Abstract
The loss of collagen organization is considered a hallmark histopathologic feature of tendinosis. At the cellular level, tenocytes have been shown to produce signal substances that were once thought to be restricted to neurons. One of the main neuropeptides implicated in tendinosis, substance P (SP), is known to influence collagen organization, particularly after injury. The aim of this study was to examine the influence of SP on collagen remodeling by primary human tendon cells cultured in vitro in three-dimensional collagen lattices. We found that SP stimulation led to an increased rate of collagen remodeling mediated via the neurokinin-1 receptor (NK-1 R), the preferred cell receptor for SP. Gene expression analysis showed that SP stimulation resulted in significant increases in MMP3 and ACTA2 mRNA levels in the collagen lattices. Furthermore, cyclic tensile loading of tendon cell cultures along with the administration of exogenous SP had an additive effect on MMP3 expression. Immunoblotting confirmed that SP increased MMP3 protein levels via the NK-1 R. This study indicates that SP, mediated via NK-1 R, increases collagen remodeling and leads to increased MMP3 mRNA and protein expression that is further enhanced by cyclic mechanical loading.
Keywords: tendinopathy, tendinosis, collagen, myofibroblasts, neurokinin-1 receptor
INTRODUCTION
Tendinosis is a chronic, degenerative musculoskeletal condition characterized by objective changes in the tendon structure (1), often seen in patients with tendinopathy (chronic tendon pain and impaired function). One of the hallmark features of tendinosis is a reduction of collagen organization accompanied by increased collagen turnover (synthesis and degradation) (1). In normal tendons, the load-bearing collagen fibres are regularly oriented and organized whereas in injured tendons, the arrangement is disorganized and frequently populated by metabolically active myofibroblastic cells with increased smooth muscle actin content (1, 2). The mechanisms leading to the loss of collagen integrity and expression of the myofibroblast phenotype are likely multifactorial. It has been postulated that the expression by tenocytes of tissue remodeling genes, including matrix metalloproteinases (MMPs), contributes to the development of the condition (3).
Recent studies on tendinosis have focused on the intrinsic properties of tenocytes as they have been shown to produce signal substances that were once thought to be restricted to neurons (4–6). Animal and clinical studies have shown that the expression of these transmitters is elevated in tendinosis tendons compared to normal tendons, and is associated with the development of early tendinosis changes (7–9). Evidence supporting this theory includes in vivo studies that demonstrate a role of the neuropeptide substance P (SP) in influencing collagen fibre reorganization (10, 11). Furthermore, SP has been known to alter the mRNA expression levels of MMP3 (stromelysin-1) in Achilles tendon and collateral ligament cells in vitro (12, 13).
The focus of the present study was to determine whether SP has a direct effect on collagen remodeling and on the expression of relevant genes, using a three dimensional cell culture assay with primary human tenocytes. In our most recent study of primary human Achilles tendon cell cultures, we established that tenocytes respond to SP primarily via SP’s preferred receptor, the Neurokinin-1 receptor (NK-1 R) (14). Thus, we aimed to determine whether collagen remodeling is in part mediated via the NK-1 R and whether there are possible associations between SP and genes that are known to influence Type I collagen organization.
METHODS
Materials and cell culture
Human hamstring and Achilles tendon biopsies were isolated and cultured as previously described (14). Ethics approval was obtained from the Regional Ethical Review Board in Umeå, Sweden, and University of British Columbia, Canada. Achilles tendon biopsies from healthy donors (Umeå) and samples of healthy hamstring tendons from patients undergoing anterior cruciate ligament reconstruction (UBC) were enzymatically digested with 2 mg/ml collagenase (Clostridopeptidase A, Sigma; C-0130) for 120 minutes or equal volumes of 0.25% trypsin (Invitrogen; 25200-056) and 2mg/ml collagenase for 30 minutes respectively and cultured in D-MEM (HyClone; SH30071.03) supplemented with 10% fetal bovine serum (HyClone; SH30071.03), 1% penicillin-streptomycin (HyClone; SV30010) and 0.2% L-Glutamine (Invitrogen; 25030-081) at 37°C in 5% CO2. All experiments were carried out with hamstring tenocytes with the exception of Flexcell experiments (see below) for which Achilles tenocytes were used.
Three dimensional primary tenocyte culture
Aliquots of 500 μl liquefied collagen gel, containing 2.5 × 105 human hamstring tendon cells, were pipetted into individual wells of 24-well tissue culture plates. The gels consisted of 70% 3.0mg/ml PureCol® collagen (Advanced BioMatrix; cat: 5005-B), 20% 5×DMEM (Invitrogen; cat: 12100-046) and 10% FBS (HyClone; SH30071.03). The gels were treated with final concentrations of either 10−7 M SP (Sigma; 85965) or 10−6 M NK-1 R antagonist for 30 mins (Sigma; code: s3144) followed by 10−7 M SP in the experimental group; these concentrations were based on pilot studies and previous work (14). PBS was used as the carrier in the control group. The gels were initially left to polymerize for 90 minutes, in the presence of carrier, SP or pre-treatment with NK1-R antagonist for 30 mins before SP treatment, in the 37°C CO2 incubator, followed by the addition of 0.5 ml DMEM/F12 (HyClone; SH30023.0) supplemented with 2% BSA (HyClone; SH30574.01) in each well. After 12 hours of initial culture, the gels were detached from the walls and the bottom of culture plate wells, and photographed at 0h, 6h, 12h, 24 h, 30h and 48h post-release using a digital scanner (Epson Perfection V5000 Photo). The areas of the contracted gels were measured using image analysis software (Image J, National Institute of Health. USA). Collagen remodeling studies were carried out on cells from passage 3 to 5, and performed in duplicates to quadruplicates for each patient. A total of ten patient samples were investigated. The average values of each treatment condition were combined to give a mean contraction at each time point. Collagen remodeling was operationally defined as the degree and rate of contraction of gels exposed to various conditions, and was evaluated by calculating the gel surface area from acquired images. To standardize comparison, results were reported as a percent of contraction: % contraction = (initial area − final area)/(initial area) × 100%) for each time point.
