Abstract
Central obesity is associated with insulin resistance and dyslipidemia. Thus, the mechanisms that control fat distribution and its impact on systemic metabolism have importance for understanding risk for diabetes and cardiovascular disease. Hypercortisolemia at the systemic (Cushing’s syndrome) or local levels (due to adipose-specific overproduction via 11β-Hydroxysteroid dehydrogenase 1) results in the preferential expansion of central, especially visceral fat depots. At the same time, peripheral subcutaneous depots can become depleted. The biochemical and molecular mechanisms underlying the depot-specific actions of glucocorticoids (GCs) on adipose tissue function remain poorly understood. GCs exert pleiotropic effects on adipocyte metabolic, endocrine and immune function, and dampen adipose tissue inflammation. GCs also regulate multiple steps in the process of adipogenesis. Acting synergistically with insulin, GCs increase the expression of numerous genes involved in fat deposition. Variable effects of GC on lipolysis are reported, and GC can improve or impair insulin action depending on the experimental conditions. Thus, the net effect of GC on fat storage appears to depend on the physiologic context. The preferential effects of GC on visceral adipose tissue have been linked to higher cortisol production and glucocorticoid receptor expression, but the molecular details of the depot-dependent actions of GCs are only beginning to be understood. In addition, increasing evidence underlines the importance of circadian variations in GCs in relationship to the timing of meals for determining their anabolic actions on the adipocyte. In summary, although the molecular mechanisms remain to be fully elucidated, there is increasing evidence that GCs have multiple, depot-dependent effects on adipocyte gene expression and metabolism that promote central fat deposition.
1. Introduction
Adipose tissue stores excess energy as triglyceride (TG) and releases it as free fatty acids (FFA) depending on body needs. Adipose tissue is also an endocrine organ that secretes numerous peptide hormones and bioactive molecules that act in auto-, para- and endocrine fashions to regulate adipose tissue as well as systemic metabolism. The higher mass of dysfunctional adipose tissue in obesity may cause or exacerbate metabolic abnormalities that lead to type 2 diabetes, cardiovascular diseases, and several types of cancer. Adipose tissues or ‘depots’ are found in multiple locations throughout the body. Each has distinct developmental, structural, and functional features [64]. Visceral depots (omental and mesenteric) are found within the abdominal cavity associated with digestive organs, while subcutaneous (sc) depots are found under the skin. Fat accumulation in both visceral and abdominal sc depots confers increased risk for metabolic disease independent of total body fat, while fat accumulation in the lower body is protective [64,108]. Similarities between metabolic abnormalities in central obesity and glucocorticoid (GC) excess, i.e. Cushing’s syndrome, have led to the hypothesis that derangements in GC metabolism and action in adipose tissue play pivotal roles in the development of obesity and pathogenesis of obesity related diseases. Indeed, visceral obesity has been appropriately described as “Cushing’s disease of the omentum” [11].
The in vivo effects of GC are complex. Pathophysiological levels cause muscle protein breakdown as well as systemic insulin resistance, while physiological levels are crucial for mobilizing stored fuel for the “fight or flight response” as well as for replenishing fat stores when insulin levels rises with feeding. Most in vivo studies involve administration of high concentrations of GC that does not provide insight into how physiological variations in systemic and tissue cortisol levels throughout the day affect systemic and tissue-specific metabolism. Additionally, analysis of the in vivo consequences of hypercortisolemia on the adipocytes is partially confounded by alterations in food intake, compensatory hyperinsulinemia and activation of the sympathetic nervous system [66]. Studies of GC action in vitro are also often difficult to interpret as they usually involve continuous treatment with very high levels of dexamethasone (Dex), a type II glucocorticoid receptor (GR) agonist.
GCs have diverse effects on adipose tissue biology. They are required for induction of lipogenic genes, regulate lipolysis [85] and adipose endocrine function [9,29,60,63], and play an important role in restraining adipose tissue inflammation in obesity [84]. GCs are also required for the differentiation of adipocyte precursors [40,80] and the maintenance of adipogenic genes in cultured adipocytes and adipose tissue [63,126]. Increasing numbers of studies address the interactions of GC and insulin, and the impact of synergisms between these two major hormones in the regulation of fat distribution and function in vivo and in vitro.
Here we review available knowledge of the actions of GC on adipose tissue, attempting to distinguish GC effects on different cell types that reside within tissues, as well as studies using cell culture models that examine the direct effects of GC on the adipocyte. We will briefly review cellular and molecular mechanisms mediating GC action at prereceptor and receptor levels, and discuss current understanding of the mechanisms through which GCs affect adiposity and fat distribution.
2. Glucocorticoids promote fat deposition and central adiposity
The powerful effects of GC on adipose tissue accumulation are clearly demonstrated by early studies showing that adrenalectomy prevents obesity in Zucker rats and this is reversed by replacement of corticosterone [28]. In mouse models, overexpression of 11-beta hydroxysteroid dehydrogenase 1 (HSD1) using an adipose specific promoter (aP2) that produces high local concentration of corticosterone within adipose tissue results in the preferential expansion of mesenteric (visceral) fat due to adipocyte hypertrophy [72]. Conversely, genetic or pharmacological inactivation of HSD1 results in a lean, insulin-sensitive phenotype [51,53,75].
In humans, excess cortisol at the systemic level results in 2 to 5-fold increases in central, especially visceral adipose tissues (Cushing’s syndrome), while peripheral sc depots waste [35,93,119]. Although the clinical literature describes peripheral fat wasting in Cushing’s syndrome, the magnitudes of the effect at different sc sites using whole body imaging methods and its reversibility with treatment remain poorly documented. One study suggested that expected depot differences in adipocyte size between the two major sc depots, i.e. smaller abdominal than gluteo-femoral adipocytes, are lost in Cushing’s syndrome [91], suggesting hypertrophy of abdominal adipocytes. The preferential expansion of abdominal sc adipose tissue in Cushing’s syndrome is mediated, at least in part, by elevated activity of lipoprotein lipase (LPL), the rate-determining step for the hydrolysis and uptake of circulating TG-fatty acids into adipocytes, together with low lipolytic rates [91] (also as reviewed in [70]). Additional in vitro as well as in vivo studies using tracer methodologies are clearly needed to understand how acute and chronic hypercortisolemia promotes central fat deposition while wasting peripheral sc depots.
3. Do systemic or local excess glucocorticoids contribute to central adiposity?
3.1. Systemic cortisol levels are normal in obesity
Individuals under chronic, uncontrollable stress are more likely to have elevated levels of visceral fat [96]. Despite an increase in overall cortisol production and rates of turnover, circulating cortisol levels have been shown to be normal or low in obesity [65,105]. Subsequent studies revealed abnormalities in the hypothalamus-pituitary-adrenal (HPA) axis of the obese which are a function of body fat distribution rather than total fat mass. Repeated measurements of salivary cortisol in free-living subjects revealed an abnormal diurnal variation of cortisol which was positively associated with an upper body fat distribution as measured by waist-hip ratio (WHR) [96]. This abnormal pattern was characterized by low variability, absent circadian rhythm, low morning cortisol, and lack of meal-induced cortisol response [65,96,97], all of which have been associated with long-term over-activation (burn-out) of the HPA axis. Lower suppression of 8 A.M. cortisol after low dose Dex is reported among subjects with elevated WHR [65,82], but other studies show enhanced cortisol suppression after Dex among abdominally obese subjects [22]. Elevated response to corticotrophin releasing hormone (CRH) stimulation [83], and increased basal [66] and stimulated response to stress [24] in subjects with abdominal obesity, suggest over-activation of the HPA axis in these subjects. A recent study [46] showing active recycling of cortisol in metabolically active tissues highlights the difficulties of understanding interactions of central and peripheral mechanisms that modulate cortisol actions in vivo.
The consequences of the circadian variations in cortisol and interactions with meals for GC action within adipose tissue also deserve consideration. For example, a pulse of cortisol increases leptin only when given together with a meal or insulin [56,57]. Indeed GCs are important for regulating circadian phenomena at the whole body level. GCs connect the central hypothalamic clock with the peripheral clocks in multiple tissues, so that diurnal functions are in step with the environmental cycles of light and nutrition [4]. At the adipocyte level, supraphysiological doses of GC can acutely affect the expression and rhythmicity of clock genes [36,124]. Given that genetic disruption of clock genes specifically in adipose tissue causes obesity [81], future studies of how variations in the pattern of cortisol secretion affect regional adipose tissue deposition and function in humans are clearly needed. GC turnover rates in adipose tissue however, are low and possibly unaffected by acute fluctuations in circulating cortisol levels [47]. Thus, the local production of GC may be more important in the physiological context, as discussed below (section 3.2).
