Abstract
The present study was designed to evaluate the specific role of protein kinase C (PKC) δ in methamphetamine (MA)-induced dopaminergic toxicity. A multiple-dose administration regimen of MA significantly increases PKCδ expression, while rottlerin, a PKCδ inhibitor, significantly attenuates MA-induced hyperthermia and behavioural deficits. These behavioural effects were not significantly observed in PKCδ antisense oligonucleotide (ASO)-treated- or PKCδ knockout (−/−)-mice. There were no MA-induced significant decreases of dopamine (DA) content or tyrosine hydroxylase (TH) expression in the striatum in rottlerin-treated-, ASO-treated- or PKCδ (−/−)-mice. The administration of MA also results in a significant decrease of TH phosphorylation at ser 40, but not ser 31, while the inhibition of PKCδ consistently and significantly attenuates MA-induced reduction in the phosphorylation of TH at ser 40. Therefore, these results suggest that the MA-induced enhancement of PKCδ expression is a critical factor in the impairment of TH phosphorylation at ser 40 and that pharmacological or genetic inhibition of PKCδ may be protective against MA-induced dopaminergic neurotoxicity in vivo.
Keywords: Methamphetamine, dopaminergic toxicity, hyperthermia, PKCδ gene, phospho-TH at ser-40, striatum
Introduction
Tyrosine hydroxylase (TH; tyrosine 3-monooxygenase, EC 1.14.16.2) catalyzes the conversion of L-tyrosine to L-3,4-dihydroxyphenylanine (L-DOPA), which is the initial and rate-limiting step in the biosynthesis of catecholamines [CA; dopamine (DA), norepinephrine, and epinephrine; Nagatsu et al., 1964]. TH also plays a central role in catecholaminergic neurotransmission (Nakashima et al., 2009) and may be related to various neuropsychiatric disorders such as Parkinson's disease (PD; Nagatsu, 1993), depressive illness (Meloni et al., 1995) and schizophrenia (Thibaut et al., 1997). Postmortem analyses of TH protein levels in nigrostriatal DA neurons have shown a marked decrease in the brains of those with PD but not those with schizophrenia (Ichinose et al., 1994). A number of phosphorylation sites have been identified on TH that influence the activity of this enzyme (Lee et al., 1989). It has been reported that TH is phosphorylated at the N-terminal serine (ser) amino sites at ser 8, ser 19, ser 31, and ser 40 by a variety of protein kinases (Dunkley et al., 2004; Nakashima et al., 2009). Thus, the phosphorylation of serine residues at the N-terminus appears to regulate the catalytic activity of TH in vivo (Dunkley et al., 2004; Hufton et al., 1995). Of the phosphorylation sites at the N-terminus of TH only ser 31 and ser 40 are readily phosphorylated and activate TH in vitro (Haycock and Wakade, 1992; Sutherland et al., 1993).
The protein kinase C (PKC) family consists of serine/threonine kinases and is broadly classified into three subgroups based on sensitivity to important cofactors, including phospholipids and Ca2+ (Dempsey et al., 2000; Gschwendt, 1999). The conventional PKC isoforms (α, βI, βII, γ) are sensitive to Ca2+ and diacylglycerol and the novel isoforms (δ, ε, η, θ, μ) are Ca2+ independent but require diacylglycerol for activation. The atypical isoforms (ζ, ι/λ) require neither Ca2+ nor diacylglycerol for activation. PKC isoforms are differentially distributed in tissues and play key roles in various cellular biological processes, including cell differentiation and growth, apoptosis, tumor suppression, and carcinogenesis. In most studies, PKC inhibitors are used to demonstrate the anti-apoptotic role of the PKC family. Of the novel isoforms, PKCδ was the first member found to be functionally modulated by tyrosine phosphorylation upon H2O2 treatment (Konishi et al., 1997; Steinberg, 2004). A number of studies have found that the proteolytic activation of PKCδ plays a key role in apoptotic cell death of dopaminergic neurons (Kaul et al., 2003; Yang et al., 2004; Kitazawa et al., 2003; Latchoumycandane et al., 2005; Kanthasamy et al., 2006).