RNA isolation, reverse transcription, and RT-PCR of 3D cultures
RNA isolation, reverse transcription and RT-PCR of three dimensional hamstring tenocyte cultures were performed as previously described (15). Briefly, collagen gels (control and SP treated) were harvested at 0h, 6h, 12h and 24h. The gels were placed in Trizol (Invitrogen) and snap frozen in liquid nitrogen. RNA was extracted using the TriSpin method, an established protocol that combines the Trizol reagent and the RNeasy Total RNA Kit (Qiagen, Mississauga, Ont.) column fractionation step (15). RNA was quantified using SybrGreen fluorescence and 1 μg of RNA from each sample was reverse transcribed using the Qiagen’s Omniscript kit (Qiagen). PCR was performed using primers for ACTA2, PTGS2, ITGA2, MMP1, MMP3, COL1A1, COL3A1, TIMP1, and 18S (Table 1). The PCR reaction mixture contained 0.75 μl each of forward and reverse primer, 3.5 μl molecular biology grade water, and 12.5 μl of Bio-Rad iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Each reaction used a volume of 7.5 μl and was performed in triplicate. Amplification and detection were performed using an iCycler Thermal Cycler (Bio-Rad). Gene expression was normalized to 18S rRNA. iCycler iQ Optical System Software version 3.0a (Bio-Rad) was used to analyze relative quantification.
Table 1.
Primer sequences used for collagen remodelling RT-PCR experiments.
| Gene | Base pairs | Primer sequences | Sequence reference |
|---|---|---|---|
| ACTA2 (smooth muscle actin) | 83 | 5′-CAGCAAACAGGAATACGATGAAG-3′ | NM_001613 |
| 5′-AGACAGAGAGGAGCAGGAAAG-3′ | |||
| PTGS2 (cyclooxyg enase 2) | 450 | 5′-GCAACACTTGAGTGGCTATC-3′ | NM_000963 |
| 5′-TCGATGTCACCATAGAGTGC-3′ | |||
| NOS2A (inducible nitric oxide synthase) | 80 | 5′-GCAGGACGAGAAGCGGAGAC-3′ | NM_153292 |
| 5′-GCATACAGGCAAAGAGCACAGC-3′ | |||
| ITGA2 (α2 integrin) | 150 | 5′-GCAAACTTCAACAAGCATTCC-3′ | NM_002203 |
| 5′-CCCGTTGTGTAATACTGATTCC-3′ | |||
| MMP1 (matrix metalloproteinase 1) | 131 | 5′-CTGCTGCTGCTGTTCTGG-3′ | NM_002421 |
| 5′-ACTTGCCTCCCATCATTCTTC-3′ | |||
| MMP3 (matrix metalloproteinase 3) | 212 | 5′-GTAGAAGGCACAATATGG-3′ | NM_002422 |
| 5′-ACTCTATGTGACAAGGTG-3′ | |||
| 18S (ribosomal RNA) | 360 | 5′-TGGTCGCTCGCTCCTCTCC-3′ | X03205 |
| 5′-CGCCTGCTGCCTTCCTTGG-3′ | |||
| COL1A1 (collagen, type I, aloha 1) | 341 | 5′CCCCCTCCCCAGCCACAAAGA-3′ | NM_000088 |
| 5′-TCTTGGTCGGTGGGTGACTCT-3′ | |||
| COL3A1 (collagen, type III, alpha 1) | 167 | 5′-GCTGTGGTGGTGTTGGAG-3′ | NM_000090 |
| 5′-AGGACTAATGAGGCTTTCTATTTG-3′ | |||
| TIMP1 (metallopeptidase inhibitor 1) | 404 | 5′-AATTCCGACCTCGTC ATC AGG-3′ | NM_003254 |
| 5′-ACTGGAAGCCCTTTTTC AGAGC-3′ |
Mechanical loading and SP stimulation of tendon cells
Human Achilles tendon cells were seeded two dimensionally on Bioflex culture plate membranes treated with collagen I (Bioflex BF-3001C) at a density of 1.5 × 105 cells per well and allowed to grow to confluence. The media was subsequently changed and replaced with either regular media or media containing a final concentration of 10−7 M SP. The wells were subsequently subdivided into loaded and unloaded groups resulting in 4 experimental groups that were repeated in quadruplicates. For the loaded groups, strain was applied equibiaxially to adherent cells via vacuum deformation of the membrane downwards across a 25mm diameter cylindrical loading post using the FlexCell© unit FX-4000 (FlexCell International Corporation, North Carolina) as previously described (14). 10% strain was applied with a frequency of 1Hz 120 minutes per day for 3 days. Samples were collected 4 hours after loading on the last day of loading.