3.2. Local production of cortisol is increased in adipose tissue of obese subjects
While serum cortisol levels are not clearly increased in human obesity, increased local activation of cortisol from cortisone within adipose tissue is associated with obesity [23,50,62,88]. Intracellular cortisol levels are regulated by HSD1 and HSD2. HSD1, a NADPH dependent enzyme, is expressed in many tissues, including liver and adipose tissue, and acts predominantly as a reductase, generating cortisol in vivo. HSD2, expressed in kidney and colon, inactivates cortisol to cortisone, protecting the mineralocorticoid receptor (MR) from activation by cortisol.
HSD1 knockout mice are protected from high-fat diet induced obesity, hyperglycemia and dyslipidemia [51,53,75], while adipose overexpression of HSD1 results in obesity with metabolic syndrome [72]. HSD1 overexpression or knockout preferentially affects intraabdominal, especially mesenteric fat, in mouse [72,75]. Similarly, administration of an HSD1 inhibitor at a dose that does not affect food intake induces preferential loss of mesenteric fat in mice [7]. The HSD1 knockout mouse also exhibits depot- and diet-dependent effects on adipose inflammation and promotes sc fat distribution [120]. It will be important to understand how the fine tuning of intracellular cortisol production in specific adipose depots impacts inflammation and metabolism in obesity.
In humans, most but not all studies that examined the relationship of obesity to adipose HSD1 mRNA and reductase activity show positive correlations with BMI and adipocyte size [23,50,62,88,111]. It is not clear however, whether high adipose HSD1 expression is a cause or a compensatory mechanism in human obesity. It has been postulated that higher cortisol synthesis in visceral fat contributes to the preferential expansion of this depot. Consistent with this idea, one study found higher HSD1 reductase activity in stromal cell cultures derived from omental than abdominal sc adipose tissue [10]. Contradictory results showing no depot differences in HSD1 activity in fresh tissue are also reported [62,111] and thus, it is not clear whether increased local production of cortisol contributes significantly to visceral fat accumulation. Studies of human adipose tissue in organ culture showed that Dex causes a depot-specific feed-forward upregulation of HSD reductase activity in omental but not abdominal sc adipose tissue [62], suggesting a mechanism through which GC promote visceral obesity in response to stress.
HSD2 is reportedly expressed in non-adipocyte cell populations within human adipose tissues. While HSD2 expression level or its activity is not increased in obesity, several studies found that HSD2 expression levels are higher in omental than sc adipose tissue [62,116], suggesting higher cortisol turnover in the visceral depot. The higher rate of turnover may provide a mechanism to fine tune GC production and hence actions in this depot in response to nutrient or hormonal cues. Potential variations in local GC levels throughout the day and possible variations with meals have not yet been systemically studied. In this context, it is noteworthy that most studies of cortisol production and action in adipose tissue are conducted in the overnight fasted state. Basu et al [5] found that meal induced cortisol production and that was due to extrasplanchnic tissue production. Studies of tissue-specific meal-induced changes in the prereceptor metabolism of GC and their receptor-mediated actions will be important in understanding the complex physiology of this hormone in the context of obesity, overnutrition and the metabolic syndrome.
4. Molecular mechanisms governing glucocorticoid actions on adipocytes
4.1. Role of the type II glucocorticoid receptor
The action of GC on target cells is thought to be mediated by the type 2 glucocorticoid receptor (GR, NR3C1), a member of the nuclear receptor superfamily that is expressed in virtually all tissues. Unliganded GR resides in the cytoplasm as a heterocomplex with several heat shock proteins and chaperone complexes [76]. Ligand binding triggers conformational changes in the receptor that result in dissociation from the heterocomplex and translocation into the nucleus. The GR homodimer binds to specific DNA locations, glucocorticoid response elements (GREs), and stimulates target gene expression. GR also associates with less well-defined negative GREs and transrepresses gene transcription. Once bound to the GREs, conformational changes in the receptor lead to chromatin remodeling and recruitment of cofactors and the transcriptional machinery.
GR is known to interact with other transcription factors through direct protein-protein interactions, mutually regulating each other's transcriptional activities. GR interaction with AP-1 and NF-κB is important for the GC-mediated transrepression of inflammatory cytokines [8] and its interaction with COUP-TFII is important for GC actions on metabolic gene expression [18]. GR is also known to associate with STAT3 [59], STAT5 [6] and estrogen receptor [17]. In addition, cytoplasmic or membrane bound GR causes rapid, nongenomic signaling [103], adding to the complexity of GC-GR mediated actions.
Recent advances in the GR biology demonstrate that multiple transcriptional (α and β) and translational isoforms (A, B, C1, 2, 3, and D1, 2, 3) are expressed in cell type or tissue specific manners [76]. The GRα and β are generated through alternative splicing of exon 9 (9α and 9β). Similar to GRα, GRβ is ubiquitously expressed, but overall at lower levels than GRα. GRβ acts as dominant negative inhibitor of GRα and its expression is increased by proinflammatory cytokines, leading to GC resistance [122]. GRβ also exhibits GRα-independent transcriptional activity [52]. Additional splice variants, GRγ, GR-A, and GR-P are reported and impact on GC signaling [76]. Cidlowski’s group demonstrated that eight GR protein subtypes are generated from GRα mRNA by alternative translation initiation [67]. Use of different translation initiation sites is tissue specific and thus, relative abundance of each isoforms differs between tissues. GR isoforms activate both unique and common genes [67], revealing complexity of GC signaling through GR translational isoforms. We have detected these translational isoforms in human adipose tissues (unpublished observation, MJL and SKF). Their roles in modulating GC action in adipocytes and other cells within adipose tissue remain to be elucidated.
GR transcriptional activity is further modulated by post-translational modifications. Human GR is phosphorylated on its N-terminus at three major sites (serine 203, 211 and 226) [48]. The phosphorylation of GR affects the transcriptional activities of GR by altering ligand- and DNA-binding affinity and cofactor recruitment. Phosphorylation at serine 211 is ligand dependent and associated with nuclear localization and activation. Serine 226 is targeted by mitogen activated kinases (MAPKs), and this mechanism links high levels of inflammatory cytokines to GC resistance in a number of disease states [48,107]. It is logical to postulate that the high inflammatory cytokine levels in obese vs. lean adipose tissues may increase the expression levels of GRβ as well as phosphorylation at serine 226 and lead to GC resistance, but no studies to our knowledge have addressed this issue.
4.2. Role of the type I glucocorticoid receptor in adipose biology
Recent studies demonstrate that the mineralocorticoid receptor (MR, NR3C2), a type 1 glucocorticoid receptor, is also expressed in adipose tissue [44] and play important roles in adipogenesis and adipose endocrine function [45,71]. The relative contribution of GC-GR and GC-MR in a specific tissue is far less than clear and depends on the relative abundance of MR and GR, circulating levels of GC and aldosterone, and local conversion of cortisone-cortisol by HSD1 and HSD2. MR binds to cortisol with 10-fold higher affinity than GR [3] and in classical MR target tissues such as kidney, MR activation by cortisol is spared by inactivation of cortisol via HSD2. Because circulating levels of aldosterone are 100–1000 times lower than cortisone and HSD2 is expressed at much lower levels than HSD1 in adipose tissue [23,62,116], it is conceivable that high local concentration of cortisol within adipose tissue may activate both GR and MR.
MR activation increases while GR activation decreases proinflammatory adipokine expression such as interleukin-6 (IL-6) and plasminogen activator inhibitor 1 [45]. The selective MR antagonist eplerenone reduces adipose expression of proinflammatory genes and infiltration of macrophages into adipose tissue with improvement in insulin sensitivity in obese rodent models [38,43,44]. In contrast, Dex (a selective agonist for GR) decreases proinflammatory cytokine expression in adipose tissue [9,29,63] and macrophage infiltration into adipose tissue in vivo [84]. Both HSD1 and MR expression levels are increased in adipose tissue of obesity, while HSD2 and GR are not [44,62,116]. Thus, the relative contribution of GC-MR pathway may be increased in obesity and contribute to adipose tissue inflammation in obesity.