However, little is known concerning the role of PKCδ during in vivo dopaminergic toxicity induced by an amphetamine analog. Thus, the involvement of PKCδ in methamphetamine (MA)-induced in vivo dopaminergic toxicity is examined here. It was observed that PKCδ is critically involved in MA-induced dopaminergic toxicity and that PKCδ inhibition using the PKCδ inhibitor rottlerin or a PKCδ gene knockout (−/−) mouse model attenuates MA-induced dopaminergic toxicity through the upregulation of TH phosphorylation at ser 40. As recent reports indicate that rottlerin-mediated pharmacological effects as a PKCδ inhibitor are somewhat controversial (Soltoff, 2007; Susarla et al., 2003; Tapia et al., 2006), an additional experiment using a PKCδ antisense oligonucleotide was performed.
Material and Methods
Animals
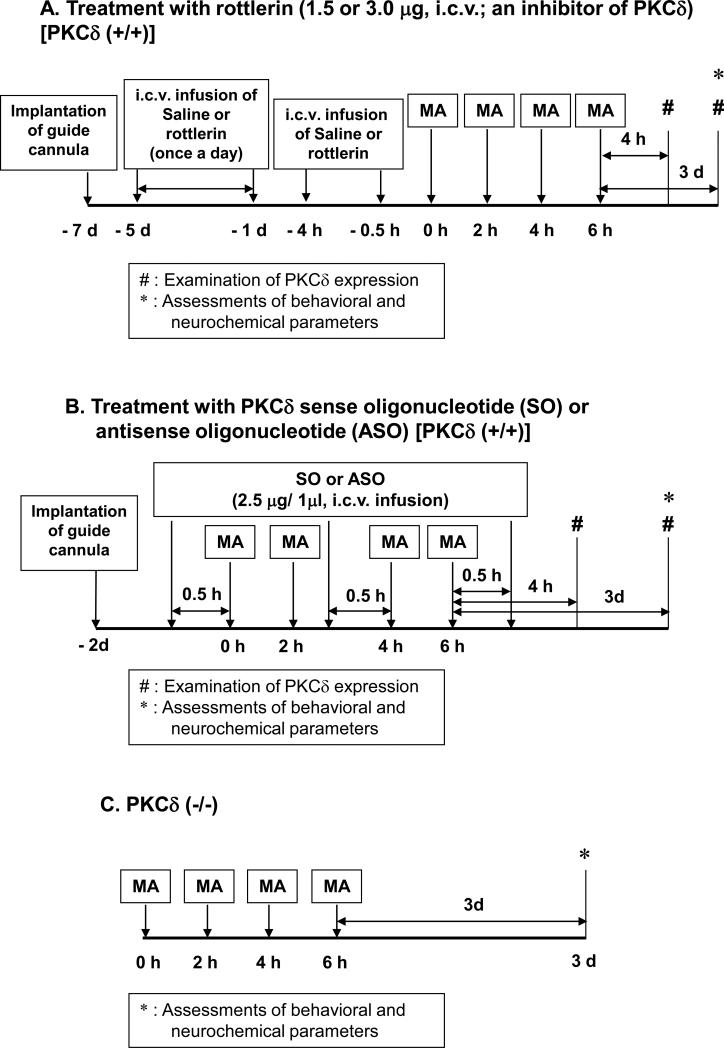
All mice were treated in accordance with the NIH Guide for the Humane Care and Use of Laboratory Animals. They were maintained on a 12/12-h light/dark cycle and fed ad libitum. They were adapted to these conditions for 2 weeks before the experiment. The experimental schedules are shown in Fig. 1.
Fig. 1.
Experimental schedule utilized to evaluate the effect of rottlerin (A). PKCδ antisense oligonucleotide (ASO) (B) or PKCδ gene knockout (C) on methamphetamine (MA)-induced dopaminergic toxicity in mice. Each animal received four injections of MA hydrochloride (8mg/kg, i.p., at 2h intervals) or saline. Rectal temperature was measured 40min after each MA treatment. Initial temperature was measured 1h before first MA injection. Ambient temperature was 21.0±1.0°C. Behavioural assessments were performed 3d after the final MA injection. Mice were sacrificed immediately after behavioural assessments for neurochemical evaluations. Additional mice were sacrificed 4 h after the final MA injection to evaluate the expression of PKCδ.