RNA isolation, reverse transcription, and qPCR of 2D cell cultures
RNA was extracted using the RNeasy Mini Kit as previously described following manufacturer’s protocol (Qiagen CA, USA) (14). Subsequently, RNA concentration was quantified using a spectrophotometer Nanodrop ND-1000 (Thermo Fisher Scientific, USA). One μg of isolated RNA from each sample was reverse transcribed into complementary DNA (cDNA) using a High Capacity cDNA Reverse Transcription kit (ABI, CA, USA). A total sample volume of 14.2μl, containing RNA and RNase free water, was added to 2μl 10x RT buffer, 0.8μl 25x dNTP, 2μl 10x RT random primers and 1μl 20x multiscribe RTase to obtain a final volume of 20μl. The synthesis cDNA from RNA was performed according to the manufacturers recommendations: 10min at 25°C followed by 120min at 37°C and 5min at 85°C before idling at 4°C on a thermal cycler (Eppendorf Mastercycler EP Gradient S, Eppendorf, North America). Quantitative PCR was performed using TaqMan® Fast Universal PCR Master Mix (2x), No AmpErase® UNG (ABI, CA, USA) according to the recommended protocol. Each sample was amplified in technical duplicates in MicroAmp® Fast 96-well Reaction Plate (ABI, USA) by splitting the starting volume of 25μl into two 10μl volumes in each well. The levels of MMP3 mRNA (ABI assay Hs0096830_m1) were determined relative to that of 18S rRNA (ABI, USA). The amplification conditions were as follow: pre-incubation at 95°C followed by 45 cycles of denaturation at 95°C for 30s and annealing/extension at 60°C for 30s using the 7500 Fast Real-Time PCR System (ABI, USA).
Immunoblotting
Hamstring tenocytes were seeded two dimensionally; 1.5 × 105 cells were added to each well of a 6-well plate. The media was subsequently replaced with either regular media, media with SP or media with 30 mins NK- 1 R inhibitor pre-treatment followed by SP for 12 hours (in concentrations mentioned above). Cells were washed with PBS before total cell extracts were obtained by lysing cells in ice-cold solubilization buffer A (20 mmol·L−1 Tris–HCL pH 8.0, 1% NP-40, 10% glycerol, 137 mmol·L−1 NaCl, 10 mmol·L−1 NaF) supplemented with protease inhibitor cocktail and 200 μmol·L−1 sodium vanadate. The cells were then sonicated for 5 s before the extracted proteins were centrifuged at 32000g for 10 min. The protein concentrations were determined by BCA protein assay. Protein extracts were resolved using SDS–polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were blocked with 5% low-fat milk for 1 h in TBST, followed by overnight incubation at 4 °C with the MMP-3 antibody (Proteintech: 17873-1-AP). Anti-rabbit antibody conjugated to horseradish peroxidase was used to detect the immune complexes by enhanced chemiluminescence using the G:BOX Chemi system (Sygene USA). Protein concentration was quantified by normalizing MMP3 protein level with vinculin using the GeneTools analysis software (Syngene USA).
Statistics
Data were analysed using the GraphPad PRISM 5 software. For comparisons of more than two treatment groups, one-way ANOVA with Bonferroni post-hoc test or independent samples t-test when two treatment groups were used. For comparison of collagen remodeling at different times, two-way ANOVA with Bonferroni post-hoc test was employed. Significance was predetermined at p<0.05.
RESULTS
Enhancement of collagen remodeling by SP
Type I collagen populated by primary human hamstring tenocytes demonstrated high initial rates of remodeling (Figure 1 and 2). While most of the remodeling occurred within 24 hours post-release for both groups, the SP treated cell cultures showed a significantly greater extent of collagen remodeling compared to the non-treated (control) cultures at all time points. Control lattices without tenocytes demonstrated no remodeling activity.
Figure 1.
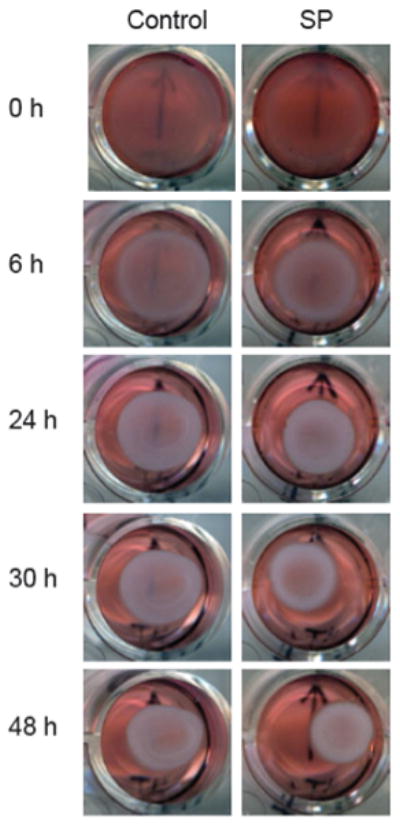
Remodeling of control and SP treated (10−7 M) tenocytes.
