Abstract
To determine whether cellular miRNAs play a role in West Nile virus (WNV) neuropathogenesis, we evaluated WNV-infected mice brain for the expression profile of miRNAs, their potential functions and their correlation with genes involved in inflammatory pathways. A total of 528 miRNAs and 168 mRNA genes were examined. One hundred thirty-nine miRNAs were significantly differentially expressed in WNV-infected mice brain. Ingenuity pathway analysis demonstrated that these miRNAs and their target genes are involved in pathways related to inflammatory response, immune-cell trafficking and cell death. Moreover, we demonstrate an inverse correlation between WNV-modulated miRNAs and their target neuroinflammatory genes in the same mice brain. We demonstrate that miR-196a, miR-202-3p, miR-449c, and miR-125a-3p target multiple genes involving cytokines, chemokines, and apoptotic genes, which belong to different signaling pathways that play critical role in WNV neuropathogenesis. Functional studies targeting specific miRNA are warranted to develop therapeutics for the management of WNV disease.
Keywords: West Nile virus, Flavivirus, miRNA, PCR array, Neuroinflammation, miR-196a, miR-202-3p, miR-449c, miR-125a-3p
Background
MicroRNAs (miRNAs) are endogenous, short, non-coding RNA molecules (≈22nts) involved in post-transcriptional regulation of gene expression (Esteller, 2011). They function by directly binding to the 3’ untranslated regions (3’UTRs) of specific target mRNA, causing a block of translation or degradation of the target mRNA (Esteller, 2011; Huang et al., 2011). miRNAs regulate gene expression in a wide range of physiological and pathological conditions such as embryogenesis, differentiation, inflammation, viral infections, diabetes and carcinogenesis (Esteller, 2011; Huang et al., 2011). miRNAs have been demonstrated to play a crucial regulatory role in neurodegenerative diseases such as Alzheimer and Parkinson (Junn and Mouradian, 2012; Meza-Sosa et al., 2012). miRNAs also play a critical role in the regulation of immune response, including the differentiation, proliferation, cell fate determination, function of immune cells, and inflammatory mediator release as well as the intracellular signaling pathways (Ha, 2011; Pauley and Chan, 2008). miRNAs have been recently demonstrated to target proteins involved in regulating inflammation, and have a significant impact on the magnitude of the inflammatory response (O'Connell et al., 2012; Sonkoly et al., 2008).
In addition to their regulatory roles in diverse biological pathways, cellular miRNAs play critical roles in virus-host interactions. Several viruses including hepatitis C virus (HCV), human immunodeficiency virus type 1 (HIV-1), influenza viruses, human T-cell lymphotropic virus type I (HTLV-I), and human cytomegalovirus (HCMV) have been demonstrated to cause dramatic changes in cellular miRNAs expression (Huang et al., 2007; Li et al., 2010; Wang et al., 2008a). miRNAs of infected cells can influence the ability of a virus to replicate or spread, since many pathways including cell cycle, apoptosis, and immune response are modulated by miRNAs. For example, miR-28, miR-125b, miR-150, miR-223, miR-198 and miR-382 inhibit HIV replication in CD4+ T cells by directly targeting HIV mRNA or by modulating cellular factors responsible for its replication (Huang et al., 2007; Sung and Rice, 2009). Furthermore, miR-122 supports HCV replication by enhancing colony formation efficiency of HCV (Jopling et al., 2005), whereas miR-196 and miR-296 substantially attenuate virus replication through type I interferon (IFN)-associated pathways in liver cells (Pedersen et al., 2007). Similarly, HCMV and HTLV-1 regulate the expression of multiple cellular miRNAs to facilitate their replication (Bellon et al., 2009; Wang et al., 2008a). Similarly, miRNA HS_154 contributes to West Nile virus (WNV)-mediated apoptosis in vitro in human neuronal cell line, SK-N-MC (Smith et al., 2012). However, in vivo role of miRNAs in WNV neuropathogenesis is largely unknown.
Since its introduction in 1999, WNV encephalitis (WNVE) has become the most common mosquito-borne flavivirus neuroinvasive disease in the U.S., with the most recent outbreak in 2012 claiming 243 lives among 5,387 confirmed cases (Beasley et al., 2013). No drugs or vaccines against WNV are approved for human use. WNV infection is subclinical in most humans. However ~20-30% patients develop symptoms of WNV disease ranging from fever and mild headaches to severe encephalitis including cognitive dysfunction, seizures and flaccid paralysis. The fatality rate is approximately 10% for hospitalized WNVE cases (Murray et al., 2006). Following peripheral infection, WNV replication is first thought to occur in skin Langerhans dendritic cells. These cells migrate to draining lymph nodes, resulting in primary viremia. By the end of the first week, the virus is largely cleared from the peripheral organs, but in a subset of patients WNV enters the brain and causes a spectrum of neurological disorders (Samuel and Diamond, 2006). Neurons are the prime target of WNV replication and virus-associated pathology is characterized by neuronal death, activation of glial cells, blood-brain barrier (BBB) disruption, increased production of pro-inflammatory mediators and infiltration of leukocytes in the perivascular space and parenchyma (Diamond and Klein, 2004; Garcia-Tapia et al., 2007; Kelley et al., 2003).
Both anti-viral and pro-inflammatory responses to WNV infection play an important role in the modulation of host defense and disease manifestation (Diamond et al., 2009; Diamond et al., 2003). The response of WNV infection in the brain is associated with massive inflammatory events including production of cytokines such as interleukin (IL)-1β, -6 and tumor necrosis factor-α (TNF-α), and chemokines (Durrant et al., 2013; Klein et al., 2005; Kumar et al., 2013). The role of the various chemokines and cytokines such as TNF-α and CXCL10, have been established in recruitment of immune cells into the brain and virus clearance (Klein et al., 2005; Shrestha et al., 2008). While, Infiltration of immune cells, specifically CD8+ T cells is essential for controlling WNV infection in the brain, it also causes immunopathology (Shrestha and Diamond, 2004; Wang et al., 2003). WNV-induced neuronal death is via apoptosis and involves induction of various apoptotic genes (Parquet et al., 2001; Samuel et al., 2007; Shrestha et al., 2003). BBB disruption is associated with disruption of tight junction proteins (TJP) and production of matrix metalloproteinases (Roe et al., 2012; Wang et al., 2008b). Thus, modulation of genes involved in various signaling pathways such as apoptosis, antigen presentation, BBB disruption and inflammation in the brain following WNV infection play a role in overall disease pathogenesis. However, the upstream regulatory pathways, which control the expression of these genes in WNV infection, are poorly understood.