Development and characterization of PKCδ(−/−) mice
A breeding pair of PKCδ (+/−) mice, originally bred into a C57BL/6 background, was a gift from Dr. K. I. Nakayama (Dept. of Molecular Genetics, Medical Institute of Bioregulation, Kyushu University, Fukuoka, Japan) (Miyamoto et al., 2002). These mice were subsequently maintained and bred into the C57BL/6 background for three to six generations in a SPF mice facility before use with wild-type mice from the same litter in our experiments. Tail DNA was evaluated and typed using polymerase chain reaction (PCR) and primers for PKCδ obtained from Bioneer Corporation (Daejeon, South Korea). PCR Primers for genotyping were as follows; 5’-GGAAGAATAAGAAACTGCATCACC-5’ and 5’-GAAGGAGCCAGAACCGAAAG-3’ for endogenous detection, and 5’-GGAAGAATAAGAAACTGCATCACC-3’ and 5’-TGGGGTGGGATTAGATAAATG-3’ for mutant detection. Brain tissue from PKCδ (−/−) mice was examined by Western blot analyses using antibodies for PKCδ and other isoforms (α, βI, βII, ζ; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) to confirm that PKCδ protein was selectively absent in PKCδ (−/−) mice and that expression of the other isoforms was normal.
Guide cannula implantation and intracerebroventricular (i.c.v.) infusion
Mice were anesthetized with pentobarbital (40 mg/kg, i.p.) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A stainless steel guide cannula (AG-4; Eicom, Kyoto, Japan) was implanted into the right lateral ventricle [stereotaxic coordinates: 0.5 mm posterior to bregma, 1 mm right to the midline, and 2 mm ventral to the dura, according to the atlas of Franklin and Paxinos (2008)] (Shin et al., 2009). No histological or mechanical disruption was produced by implantation of the infusion cannula (data not shown). Microinfusion into the lateral ventricle was performed through a microinfusion cannula (AMI-4, Eicom, Kyoto, Japan) at a rate of 1 μL/min using a microinjection pump (CMA/100, CMA, Solna, Sweden). The microinfusion cannula was kept in place for 1 min after infusion to avoid backflow.
Guide cannula implantation did not affect the behaviour of the subjects. Subsequent to guide cannula implantation, each animal was housed in a single cage in order to safely maintain the integrity of the implantation during the experimental period.
Intracerebroventricular infusion of rottlerin and treatment with methamphetamine (MA)
Rottlerin (PKCδ inhibitor; Biomol Research Laboratories Inc., Plymouth, PA, USA) was dissolved in DMSO as a stock solution and then stored at −20°C. Rottlerin was diluted in sterile saline immediately before use at a concentration of 1.0 μg/μL. The final DMSO concentration was 10% (v/v). After 2 days of recovery from the guide cannula implantation, mice were microinfused into the lateral ventricle with rottlerin (1.5 or 3.0 μg) once a day for 5 days. On the next day, mice received four doses of MA (8 mg/kg, i.p.) or saline at 2-h intervals. Additional microinfusion of rottlerin was performed 4 h and 0.5 h before the first MA injection (Fig. 1A). The dose of rottlerin was determined based on previous studies (Smith et al., 2007; Smith et al., 2006) and our pilot study (Shin et al., 2010).
Application of PKCδ antisense oligonucleotide (ASO) or PKCδ sense oligonucleotide (SO) and treatment with MA
ASO was complementary to the transition initiation region of mouse PKCδ mRNA (5’-AGGGTGCCATGATGGA) (Liedtke and Cole, 1997). As a control, SO was used (5’-TCGATCATGGCACCCT). SO and ASO used here were phosphorothioated on the two terminal bases of the 5’-end and three terminal bases of the 3’-end (Bioneer Corporation). Each oligonucleotide was dissolved in sterile saline immediately before use. After 2 days of recovery from the guide cannula implantation, PKCδ (+/+) mice received four doses of MA (8 mg/kg, ip) or saline at 2-h intervals. Then, 30 min before the first and third injections of MA and 30 min after the final injection of MA, ASO (2.5 μg/μL) or SO (2.5 μg/μL) was microinfused into the lateral ventricle (Fig. 1B). To confirm the decrease in PKCδ protein expression by ASO treatment, Western blot analysis was performed using an antibody against PKCδ (Santa Cruz Biotechnology, Inc.; Fig. 3).