Figure 2.
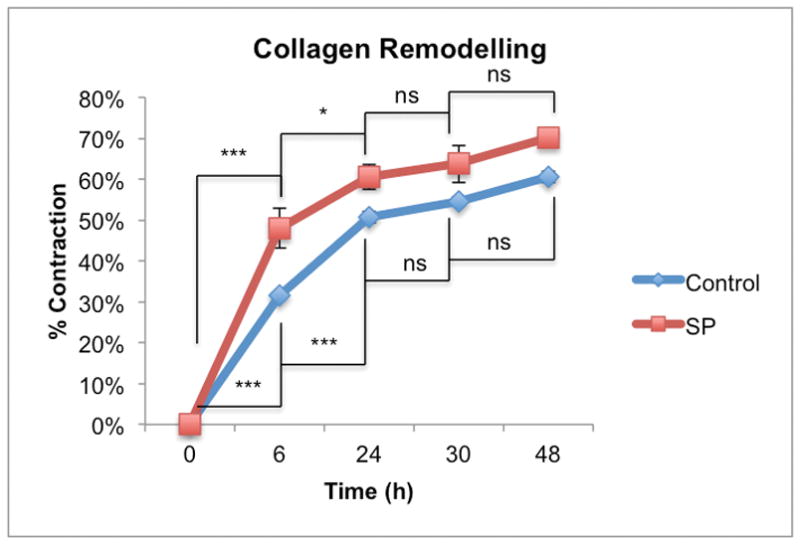
Remodeling by control and SP treated (10−7 M) tenocytes increased in a time-dependent manner (p<0.001, two-way ANOVA). The rate of remodeling by SP treated tenocytes was greater than the control group (p<0.01, two-way ANOVA). For the control and SP treated groups most of the remodeling occurred within 24 hours post-release (0–6h*** p<0.001 for both control and SP treated groups and 6–24h ***p<0.001 and *p<0.05 for control and SP treated respectively, two-way ANOVA, followed by Bonferroni post-hoc test) after which there was no difference between the two groups at the 30 and 48-hour time points.
Association of collagen remodeling with specific mRNA levels
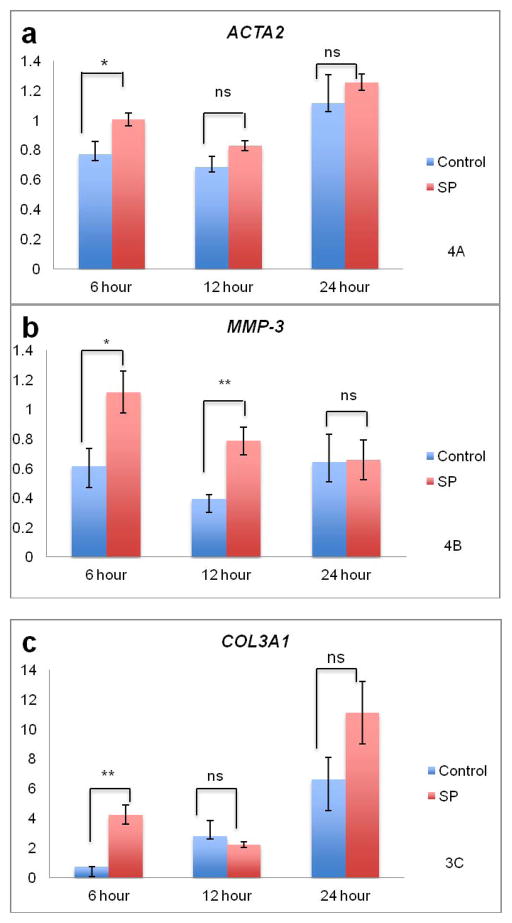
The mRNA levels for ACTA2, MMP1, MMP3, PTGS2, NOS2A, ITGA2, COL1A1, COL3A1 and TIMP1 were determined by real time RT-qPCR. The relative mRNA levels for each gene are summarized in Table 2. The mRNA levels for MMP1, PTGS2, ITGA2 and COL1A1 were upregulated during collagen remodeling, but were not influenced by SP treatment (***p<0.001, ***p<0.001, *p<0.05 and ***p<0.001, two-way ANOVA, time variable, respectively). Of the nine genes investigated, mRNA for ACTA2 (*p<0.05), MMP3 (**p<0.01), COL3A1 (*p<0.05) and TIMP1 (***p<0.001) showed differences in expression upon SP treatment compared to the control (two-way ANOVA, SP treatment variable). NOS2A and COL1A1 showed downregulation for both SP and control groups based on time, and independently of SP treatment (*p<0.05 and ***p<0.001, two-way ANOVA, time variable, respectively). ACTA2 was upregulated over time and in response to SP (**p<0.01 time, *p<0.05 SP treatment, two-way ANOVA) (Figure 3A). The mRNA level for MMP3 was increased with SP treatment but the interaction with time was not significant (**p<0.01, SP treatment, two-way ANOVA) (Figure 3B). COL3A1 was upregulated over time and in response to SP (***p<0.001 time, *p<0.05 SP treatment, two-way ANOVA) (Figure 3C). The mRNA level for TIMP1 was increased with SP treatment but the interaction with time was not significant (***p<0.001, SP treatment, two-way ANOVA) (Figure 3D).