Since, miRNAs, by modulating networks of hundreds to thousands of mRNAs regulate multiple signaling pathways involving inflammatory response, we analyzed the cellular miRNA expression profiles in mice brain following WNV infection. We assessed the potential functions of differentially expressed miRNAs by analyzing the predicted target genes and associated signaling pathways. We also examined the mRNA gene expression data from the same mice brain to assess functional associations of differentially expressed cellular miRNAs with their differentially expressed targets and their role in WNV neuropathogenesis. To our knowledge, this is the first study to evaluate modulation of miRNAs in the mice brain following WNV infection.
Results and Discussions
WNV modulates cellular miRNAs expression in mice brain
Several studies have demonstrated changes in cellular miRNAs expression levels in response to viral infection (Bhomia et al., 2010; Li et al., 2010; Zhou et al., 2012). To identify differentially expressed miRNAs associated with WNV infection in the mice brain, we evaluated cellular miRNA expression profiles in the brain of mock- and WNV-infected mice at day 8 after infection. WNV is first detected in the brain between days 4 and 6 after footpad inoculation and peak virus load is observed at day 8 after infection (Kumar et al., 2012; Roe et al., 2012). Therefore, to understand the miRNA changes occurring at the time of high WNV replication in brain, we examined miRNAs expression at day 8 after infection. No virus was detected in the mock-infected brains. Virus titers were similar (6 × 103 ± 1,178 PFU/g of tissue, n=4) in the brains of all WNV-infected mice at day 8 after infection as measured by plaque assay (Kumar et al., 2012).
We used quantitative RT-PCR array to evaluate the miRNA expression profiles in the brain of mock- and WNV-infected mice. Among 528 miRNAs present on the array, 139 miRNAs were significantly modulated. miRNAs that were altered at least 2-fold were considered significant. Thirty-six miRNAs were significantly up-regulated, and 103 were down-regulated in WNV-infected brains when compared to mock brains (Tables 1 and 2). The number of down-regulated miRNAs was approximately three times more than the up-regulated miRNAs. Since miRNA negatively regulate gene expression, this pattern correlates with previously reported WNV data, where the number of inflammatory genes was up-regulated in the brain at day 8 after infection (Glass et al., 2005; Klein et al., 2005; Kumar et al., 2013). Ten miRNAs were up-regulated more than 10-fold. Among these, let-7a was profoundly increased by more than 1,000-fold and miR-99a was up regulated more than 100-fold in WNV-infected brain when compared to mock brain (Table 1). Overall, significant increase in expression of let-7 family members (between 7 to 1,000-fold change) was observed with the exception of let-7c, which was reduced by 2.1-fold in WNV-infected brain. Let 7 family members are among the highly abundant regulators of gene expression in the brain (Lehmann et al., 2012). Let-7 is observed to increase among patients with Down syndrome, Parkinson's disease (PD) as well as Alzheimer's disease (AD) (Kuhn et al., 2008; Lehmann et al., 2012). It has been demonstrated that let-7 induces neurodegeneration through activation of neuronal toll-like receptor 7 (Lehmann et al., 2012). Among down-regulated miRNAs, miR-196a, miR-470, miR-21*, miR-208a, miR-683, miR-184, miR-693-3p, miR-202-3p, miR-429, miR-878-3p, and miR-327 were down-regulated more than 10-fold. miRNA-196a is associated with cell survival and protection in neurodegenerative diseases such as Huntington's disease (HD) (Cheng et al., 2013). In our study, miRNA-196a was down-regulated by more than 13,000-fold (Table 2), which correlates with neuronal apoptosis observed in WNV-infected mice (Kumar et al., 2013).
Table 1.
Up-regulated miRNAs#
| miRNA | Fold-change | miRNA | Fold-change | miRNA | Fold-change |
|---|---|---|---|---|---|
| let-7a | 1024 | let-7i | 7 | miR-30a | 2.8 |
| miR-99a | 128 | miR-1187 | 4.8 | miR-181c | 2.8 |
| miR-30e | 91 | miR-30c-1* | 4.4 | miR-133b | 2.5 |
| let-7e | 60 | miR-201 | 4.2 | miR-221 | 2.4 |
| miR-142-5p | 26 | miR-503 | 4.0 | miR-219 | 2.4 |
| let-7g | 24 | miR-122 | 3.4 | miR-1900 | 2.4 |
| miR-142-3p | 21 | miR-193 | 3.4 | miR-20a | 2.3 |
| miR-155 | 15 | miR-500 | 3.2 | miR-465a-3p | 2.3 |
| miR-27b | 13 | miR-449a | 3.0 | miR-770-3p | 2.2 |
| miR-19a | 11 | miR-133a* | 3.0 | miR-18a | 2.1 |
| miR-101a | 9 | miR-196a* | 2.9 | miR-374 | 2.1 |
| miR-32 | 8 | miR-879 | 2.9 | miR-217 | 2.1 |
Change in the levels of each gene was first normalized to the housekeeping genes and then the fold-change in WNV-infected brain was calculated in comparison to mock-infected brain.
miRNA strands that are the complementary strands of the functional single-stranded miRNAs.
Table 2.
Down-regulated miRNAs#
| miRNA | Fold-change | miRNA | Fold-change | miRNA | Fold-change |
|---|---|---|---|---|---|
| miR-196a | −13308 | miR-672 | −4.7 | miR-98 | −3.0 |
| miR-470 | −28 | miR-423-5p | −4.7 | miR-100 | −3.0 |
| miR-21* | −28 | miR-302b | −4.7 | miR-673-5p | −3.0 |
| miR-208a | −20 | miR-546 | −4.5 | miR-141* | −2.9 |
| miR-683 | −13 | miR-200b* | −4.4 | miR-466f-3p | −2.9 |
| miR-184 | −12 | miR-295* | −4.4 | miR-877 | −2.9 |
| miR-693-3p | −12 | miR-106a | −4.4 | miR-380-5p | −2.9 |
| miR-202-3p | −12 | miR-141 | −4.3 | miR-324-5p | −2.9 |
| miR-429 | −12 | miR-200c* | −4.1 | miR-30b | −2.8 |
| miR-878-3p | −11 | miR-130b | −4.1 | miR-708 | −2.8 |
| miR-327 | −10 | miR-145* | −3.9 | miR-322 | −2.8 |
| miR-466d-5p | −8.7 | miR-574-3p | −3.9 | miR-132 | −2.7 |
| miR-654-5p | −8.3 | miR-671-3p | −3.9 | miR-149 | −2.7 |
| miR-125a-3p | −7.6 | miR-135a | −3.8 | miR-370 | −2.7 |
| miR-20b | −7.6 | miR-467a* | −3.8 | miR-101b | −2.7 |
| miR-183 | −7.5 | miR-375 | −3.8 | miR-7a | −2.7 |
| miR-200c | −7.4 | miR-293 | −3.6 | miR-191 | −2.6 |
| miR-181a-2* | −7.3 | miR-151-5p | −3.5 | miR-96 | −2.6 |
| miR-675-5p | −7.2 | miR-31 | −3.5 | miR-129-5p | −2.5 |
| miR-590-5p | −7.2 | miR-466g | −3.4 | miR-151-3p | −2.5 |
| miR-471 | −7.1 | miR-452 | −3.4 | miR-126-5p | −2.5 |
| miR-465a-5p | −6.6 | miR-188-5p | −3.3 | miR-1-2-as | −2.4 |
| miR-197 | −5.9 | miR-103 | −3.3 | miR-679 | −2.4 |
| miR-290-3p | −5.8 | miR-291a-5p | −3.3 | miR-153 | −2.3 |
| miR-296-3p | −5.8 | miR-183* | −3.2 | miR-222 | −2.3 |
| miR-335-3p | −5.5 | miR-693-5p | −3.2 | miR-378 | −2.3 |
| miR-128 | −5.4 | miR-470* | −3.1 | miR-300 | −2.3 |
| miR-292-3p | −5.4 | miR-183* | −3.1 | miR-9* | −2.2 |
| miR-298 | −5.4 | miR-212 | −3.1 | miR-106b | −2.2 |
| miR-574-5p | −5.1 | miR-195 | −3.1 | miR-449b | −2.2 |
| miR-678 | −5.1 | miR-29c | −3.1 | let-7c | −2.1 |
| miR-449c | −4.9 | miR-148a | −3.1 | miR-138 | −2.1 |
| miR-1938 | −4.8 | miR-152 | −3.1 | miR-425 | −2.1 |
| miR-200a* | −4.8 | miR-381 | −3.1 | ||
| miR-687 | −4.8 | miR-210 | −3.0 |
Change in the levels of each gene was first normalized to the housekeeping genes and then the fold-change in WNV-infected brain was calculated in comparison to mock-infected brain.