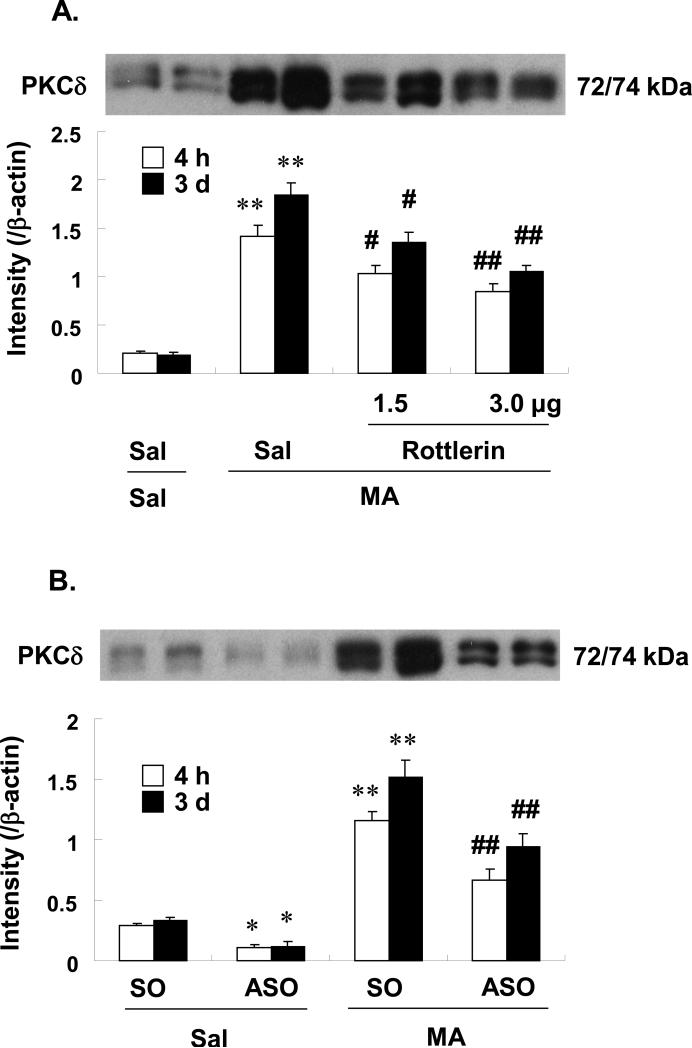
Fig. 3.
Effects of rottlerin (3.0μg, i.c.v.) (A) or ASO (2.5μg, i.c.v.) (B) on the protein expression of PKCδ 4h and 3d after the final methamphetamine (MA) administration in the striatum of the PKCδ (+/+) mice. Sal = Saline. SO = PKCδ sense oligonucleotide. ASO = PKCδ antisense oligonucleotide. Each value is the mean ± S.E.M of 5 animals. *P<0.05, **P<0.01 vs. Sal+Sal or SO+Sal, #P<0.05, ##P<0.01 vs. Sal+MA or SO+MA (one-way ANOVA followed by Fisher's PLSD test).
Measure of rectal temperature
Rectal temperature was measured in the MA- and saline-treated mice. Measurement was performed at constant daytime intervals starting at 9:00 AM to avoid any influence of circadian variation. Rectal temperature (under ambient temperature: 21 ± 1°C) was measured by inserting a thermometer probe lubricated with oil at least 3 cm into the rectum of the mice. To prevent sudden movements, especially in MA-treated mice, animals were gently handled with a wool glove while their tail was moved to allow probe insertion. This was done to reduce any effect of restraint stress on rectal temperature. When the attempt to insert probe was not successful (i.e., sudden movements of the animal or the need to restrain the mouse), the animal was excluded from the group.
