thereby, elucidating the progression of disease.
Materials and Methods
The study included 45 diagnosed cases of OSMF retrieved from the archives of Oral Pathology Laboratory and 10 subjects in the control group. Two sections (serial sections) of 5μm thickness from each paraffin embedded tissue block were prepared. One of the sections was stained with Hematoxylin and Eosin (H&E) and other with modified picrosirius red procedure. 12
Picrosirius red stain for collagen
5-micron thick sections were floated on to micro slides and incubated for 1 hour at 480C on a slide warmer for proper adhesion of the section on to the slide. Sections were then deparaffinized in xylene and hydrated through decreasing grades of alcohol. These sections were incubated in 0.1 % (W/V) Sirius red F3B (BX 10934, Bayer chemicals) in saturated Picric acid solution for 1 hour at room temperature. This was followed by rinsing with distilled water, staining with Mayer’s haematoxylin, differentiation in 1% Hcl, alkalinization with tap water, dehydration and mounting. Sections were then examined using Polarizing microscope.
Evaluation
The H&E stained slides were observed to record the following parameters:
Presence or absence of hyalinization in the lamina propria
Dynamic state of the blood vessels (dilated/constricted)
Density of connective tissue (loose fibrillar/ dense bundular)
Deposition of collagen around muscles
Deposition of collagen around blood vessels
The picrosirius red stained slides were reviewed for different polarizing colors of collagen in lamina propria, around muscle and around blood vessel. Four groups of polarizing colors were observed which included greenish yellow (GY), Yellowish orange (YO) and reddish orange
(RO). Results were then statistically analyzed using Chisquare test.
Results
Normal buccal mucosa
On evaluating the H&E stained control sections, rete ridges, absence of hyalinization and dilated blood vessels were observed. ( Figure 1 ) Loose fibrillar collagen towards
Figure 1: Shows normal buccal mucosa with loose fibrillar collagen towards the basement membrane and dense collagen bundles in the deeper lamina propria. (H&E stain-10X).
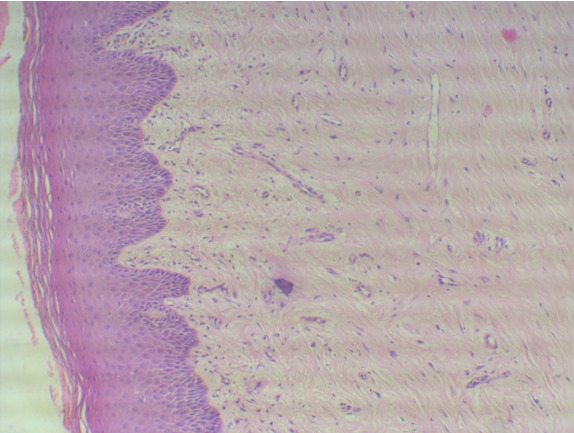
the basement membrane and dense collagen bundles in the deeper lamina propria with absence of collagen deposition around blood vessels and muscles were noted. With picrosirius red stain, normal buccal mucosa showed predominantly greenish yellow birefringence towards basement membrane, around muscles and blood vessels while the deeper lamina propria showed predominantly yellowish or reddish orange birefringence.
Oral Submucous Fibrosis
On evaluation of 45 cases of OSMF, 43 cases exhibited juxta-epithelial hyalinization. While considering the density of connective tissue in lamina propria, 39 (86.6%) cases showed bundular arrangement of collagen and delicate fibrillar collagen in 6 (13.3%) cases. On assessing
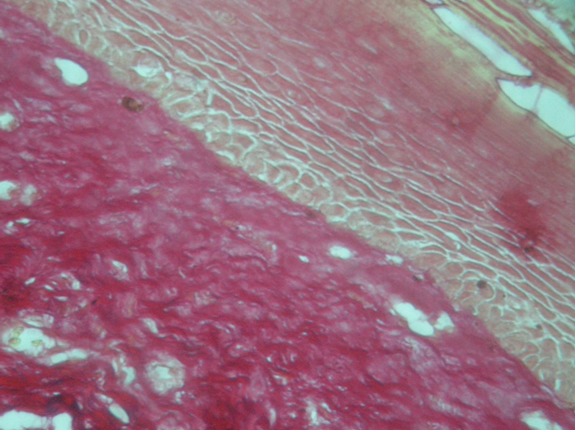
the dynamic state of blood vessels in lamina propria, 20 (44.45%) cases showed dilated vessels and 25 (55.5%) cases revealed constricted lumen. However, deposition of collagen fibers were abundant around the muscle tissue (71.1%) and around the blood vessels (64.4%) (Table-1, Figure 2 )
Table 1: Parameters in OSMF using H&E stain
| Parameters | Cases (45) | ||
| No. | % | ||
| Hyalinization in lamina propria | Present | 43 | 95.55 |
| Absent | 2 | 4.45 | |
| Density of connective tissue | Dense bundle like | 39 | 86.66 |
| Loose fibril like | 6 | 13.34 | |
| Blood vessels in lamina propria | Constricted | 25 | 55.55 |
| Dilated | 20 | 44.45 | |
| Collagen deposition around muscle | Dense | 32 | 71.11 |
| Sparse | 13 | 28.89 | |
| Collagen deposition around blood vessel in sub mucosa | Dense | 29 | 64.44 |
| Sparse | 16 | 35.56 | |
Figure 2: Shows OSMF with atrophic epithelium, constricted blood vessels and perivascular inflammatory infiltrate, collagen deposition around muscle and blood vessel. (H&E stain-20X).