4.3. Depot dependent actions of glucocorticoids: role of GR and MR
Intraabdominal fat is thought to be more responsive to GCs than is sc adipose tissue, explaining the preferential accumulation of fat in this depot with chronic GC excess. Accordingly, studies found that GC binding to tissue homogenates and GR mRNA expression levels are 2 to 4-fold greater in omental than sc adipose tissue [73,86,90,116]. However, recent developments in our knowledge of GR biology reveal the more complex nature of GC signaling in adipose tissue. Thus, the relative abundance of GRα may not completely explain the depot-differences in GC actions. Rather, levels of transcriptional and translational GR isoforms, the phosphorylation status of GR, and expression levels of MR and GR-interacting partners, are likely to contribute to the depot-dependent effects of GCs. Inflammatory cytokines including TNF increase GRβ expression [122] and phosphorylation of GRα at serine 226 and decrease its transcriptional activity [64]. The higher inflammatory environment in visceral vs. sc may well play a role in depot differences in GC action. Further, mRNA expression levels of MR are also higher in omental than sc depot ([44] and our unpublished observation, MJL and SKF). Thus, we postulate that the relative contribution of the MR compared to the GR pathway may be amplified in human visceral adipose tissues.
5. Glucocorticoid actions on adipose tissue function
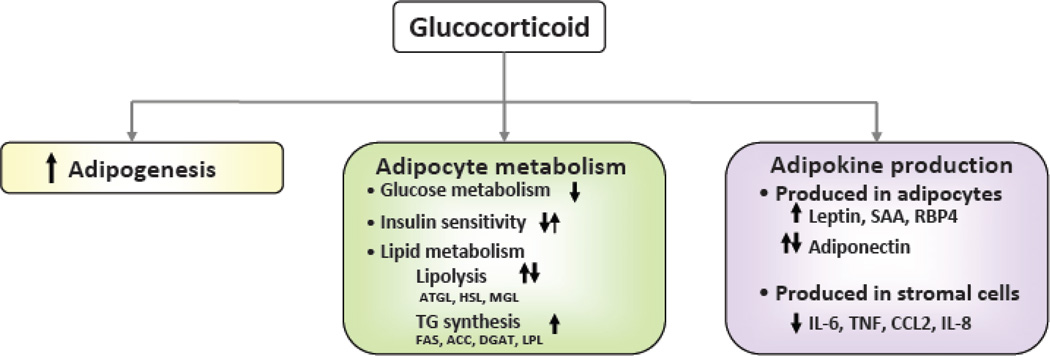
GCs affect almost every aspect of adipose tissue, including development, metabolism, and endocrine function (Figure 1).
Figure 1. Pleiotropic actions of glucocorticoids in adipose tissue.
GCs influence multiple aspects of adipose tissue biology: adipogenesis, metabolism, inflammation and adipokine production. Arrows indicates the direction of reported changes in experiments conducted under different conditions. Specifying these conditions, as discussed in the text, is essential for developing a full understanding of GC action.
5.1. Glucocorticoids and adipogenesis
GCs are required for full differentiation of adipocytes. GC actions on adipogenesis are explained by their anti-proliferative effects on preadipocytes [15,40], induction of a key adipogenic transcription factor, C/EBPbeta, as well as suppression of Pref-1 and Runx2 [110,123,127]. Several differences are noted for the mechanisms through which GCs enhance adipogenesis between the two most commonly used preadipocyte models, 3T3-L1 and primary human preadipocytes. GCs are required for cell survival during the clonal expansion that precedes terminal differentiation in 3T3-L1 but not in human preadipocytes [110]. GCs sensitize insulin signaling in human preadipocytes and this ‘priming’ action enhances their responses to adipogenic stimuli [109]. In contrast, this does not occur in 3T3-L1 cells [109], which are less committed to an adipocyte cell fate compared to human preadipocytes. Whether visceral and sc preadipocytes are differentially sensitive to this ‘priming’ action of GC to induce adipogenesis is unknown.
While the proadipogenic effects of GCs are well accepted, whether their effects are MR or GR is not clear. Recent studies demonstrate that MR rather than GR is important for the proadipogenic effects of GCs in 3T3-L1 and mouse and human preadipocytes [13,14,45]. In primary human preadipocytes however, we found contradictory results. MR is expressed at much lower levels than GR, and RNAi-mediated suppression of GR but not MR blocked GC-stimulation of adipogenesis in primary human preadipocytes (unpublished observation, MJL and SKF). Adipose-specific GR or MR knockout mouse models will be able to determine whether MR or GR mediates GC actions on adipogenesis in vivo.
Depot-dependent actions of GC on adipogenesis have been implicated in rodent models. Corticosterone administration to rats increases intraabdominal depots (mesenteric and epididymal) but not sc ones [12,92]. The appearance of smaller and more numerous adipocytes in the mesenteric fat after corticosterone administration [12] suggest that increases in adipose tissue mass occurred through preadipocyte differentiation rather than adipocyte hypertrophy. Data on humans are scarce. Whether the preferential increase in visceral depot that is observed in subjects with Cushing’s syndrome [35,119] is due to increase in adipogenesis or hypertrophy of existing adipocytes or both is not known. In addition, whether GCs differentially affect differentiation of preadipocytes derived from visceral vs. abdominal and limb sc adipose tissues is also not established. Omental preadipocytes from females but not males contain lower levels of GR mRNA and GC binding, potentially contributing to depot, sex-dependent differences in preadipocyte differentiation [49].
5.2. Glucocorticoid regulation of adipose gene networks
GCs are known to affect up to 20% of adipose expressed genes in microarray studies [63,126]. GCs induce genes in metabolic pathways (expressed mainly in adipocytes) while suppressing genes in immune/inflammatory and proapoptotic pathways. As microarray studies cannot identify whether GC affected genes are primary or secondary targets of GR, ChIP-seq technologies have been applied [126]. More than 8840 genome locations are identified as GR binding regions (GBR) upon Dex treatment in 3T3-L1 adipocytes. When ChIP-seq data are compared with transcriptome analysis, 54% of genes that are regulated by 6-h Dex treatment contain or are located nearby GBRs. There is little overlap between the GR targets identified with ChIP-seq between different cell types [87], underlining the cell type specific effects of the GC-GR pathway. The specificity of GC actions may be determined by expression levels of GR isoforms and the phosphorylation state of GR, but no studies to date have addressed this possibility.
5.3. Glucocorticoids and adipose tissue metabolism
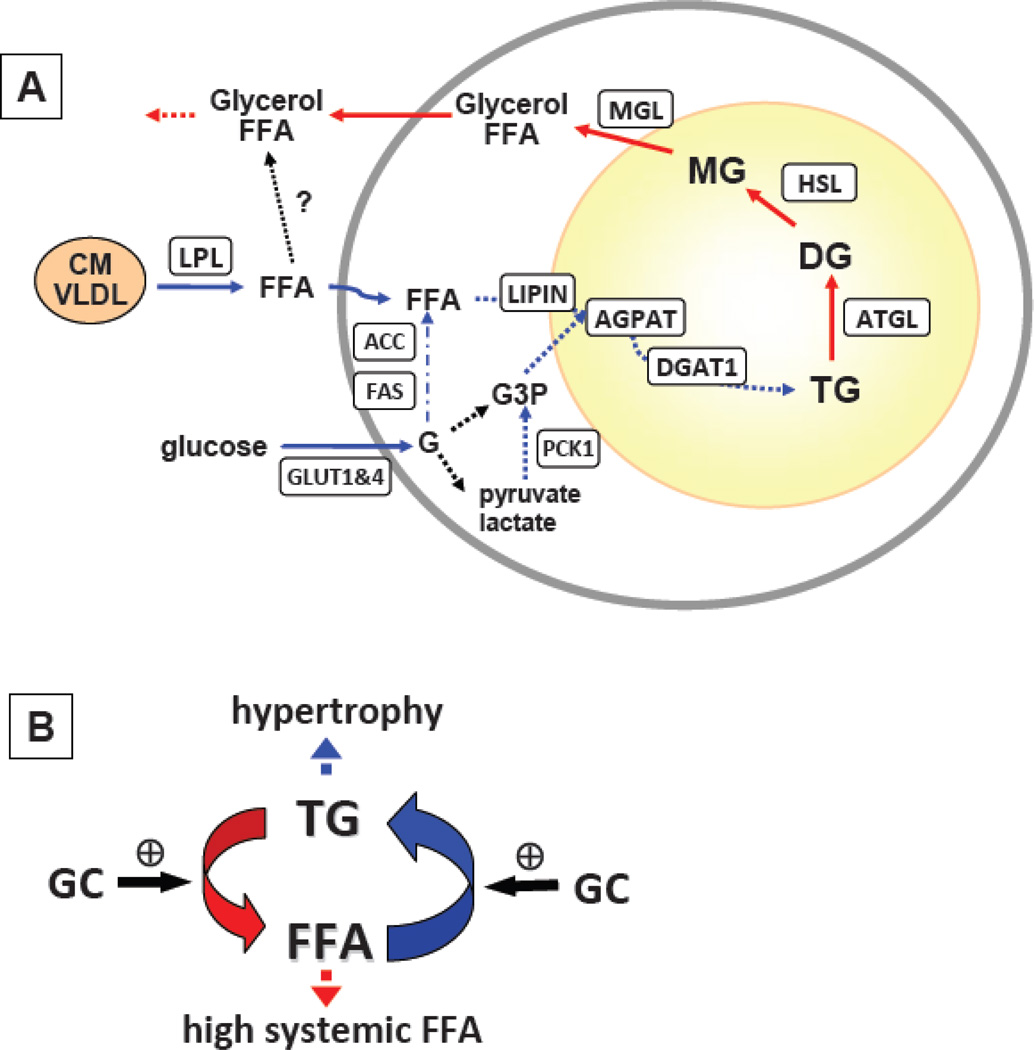
Adipose tissues store excess energy as neutral lipids, TG, and release it as FFA and glycerol in highly regulated fashion. TG is synthesized from FFA esterified to a glyceride-glycerol backbone. The glyceride-glycerol backbone is derived from glucose and the majority of FFA is delivered through lipoprotein lipase (LPL) breakdown of circulating TG in chylomicron or very low density lipoprotein (VLDL), as de novo lipogenesis (DNL) is minimal in normal situation [42]. GCs are known to affect almost every aspect of these adipose metabolic processes (Figure 2A). The effects of GC on adipocyte glucose metabolism [1] and lipid metabolism [85] have been previously reviewed so we only briefly discuss them here.