Table 2.
Summary of two-way ANOVA results for gene-expression analysis of collagen gels.
| Gene | Time Lapsed | Treatment with SP |
|---|---|---|
| ACTA2 |
** p<0.01
|
* p<0.05
|
| PTGS2 |
*** p<0.001
|
n.s. |
| NOS2A |
* p<0.05
|
n.s |
| ITGA2 |
* p<0.05
|
n.s. |
| MMP1 |
*** p<0.001
|
n.s. |
| MMP3 | n.s. |
** p<0.01
|
| COL1A1 |
*** p<0.001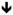
|
n.s. |
| COL3A1 |
*** p<0.001
|
* p<0.05
|
| TIMP1 | n.s. |
*** p<0.001
|
p < 0.05,
p < 0.01,
p < 0.001.
Figure 3.
Effects of SP on collagen remodeling and mRNA levels. Figure 3A. For ACTA2, expression increased in both control and SP treated tenocytes (p<0.01, two-way ANOVA) while SP further increased ACTA2 expression compared to control (p<0.05, two-way ANOVA). There was an interaction between time and treatment. At the 6 hour time point, SP significantly upregulated ACTA2 compared to control (*p<0.05, independent samples t-test). Figure 3B. There was an increase in MMP3 expression due to SP treatment (p<0.001, two-way ANOVA). There was no interaction between time and treatment. SP led to significant upregulation of MMP3 at 6 and 12 hours compared to the control (*p<0.05 and **p<0.01, independent samples t-test respectively). Figure 3C. For COL3A1, expression increased in both control and SP treated tenocytes (p<0.05, two-way ANOVA). There was an interaction between time and treatment. At the 6 hour time point, SP significantly upregulated COL3A1 compared to the control (**p<0.01, independent samples t-test). Figure 3D. There was an increase in TIMP1 expression due to SP treatment (p<0.001, two-way ANOVA). There was an interaction between time and treatment. SP led to significant upregulation of TIMP1 at the 24 hour time point (*p<0.05, independent samples t-test).
Evidence that NK-1 R mediates SP enhancement of collagen remodeling
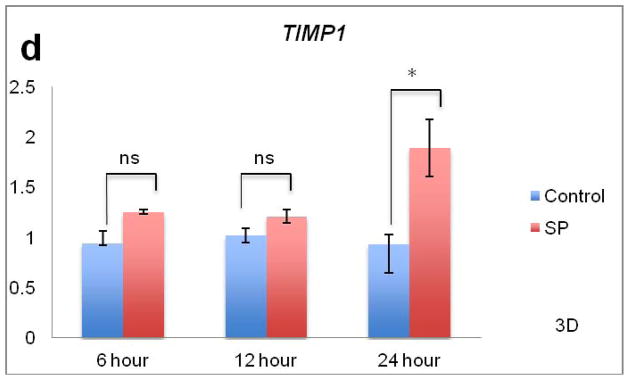
To confirm that the effect of enhanced collagen remodeling was mediated via NK-1 R, an NK-1 R antagonist was used (Figure 4). Exposure of human hamstring tenocytes to the NK-1 R antagonist began to inhibit collagen remodeling at 6h and completely abolished collagen remodeling at 12h.
Figure 4.
Collagen remodeling 6, 12 and 24 hours after release of lattices (control-, SP- (10−7 M), and NK-1 R inhibitor (10−6 M) & SP- (10−7 M)- treated). The extent of remodeling in the SP treated group was increased compared to control at 6 and 12 hours (6h ***p<0.001, 12h ***p<0.001, 2-way ANOVA, followed by Bonferroni post-hoc test). The inhibitor temporary blocked the effects of SP at 12h but the effect was incomplete at 6h compared to control (*p<0.05, 2-way ANOVA, followed by Bonferroni post-hoc test). At 12h, treatment with SP resulted in significant increase in remodeling while treatment of the inhibitor blocked the effect (***p<0.001, 2-way ANOVA, followed by Bonferroni post-hoc test).
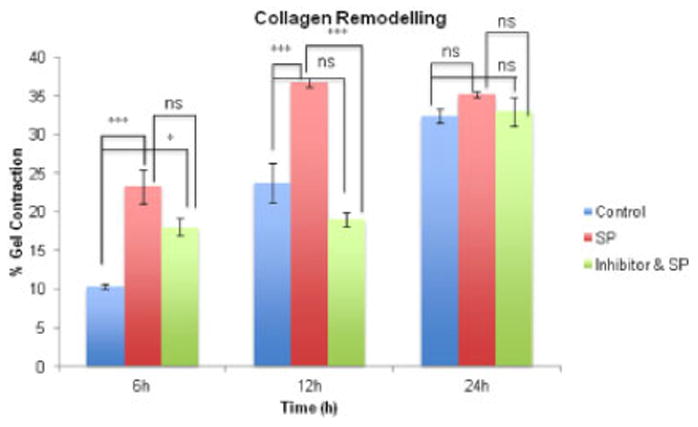
SP stimulation of MMP3 expression is additive with cyclic loading
SP expression in models of early tendinosis occurs in an environment where cells are exposed to cyclic tensile loading. Thus, we next examined whether SP stimulation would increase MMP3 expression in primary human Achilles tenocytes which were simultaneously exposed to cyclic load. Interestingly, we found that SP and cyclic loading had an additive effect on MMP3 expression and that MMP3 expression is significantly different between unloaded and loaded tenocytes (p<0.01, two-way ANOVA) (Figure 5). The mean level of MMP3 expression is elevated for both unloaded and loaded samples following SP (10−7 M) stimulation (p<0.001, two-way ANOVA). For both the unloaded and loaded groups, there was approximately a two-hold increase in MMP3 expression due to SP (*p<0.5 and ***p<0.001 respectively, two-way ANOVA, followed by Bonferroni post-hoc test).