Polarizing Microscopy
On evaluation of polarizing colors and intensities of birefringence of collagen fibres in lamina propria, 9 (20%) cases displayed different intensities of birefringence varying from greenish yellow to yellowish orange or reddish orange and few sections displayed only yellowish
orange in 21 (46.7%) and about 11 (24.4%) cases reddish
orange. Around 4 (8.9%) cases exhibited exclusive greenish yellow birefringence in lamina propria. Statistical analysis was done using chi-square (χ2) test and the results obtained were significant. (p=0.0012) (Table-2, Figure 3 & 4 )
Table 2: Polarizing colors in Lamina Propria, around muscle and blood vessels
| GY | GY=YO/RO | YO | RO | Total No.of Cases | χ2 | P | |
| Collagen in Lamina Propria | 4(8.9%) | 9(20%) | 21(46.7) | 11(24.4%) | 45 | 22.478 | 0.0012 |
| Collagen deposition around the muscle | 8(17.8%) | 12(26.7%) | 1533.3%) | 10(22.2%) | 45 | 18.978 | 0.0024 |
| Collagen deposition around the blood vessels | 6(13.3%) | 17(37.8%) | 1431.1%) | 8(17.8%) | 45 | 16.555 | 0.0019 |
x2 = Chi-Square test
GY= Greenish yellow
GY=YO / RO (Equal distribution)
YO=Yellowish orange.
RO= Reddish orange
P < 0.001 = VHS (VERY HIGHLY SIGNIFICANT)
P < 0.01 = HS (HIGHLY SIGNIFICANT)
P < 0.05 = S (SIGNIFICANT)
Figure 3: Shows OSMF with predominantly greenish yellow to reddish orange birefringence in the lamina propria. (Picrosirius red stain-10X).
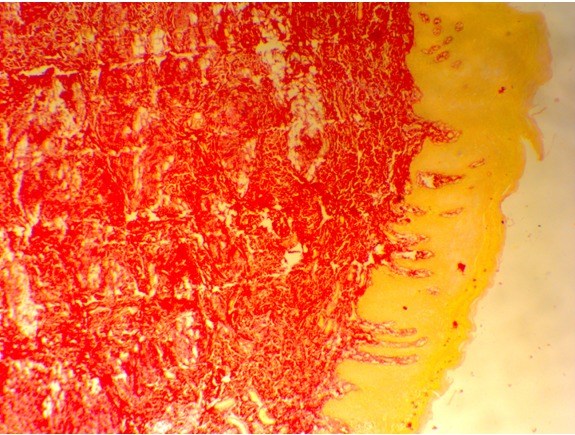
Figure 4: Shows OSMF with predominantly yellowish orange birefringence in the lamina propria. (Picrosirius red stain-10X).