Figure 2. Glucocorticoids simultaneously increase pathways of TG synthesis and breakdown.
A. GCs especially when added in the presence of insulin, mimicking the fed state, induce the expression of genes involved in lipid uptake (LPL), TG synthesis (LIPIN, AGPAT, DGAT1) and de novo lipogenesis (ACC, FAS). GCs also increase the expression of genes involved in TG hydrolysis (ATGL, HSL, MGL). GC induction of PCK1 expression is likely to play a role in glyceroneogenesis, contributing to TG synthetic pathway. B. GCs increase lipid turnover in adipose tissue. While GCs are widely cited as lipolytic and thought to increase systemic FFA, they also increase lipid uptake and TG synthesis. Factors that tip this balance toward TG synthesis in a depot-dependent manner may lead to hypertrophy of existing adipocytes and hence affect regional adiposity.
5.3.1. Glucocorticoid effects on adipocyte glucose metabolism
GC treatment in vitro, in the absence of insulin, have been shown to decrease glucose uptake and metabolism (oxidation into CO2 and to glyceride-glycerol and FA synthesis) [27,31,37,77] with a time lag of 1–2 hours. In addition, pretreatment with GC antagonizes the ability of insulin to stimulate glucose uptake in adipocytes through different mechanisms depending on the exposure time. Acutely, GCs do not affect insulin binding, thus short-term effects of GC might be explained by glucose transporter translocation [31,77]. Chronic Dex-impairment of insulin-stimulated glucose uptake is explained through decreases in insulin binding, expression of insulin receptor and insulin receptor substrate 1 (IRS1), and translocation of glucose transporter (GLUT4) [31,37,77,98,113]. Dex effects on basal glucose uptake however, are more pronounced than its antagonism of the insulin-stimulated glucose uptake. Thus, Dex actually increases fold over stimulation at maximal insulin concentrations [31]. Similarly, enhanced insulin signaling and glucose uptake following pretreatment with GC in human adipocytes [32,34] and preadipocytes [109] have been reported.
GCs also exert depot-dependent effects on insulin signaling, glucose uptake and the expression of insulin signaling proteins [41,69]. GCs increase IRS2 expression and insulin stimulation of AKT phosphorylation in abdominal sc but not omental adipocytes [41], while chronically GCs down-regulate components of the insulin signaling in omental but not abdominal sc adipose tissue [69].
5.3.2. Glucocorticoids and lipid storage, additive or synergistic effects with insulin to increase lipid synthesis
GCs differentially affect lipid storage depending on nutritional or hormonal conditions. While GCs decrease lipogenesis and FFA uptake in basal or fasting conditions [33,118], GCs act additively or synergistically with insulin to upregulate lipogenesis [33,74,121]. GC induction of DNL is explained by stimulation of key enzymes, acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) [63,121] (Figure 2A). DNL in adipose tissue is a minor contributor to total TG-FA [42] in humans eating moderate fat diets and the majority of FFA is derived from LPL breakdown of circulating TG-rich lipoproteins. GCs induce adipose LPL mRNA expression and activity and enzymes of TG synthesis with greater effects in omental than abdominal sc [2,30,63,79], and this mechanism may contribute to the hypertrophy of omental adipocytes with excess GC. The sensitivity and responsiveness of different sc depots, i.e. abdominal vs. gluteo-femoral, to GCs have not been studied, but may contribute to variations in fat distribution.
5.3.3. Glucocorticoids and lipolysis
GCs are widely cited as being lipolytic. However, results differ depending on the concentration and duration of treatment and the experimental model systems used. Although infusion of fairly high concentrations of GC increases systemic FFA and glycerol within several hours in overnight fasted humans [19–21,41,99,100], cortisol infusion actually decreases abdominal sc adipose tissue lipolysis as measured by the arterio-venous difference across this depot [99]. The authors suggested that depot-specific effect of cortisol on the enzymes of intracellular lipolysis (hormone-sensitive lipase, HSL) and intravascular lipolysis (LPL) may explain how cortisol could acutely increase systemic lipolysis (glycerol and FA rate of appearance) while chronically higher levels would promote net central fat deposition over the long-term. A recent study demonstrated that systemic hydrocortisone infusion increases lipolysis, but the magnitude of suppression by insulin is actually increased [41].
Analysis of data from long-term (days to weeks) infusion of GC [12,114,125,126] is complicated by compensatory hyperinsulinemia which may chronically promote higher FA flux via induction of insulin resistance and activation of the sympathetic nervous system. In addition, GC-stimulation of beta adrenergic receptors and responsiveness to beta-adrenergic agonists [55,58,68,94] also contribute to high lipolysis after chronic GC treatment.
Results from in vitro studies testing the effects of GC on lipolysis using adipocytes and adipose tissue in culture are conflicting. In 3T3-L1 adipocytes and rat adipocytes, GCs increase lipolysis [12,125]. Adipocytes isolated after in vivo GC treatment also exhibit higher rates of lipolysis in vitro [12,54]. The stimulatory effects of GC on lipolysis have been attributed to its induction of adipose triglyceride lipase (ATGL) and HSL, and down-regulation of phosphodiesterase 3B (PDE3B) [12,54,125]. In contrast, we did not find any significant effects of GC (Dex) on lipolysis in primary cultures of newly-differentiated human adipocytes [61]. In human adipose tissue explants, GC does not affect [26], inhibit [78], or antagonize insulin-stimulated lipolysis ([16] and our unpublished observation, MJL and SKF).
GCs have permissive effects on growth hormone (GH) and catecholamines to increase lipolysis. The permissive effects of GC on GH action are demonstrated in vivo [21], in rat adipocytes [27], and in human adipose tissue [26,78]. GCs are also required for normal response to catecholamine or fasting induction of lipolysis [25]. Similarly, in primary human adipocytes, we found that Dex enhances responses to beta-adrenergic stimulation [61].
Several studies showed that chronic exposure of human adipose tissue and 3T3-L1 adipocytes or mice in vivo to GCs increases genes involved in lipid storage and mobilization simultaneously [26,63,126]. Recently, Hellerstein’s group demonstrated that increased cycling between TG and FFAs in adipose tissues of Cushingoid CRH-Tg mice in vivo [39]. Thus, it appears that GCs, at least in long-term, increase lipid turnover in adipose tissue (Figure 2B). Factors that tip the balance toward TG synthesis over breakdown may promote the hypertrophy of existing adipocytes in a depot-dependent manner and hence affect regional adiposity.