Western blot analysis
The western blot assay was performed as described previously (Jung et al., 2010; Liu et al., 2008; Nguyen et al., 2009). Tissues were homogenized in lysis buffer, containing 200 mM Tris HCl (pH 6.8), 1% SDS, 5 mM ethylene glycol-bis(2-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA), 5 mM ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 1X phosphatase inhibitor cocktail I (Sigma, St. Louis, MO, USA), 1 × protease inhibitor cocktail (Sigma). Lysate was centrifuged at 12,000 × g for 30 min and supernatant fraction was used for Western blot analysis. Proteins (20-50ug/lane) were separated by 8% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto the PVDF membranes. Following transfer, the membranes were preincubated with 5% non-fat milk for 30 min and incubated overnight at 4°C with primary antibody against β-actin (1:50000, Sigma), TH (1:5000, Chemicon), phospho-TH at ser 31 (1:500, Chemicon) or phospho-TH st ser 40 (1:500, Chemicon). And then, membranes were incubated with HRP-conjugated secondary anti-rabbit IgG (1:1000, GE healthcare, Piscataway, NJ, USA), anti-mouse IgG (1:1000, Sigma) or anti-goat IgG (1:1000, Sigma) for 2h. Subsequent visualization was performed using enhanced chemiluminescence system (ECL plus®, GE healthcare). Relative intensities of the bands were quantified by PhotoCapt MW (version 10.01 for Windows; Vilber Lourmat, Marne la Vallée, France), and then quantification of TH, phospho-TH at ser 31 or phospho-TH at ser 40 was normalized to β-actin intensity. In addition, intensity of phospho-TH at ser 31 or phospho-TH at ser 40 was further analyzed as the ratio of phospho-TH at ser 31 to pan-TH or phospho-TH at ser 40 to pan-TH (Nguyen et al., 2009).
Immunocytochemistry
Animals were sacrificed at 3 days after the last MA injection. They were anesthetized with 60% urethane and perfused transcardially with 50 ml of 50 mM phosphate buffered saline (PBS), followed by 50 ml of a mixture of 4% paraformaldehyde in PBS. The rate of perfusion was 10 ml/min. The brains were removed, post-fixed at 4°C for 24h in the same fixative and then cryoprotected in 30% sucrose in PBS. The brains were cut on a horizontal sliding microstome into 30 μm transverse free-floating sections (Choi et al., 2009; Hunter et al., 2007; Jung et al., 2010; Li et al., 2008; Liu et al., 2008). Every sixth section throughout the entire extent of the striatum was collected for immunostaining. Briefly, prior to incubation with the primary antibodies, sections were preincubated with 0.3 % hydrogen peroxide in PBS for 30 min (to block endogenous peroxidase activity), then in PBS containing 0.4 % Triton X-100 and 1 % normal serum for 20 min. The sections were then incubated for 48 h at 4°C in primary antibody against phospho-TH at ser 40 (1:500, Chemicon). The sections were further incubated with secondary biotinylated antisera (1:1000 dilution; Vector, Burlingame, CA) for 1 h, and immersed in avidin-biotin-peroxidase complex (ABC Elite kit, Vector) for 1 h. Sections were always washed three times with PBS (pH 7.4) between each incubation step. 3, 3’-diaminobenzidine (DAB) was used as a chromogen.
Digital images were acquired on an Olympus microscope (BX51, Olympus®, Tokyo, Japan) using digital microscope camera (DP72, Olympus®, Tokyo, Japan), and IBM PC. A region of interest (ROI) was created and the immunoreactive density of striatal phospho-TH at ser 40 was measured by Optimas® version 6.51 software (Media Cybernetics, Inc. Silver Spring, MD, USA).