miRNA strands that are the complementary strands of the functional single-stranded miRNAs.
WNV-modulated specific miRNAs are also involved in other neurological diseases
Specific miRNAs have been identified in various human brain disorders (Meza-Sosa et al., 2012; Rege et al., 2013). Interestingly, several miRNAs differentially modulated following WNV infection have been implicated to play important role in the pathogenesis of other virus-mediated neurological diseases and classical neurodegenerative diseases such as HD, AD, and PD. For example, up-regulation of miR-30e and down-regulation of miR-196, miR-128, miR-29c, miR-132, miR-222, and miR-9* observed in WNV-infected mice brain in our study, have been associated with HD, while reduced expression of miR-7 and up-regulation of miR-101 are associated with PD and AD, respectively (Meza-Sosa et al., 2012; Rege et al., 2013). Similarly, increased expression of miR-155 was observed in Down syndrome (Kuhn et al., 2008). Moreover, increased expression of miR-155, miR-101a, miR-449a, and miR-879, and reduced expression of miR-130b, miR-195, miR-381, miR-322, miR-129-5p, and miR-126-5p observed in our study have been also demonstrated in the brain of CD-1 mice infected with Venezuelan equine encephalitis virus (VEEV) (Bhomia et al., 2010). Interestingly, down-regulation of let-7 family miRNAs, the most abundant miRNAs in mouse brain, was observed in both classical neurodegenerative disease such as HD and virus-induced encephalitis such as VEE in mice (Bhomia et al., 2010; Lagos-Quintana et al., 2002; Rege et al., 2013). In contrast, we observed a significant increase in the expression of let-7 family members in WNV-infected mice brain, suggesting that up-regulation of let-7 family miRNAs is specific for WNV infection in the brain.
However, no studies have previously reported modulation of miRNAs in mice brain after WNV infection. Recently Cho et. al. demonstrated high expression of miRNA-132 in naïve cortical neurons compared to naïve granule cell neurons (Cho et al., 2013). They further demonstrated that ectopic expression of miRNA-132 in naïve granule cell neurons decreased the expression of interferon stimulating genes (ISGs). Moreover, they also observed increased WNV replication in cortical neurons compared to granule cell neurons. Collectively, based on these data, Cho et. al. concluded that high expression of miR-132 in naïve cortical neurons might enhance WNV infection by negatively regulating IFN-β signaling and anti-WNV immunity (Cho et al., 2013). However, this study has not examined the change in the miRNA-132 expression in the neurons after WNV infection. Our data demonstrate down-regulation of miR-132 after WNV infection in the brain (Table 2), which correlates with previously published reports of increased production of ISGs and IFN-β in the mice brain at day 8 after infection (Lazear et al., 2011; Suthar et al., 2010). Another study by Smith el. al. has demonstrated that WNV infection modulates the expression of several human cellular miRNAs in human embryonic kidney, HEK293, and human neuronal epithelioma, SK-N-MC, cell lines (Smith et al., 2012). They also demonstrated that WNV-induced cellular miRNA, Hs_154, contributes to virus-mediated apoptosis in SK-N-MC cell line (Smith et al., 2012). Although, a hairpin structure homologous to that encoding Hs_154 in humans has been identified in mice but was not evaluated in this study (Berezikov et al., 2006). Taken together, differential regulation of similar miRNAs in virus-mediated neurological as well as HD, AD and PD suggests that viruses possibly modulate common neurological pathways, which are also involved in classical neurodegenerative diseases.
Functional analysis of WNV-modulated miRNAs and their predicted targets
To characterize the function of differentially expressed miRNAs, ingenuity pathways analysis (IPA) was conducted using the predicted gene targets to analyze their potential biological function. Biofunctional analysis of WNV-modulated miRNAs and their targets revealed cell death and survival, immune-cell trafficking, cell-mediated immune response, and inflammatory response as the top pathways in signaling pathways category (Fig. 1A). Since WNV infection of the mice brain is associated with enhanced infiltration of immune cells into the brain, increased production of pro-inflammatory cytokines and chemokines and apoptosis of neurons, these miRNAs may play an important role in neuroinflammation and neuronal death following WNV infection (Glass et al., 2005; Klein et al., 2005; Kumar et al., 2013; Shrestha et al., 2003).
Fig. 1.
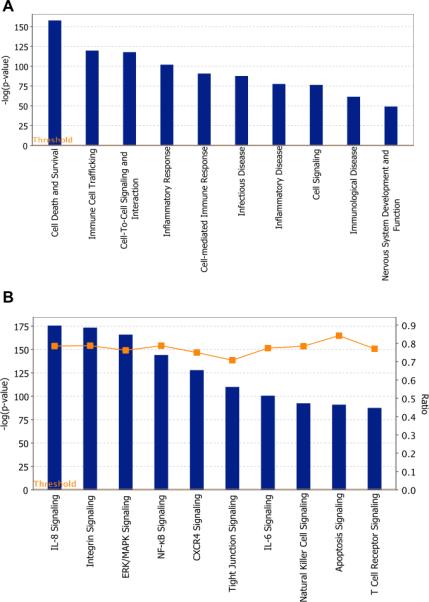
Core functional pathway analysis of WNV-modulated miRNAs using IPA. (A) Top biological functions regulated by significantly modulated miRNAs. Threshold bar indicates cut-off point of significance P < 0.05, using Fisher's exact test. (B) Top canonical signaling pathways regulated by significantly modulated miRNAs. Threshold bar indicates cut-off point of significance P < 0.05, using Fisher's exact test. Line indicates ratio of genes in network to total number of genes in the canonical pathway.