5.4. Glucocorticoid modulation of adipose endocrine function
Adipose tissue secretes numerous peptide hormones and cytokines. The adipocytes themselves produce many of these, including leptin, adiponectin, retinol binding protein 4 (RBP4), and serum amyloid A (SAA), while most proinflammatory cytokines and chemokines are produced by non-adipose cells within the adipose tissue [112]. GCs regulate the production of many of these adipokines. GCs increase leptin expression [60], while thier effects on adiponectin are still controversial [106]. As expected from their well known anti-inflammatory actions, GCs decrease inflammatory cytokines, namely IL-6, IL-8, and TNF in intact samples of adipose tissue [9,29]. Accordingly, the most significantly suppressed pathway after Dex treatment in human adipose tissue is immune/inflammatory responses [63]. Dex however, induces some immune/inflammatory genes that are known to be involved in acute phase response or innate immunity (SAA, Complement factor 7, 1q, and D, RBP4, and metallothioneins). Many of these Dex-induced immune genes are expressed in mature adipocytes, while suppressed genes are mainly expressed in stromal cells [112], demonstrating cell type specific effects of Dex within adipose tissue.
6. Glucocorticoids and brown adipose tissue
Metabolically active brown adipose tissue is believed to be present in adult humans and may play role in energy homeostasis and weight control [89]. While GCs promote brown preadipocyte differentiation [101], they inhibit uncoupling protein 1 (UCP1) expression and activity in brown adipocytes [102,117]. Accordingly, corticosterone administration to rats decreases thermogenic activity and UCP1 expression while increasing lipid accumulation in BAT [104]. These data demonstrate that GCs impair brown adipose tissue function and promote a ‘brown’ to ‘white’ phenotypic conversion.
7. Glucocorticoid regulated pathways as therapeutic targets
GCs are widely prescribed due to their anti-inflammatory or immunosuppressive effects. Chronic GCs, however, result in weight gain, hypertension and increased risk for diabetes. GC-transactivated genes are implicated in the pathogenesis of such side effects, while GR interactions with AP-1 and NF-κB are important in its anti-inflammatory actions. Thus, selective GR modulators that favor transrepression over transactivation are in development [115]. In addition, clinical trials testing the effectiveness of HSD1 inhibitors for the treatment for obesity and metabolic diseases are under the investigation [95].
8. Conclusion
GCs have profound effects on adipose tissue, adipogenesis and adipose tissue metabolic and endocrine function. With chronic excess GC, produced systemically or through local adipose tissue conversion, fat accumulates in central adipose depots and contributes to metabolic derangements. The mechanisms through which high GCs promote the preferential expansion of visceral adipose depots remain incompletely understood. Intraabdominal depots are thought to be more responsive to GC due to high GR expression, leading to expansion of this depot. In addition, the type 1 glucocorticoid receptor, MR is expressed in adipose tissue, and mediates several actions of GC. The molecular complexity in GR biology in adipocytes, specifically the roles of multiple GR isoforms, interactions of GR with other transcription factors in adipocytes are emerging. Understanding the cross-talk of GR and inflammatory pathways via post-translational modifications of GR, is beginning to be understood and hold promise for the development of novel therapies for abdominal obesity and its deleterious metabolic consequences.
Highlights.
Glucocorticoids (GC) regulate how much fat is stored in specific fat depots.
Gucocorticoids regulate adipogenesis, and adipose metabolic and endocrine function.
Central fat depots are more responsive glucocorticoids.
The prereceptor activation of cortisone to cortisol regulates local fat distribution.
Type I and II glucocorticoid receptors are both important in adipocytes.
Acknowledgements
The authors’ work is supported by NIH R01 DK080448, R01 DK52398, and P30 DK046200.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin. Sci. (Lond) 1999;96:513–523. doi: 10.1042/cs0960513. [DOI] [PubMed] [Google Scholar]
- 2.Appel B, Fried SK. Effects of insulin and dexamethasone on lipoprotein lipase in human adipose tissue. Am. J. Physiol. 1992;262:E695–E699. doi: 10.1152/ajpendo.1992.262.5.E695. [DOI] [PubMed] [Google Scholar]
- 3.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- 4.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- 5.Basu R, Singh R, Basu A, Johnson CM, Rizza RA. Effect of nutrient ingestion on total-body and splanchnic cortisol production in humans. Diabetes. 2006;55:667–674. doi: 10.2337/diabetes.55.03.06.db05-1335. [DOI] [PubMed] [Google Scholar]
- 6.Baugh JE, Jr, Floyd ZE, Stephens JM. The modulation of STAT5A/GR complexes during fat cell differentiation and in mature adipocytes. Obesity (Silver. Spring) 2007;15:583–590. doi: 10.1038/oby.2007.500. [DOI] [PubMed] [Google Scholar]
- 7.Berthiaume M, Laplante M, Festuccia W, Gelinas Y, Poulin S, Lalonde J, Joanisse DR, Thieringer R, Deshaies Y. Depot-specific modulation of rat intraabdominal adipose tissue lipid metabolism by pharmacological inhibition of 11beta-hydroxysteroid dehydrogenase type 1. Endocrinology. 2007;148:2391–2397. doi: 10.1210/en.2006-1199. [DOI] [PubMed] [Google Scholar]
- 8.Brogan IJ, Murray IA, Cerillo G, Needham M, White A, Davis JR. Interaction of glucocorticoid receptor isoforms with transcription factors AP-1 and NF-kappaB: lack of effect of glucocorticoid receptor beta. Mol. Cell Endocrinol. 1999;157:95–104. doi: 10.1016/s0303-7207(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 9.Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiott KM, Fain JN, Richelsen B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am. J. Physiol Endocrinol. Metab. 2004;286:E8–E13. doi: 10.1152/ajpendo.00269.2003. [DOI] [PubMed] [Google Scholar]
- 10.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrin. 1999;140:3188–3196. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 11.Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect "Cushing's disease of the omentum"? Lancet. 1997;349:1210–1213. doi: 10.1016/S0140-6736(96)11222-8. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JE, Peckett AJ, D'souza AM, Hawke TJ, Riddell MC. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. Am. J. Physiol Cell Physiol. 2011;300:C198–C209. doi: 10.1152/ajpcell.00045.2010. [DOI] [PubMed] [Google Scholar]
- 13.Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, Fabbri A, Zennaro MC, Feve B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152:113–125. doi: 10.1210/en.2010-0674. [DOI] [PubMed] [Google Scholar]
- 14.Caprio M, Feve B, Claes A, Viengchareun S, Lombes M, Zennaro MC. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007;21:2185–2194. doi: 10.1096/fj.06-7970com. [DOI] [PubMed] [Google Scholar]
- 15.Chapman AB, Knight DM, Ringold GM. Glucocorticoid regulation of adipocyte differentiation: hormonal triggering of the developmental program and induction of a differentiation-dependent gene. J. Cell Biol. 1985;101:1227–1235. doi: 10.1083/jcb.101.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cigolini M, Smith U. Human adipose tissue in culture. VIII. Studies on the insulin-antagonistic effect of glucocorticoids. Metabolism. 1979;28:502–510. doi: 10.1016/0026-0495(79)90189-6. [DOI] [PubMed] [Google Scholar]
- 17.Cvoro A, Yuan C, Paruthiyil S, Miller OH, Yamamoto KR, Leitman DC. Cross talk between glucocorticoid and estrogen receptors occurs at a subset of proinflammatory genes. J. Immunol. 2011;186:4354–4360. doi: 10.4049/jimmunol.1002205. [DOI] [PubMed] [Google Scholar]
- 18.De Martino MU, Alesci S, Chrousos GP, Kino T. Interaction of the glucocorticoid receptor and the chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII): implications for the actions of glucocorticoids on glucose, lipoprotein, and xenobiotic metabolism. Ann. N. Y. Acad. Sci. 2004;1024:72–85. doi: 10.1196/annals.1321.006. pp. 72–85. [DOI] [PubMed] [Google Scholar]
- 19.Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]