Measurement of dopamine
Mice were sacrificed 3 days after the last MA injection. The striatum was dissected out and immediately frozen on dry ice, and stored at −70 °C until extraction. The striatum obtained from each animal was weighed, ultrasonicated in 10 % perchloric acid containing 10 ng/mg of the internal standard dihydroxybenzylamine, and centrifuged at 20,000 × g for 10 min. The level of dopamine was determined by HPLC coupled with electrochemical detection as described (Jung et al., 2010; Nakajima et al., 2004; Liu et al., 2010). The 20 μl aliquot of the supernatant was injected into the HPLC equipped with a 3 μm C18 column. The mobile phase was comprised of 26 ml of acetonitrile, 21 ml of tetrahydrofuran and 960 ml of 0.15 M monochloroacetic acid (pH 3.0) containing 50 mg/l of EDTA and 200 mg/l of sodium octyl sulfate. The amount of dopamine was determined by comparison of peak area of tissue sample with standard, and was expressed in micrograms per gram of wet tissue.
Locomotor activity
Locomotor activity was measured for 30 min 3 days after the last MA administration using an automated video-tracking system (Noldus Information Technology, Wagenin, The Netherlands). Four test boxes (40 × 40 × 30 cm high) were operated simultaneously by an IBM computer. Mice were studied individually during locomotion in each test box, where they were adapted for 5 min before starting the experiment. A printout for each session showed the pattern of the ambulatory movements of the test box. The distance traveled in cm by the animals in horizontal locomotor activity was analyzed. Data were collected and analyzed between 09:00 and 17:00 h (Jung et al., 2010).
Rota-rod test
Rota-rod test was performed 1 hour after locomotor activity measurement. The apparatus (Ugo Basile model 7650, Comerio, VA, Italy) consisted of a base platform and a rotating rod with a non-slippery surface. The rod was placed at a height of 15 cm from the base. The rod, 30 cm in length, was divided into 5 equal sections by 6 opaque disks (so that the subjects cannot be distracted by one another). To assess motor performance, the mice first trained on the apparatus for 2 minutes at a constant rate of 4 r.p.m. The test was performed 30 minutes after training and an accelerating paradigm was applied, starting from a rate of 4 r.p.m. to a maximum speed of 40 r.p.m., then the rotation speed was kept constant at 40 r.p.m. for a maximum of 300 s. The duration for which the animal could maintain balance on the rotating drum was measured as the latency to fall, with a maximal cut-off time of 300 s (Jung et al., 2010).
Statistical analyses
Data were analyzed using a one-way ANOVA followed by Fischer's PLSD test or a oneway ANOVA for repeated measures followed by Fischer's PLSD test. P-values of less than 0.05 were deemed to indicate statistical significance.
Results
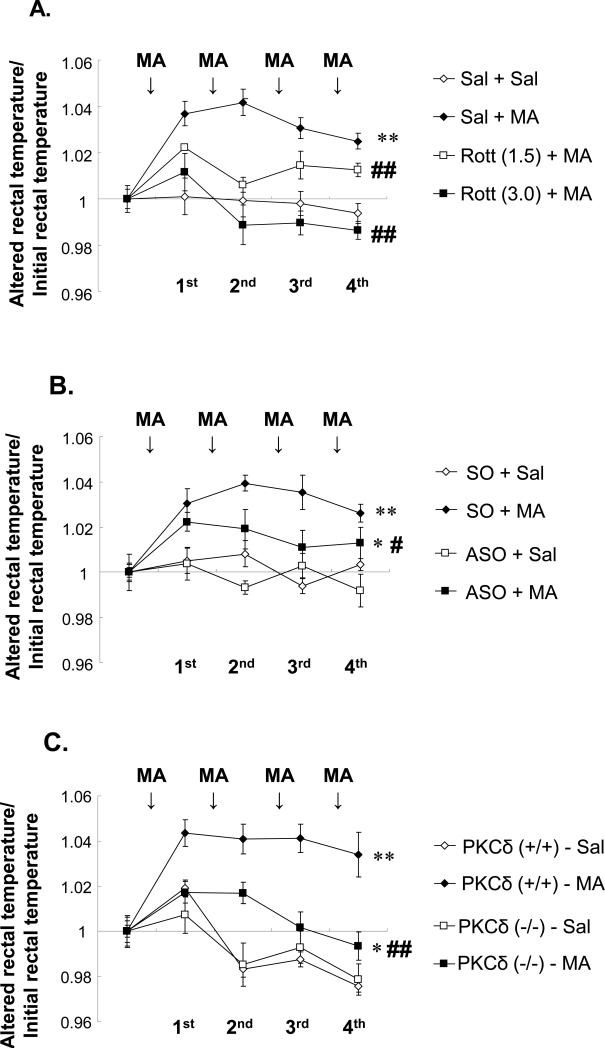
Effects of PKCδ inhibition on MA-induced hyperthermia in mice