Our pathway-based analysis further demonstrated that WNV-modulated miRNAs and their targets regulate important canonical signaling pathways such as integrin, NF-kB and ERK/MAPK, CXCR4, TJP, and apoptosis signaling pathways (Fig. 1B). Integrins have been demonstrated to enhance WNV replication in various cells including Vero, baby hamster kidney, and mouse embryo fibroblast cells (Chu and Ng, 2004; Schmidt et al., 2013). WNV infection is associated with activation of NF-kB and ERK/MAPK signaling pathways, which regulates antigen presentation, production of pro-inflammatory cytokines and cell survival after infection (Kesson and King, 2001; Scherbik and Brinton, 2010; Suthar et al., 2013). WNV-induced expression of CXCR4 prevents T cell trafficking into the mice brain (McCandless et al., 2008). Migration of T cells into the brain is essential for protection against WNV infection by clearing virus in the brain (McCandless et al., 2008; Shrestha and Diamond, 2004). We have previously demonstrated that WNV-induced disruption of TJP leads to the opening of the BBB allowing unrestricted entry of virus and immune cells into the mice brain (Roe et al., 2012). Apoptosis is a principal cause of neuronal death in WNV infection and involves activation of various apoptotic-signaling molecules such as caspase3/9 and Bcl2 family genes (Parquet et al., 2001; Samuel and Diamond, 2006; Shrestha et al., 2003). Thus, by regulating these canonical pathways, these differentially modulated miRNAs may play an important role in WNV disease pathogenesis of brain.
WNV modulates neuroinflammatory mRNA gene expression in mice brain
We further analyzed the mRNA expressions of key neuroinflammatory molecules in the same mice brain to examine whether differentially expressed miRNAs could regulate their target genes. Consistent with the earlier studies WNV infection in the brain significantly induced the expression of pro-inflammatory cytokines, chemokines and their receptors, genes involved in apoptosis signaling and antigen presentation pathways (Garcia-Tapia et al., 2007; Glass et al., 2005; Kumar et al., 2013) (Table 3). Only mRNA genes that were altered at least 2-fold were considered significant. To examine that this increase in mRNA expression also lead to increased protein levels, we measured protein levels of key chemokines and cytokines in the brain homogenates using Luminex assay. Similar to the mRNA expression, protein levels of the key chemokines such as CXCL10, CCL2, CCL5, CCL3, CXCL1 and cytokines such as IL10, IFNγ, TNF-α, and IL-1β were significantly increased in the brain of WNV-infected mice as compared to mock-infected mice (Fig. 3). Many of these genes are known to play a significant role in WNV neuropathogenesis (Diamond et al., 2009; Diamond et al., 2003).
Table 3.
Significantly modulated mRNA genes#
| mRNA | Fold-change | mRNA | Fold-change | mRNA | Fold-change |
|---|---|---|---|---|---|
| CXCL10 | 641.36 | IL1R2 | 4.36 | CCR8 | −2.11 |
| CXCL9 | 394.81 | ITGB2 | 4.36 | CCR4 | −2.11 |
| CCL2 | 279.17 | CXCL1 | 4.07 | CCL1 | −2.11 |
| CCL5 | 113.38 | CCR7 | 3.80 | CCL20 | −2.11 |
| CCL7 | 85.92 | NFKBIA | 3.36 | CCL24 | −2.11 |
| CCL12 | 65.12 | HMOX1 | 3.36 | IL13 | −2.11 |
| CCR2 | 40.09 | IL1β | 3.31 | IL1F6 | −2.11 |
| CCL8 | 21.48 | FOS | 3.14 | IL1F8 | −2.11 |
| CCL4 | 20.04 | RUNX1 | 2.93 | IL20 | −2.11 |
| CCL3 | 17.45 | S100A8 | 2.73 | XCR1 | −2.11 |
| CXCL13 | 17.45 | BCL2L1 | 2.56 | LTA | −2.21 |
| IL10 | 12.34 | TXNIP | 2.55 | IL3 | −2.21 |
| IL2RG | 12.34 | IL1R1 | 2.54 | IL4 | −2.21 |
| IFNγ | 10.02 | CCR9 | 2.51 | IL5RA | −2.21 |
| CXCL11 | 7.59 | WFS1 | 2.38 | BDNF | −2.38 |
| CCL9 | 6.61 | CEBPB | 2.38 | IL16 | −2.42 |
| CCR1 | 6.17 | TNFRSF1B | 2.34 | IL17B | −2.42 |
| TNF-α | 6.17 | CD40LG | 2.34 | PLG | −2.55 |
| IL2RB | 5.76 | PTGS2 | 2.22 | MYOD1 | −2.55 |
| C3 | 5.37 | THBS1 | 2.22 | AIPL1 | −2.55 |
| TNFSF10 | 5.10 | CCR5 | 2.18 | SMAD6 | −2.56 |
| CXCR3 | 5.01 | MYC | 2.08 | EPHB2 | −2.68 |
| CCR3 | 5.01 | BCL6 | 2.04 | MSX2 | −2.73 |
| LTB | 5.01 | SERPINE1 | 2.03 | PF4 | −2.98 |
| RBL1 | 4.44 | SNAI1 | 2.03 | CCR6 | −5.56 |
| CASP1 | 4.36 | CRP | −2.11 | ATF3 | −6.73 |
| CCL19 | 4.36 | CXCL15 | −2.11 |
Change in the levels of each gene was first normalized to the housekeeping genes and then the fold-change in WNV-infected brain was calculated in comparison to mock-infected brain.
Fig. 3.

Networks of the interactions of the miRNA target genes. IPA tool was used to generate the miRNA-mRNA interaction network using (A) miR-196a, (B) miR-202-3p, (C) miR-449c, and (D) miR-125a-3p and neuroinflammatory mRNAs genes analyzed in our study. Solid lines represent direct interactions and dashed lines indirect interactions. Red (increased expression) and green (decreased expression).