- 20.Djurhuus CB, Gravholt CH, Nielsen S, Mengel A, Christiansen JS, Schmitz OE, Moller N. Effects of cortisol on lipolysis and regional interstitial glycerol levels in humans. Am. J. Physiol Endocrinol. Metab. 2002;283:E172–E177. doi: 10.1152/ajpendo.00544.2001. [DOI] [PubMed] [Google Scholar]
- 21.Djurhuus CB, Gravholt CH, Nielsen S, Pedersen SB, Moller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am. J. Physiol Endocrinol. Metab. 2004;286:E488–E494. doi: 10.1152/ajpendo.00199.2003. [DOI] [PubMed] [Google Scholar]
- 22.Duclos M, Gatta B, Corcuff JB, Rashedi M, Pehourcq F, Roger P. Fat distribution in obese women is associated with subtle alterations of the hypothalamic-pituitary-adrenal axis activity and sensitivity to glucocorticoids. Clin. Endocrinol. (Oxf) 2001;55:447–454. doi: 10.1046/j.1365-2265.2001.01384.x. [DOI] [PubMed] [Google Scholar]
- 23.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Heintze U, Janke J, Luft FC, Sharma AM. Regulation of 11beta-HSD genes in human adipose tissue: influence of central obesity and weight loss. Obes. Res. 2004;12:9–17. doi: 10.1038/oby.2004.3. [DOI] [PubMed] [Google Scholar]
- 24.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom. Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Exton JH, Friedmann N, Wong EH, Brineaux JP, Corbin JD, Park CR. Interaction of glucocorticoids with glucagon and epinephrine in the control of gluconeogenesis and glycogenolysis in liver and of lipolysis in adipose tissue. J. Biol. Chem. 1972;247:3579–3588. [PubMed] [Google Scholar]
- 26.Fain JN, Cheema P, Tichansky DS, Madan AK. Stimulation of human omental adipose tissue lipolysis by growth hormone plus dexamethasone. Mol. Cell Endocrinol. 2008;295:101–105. doi: 10.1016/j.mce.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Fain JN, Kovacev VP, Scow RO. Effect of growth hormone and dexamethasone on lipolysis and metabolism in isolated fat cells of the rat. J. Biol. Chem. 1965;240:3522–3529. [PubMed] [Google Scholar]
- 28.Freedman MR, Horwitz BA, Stern JS. Effect of adrenalectomy and glucocorticoid replacement on development of obesity. Am. J. Physiol. 1986;250:R595–R607. doi: 10.1152/ajpregu.1986.250.4.R595. [DOI] [PubMed] [Google Scholar]
- 29.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 30.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J. Clin. Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garvey WT, Huecksteadt TP, Monzon R, Marshall S. Dexamethasone regulates the glucose transport system in primary cultured adipocytes: different mechanisms of insulin resistance after acute and chronic exposure. Endocrinology. 1989;124:2063–2073. doi: 10.1210/endo-124-5-2063. [DOI] [PubMed] [Google Scholar]
- 32.Gathercole LL, Bujalska IJ, Stewart PM, Tomlinson JW. Glucocorticoid modulation of insulin signaling in human subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 2007;92:4332–4339. doi: 10.1210/jc.2007-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gathercole LL, Morgan SA, Bujalska IJ, Hauton D, Stewart PM, Tomlinson JW. Regulation of lipogenesis by glucocorticoids and insulin in human adipose tissue. PLoS. One. 2011;6:e26223. doi: 10.1371/journal.pone.0026223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gathercole LL, Morgan SA, Bujalska IJ, Stewart PM, Tomlinson JW. Short- and long-term glucocorticoid treatment enhances insulin signalling in human subcutaneous adipose tissue. Nutr. Diabetes. 2011;1:e3. doi: 10.1038/nutd.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geer EB, Shen W, Gallagher D, Punyanitya M, Looker HC, Post KD, Freda PU. MRI assessment of lean and adipose tissue distribution in female patients with Cushing's disease. Clin. Endocrinol. (Oxf) 2010;73:469–475. doi: 10.1111/j.1365-2265.2010.03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Abellan P, Diez-Noguera A, Madrid JA, Lujan JA, Ordovas JM, Garaulet M. Glucocorticoids Affect 24 h Clock Genes Expression in Human Adipose Tissue Explant Cultures. PLoS. One. 2012;7:e50435. doi: 10.1371/journal.pone.0050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunfeld C, Baird K, Van OE, Kahn CR. Glucocorticoid-induced insulin resistance in vitro: evidence for both receptor and postreceptor defects. Endocrinology. 1981;109:1723–1730. doi: 10.1210/endo-109-5-1723. [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–2261. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris C, Roohk DJ, Fitch M, Boudignon BM, Halloran BP, Hellerstein MK. Large Increases in Adipose Triacylglycerol Flux in Cushingoid CRH-Tg Mice is Explained by Futile Cycling. Am. J. Physiol Endocrinol. Metab. 2012 doi: 10.1152/ajpendo.00154.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 1987;64:832–835. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- 41.Hazlehurst JM, Gathercole LL, Nasiri M, Armstrong MJ, Borrows S, Yu J, Wagenmakers AJ, Stewart PM, Tomlinson JW. Glucocorticoids fail to cause insulin resistance in human subcutaneous adipose tissue in vivo. J. Clin. Endocrinol. Metab. 2013;98:1631–1640. doi: 10.1210/jc.2012-3523. [DOI] [PubMed] [Google Scholar]
- 42.Hellerstein MK. De novo lipogenesis in humans: metabolic and regulatory aspects. Eur. J. Clin. Nutr. 1999;53(Suppl 1):S53–S65. doi: 10.1038/sj.ejcn.1600744. [DOI] [PubMed] [Google Scholar]
- 43.Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc. Res. 2009;84:164–172. doi: 10.1093/cvr/cvp191. [DOI] [PubMed] [Google Scholar]
- 44.Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, Shimomura I. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem. Biophys. Res. Commun. 2012;419:182–187. doi: 10.1016/j.bbrc.2012.01.139. [DOI] [PubMed] [Google Scholar]
- 45.Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, Klein J. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J. Endocrinol. 2010;204:153–164. doi: 10.1677/JOE-09-0292. [DOI] [PubMed] [Google Scholar]
- 46.Hughes KA, Manolopoulos KN, Iqbal J, Cruden NL, Stimson RH, Reynolds RM, Newby DE, Andrew R, Karpe F, Walker BR. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes. 2012;61:1357–1364. doi: 10.2337/db11-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes KA, Reynolds RM, Andrew R, Critchley HO, Walker BR. Glucocorticoids turn over slowly in human adipose tissue in vivo. J. Clin. Endocrinol. Metab. 2010;95:4696–4702. doi: 10.1210/jc.2010-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann. N. Y. Acad. Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- 49.Joyner JM, Hutley LJ, Cameron DP. Glucocorticoid receptors in human preadipocytes: regional and gender differences. J. Endocrinol. 2000;166:145–152. doi: 10.1677/joe.0.1660145. [DOI] [PubMed] [Google Scholar]
- 50.Kannisto K, Pietilainen KH, Ehrenborg E, Rissanen A, Kaprio J, Hamsten A, Yki-Jarvinen H. Overexpression of 11beta-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J. Clin. Endocrinol. Metab. 2004;89:4414–4421. doi: 10.1210/jc.2004-0153. [DOI] [PubMed] [Google Scholar]
- 51.Kershaw EE, Morton NM, Dhillon H, Ramage L, Seckl JR, Flier JS. Adipocyte-specific glucocorticoid inactivation protects against diet-induced obesity. Diabetes. 2005;54:1023–1031. doi: 10.2337/diabetes.54.4.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kino T, Su YA, Chrousos GP. Human glucocorticoid receptor isoform beta: recent understanding of its potential implications in physiology and pathophysiology. Cell Mol. Life Sci. 2009;66:3435–3448. doi: 10.1007/s00018-009-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotelevtsev Y, Holmes MC, Burchell A, Houston PM, Schmoll D, Jamieson P, Best R, Brown R, Edwards CR, Seckl JR, Mullins JJ. 11beta-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc. Natl. Acad. Sci. U. S. A. 1997;94:14924–14929. doi: 10.1073/pnas.94.26.14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11160–11165. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lacasa D, Agli B, Giudicelli Y. Permissive action of glucocorticoids on catecholamine-induced lipolysis: direct "in vitro" effects on the fat cell beta-adrenoreceptor-coupled-adenylate cyclase system. Biochem. Biophys. Res. Commun. 1988;153:489–497. doi: 10.1016/s0006-291x(88)81121-5. [DOI] [PubMed] [Google Scholar]