MA-induced temperature dysregulation plays a key role in the degenerative effects of the drug. Thus, the body temperature of the animals was measured after PKCδ inhibition (Fig. 2). Treatment with the PKCδ inhibitor rottlerin dose-dependently blocked (P<0.01 vs. Sal+MA) MA-induced hyperthermia in PKCδ (+/+) mice. Because the i.c.v. route is more effective than the p.o. route in producing the neuroprotective effects of rottlerin (Shin et al., 2010) an i.c.v. infusion was utilized (Fig.2A). MA significantly induced hyperthermia in sense oligonucleotide (SO)-infused PKCδ (+/+) mice (P<0.01 vs. SO-infused saline) (Fig. 2B) but MA-induced hyperthermia was significantly attenuated (P<0.05) by antisense oligonucleotide (ASO) infusion (2.5μg, i.c.v). This finding is comparable to that in MA-treated PKCδ (−/−) mice. MA-induced hyperthermia in PKCδ (+/+) mice was consistently and significantly weakened in MA-treated PKCδ (−/−) mice (P<0.01; Fig. 2C).
Fig. 2.
Effects of rottlerin (1.5 or 3.0μg, i.c.v.) (A). ASO (2.5μg, i.c.v.) (B) or PKCδ gene knockout (C) on methamphetamine (MA)-induced hyperthermia in mice. Initial rectal temperature (°C) in each group was as follows; A) 37.21±0.23 (Sal+Sal), 36.97±0.16 (Sal+MA), 37.37±0.17 [Rott (1.5)+MA], and 37.54±0.22 [Rott (3.0)+MA], B) 37.21±0.24 (SO+Sal), 36.75±0.19 (SO+MA), 36.84±0.16 (ASO+Sal), and 37.14±0.20 (ASO+MA), C) 37.78±0.16 (Sal-treated PKCδ (+/+) mice), 37.21±0.21 (MA-treated PKCδ (+/+) mice), 37.07±0.23 (Sal-treated PKCδ (−/−) mice), and 37.68±0.15 (MA-treated PKCδ (−/−) mice). Each value is the mean ± S.E.M of 12 animals. *P<0.05, **P<0.01 vs. Sal+Sal, SO+Sal, ASO+Sal, Sal-treated PKCδ (+/+) mice or Sal-treated PKCδ (−/−) mice, #P<0.05, ##P<0.01 vs. Sal+MA, SO+MA or MA-treated PKCδ (+/+) mice (ANOVA for repeated measures followed by Fisher's PLSD test).
Effects of MA on the expression of PKCδ
It is known that PKCδ modulates the release of DA in the nigrostriatal pathway. This is supported by a number of findings, which report that the in vivo administration of drugs that affect DA release result in changes in PKC activity in the striatum (Giambalvo, 1988; Giambalvo, 1989). Thus, the striatal expression of PKCδ after the final MA dose was examined (Fig.3).
Some PKCδ expression was observed in the absence of MA in PKCδ (+/+) mice although treatment with MA significantly increased PKCδ expression (P<0.01). Rottlerin (1.5 or 3.0μg, i.c.v.) significantly attenuated MA-induced PKCδ expression in a dose- and time-dependent manner (4h and 3d post-MA; Sal+MA vs. rottlerin 1.5μg+MA or rottlerin 3.0μg+MA; P<0.05 or P<0.01; Fig. 3A). The infusion of ASO (2.5μg, i.c.v.) consistently and significantly decreased striatal PKCδ protein levels in the absence or presence of MA (ASO+Saline vs. SO+Saline, P<0.05; ASO+MA vs. SO+MA, P<0.01; Fig. 3B). Little PKCδ expression was observed in the absence or presence of MA in PKCδ (−/−) mice (data not shown).