Network analysis of expression of miRNAs and mRNAs from WNV-infected mice brain
We next sought to determine whether mRNA gene expression changes might be influenced by the differential expression of cellular miRNAs during WNV infection in the brain. To analyze the direct and indirect miRNA-mRNA gene interactions, we conducted IPA expression pairing analysis with mRNA expression data and significantly modulated miRNAs. Several miRNAs were found to directly or indirectly target multiple genes analyzed in our study and demonstrated an inverse correlation with mRNA gene expression of inflammatory molecules induced by WNV in the same brain sample and the same time point after infection, day 8, suggesting their role in regulating inflammation following WNV infection. We focused on four miRNAs, miR-196a, miR-202-3p, miR-449c, and miR-125a-3p, which regulate multiple genes that play key role in WNV-induced neuroinflammation (Fig. 3A-D).
miR-196a is the topmost down-regulated miRNA, which targets CCR2, NFKBIA, and SMAD6 (Fig. 3A). While, CCR2 is a chemokine receptor critical for monocytes accumulation in the mice brain and enhanced survival in WNVE, NFKBIA is a member of NF-kB inhibitor family, which inhibits NF-kB function (Lim et al., 2011). NF-kB is a transcription factor essential for antigen presentation and production of type I IFNs and pro-inflammatory cytokines (Fredericksen, 2013; Kesson and King, 2001; Suthar et al., 2013). Our data suggest that down-regulation of miR-196a led to increased expression of CCR2 and NFKBIA in WNV-infected mice brain. miR-196 has been previously demonstrated to play diverse biological functions involving immune response, inflammation and virus defense (Chen et al., 2011). Furthermore, miR-202-3p is down-regulated and its targets TNFRSF1B, CCR7, BCL2L1, S100A8, THBS1, CCL7 and IL10 are up-regulated in WNV-infected mice brain (Fig. 3B). Similarly, down-regulation of miR-449c led to up-regulation of its targets CXCL10, CXCL11, NFKBIA, SERPINE1, IL2RB, CCR1, MYC, SNAI1, and BCL6 (Fig. 3C). Along with mRNA, our data also demonstrated increase in the protein levels of the target genes such as IL10 and CXCL10 (Fig. 2). These targets involve genes belonging to immune-cell trafficking, inflammatory response and apoptosis signaling pathways which mediates multiple effectors function such as trafficking of immune cells into the brain, production of pro-inflammatory mediators and neuronal apoptosis after WNV infection (Bai et al., 2009; Kesson and King, 2001; Klein et al., 2005; Parquet et al., 2001). It is interesting to see correlation between WNV-modulated miRNAs and target genes at both mRNA and protein level, which is consistent with the miRNA-mRNA-protein triad and demonstrate functional importance of our results.
Fig. 2.

Enhanced production of cytokines and chemokines in WNV-infected mice brains. Brains were harvested from mock and WNV-infected WT mice at day 8 after infection and homogenized as described in materials and methods. Levels of chemokines and cytokines as noted in the figure were measured using multiplex Luminex assay and are expressed as the mean concentration (pg/g of tissue) ± SEM (n=4 per group). *p < 0.05, **p < 0.001, using unpaired student t-test.
We further demonstrate that miR-125a-3p may play an important role in regulating anti-viral cytokines in the brain following WNV infection. miR-125a-3p targets PTGS2, IL1R1, IL10 and CCL4, which play significant role in modulating host immune responses to WNV infection in the brain (Fig. 3D). IL1R1 signaling is required to restrict WNV infection in the mice brain by T cell reactivation and limiting inflammation (Durrant et al., 2013). In contrast, IL10 signaling facilitates WNV infection in the mice by suppressing antiviral cytokines production (Bai et al., 2009). We previously demonstrated that WNV-induced PTGS2 or COX-2 is involved in modulating the expression of multiple neuroinflammatory mediators in human brain astrocytes (Verma et al., 2011). While proinflammatory cytokines response is important in anti-WNV immunity, increased production of these cytokines also contributes to the overall disease pathogenesis (Bai et al., 2010; Diamond et al., 2009; Wang et al., 2003). Therefore, down-regulation of miR-125a-3p after WNV infection in the brain may be a protective host response to balance between an effective immune response and immunopathology due to excessive inflammation. miR-125a has been previously demonstrated to constitutively activate NF-κB pathway (Kim et al., 2012). WNV infection is also associated with activation of NF-kB pathway, which regulates antigen presentation and production of type I IFNs and pro-inflammatory mediators (Fredericksen, 2013; Kesson and King, 2001; Suthar et al., 2013). Therefore, down-regulation of miR-125a-3p could control WNV-induced inflammation in the brain by regulating activation of NF-kB pathway. miR-125a-3p has also been demonstrated to interfere with hepatitis B virus translation and down regulates the expression of the virus surface antigen (Potenza et al., 2011).
Similar to the down-regulated miRNAs, several up-regulated miRNAs were found to directly or indirectly target multiple genes analyzed in our study. Up-regulation of specific miRNAs such as miR-155 and miR-32 was well correlated with neuroinflammatory molecules (Fig. 4). miR-155 target genes were IL-13, BDNF, and CCR9 (Fig. 4A). One of the target genes, IL13, is involved in cell survival and reduced levels of IL13 observed in WNV-infected mice may promote apoptosis (Park et al., 2009). Also, miR-155 has been previously demonstrated to regulate immune response to bacterial and viral Infections (O'Connell et al., 2012). Recently, aberrant expression of miR-155 was observed in many autoimmune conditions, including rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus (Leng et al., 2011; O'Connell et al., 2012). Furthermore, miR-32 targets SMAD6, SOX4, IL36B, and CCR9 genes (Fig. 4B) and it has been demonstrated recently that TGF-β1/Smad6–signaling pathway contributes to WNV pathogenesis (Sultana et al., 2012). However, the role of other target genes, SOX4, IL36B, and CCR9 genes in WNV neuroinflammation remains to be determined.
Fig. 4.
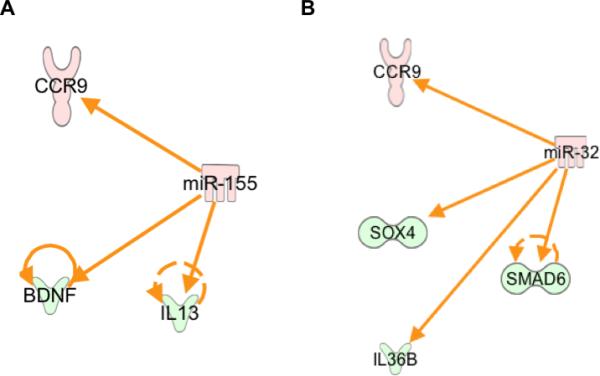
Networks of the interactions of the miRNA target genes. IPA tool was used to generate the miRNA-mRNA interaction network using (A) miR-155, (B) miR-32 and neuroinflammatory mRNAs genes analyzed in our study. Solid lines represent direct interactions and dashed lines indirect interactions. Red (increased expression) and green (decreased expression).