- 56.Laferrere B, Caixas A, Fried SK, Bashore C, Kim J, Pi-Sunyer FX. A pulse of insulin and dexamethasone stimulates serum leptin in fasting human subjects. Eur. J Endocrinol. 2002;146:839–845. doi: 10.1530/eje.0.1460839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laferrere B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. Journal of Clinical Endocrinology and Metabolism. 1998;83:3742–3745. doi: 10.1210/jcem.83.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamberts SW, Timmermans HA, Kramer-Blankestijn M, Birkenhager JC. The mechanism of the potentiating effect of glucocorticoids on catecholamine-induced lipolysis. Metabolism. 1975;24:681–689. doi: 10.1016/0026-0495(75)90035-9. [DOI] [PubMed] [Google Scholar]
- 59.Langlais D, Couture C, Balsalobre A, Drouin J. The Stat3/GR interaction code: predictive value of direct/indirect DNA recruitment for transcription outcome. Mol. Cell. 2012;47:38–49. doi: 10.1016/j.molcel.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 60.Lee MJ, Fried SK. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol Endocrinol. Metab. 2009;296:E1230–E1238. doi: 10.1152/ajpendo.90927.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee MJ, Fried SK. Glucocorticoids antagonize tumor necrosis factor-alpha-stimulated lipolysis and resistance to the antilipolytic effect of insulin in human adipocytes. Am. J. Physiol Endocrinol. Metab. 2012;303:E1126–E1133. doi: 10.1152/ajpendo.00228.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee MJ, Fried SK, Mundt SS, Wang Y, Sullivan S, Stefanni A, Daugherty BL, Hermanowski-Vosatka A. Depot-specific regulation of the conversion of cortisone to cortisol in human adipose tissue. Obesity. (Silver. Spring) 2008;16:1178–1185. doi: 10.1038/oby.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee MJ, Gong DW, Burkey BF, Fried SK. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: a microarray study. Am. J. Physiol Endocrinol. Metab. 2011;300:E571–E580. doi: 10.1152/ajpendo.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 2012 doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ljung T, Andersson B, Bengtsson BA, Bjorntorp P, Marin P. Inhibition of cortisol secretion by dexamethasone in relation to body fat distribution: a dose-response study. Obes. Res. 1996;4:277–282. doi: 10.1002/j.1550-8528.1996.tb00546.x. [DOI] [PubMed] [Google Scholar]
- 66.Ljung T, Holm G, Friberg P, Andersson B, Bengtsson BA, Svensson J, Dallman M, McEwen B, Bjorntorp P. The activity of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in relation to waist/hip circumference ratio in men. Obes. Res. 2000;8:487–495. doi: 10.1038/oby.2000.61. [DOI] [PubMed] [Google Scholar]
- 67.Lu NZ, Cidlowski JA. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell. 2005;18:331–342. doi: 10.1016/j.molcel.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 68.Lundgren M, Buren J, Lindgren P, Myrnas T, Ruge T, Eriksson JW. Sex- and depot-specific lipolysis regulation in human adipocytes: interplay between adrenergic stimulation and glucocorticoids. Horm. Metab Res. 2008;40:854–860. doi: 10.1055/s-0028-1087168. [DOI] [PubMed] [Google Scholar]
- 69.Lundgren M, Buren J, Ruge T, Myrnas T, Eriksson JW. Glucocorticoids down-regulate glucose uptake capacity and insulin-signaling proteins in omental but not subcutaneous human adipocytes. J. Clin. Endocrinol. Metab. 2004;89:2989–2997. doi: 10.1210/jc.2003-031157. [DOI] [PubMed] [Google Scholar]
- 70.Macfarlane DP, Forbes S, Walker BR. Glucocorticoids and fatty acid metabolism in humans: fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008;197:189–204. doi: 10.1677/JOE-08-0054. [DOI] [PubMed] [Google Scholar]
- 71.Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol. Cell Endocrinol. 2012;350:281–288. doi: 10.1016/j.mce.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, Flier JS. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- 73.Miller LK, Kral JG, Strain GW, Zumoff B. Differential binding of dexamethasone to ammonium sulfate precipitates of human adipose tissue cytosols. Steroids. 1987;49:507–522. doi: 10.1016/0039-128x(87)90091-2. [DOI] [PubMed] [Google Scholar]
- 74.Minshull M, Strong CR. The stimulation of lipogenesis in white adipose tissue from fed rats by corticosterone. Int. J. Biochem. 1985;17:529–532. doi: 10.1016/0020-711x(85)90151-x. [DOI] [PubMed] [Google Scholar]
- 75.Morton NM, Paterson JM, Masuzaki H, Holmes MC, Staels B, Fievet C, Walker BR, Flier JS, Mullins JJ, Seckl JR. Novel adipose tissue-mediated resistance to diet-induced visceral obesity in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2004;53:931–938. doi: 10.2337/diabetes.53.4.931. [DOI] [PubMed] [Google Scholar]
- 76.Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 2011;286:3177–3184. doi: 10.1074/jbc.R110.179325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olefsky JM. Effect of dexamethasone on insulin binding, glucose transport, and glucose oxidation of isolated rat adipocytes. J. Clin. Invest. 1975;56:1499–1508. doi: 10.1172/JCI108231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ottosson M, Lonnroth P, Bjorntorp P, Eden S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J. Clin. Endocrinol. Metab. 2000;85:799–803. doi: 10.1210/jcem.85.2.6358. [DOI] [PubMed] [Google Scholar]
- 79.Ottosson M, Marin P, Karason K, Elander A, Bjorntorp P. Blockade of the glucocorticoid receptor with RU 486: effects in vitro and in vivo on human adipose tissue lipoprotein lipase activity. Obes. Res. 1995;3:233–240. doi: 10.1002/j.1550-8528.1995.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 80.Pantoja C, Huff JT, Yamamoto KR. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell. 2008;19:4032–4041. doi: 10.1091/mbc.E08-04-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat. Med. 2012;18:1768–1777. doi: 10.1038/nm.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del RG, de PG, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J. Clin. Endocrinol. Metab. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- 83.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, Labate AM, Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J. Clin. Endocrinol. Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 84.Patsouris D, Neels JG, Fan W, Li PP, Nguyen MT, Olefsky JM. Glucocorticoids and thiazolidinediones interfere with adipocyte-mediated macrophage chemotaxis and recruitment. J. Biol. Chem. 2009;284:31223–31235. doi: 10.1074/jbc.M109.041665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011 doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 86.Pedersen SB, Jonler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J. Clin. Endocrinol. Metab. 1994;78:1354–1359. doi: 10.1210/jcem.78.6.8200937. [DOI] [PubMed] [Google Scholar]
- 87.Polman JA, Welten JE, Bosch DS, de Jonge RT, Balog J, van der Maarel SM, de Kloet ER, Datson NA. A genome-wide signature of glucocorticoid receptor binding in neuronal PC12 cells. BMC. Neurosci. 2012;13:118. doi: 10.1186/1471-2202-13-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rask E, Walker BR, Soderberg S, Livingstone DE, Eliasson M, Johnson O, Andrew R, Olsson T. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J. Clin. Endocrinol. Metab. 2002;87:3330–3336. doi: 10.1210/jcem.87.7.8661. [DOI] [PubMed] [Google Scholar]
- 89.Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu. Rev. Nutr. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rebuffe-Scrive M, Bronnegard M, Nilsson A, Eldh J, Gustafsson JA, Bjorntorp P. Steroid hormone receptors in human adipose tissues. J. Clin. Endocrinol. Metab. 1990;71:1215–1219. doi: 10.1210/jcem-71-5-1215. [DOI] [PubMed] [Google Scholar]
- 91.Rebuffe-Scrive M, Krotkiewski M, Elfverson J, Bjorntorp P. Muscle and adipose tissue morphology and metabolism in Cushing's syndrome. J. Clin. Endocrinol. Metab. 1988;67:1122–1128. doi: 10.1210/jcem-67-6-1122. [DOI] [PubMed] [Google Scholar]
- 92.Rebuffe-Scrive M, Walsh UA, McEwen B, Rodin J. Effect of chronic stress and exogenous glucocorticoids on regional fat distribution and metabolism. Physiol Behav. 1992;52:583–590. doi: 10.1016/0031-9384(92)90351-2. [DOI] [PubMed] [Google Scholar]