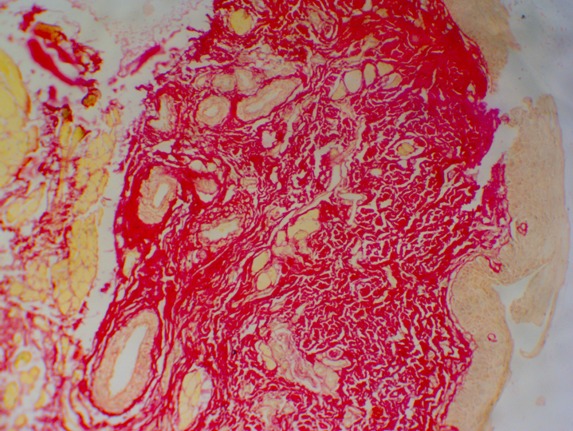
On assessing the intensity of birefringence of collagen in submucosa, particularly around the muscle tissue, 15 (33.3%) sections displayed yellowish-orange birefringence, 12 (26.7%) sections mixed intensities of greenish yellow and yellowish orange or reddish orange while 8 (17.8%) sections only greenish yellow birefringence and the remaining 10 sections (22.2%) only reddish orange birefringence. Chi-square test was applied on the results to compare the collagen deposition with the polarizing colors around muscle. p value was found to be very highly significant (p=0.0024) (Table-2).
When the intensities of birefringence in collagen fibers around the blood vessels were evaluated, 17 (37.8%) sections showed mixed colors of greenish yellow and yellowish orange or reddish orange, 14 (31.1%) sections yellowish orange, 8 (17.8%) sections reddish orange and 6
(13.3%) sections greenish yellow. When collagen deposition around blood vessels, was compared with polarizing colors and subjected to value was found to be very highly significant (p= 0.0019) (Table-2).
Discussion
Abnormal collagen deposition in the connective tissue of OSMF is directly related to areca nut (betel nut), a component of the betel quid, which induces an increase in the turnover of collagen. 13 , 14 Fibroblasts are stimulated by the alkaloids and the flavanoids present in the areca nut resulting in the accumulation of collagen. The collagen thus accumulated, exhibits increased cross linking, along with reduced collagenase activity results in decreased collagen degradation. 15
Currently many researchers have observed that Sirius red stain enhances the normal birefringence of collagen fibers in tissue sections. 16 Thin collagen fibers exhibit green to greenish yellow polarizing colors, whereas thick fibers Chi-square-χ2 test, p reveal yellowish-orange through orange to red polarizing colors with picrosirius red stain. Green to greenish-yellow
colors implicates poorly packed collagen whereas orange red color denotes tightly packed fibers. 17 , 18 The color and intensity of birefringence are due to the difference in their pattern of physical aggregation and thickness of collagen fibres. 16
In the present study, the color intensities of birefringence in control sections were predominantly greenish-yellow towards basement membrane, around muscle and around blood vessel. While the deeper lamina propria showed predominantly yellowish-orange to reddish-orange birefringence as observed by Montes et al 19 and Junqueira et al 18 in their respective studies.
The density of collagen fibers increased as the disease progressed which is in accordance to the previous observations made by several authors who postulated that fibroblastic activity is modulated by interaction of these cells with local factors present in the oral mucosa. In
addition to the inflammatory cells, arecoline the main alkaloid in betel nut extracts could stimulate fibroblastic proliferation and collagen synthesis. Furthermore Jeng et al , in their study stated that these extracts can cause genetic alterations in human fibroblasts, in turn these fibroblasts can persist for several generations and are responsible for increased collagen synthesis. 20 It was also found that the
activity of lysyl oxidase is increased in OSMF fibroblasts compared to normal fibroblasts. 21
Although considerable amount of work has been done on collagen, not many techniques have been proposed to demonstrate this protein adequately in tissue sections. Picrosirius red stain was proved to be excellent in demonstrating both thick and thin collagen fibres by
enhancing their birefringence. 16
The polarization colors in most of the cases of OSMF were greenish yellow and yellowish orange in the lamina propria, deeper in the sub mucosa around the muscle and blood vessel. This progressive alteration in the optical densities from greenish yellow to yellowish orange
presumably are due to the varying thickness of collagen fibers and also the physical aggregation of the fibers, which was supported by Perez Tamayo et al , who observed a loosely packed thin fibrils of type III collagen imparting greenish yellow color. 22 Usually the thin fibrils form a loose mesh work interacting with ground substance. Probably this type of physical aggregation results in a weak greenish yellow birefringence and a variable colors from greenish yellow to yellowish or reddish orange.