In conclusion, this study for the first time demonstrates that WNV infection causes modulation of miRNAs expression in the mice brain. Biofunctional analysis demonstrates that WNV-modulated miRNAs and their target genes regulate the pathways that are important for WNV disease pathogenesis. These data demonstrate an inverse correlation between WNV-modulated miRNAs and target neuroinflammatory genes induced by WNV infection in the mice brain. Moreover, positive correlation of WNV-modulated miRNAs with protein levels of neuroinflammatory genes, which are known to play a significant role in WNV neuropathogenesis, further emphasize functional importance of these miRNAs in WNV disease pathogenesis. Furthermore, our analysis also demonstrates that individual miRNAs including miR-196a, miR-202-3p, miR-449c, and miR-125a-3p can modulate multiple upstream regulatory genes and down-regulation of these miRNAs following WNV infection triggers the pathways leading to neuroinflammation and neuronal death in the mice brain. Collectively these data suggest that miRNAs regulate downstream gene expression, important in WNV disease pathogenesis, and can be targeted in the future to develop therapeutics for the management of WNV disease.
Materials and Methods
Animal experiments
Nine-week old C57BL/6J mice were purchased from The Jackson Laboratory. Animals were housed four per cage and allowed to eat and drink ad libitum. The animal suite was maintained at 72°F, 45% humidity and on 12/12-light/dark cycles. Sawdust bedding was provided along with paper towel. This study was approved by the University of Hawaii Institutional Animal Care and Use Committee (IACUC) (protocol number 10-948), and conducted in strict accordance with guidelines established by the National Institutes of Health and the University of Hawaii IACUC. All animal experiments were conducted in consultation with veterinary and animal care staff at the University of Hawaii in the animal biosafety level-3 laboratory.
Mice (four animals per group) were inoculated via the footpad route with 10 plaque forming units (PFU) of WNV (NY99) or PBS (mock). A power calculation assuming a type I error of 0.05 gave a power of 92% using four mice per group. At day 8 after infection, mice were anesthetized using isoflurane and perfused with cold PBS as described previously (Kumar et al., 2012). Brains were harvested and flash frozen in 2-methylbutane (Sigma) and stored at −80° C until further use.
Plaque assay
One-half of the frozen brain of four mice was weighed and homogenized in a bullet blender (Next Advance) using glass or zirconium beads, and plaque assay was conducted for four brain homogenates using Vero cells for analysis of WNV titer as described previously (Kumar et al., 2012; Kumar et al., 2013).
Luminex assay
Levels of cytokines and chemokines in the above mentioned four brain homogenates were measured by Luminex assay using MILLIPLEX MAP Mouse Cytokine/Chemokine kit (Millipore) as described previously (Kumar et al., 2013).
miRNA PCR array
One-half of the frozen brain of four mice was powdered over dry ice to obtain a homogenous sampling. An aliquot of the frozen powder was used to isolate total miRNA using miRNeasy Mini Kit (Qiagen, Cat # 217004) according to the manufacturer's protocol. Genomic DNA contamination was eliminated by digesting the RNA with RNase-free DNase (Ambion, Austin, TX). RNA was quantitated using Nanodrop (Thermo Scientific, Wilmington, DE) and the 28S/18S RNA ratios of all RNA samples were between 1.8 and 2. Total RNA (1.5 μg) was reverse-transcribed into cDNA using RT2 miRNA First Strand Kit (Qiagen, Cat # 331401) as per manufacturer's protocol. cDNA from all four animals from each group was pooled.
The expression profile of miRNAs was measured by quantitative real-time polymerase chain reaction (qPCR) using the Mouse miRNome RT2 miRNA PCR Array (SABiosciences, Cat # 331213) as per manufacturer's protocol. Briefly, 100 μL (6 μg) of total cDNA was mixed with 5 mL of RT2 SYBR Green Fluor qPCR mastermix (Qiagen, Cat # 330513) and 4.9 mL of nuclease free water. Twenty five-μL of this mixture was loaded in each well of a 96-well plate and qPCR was conducted using Bio-Rad iCycler real-time PCR machine as per manufacturer's protocol (Kumar et al., 2013). The miRNA PCR array contains 528 unique rodent miRNAs in six 96-well plates. Each plate also includes four housekeeping genes (Snord85, Snord68, Snord66, and Rnu6), two positive PCR controls (PPC) and two reverse transcription controls (RTC).
mRNA PCR array
As mentioned above, an aliquot of same frozen brain powder was used to extract total RNA, reverse-transcribed into cDNA and expression profile of multiple cytokines, chemokines and their receptors was analyzed using a commercial Profiler Inflammatory Cytokines and Receptors PCR Array (SABiosciences, Cat # PAMM-011Z) and RT2 Profiler TGFβ Signaling Targets PCR Array (SABiosciences, Cat # PAMM-235Z) as described previously (Kumar et al., 2013). cDNA from four animals from each group was pooled.
Statistical Analysis
qPCR data obtained from the miRNA and mRNA PCR arrays was analyzed for the modulation of statistically significant miRNAs and mRNAs using RT2 Profiler PCR Array Data Analysis version 3.5 (SABiosciences) as described previously (Kumar et al., 2013). Cycle threshold (Ct) values of >35 was considered to be non-specific and miRNAs and mRNAs with raw Ct value of >35 were excluded from the analysis. The fold change in WNV-infected mice brain as compared to mock-infected brain was calculated after normalizing to the housekeeping genes. miRNAs and mRNAs with a fold change of ±2 were considered significant.
Target prediction and pathway analysis was conducted using IPA (Ingenuity Systems Inc., Redwood City, CA) as described previously (Shin et al., 2013). Fisher's exact test, using IPA, was used to calculate cut-off point of significance. p < 0.05 is considered significant. We also conducted correlation pairing with mRNA expression data and significantly modulated miRNAs using IPA as described previously (Shin et al., 2013).
For Luminex analysis, unpaired student t-test using Graphpad was used to calculate p values as described previously (Kumar et al., 2012). p < 0.05 is considered significant.
Research Highlights (For Review).
WNV infection causes modulation of miRNAs expression in the mice brain.
miRNAs and their target genes regulate the pathways important for WNV pathogenesis.
We demonstrate inverse correlation between WNV-modulated miRNAs and target genes.
WNV-modulated miRNAs directly correlate with protein levels of inflammatory genes.