- 93.Rockall AG, Sohaib SA, Evans D, Kaltsas G, Isidori AM, Monson JP, Besser GM, Grossman AB, Reznek RH. Computed tomography assessment of fat distribution in male and female patients with Cushing's syndrome. Eur. J. Endocrinol. 2003;149:561–567. doi: 10.1530/eje.0.1490561. [DOI] [PubMed] [Google Scholar]
- 94.Ros M, Northup JK, Malbon CC. Adipocyte G-proteins and adenylate cyclase. Effects of adrenalectomy. Biochem. J. 1989;257:737–744. doi: 10.1042/bj2570737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenstock J, Banarer S, Fonseca VA, Inzucchi SE, Sun W, Yao W, Hollis G, Flores R, Levy R, Williams WV, Seckl JR, Huber R. The 11-beta-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care. 2010;33:1516–1522. doi: 10.2337/dc09-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J. Clin. Endocrinol. Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 97.Rosmond R, Holm G, Bjorntorp P. Food-induced cortisol secretion in relation to anthropometric, metabolic and haemodynamic variables in men. Int. J. Obes. Relat Metab Disord. 2000;24:416–422. doi: 10.1038/sj.ijo.0801173. [DOI] [PubMed] [Google Scholar]
- 98.Sakoda H, Ogihara T, Anai M, Funaki M, Inukai K, Katagiri H, Fukushima Y, Onishi Y, Ono H, Fujishiro M, Kikuchi M, Oka Y, Asano T. Dexamethasone-induced insulin resistance in 3T3-L1 adipocytes is due to inhibition of glucose transport rather than insulin signal transduction. Diabetes. 2000;49:1700–1708. doi: 10.2337/diabetes.49.10.1700. [DOI] [PubMed] [Google Scholar]
- 99.Samra JS, Clark ML, Humphreys SM, Macdonald IA, Bannister PA, Frayn KN. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J. Clin. Endocrinol. Metab. 1998;83:626–631. doi: 10.1210/jcem.83.2.4547. [DOI] [PubMed] [Google Scholar]
- 100.Samra JS, Clark ML, Humphreys SM, Macdonald IA, Matthews DR, Frayn KN. Effects of morning rise in cortisol concentration on regulation of lipolysis in subcutaneous adipose tissue. Am. J. Physiol. 1996;271:E996–E1002. doi: 10.1152/ajpendo.1996.271.6.E996. [DOI] [PubMed] [Google Scholar]
- 101.Shima A, Shinohara Y, Doi K, Terada H. Normal differentiation of rat brown adipocytes in primary culture judged by their expressions of uncoupling protein and the physiological isoform of glucose transporter. Biochim. Biophys. Acta. 1994;1223:1–8. doi: 10.1016/0167-4889(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 102.Soumano K, Desbiens S, Rabelo R, Bakopanos E, Camirand A, Silva JE. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol. Cell Endocrinol. 2000;165:7–15. doi: 10.1016/s0303-7207(00)00276-8. [DOI] [PubMed] [Google Scholar]
- 103.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pract. Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 104.Strack AM, Bradbury MJ, Dallman MF. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am. J. Physiol. 1995;268:R183–R191. doi: 10.1152/ajpregu.1995.268.1.R183. [DOI] [PubMed] [Google Scholar]
- 105.Strain GW, Zumoff B, Kream J, Strain JJ, Levin J, Fukushima D. Sex difference in the influence of obesity on the 24 hr mean plasma concentration of cortisol. Metabolism. 1982;31:209–212. doi: 10.1016/0026-0495(82)90054-3. [DOI] [PubMed] [Google Scholar]
- 106.Sukumaran S, Dubois DC, Jusko WJ, Almon RR. Glucocorticoid effects on adiponectin expression. Vitam. Horm. 2012;90:163–186. doi: 10.1016/B978-0-12-398313-8.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szatmary Z, Garabedian MJ, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J. Biol. Chem. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- 108.Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 109.Tomlinson JJ, Boudreau A, Wu D, Abdou SH, Carrigan A, Gagnon A, Mears AJ, Sorisky A, Atlas E, Hache RJ. Insulin sensitization of human preadipocytes through glucocorticoid hormone induction of forkhead transcription factors. Mol. Endocrinol. 2010;24:104–113. doi: 10.1210/me.2009-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomlinson JJ, Boudreau A, Wu D, Atlas E, Hache RJ. Modulation of early human preadipocyte differentiation by glucocorticoids. Endocrinology. 2006;147:5284–5293. doi: 10.1210/en.2006-0267. [DOI] [PubMed] [Google Scholar]
- 111.Tomlinson JW, Sinha B, Bujalska I, Hewison M, Stewart PM. Expression of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue is not increased in human obesity. J. Clin. Endocrinol. Metab. 2002;87:5630–5635. doi: 10.1210/jc.2002-020687. [DOI] [PubMed] [Google Scholar]
- 112.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 113.Turnbow MA, Keller SR, Rice KM, Garner CW. Dexamethasone down-regulation of insulin receptor substrate-1 in 3T3-L1 adipocytes. J. Biol. Chem. 1994;269:2516–2520. [PubMed] [Google Scholar]
- 114.van Raalte DH, Brands M, van der Zijl NJ, Muskiet MH, Pouwels PJ, Ackermans MT, Sauerwein HP, Serlie MJ, Diamant M. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia. 2011 doi: 10.1007/s00125-011-2174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur. J. Clin. Invest. 2009;39:81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 116.Veilleux A, Laberge PY, Morency J, Noel S, Luu-The V, Tchernof A. Expression of genes related to glucocorticoid action in human subcutaneous and omental adipose tissue. J. Steroid Biochem. Mol. Biol. 2010;122:28–34. doi: 10.1016/j.jsbmb.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 117.Viengchareun S, Penfornis P, Zennaro MC, Lombes M. Mineralocorticoid and glucocorticoid receptors inhibit UCP expression and function in brown adipocytes. Am. J. Physiol Endocrinol. Metab. 2001;280:E640–E649. doi: 10.1152/ajpendo.2001.280.4.E640. [DOI] [PubMed] [Google Scholar]
- 118.Volpe JJ, Marasa JC. Hormonal regulation of fatty acid synthetase, acetyl-CoA carboxylase and fatty acid synthesis in mammalian adipose tissue and liver. Biochim. Biophys. Acta. 1975;380:454–472. doi: 10.1016/0005-2760(75)90113-7. [DOI] [PubMed] [Google Scholar]
- 119.Wajchenberg BL, Bosco A, Marone MM, Levin S, Rocha M, Lerario AC, Nery M, Goldman J, Liberman B. Estimation of body fat and lean tissue distribution by dual energy X-ray absorptiometry and abdominal body fat evaluation by computed tomography in Cushing's disease. J. Clin. Endocrinol. Metab. 1995;80:2791–2794. doi: 10.1210/jcem.80.9.7673425. [DOI] [PubMed] [Google Scholar]
- 120.Wamil M, Battle JH, Turban S, Kipari T, Seguret D, de Sousa PR, Nelson YB, Nowakowska D, Ferenbach D, Ramage L, Chapman KE, Hughes J, Dunbar DR, Seckl JR, Morton NM. Novel fat depot-specific mechanisms underlie resistance to visceral obesity and inflammation in 11 beta-hydroxysteroid dehydrogenase type 1-deficient mice. Diabetes. 2011;60:1158–1167. doi: 10.2337/db10-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Jones VB, Urs S, Kim S, Soltani-Bejnood M, Quigley N, Heo YR, Standridge M, Andersen B, Dhar M, Joshi R, Wortman P, Taylor JW, Chun J, Leuze M, Claycombe K, Saxton AM, Moustaid-Moussa N. The human fatty acid synthase gene and de novo lipogenesis are coordinately regulated in human adipose tissue. J. Nutr. 2004;134:1032–1038. doi: 10.1093/jn/134.5.1032. [DOI] [PubMed] [Google Scholar]
- 122.Webster JC, Oakley RH, Jewell CM, Cidlowski JA. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiper-Bergeron N, Salem HA, Tomlinson JJ, Wu D, Hache RJ. Glucocorticoid-stimulated preadipocyte differentiation is mediated through acetylation of C/EBPbeta by GCN5. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2703–2708. doi: 10.1073/pnas.0607378104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, Eckel RH, Mynatt RL, Gimble JM. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity (Silver. Spring) 2007;15:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- 125.Xu C, He J, Jiang H, Zu L, Zhai W, Pu S, Xu G. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 2009 doi: 10.1210/me.2008-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu CY, Mayba O, Lee JV, Tran J, Harris C, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor binding regions in adipocytes reveal gene network involved in triglyceride homeostasis. PLoS. One. 2010;5:e15188. doi: 10.1371/journal.pone.0015188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang YY, Li X, Qian SW, Guo L, Huang HY, He Q, Liu Y, Ma CG, Tang QQ. Down-regulation of type I Runx2 mediated by dexamethasone is required for 3T3-L1 adipogenesis. Mol. Endocrinol. 2012;26:798–808. doi: 10.1210/me.2011-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]