Figure 5.
qPCR of MMP3 mRNA in cultured human primary Achilles tendon cells. MMP3 expression is significantly different between unloaded and loaded tenocytes (p<0.01, two-way ANOVA). The mean level of MMP3 expression is elevated for both unloaded and loaded samples following SP (10−7 M) stimulation (p<0.001, two-way ANOVA). For both the unloaded and loaded groups, there was approximately a two-hold increase in MMP3 expression due to SP (*p<0.5 and ***p<0.001 respectively, two-way ANOVA, followed by Bonferroni post-hoc test).
Finally, we confirmed that SP administration led to increased MMP3 at the protein level (***p<0.001, one-way ANOVA, followed by Bonferroni post-hoc test), as quantified by protein band intensities. This increase in MMP3 was inhibited by the NK-1 R blocker (***p<0.001, one-way ANOVA, followed by Bonferroni post-hoc test). (Figure 6).
Figure 6.
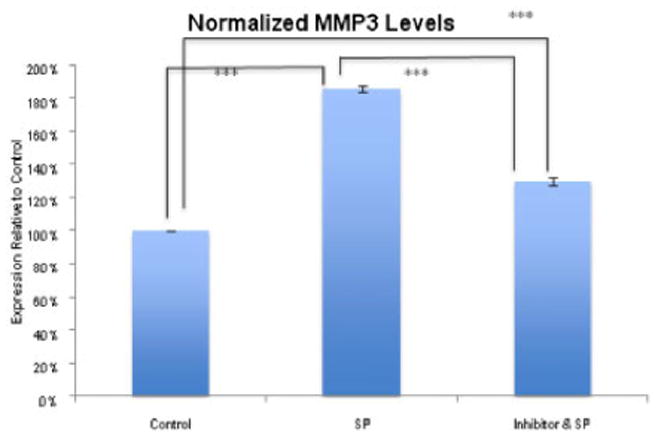
Effect of SP on MMP3 protein level. Analysis of MMP3 after 12 hours of incubation with SP (10−7 M), without SP (control), and with SP and the NK-1 R blocker (10−6 M), as measured by normalized band intensity. MMP3 was significantly increased after incubation with SP (***p<0.001, one-way ANOVA, followed by Bonferroni post-hoc test) and this increase was much less obvious in the presence of the NK-1 R antagonist (***p<0.001, one-way ANOVA, followed by Bonferroni post-hoc test) when both are compared to the control. As well, the NK-1 R antagonist resulted in significant decreased in MMP3 protein level relative to treatment with SP (***p<0.001, one-way ANOVA, followed by Bonferroni post-hoc test).
DISCUSSION
Collagen remodeling is enhanced by SP stimulation
The present study established that SP influences collagen organization and the mRNA levels for the specific type of collagen being produced by tenocytes, and leads to a greater rate of collagen remodeling compared to control. The gel contraction assay demonstrated the intrinsic ability of tenocytes to reorganize Type I collagen lattices in a time dependent manner. Tenocytes cultured three-dimensionally in collagen treated with SP showed a greater rate and extent of remodeling compared to control, with most of the change typically occurring during the first 24 hours following the release of tension from the extracellular matrix (Figure 5). Furthermore, inhibition of NK-1 R reduced the effect of SP on collagen remodeling. The current results reinforce the existence of biologically relevant effects of SP in human tendon which may play a role in either recovery from injury, or the development of overuse pathology like tendinosis. While SP is suggested to promote early tissue proliferation that is essential in the initial repair of normal tendons, the sustained upregulation of SP seen in tendinosis might have negative effects on tendon healing instead, leading to prolonged or excessive collagen remodeling activity (11, 16).