In the present study mixed birefringence was observed (9 cases) in the lamina propria. While in few cases of OSMF predominantly reddish (11 cases) and yellowish orange (21 cases) birefringence was noted in the lamina propria. Around the muscle yellowish orange (15 cases) and reddish orange (10 cases) colours were observed along with mixed birefringence (8 cases). Around the vessel wall predominantly mixed birefringence (17 cases) was observed followed by yellowish orange (14 cases) (Table 2), implying that the closely packed thick fibrils of type I collagen are more evident. Kuo et al , in their study stated that, the betel nut extracts and cytokines from
inflammatory cells could stimulate fibroblastic proliferation and activate one or more subtypes of fibroblasts to produce type I collagen. 23
Furthermore, in 25 (H&E) sections, blood vessels were seen in various stages of narrowing which was apparent in smaller vessels in upper lamina propria and gradual spread to deeper larger vessels (Table 1). This could be elucidated by the fact as put forward by Sirsat and Pindborg, 24 that hyalinization starts initially in the juxta-epithelial zone and spreads downwards with increasing intensity. The persistent dilatation of blood vessels in the present study may be due to a rise in mast cells, a feature of delayed reaction.
Thus the existence of mixed birefringence implicates that there is an increase in both type I and type III collagen while yellowish orange to reddish orange as type I collagen which is usually arranged in thick bundles which signify a biochemical and quantitative alterations in collagen.
Conclusion
Oral submucous fibrosis being the most common potentially malignant disorder, with changes in the structural integrity of collagen leading to the dysplastic changes in the epithelium resulting in carcinoma. Picrosirius red staining is a most simple technique to visualize changes in the collagen both quantitatively and qualitatively.
Though the procedure is lucid, variations in the results might occur on factors like pH, concentration of the stain and duration of staining. Hence, future studies must emphasise on the ultrastructural features of connective tissue in different stages of OSMF.
Footnotes
Source of Support: Nil
Conflict of Interest: None
Contributor Information
Surekha Velidandla, Department of Oral & Maxillofacial Pathology, M N R Dental College, Andhra Pradesh, India.
Pravin Gaikwad, Department of Oral & Maxillofacial Pathology, Surendera Dental College, Rajasthan, India.
Kranti Kiran Reddy Ealla, Department of Oral & Maxillofacial Pathology, M N R Dental College, Andhra Pradesh, India.
Kavitha D Bhorgonde, Department of Oral & Maxillofacial Pathology, S B Patil Dental College, Karnataka, India.
Prahalad Hunsingi, Department of Oral & Maxillofacial Pathology, A M E’s Dental College, Karnataka, India.
Anoop Kumar, Department of Oral & Maxillofacial Pathology, PSM Dental College, Kerala, India.
References
- 1.R Rajendran. Oral submucous fibrosis: Etiology, pathogenesis and future research. Bull World Health Organ. 1994;72:985–996. [PMC free article] [PubMed] [Google Scholar]
- 2.WM Tilakaratne, MF Klinikowski, T Saku, TJ Peters, S Warnakulasuriya. Oral submucous fibrosis: Review on aetiology and pathogenesis. Oral Oncol. 2006;42(6):561–568. doi: 10.1016/j.oraloncology.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.PR Murti, RB Bhonsle, JJ Pindborg, DK Daftary, PC Gupta, FS Mehta. Malignant transformation rate in oral submucous fibrosis over a 17-year period. Community Dent Oral Epidemiol. 1985;13:340–341. doi: 10.1111/j.1600-0528.1985.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 4.JC Paymaster. Cancer of the buccal mucosa; a clinical study of 650 cases in Indian patients. Cancer. 1956;9:431–435. doi: 10.1002/1097-0142(195605/06)9:3<431::aid-cncr2820090302>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.CB More, S Gupta, J Joshi, SN Varma. Classification system for Oral Submucous Fibrosis. J Indian Acad Oral Med Radiol. 2012;24(1):24–29. [Google Scholar]