Acknowledgments
This work was supported by Institutional funds and grant (P20GM103516) from the Centers of Biomedical Research Excellence, National Institute of General Medicine, National Institutes of Health. We thank Dr. Gordon Okimoto and Mike Loomis of University of Hawaii Cancer Center and COBRE Bioinformatics Core for assistance with Ingenuity analysis. We thank Dr. James Davis of the JABSOM Biostatistics and Data Management Core supported by the National Institute on Minority Health and Health Disparities (U54MD007584), NIH for statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bai F, Kong KF, Dai J, Qian F, Zhang L, Brown CR, Fikrig E, Montgomery RR. A paradoxical role for neutrophils in the pathogenesis of West Nile virus. J Infect Dis. 2010;202:1804–1812. doi: 10.1086/657416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Town T, Qian F, Wang P, Kamanaka M, Connolly TM, Gate D, Montgomery RR, Flavell RA, Fikrig E. IL-10 signaling blockade controls murine West Nile virus infection. PLoS Pathog. 2009;5:e1000610. doi: 10.1371/journal.ppat.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beasley DW, Barrett AD, Tesh RB. Resurgence of West Nile neurologic disease in the United States in 2012: What happened? What needs to be done? Antiviral Res. 2013;99:1–5. doi: 10.1016/j.antiviral.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113:4914–4917. doi: 10.1182/blood-2008-11-189845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezikov E, Thuemmler F, van Laake LW, Kondova I, Bontrop R, Cuppen E, Plasterk RH. Diversity of microRNAs in human and chimpanzee brain. Nat Genet. 2006;38:1375–1377. doi: 10.1038/ng1914. [DOI] [PubMed] [Google Scholar]
- Bhomia M, Balakathiresan N, Sharma A, Gupta P, Biswas R, Maheshwari R. Analysis of microRNAs induced by Venezuelan equine encephalitis virus infection in mouse brain. Biochem Biophys Res Commun. 2010;395:11–16. doi: 10.1016/j.bbrc.2010.03.091. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang Y, Zhang L, Weakley SM, Yao Q. MicroRNA-196: critical roles and clinical applications in development and cancer. J Cell Mol Med. 2011;15:14–23. doi: 10.1111/j.1582-4934.2010.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PH, Li CL, Chang YF, Tsai SJ, Lai YY, Chan AW, Chen CM, Yang SH. miR-196a ameliorates phenotypes of Huntington disease in cell, transgenic mouse, and induced pluripotent stem cell models. Am J Hum Genet. 2013;93:306–312. doi: 10.1016/j.ajhg.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Proll SC, Szretter KJ, Katze MG, Gale M, Jr., Diamond MS. Differential innate immune response programs in neuronal subtypes determine susceptibility to infection in the brain by positive-stranded RNA viruses. Nat Med. 2013;19:458–464. doi: 10.1038/nm.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JJ, Ng ML. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J Biol Chem. 2004;279:54533–54541. doi: 10.1074/jbc.M410208200. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Klein RS. West Nile virus: crossing the blood-brain barrier. Nat Med. 2004;10:1294–1295. doi: 10.1038/nm1204-1294. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Mehlhop E, Oliphant T, Samuel MA. The host immunologic response to West Nile encephalitis virus. Front Biosci. 2009;14:3024–3034. doi: 10.2741/3432. [DOI] [PubMed] [Google Scholar]
- Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- Durrant DM, Robinette ML, Klein RS. IL-1R1 is required for dendritic cell-mediated T cell reactivation within the CNS during West Nile virus encephalitis. J Exp Med. 2013;210:503–516. doi: 10.1084/jem.20121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- Fredericksen BL. The neuroimmune response to West Nile virus. J Neurovirol. 2013 doi: 10.1007/s13365-013-0180-z. DOI 10.1007/s13365-013-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tapia D, Hassett DE, Mitchell WJ, Jr., Johnson GC, Kleiboeker SB. West Nile virus encephalitis: sequential histopathological and immunological events in a murine model of infection. J Neurovirol. 2007;13:130–138. doi: 10.1080/13550280601187185. [DOI] [PubMed] [Google Scholar]
- Glass WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TY. The Role of MicroRNAs in Regulatory T Cells and in the Immune Response. Immune Netw. 2011;11:11–41. doi: 10.4110/in.2011.11.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wang F, Argyris E, Chen K, Liang Z, Tian H, Huang W, Squires K, Verlinghieri G, Zhang H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat Med. 2007;13:1241–1247. doi: 10.1038/nm1639. [DOI] [PubMed] [Google Scholar]
- Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- Junn E, Mouradian MM. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol Ther. 2012;133:142–150. doi: 10.1016/j.pharmthera.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley TW, Prayson RA, Ruiz AI, Isada CM, Gordon SM. The neuropathology of West Nile virus meningoencephalitis. A report of two cases and review of the literature. Am J Clin Pathol. 2003;119:749–753. doi: 10.1309/PU4R-76JJ-MG1F-81RP. [DOI] [PubMed] [Google Scholar]
- Kesson AM, King NJ. Transcriptional regulation of major histocompatibility complex class I by flavivirus West Nile is dependent on NF-kappaB activation. J Infect Dis. 2001;184:947–954. doi: 10.1086/323603. [DOI] [PubMed] [Google Scholar]
- Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC. MicroRNAs miR-125a and miR-125b constitutively activate the NF-kappaB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20). Proc Natl Acad Sci U S A. 2012;109:7865–7870. doi: 10.1073/pnas.1200081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, Engle M, Diamond MS. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DE, Nuovo GJ, Martin MM, Malana GE, Pleister AP, Jiang J, Schmittgen TD, Terry AV, Jr., Gardiner K, Head E, Feldman DS, Elton TS. Human chromosome 21-derived miRNAs are overexpressed in down syndrome brains and hearts. Biochem Biophys Res Commun. 2008;370:473–477. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kumar M, Roe K, Nerurkar PV, Namekar M, Orillo B, Verma S, Nerurkar VR. Impaired virus clearance, compromised immune response and increased mortality in type 2 diabetic mice infected with west nile virus. PLoS One. 2012;7:e44682. doi: 10.1371/journal.pone.0044682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M, Roe K, Orillo B, Muruve DA, Nerurkar VR, Gale M, Jr., Verma S. Inflammasome adaptor protein Apoptosis-associated speck-like protein containing CARD (ASC) is critical for the immune response and survival in west Nile virus encephalitis. J Virol. 2013;87:3655–3667. doi: 10.1128/JVI.02667-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- Lazear HM, Pinto AK, Vogt MR, Gale M, Jr., Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. J Virol. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann SM, Kruger C, Park B, Derkow K, Rosenberger K, Baumgart J, Trimbuch T, Eom G, Hinz M, Kaul D, Habbel P, Kalin R, Franzoni E, Rybak A, Nguyen D, Veh R, Ninnemann O, Peters O, Nitsch R, Heppner FL, Golenbock D, Schott E, Ploegh HL, Wulczyn FG, Lehnardt S. An unconventional role for miRNA: let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat Neurosci. 2012;15:827–835. doi: 10.1038/nn.3113. [DOI] [PubMed] [Google Scholar]