SP increases influences gene expression in human tenocytes
The addition of SP enhanced the remodeling of the collagen matrix and led to significant regulation of genes relevant to this process. In the present study, specific elevations in ACTA2, MMP3, COL3A1 and TIMP1 levels accompanied administration of SP. ACTA2 encodes α-SMA, which is sometimes taken as an indicator of a “myofibroblast” phenotype which is a hallmark of tendinosis lesions (1). The upregulation of genes for ACTA2 and MMP3 as a result of SP administration is of interest since elevated levels of SP in tendinosis tissue have been implicated in the development of pathology (7, 9). Tendinosis pathology is also associated with elevated type III collagen levels and, correspondingly, smaller collagen fibrils (1,3). Interestingly, in our study, we found that SP administration results in increased level of MMP3 mRNA and its protein, a key member of the MMP family that is also associated with tendinosis. Conversely, surgical biopsies of chronically painful tendons have found increased levels of SP but decreased mRNA levels of MMP3 (17, 18). In our study, the time course of MMP3 upregulation appeared to be quite short (having returned to baseline by 24 hours). SP also increased ACTA2 expression, which is consistent with an increased alpha smooth muscle actin (α-SMA) in chronic tendinosis biopsies (1). The increased remodeling in the SP treated gels compared to the control may be in part the result of increased ACTA2 expression following SP stimulation. While treated and untreated tenocytes both exhibited increased ACTA2 as time lapsed, this expression was higher in the SP treated group. Previous work has reported a positive correlation between the expression of ACTA2 and collagen matrix remodeling in fibroblasts from Dupuytren’s contractures (19).
SP stimulation and cyclic mechanical loading both induce increased MMP3 expression
Mechanical loading has previously been reported to increase MMP3 expression in human tendon cells (20). The present study involving primarily tenocytes, in keeping with previous experiments with human osteoblast-like cells, shows that the Type I collagen remodeling assay also led to increases in MMP3 mRNA (14, 21). Considering that administration of SP, cyclic mechanical loading and ‘relaxed loading’ all result in increases in MMP3 expression, we decided to investigate whether the simultaneous application of SP and cyclic loading would result in further enhancement of MMP3 expression. It would have been interesting to conduct these experiments in the three-dimensional culture system (e.g. superimposing mechanical loading on “relaxed loaded” collagen lattices), but this was not feasible given our current experimental facilities. A possible mechanism that could explain why SP and cyclic loading both drive increases in MMP3 mRNA, is that both pathways converge with phosphorylation of ERK1/2. We have previously shown that ERK1/2 activation is increased by SP and is specifically mediated by the NK-1 R and that ERK1/2 activation is increased in mechanically loaded tenocytes in vivo (14, 22). Another promising direction to pursue in the future is whether there is a potential link between SP and MMP3 via TGF-β, a potent factor known to promote collagen remodeling. We have recently found that the administration of exogenous TGF-β1 to tenocytes in collagen lattices leads to marked collagen remodeling that surpasses the effects caused by SP (unpublished data). The effect of enhanced collagen remodeling could be attributed to increased α-SMA expression mediated by TGF-β. A study involving fibroblasts has shown that TGF-β-antagonizing agents reduced α-SMA expression and subsequently matrix remodeling (23). As well, TGF-β is known to be involved in the SMAD3 pathway as well as the ERK1/2 pathway that results in enhancing cell proliferation (24).
Study Limitations
While the results of the current study support the conclusion that SP is an effective mediator of collagen remodeling by tenocytes, and leads to increased ACTA2 and MMP3 expression, there are several limitations to this study. First, there was some inter-individual variability in the responsiveness of cells cultured from different patients to the experimental conditions. Out of the ten patients that were investigated on separate occasions with the collagen remodeling assay, three of these patients showed no significant difference between SP and no treatment. Additionally, the tenocytes derived from these three patients failed to exhibit the usual high rate of remodeling compared to their responsive counterparts, even in the control condition. It is suggested that the inability for some patient-derived cell cultures to respond as expected to the experimental conditions might be due to slight proximal-distal variation in sampling location, since these tenocytes are derived from otherwise discarded hamstring tendons from ACL reconstructions. As well, there may be slight variations in ACTA2 levels amongst different patients that can affect the remodeling rate of collagen. There have been studies showing that when ACTA2 is repressed, for example by TGF-β-antagonizing agents, the rate of collagen remodeling is decreased (23). Nevertheless, the main conclusion of the study showing SP, via NK-1 R, plays a significant role in enhancing collagen remodeling in primary tenocytes in vitro is a novel finding that complements existing in vivo studies.
Summary
The present study shows an increase in Type I collagen remodeling, and an elevation in MMP3, ACTA2, TIMP1 and COL3A1 expression in response to SP stimulation in human tenocytes. These findings provide further support that SP may play a role in the response of tendon to injury, or to the development or progression of tendinosis.
Acknowledgments
The authors thank Ms. Carolyn Hewitt for excellent technical services and Prof. Håkan Alfredson for surgical Achilles tendon biopsies (Umeå). This project was primarily funded by National Sciences and Engineering Research Council, and WorksafeBC. This study was also supported by the Canadian Institutes of Health Research. A Scott received a Michael Smith Scholar award. P Danielson received grants from the national Swedish Research Council (521-2009-2921; co-applicant: A Scott), the Swedish National Centre for Research in Sports (54/10 and P2011-0170), and the Swedish Society of Medicine (SLS-176511), which contributed to the studies on the human Achilles tendon cells.