- 6.ML Noorlander, P Melis, A Jonker, CJ Van Noorden. A Quantitative Method to determine the Orientation of Collagen Fibres in the Dermis. J Histochem Cytochem. 2002;50(11):1469–1474. doi: 10.1177/002215540205001106. [DOI] [PubMed] [Google Scholar]
- 7.L Rich, P Whittaker. Collagen and Picrosirius Red staining: A polarized Light Assessment of Fibrillar hue and spatial distribution. Braz J Morphol Sci. 2005;22(2):97–104. [Google Scholar]
- 8.CJ Chiu, ML Chang, CP Chiang, LJ Hahn, LL Hsieh, CJ Chen. Interaction of Collagen-related genes and susceptibility to Betel Quid-induced Oral Sub mucous Fibrosis. Cancer Epidemiol Biomarkers Prev. 2002;11(7):646–653. [PubMed] [Google Scholar]
- 9.DE Ceena, TS Bastian, L Ashok, RG Annigeri. Comparative study of clinicofunctional staging of oral sub mucous fibrosis with qualitative analysis of collagen fibers under polarizing microscopy. Indian J Dent Res. 2009;20(3):271–276. doi: 10.4103/0970-9290.57356. [DOI] [PubMed] [Google Scholar]
- 10.CW Van Wyk, HA Seedat, VM Philips. Collagen in submucous fibrosis: an electron-microscopic study. J Oral Pathol Med. 1990;19(4):182–187. doi: 10.1111/j.1600-0714.1990.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 11.GS Montes, LC Junquiera. The use of the picrosirius-polarization method for the Study of the biopathology of Collagen. Mem Inst Oswaldo Cruz. 1991;86 Suppl 3:1–11. doi: 10.1590/s0074-02761991000700002. [DOI] [PubMed] [Google Scholar]
- 12.D Dayan, Y Hiss, A Hirshberg, JJ Bubis, M Wolman. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry. 1989;93:27–29. doi: 10.1007/BF00266843. [DOI] [PubMed] [Google Scholar]
- 13.JP Canniff, W Harvey. The aetiology of oral submucous fi brosis: The stimulation of collagen synthesis by extracts of areca nut. Int J Oral Surg. 1981;10:163–167. [PubMed] [Google Scholar]
- 14.W Harvey, A Scutt, S Meghji, JP Canniff. Stimulation of human buccal mucosa fibroblasts in vitro by betel-nut alkaloids. Arch Oral Biol. 1986;31:45–49. doi: 10.1016/0003-9969(86)90112-3. [DOI] [PubMed] [Google Scholar]
- 15.P Rajalalitha, S Vali. Molecular pathogenesis of oral submucous fibrosis - a collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–328. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.LC Junqueira, G Bignolas, RR Brentani. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- 17.A Hirshberg, S Sherman, A Buchner, D Dayan. Collagen fibers in the wall of odontogenic keratocysts: A study with picrosirius red and polarizing microscopy. J Oral Pathol Med. 1999;28(9):410–412. doi: 10.1111/j.1600-0714.1999.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 18.LC Junqueira, GS Montes, EM Sanchez. The influence of tissue section thickness on the study of collagen by the Picrosirius polarization method. Histochemistry. 1982;74(1):153–156. doi: 10.1007/BF00495061. [DOI] [PubMed] [Google Scholar]
- 19.GS Montes, RM Krisztan, KM Shigihara, R Tokoro, PA Mourao, LC Junqueira. Histochemical and morphological characterization of reticular fibers. Histochemistry. 1980;65(2):131–141. doi: 10.1007/BF00493161. [DOI] [PubMed] [Google Scholar]
- 20.JH Jeng, ML Kuo, LJ Hahn, MY Kuo. Genotoxic and non-genotoxic effects of betel quid ingredients on oral mucosal fibroblasts in vitro. J Dent Res. 1994;73(5):1043–1049. doi: 10.1177/00220345940730050501. [DOI] [PubMed] [Google Scholar]
- 21.RH Ma, CC Tsai, TY Shieh. Increased lysyl oxidase activity in fibroblasts cultured from oral submucous fibrosis associated with betel nut chewing in Taiwan. J Oral Pathol Med. 1995;24(9):407–412. doi: 10.1111/j.1600-0714.1995.tb01210.x. [DOI] [PubMed] [Google Scholar]
- 22.R Perez Tamayo, I Montfort. The susceptibility of hepatic collagen to homologous collagenase in human and experimental cirrhosis of liver. Am J Pathol. 1980;100(2):427–442. [PMC free article] [PubMed] [Google Scholar]
- 23.MY Kuo, HM Chen, LJ Hahn, CC Hsieh, CP Chiang. Collagen biosynthesis in human oral submucous fibrosis fibroblast culture. J Dent Res. 1995;74(11):1783–1788. doi: 10.1177/00220345950740111101. [DOI] [PubMed] [Google Scholar]
- 24.SM Sirsat, JJ Pindborg. The vascular response in early and advanced oral submucous fibrosis. Acta Pathol Microbiol Scand. 1967;70(2):179–184. doi: 10.1111/j.1699-0463.1967.tb01280.x. [DOI] [PubMed] [Google Scholar]