- Leng RX, Pan HF, Qin WZ, Chen GM, Ye DQ. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 2011;22:141–147. doi: 10.1016/j.cytogfr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Li Y, Chan EY, Li J, Ni C, Peng X, Rosenzweig E, Tumpey TM, Katze MG. MicroRNA expression and virulence in pandemic influenza virus-infected mice. J Virol. 2010;84:3023–3032. doi: 10.1128/JVI.02203-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JK, Obara CJ, Rivollier A, Pletnev AG, Kelsall BL, Murphy PM. Chemokine receptor Ccr2 is critical for monocyte accumulation and survival in West Nile virus encephalitis. J Immunol. 2011;186:471–478. doi: 10.4049/jimmunol.1003003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-Sosa KF, Valle-Garcia D, Pedraza-Alva G, Perez-Martinez L. Role of microRNAs in central nervous system development and pathology. J Neurosci Res. 2012;90:1–12. doi: 10.1002/jnr.22701. [DOI] [PubMed] [Google Scholar]
- Murray K, Baraniuk S, Resnick M, Arafat R, Kilborn C, Cain K, Shallenberger R, York TL, Martinez D, Hellums JS, Hellums D, Malkoff M, Elgawley N, McNeely W, Khuwaja SA, Tesh RB. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect. 2006;134:1325–1332. doi: 10.1017/S0950268806006339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Ann rev immunol. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- Park KW, Baik HH, Jin BK. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J Immunol. 2009;183:4666–4674. doi: 10.4049/jimmunol.0803392. [DOI] [PubMed] [Google Scholar]
- Parquet MC, Kumatori A, Hasebe F, Morita K, Igarashi A. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 2001;500:17–24. doi: 10.1016/s0014-5793(01)02573-x. [DOI] [PubMed] [Google Scholar]
- Pauley KM, Chan EK. MicroRNAs and their emerging roles in immunology. Ann N Y Acad Sci. 2008;1143:226–239. doi: 10.1196/annals.1443.009. [DOI] [PubMed] [Google Scholar]
- Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza N, Papa U, Mosca N, Zerbini F, Nobile V, Russo A. Human microRNA hsa-miR-125a-5p interferes with expression of hepatitis B virus surface antigen. Nucleic Acids Res. 2011;39:5157–5163. doi: 10.1093/nar/gkr067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege SD, Geetha T, Pondugula SR, Zizza CA, Wernette CM, Babu JR. Noncoding RNAs in Neurodegenerative Diseases. ISRN Neurol. 2013;2013:375852. doi: 10.1155/2013/375852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe K, Kumar M, Lum S, Orillo B, Nerurkar VR, Verma S. West Nile virus-induced disruption of the blood-brain barrier in mice is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. J Gen Virol. 2012;93:1193–203. doi: 10.1099/vir.0.040899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel MA, Morrey JD, Diamond MS. Caspase 3-dependent cell death of neurons contributes to the pathogenesis of West Nile virus encephalitis. J Virol. 2007;81:2614–2623. doi: 10.1128/JVI.02311-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbik SV, Brinton MA. Virus-induced Ca2+ influx extends survival of west nile virus-infected cells. J Virol. 2010;84:8721–8731. doi: 10.1128/JVI.00144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Keller M, Bader BL, Korytar T, Finke S, Ziegler U, Groschup MH. Integrins modulate the infection efficiency of West Nile virus into cells. J Gen Virol. 2013;94:1723–1733. doi: 10.1099/vir.0.052613-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin OS, Kumar M, Yanagihara R, Song JW. Hantaviruses induce cell type- and viral species-specific host microRNA expression signatures. Virology. 2013;446:217–224. doi: 10.1016/j.virol.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Gottlieb D, Diamond MS. Infection and injury of neurons by West Nile encephalitis virus. J Virol. 2003;77:13203–13213. doi: 10.1128/JVI.77.24.13203-13213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha B, Zhang B, Purtha WE, Klein RS, Diamond MS. Tumor necrosis factor alpha protects against lethal West Nile virus infection by promoting trafficking of mononuclear leukocytes into the central nervous system. J Virol. 2008;82:8956–8964. doi: 10.1128/JVI.01118-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JL, Grey FE, Uhrlaub JL, Nikolich-Zugich J, Hirsch AJ. Induction of the cellular microRNA, Hs_154, by West Nile virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors. J Virol. 2012;86:5278–5287. doi: 10.1128/JVI.06883-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkoly E, Stahle M, Pivarcsi A. MicroRNAs and immunity: novel players in the regulation of normal immune function and inflammation. Semin Cancer Biol. 2008;18:131–140. doi: 10.1016/j.semcancer.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Sultana H, Neelakanta G, Foellmer HG, Montgomery RR, Anderson JF, Koski RA, Medzhitov RM, Fikrig E. Semaphorin 7A contributes to West Nile virus pathogenesis through TGF-beta1/Smad6 signaling. J Immunol. 2012;189:3150–3158. doi: 10.4049/jimmunol.1201140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TL, Rice AP. miR-198 inhibits HIV-1 gene expression and replication in monocytes and its mechanism of action appears to involve repression of cyclin T1. PLoS Pathog. 2009;5:e1000263. doi: 10.1371/journal.ppat.1000263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthar MS, Diamond MS, Gale M., Jr. West Nile virus infection and immunity. Nat Rev Microbiol. 2013;11:115–128. doi: 10.1038/nrmicro2950. [DOI] [PubMed] [Google Scholar]
- Suthar MS, Ma DY, Thomas S, Lund JM, Zhang N, Daffis S, Rudensky AY, Bevan MJ, Clark EA, Kaja MK, Diamond MS, Gale M., Jr. IPS-1 is essential for the control of West Nile virus infection and immunity. PLoS Pathog. 2010;6:e1000757. doi: 10.1371/journal.ppat.1000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Kumar M, Nerurkar VR. Cyclooxygenase-2 inhibitor blocks the production of West Nile virus-induced neuroinflammatory markers in astrocytes. J Gen Virol. 2011;92:507–515. doi: 10.1099/vir.0.026716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FZ, Weber F, Croce C, Liu CG, Liao X, Pellett PE. Human cytomegalovirus infection alters the expression of cellular microRNA species that affect its replication. J Virol. 2008a;82:9065–9074. doi: 10.1128/JVI.00961-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Dai J, Bai F, Kong KF, Wong SJ, Montgomery RR, Madri JA, Fikrig E. Matrix metalloproteinase 9 facilitates West Nile virus entry into the brain. J Virol. 2008b;82:8978–8985. doi: 10.1128/JVI.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lobigs M, Lee E, Mullbacher A. CD8+ T cells mediate recovery and immunopathology in West Nile virus encephalitis. Journal of virology. 2003;77:13323–13334. doi: 10.1128/JVI.77.24.13323-13334.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Pupo GM, Gupta P, Liu B, Tran SL, Rahme R, Wang B, Rua R, Rizos H, Carroll A, Cairns MJ, Saksena NK. A parallel genome-wide mRNA and microRNA profiling of the frontal cortex of HIV patients with and without HIV-associated dementia shows the role of axon guidance and downstream pathways in HIV-mediated neurodegeneration. BMC Genomics. 2012;13:677. doi: 10.1186/1471-2164-13-677. [DOI] [PMC free article] [PubMed] [Google Scholar]