References
- 1.Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies. Update and implications for clinical management. Sports Med. 1999;27:393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 2.Sivaguru M, Durgam S, Ambekar R, et al. Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging. Opt Express. 2010;24:24983–93. doi: 10.1364/OE.18.024983. [DOI] [PubMed] [Google Scholar]
- 3.Riley G. Chronic tendon pathology: molecular basis and therapeutic implications. Expert Reviews in Molecular Medicine. 2005;5:1–25. doi: 10.1017/S1462399405008963. [DOI] [PubMed] [Google Scholar]
- 4.Forsgren S, Alfredson H, Bjur D, et al. Novel information on the non-neuronal cholinergic system in orthopedics provides new possible treatment strategies for inflammatory and degenerative diseases. Orthop Rev (Pavia) 2009;1:e11. doi: 10.4081/or.2009.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjur D, Danielson P, Alfredson H, Forsgren S. Presence of a non-neuronal cholinergic system and occurrence of up- and down-regulation in expression of M2 muscarinic acetylcholine receptors: new aspects of importance regarding Achilles tendon tendinosis (tendinopathy) Cell Tissue Res. 2008;2:385–400. doi: 10.1007/s00441-007-0524-1. [DOI] [PubMed] [Google Scholar]
- 6.Andersson G, Danielson P, Alfredson H, Forsgren S. Presence of substance P and the neurokinin-1 receptor in tenocytes of the human Achilles tendon. Regul Pept. 2008;1–3:81–7. doi: 10.1016/j.regpep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Andersson G, Backman LJ, Scott A, et al. Substance P accelerates hypercellularity and angiogenesis in tendon tissue and enhances paratendinitis in response to Achilles tendon overuse in a tendinopathy model. Br J Sports Med. 2011;13:1017–22. doi: 10.1136/bjsm.2010.082750. [DOI] [PubMed] [Google Scholar]
- 8.Steyaert A, Burssens P, Forsyth R, Vanderstraeten G. Qualitative analysis of substance P, NK1-receptor and nerve ingrowth in substance P-treated ruptured rat Achilles tendon. Acta Orthop Belg. 2010;3:387–95. [PubMed] [Google Scholar]
- 9.Lui PP, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sports Med. 2010;4:757–64. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 10.Burssens P, Steyaert A, Forsyth R, et al. Exogenously administered substance P and neutral endopeptidase inhibitors stimulate fibroblast proliferation, angiogenesis and collagen organization during Achilles tendon healing. Foot Ankle Int. 2005;10:832–9. doi: 10.1177/107110070502601008. [DOI] [PubMed] [Google Scholar]
- 11.Carlsson O, Schizas N, Li J, Ackermann PW. Substance P injections enhance tissue proliferation and regulate sensory nerve ingrowth in rat tendon repair. Scand J Med Sci Sports. 2011;4:562–9. doi: 10.1111/j.1600-0838.2009.01080.x. [DOI] [PubMed] [Google Scholar]
- 12.Hart DA, Reno C. Pregnancy alters the in vitro responsiveness of the rabbit medial collateral ligament to neuropeptides: effect on mRNA levels for growth factors, cytokines, iNOS, COX-2, metalloproteinases and TIMPs. Biochimica et Biophysica Acta. 1998;(1):35–43. doi: 10.1016/s0925-4439(98)00051-9. [DOI] [PubMed] [Google Scholar]
- 13.Hart DA, Kydd A, Reno C. Gender and pregnancy affect neuropeptide responses of the rabbit Achilles tendon. Clin Orthop Relat Res. 1999;365:237–46. doi: 10.1097/00003086-199908000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Backman LJ, Fong G, Andersson G, et al. Substance P is a mechanoresponsive, autocrine regulator of human tenocyte proliferation. PLoS One. 2011;11:e27209. doi: 10.1371/journal.pone.0027209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reno C, Marchuk L, Sciore P, Frank CB, Hart DA. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;6:1082–6. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 16.Lui PPY, Chan LS, Fu SC, Chan KM. Expression of sensory neuropeptides in tendon is associated with failed healing and activity-related tendon pain in collagenase-induced tendon injury. Am J Sport Med. 2010;4:757–64. doi: 10.1177/0363546509355402. [DOI] [PubMed] [Google Scholar]
- 17.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biol. 2001;3:159–69. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 18.Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human Achilles tendon. Arthritis Rheum. 2006;3:832–42. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- 19.Tomasek J, Rayan GM. Correlation of alpha-smooth muscle actin expression and contraction in Dupuytren’s disease fibroblasts. J Hand Surg Am. 1995;3:450–5. doi: 10.1016/S0363-5023(05)80105-4. [DOI] [PubMed] [Google Scholar]
- 20.Tsuzaki M, Bynum D, Almekinders L, et al. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;3:556–62. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- 21.Parreno J, Buckley-Herd G, de-Hemptinne I, Hart DA. Osteoblastic MG-63 cell differentiation, contraction, and mRNA expression in stress-relaxed 3D collagen I gels. Mol Cell Biochem. 2008;1–2:21–32. doi: 10.1007/s11010-008-9801-x. [DOI] [PubMed] [Google Scholar]
- 22.Scott A, Cook JL, Hart DA, et al. Tenocyte responses to mechanical loading in vivo: a role for local insulin-like growth factor 1 signaling in early tendinosis in rats. Arth Rheum. 2007;3:871–81. doi: 10.1002/art.22426. [DOI] [PubMed] [Google Scholar]
- 23.Hinz B, Celetta G, Tomasek JJ, et al. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;9:2730–41. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-β-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;6:1162–9. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]