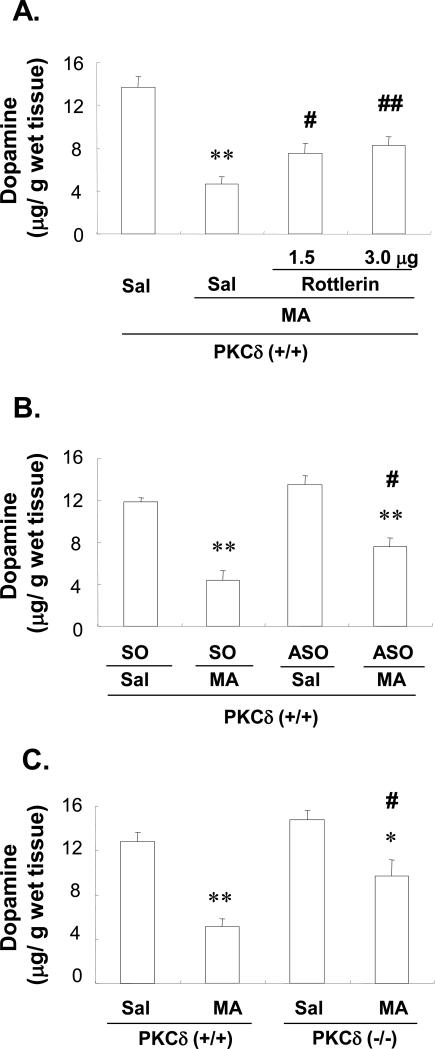
PKCδ participates in MA-induced striatal DA depletion
MA treatment significantly decreased striatal DA levels (P<0.01 vs. Saline-treated PKCδ (+/+)-mice) and rottlerin (1.5 or 3.0μg, i.c.v) significantly attenuated the MA-induced decrease of DA in a dose-dependent manner (Sal+MA vs. rottlerin 1.5 or 3.0μg +MA, P<0.05 or P< 0.01; Fig. 4A). To further confirm the role of PKCδ in this process, SO or ASO was infused into the brains of PKCδ (+/+) mice. There were no significant changes observed between SO-infused and ASO-infused PKCδ (+/+) mice in the absence of MA (Saline treatment; Fig. 4B). An MA-induced decrease in DA was observed in SO-infused PKCδ (+/+) mice (P<0.01 vs. SO-infused Saline group) while ASO-infused mice exhibited less of an MA-induced effect (DA level; SO-infused MA-treatment vs. ASO-infused MA-treatment, P<0.05; Fig. 4B).
Fig. 4.
Effect of rottlerin (A), ASO (B) or PKCδ gene knockout (C) on changes in dopamine 3d after the final methamphetamine (MA) administration in the striatum of the mice. Sal = Saline. Rottlerin 1.5 or Rottlerin 3.0 = rottlerin, a PKCδ inhibitor at the doses of 1.5 or 3.0 μg, i.c.v.. SO = PKCδ sense oligonucleotide. ASO = PKCδ antisense oligonucleotide. Each value is the mean ± S.E.M of 6 animals. *P<0.05, **P<0.01 vs. Sal+Sal, SO+Sal, ASO+Sal, Sal-treated PKCδ (+/+) mice or Sal-treated PKCδ (−/−) mice, #P<0.05, ##P<0.01 vs. Sal+MA, SO+MA or MA-treated PKCδ (+/+) mice (one-way ANOVA followed by Fisher's PLSD test).
PKCδ (−/−) mice were also utilized to investigate whether genetic alterations of PKC activity might protect against MA-induced toxicity. The PKCδ (−/−) mice showed a smaller decrease in striatal DA levels, as compared to MA-treated PKCδ (+/+) mice (MA-treated PKCδ (+/+) vs. MA-treated PKCδ (−/−), P<0.05; Fig. 4C).
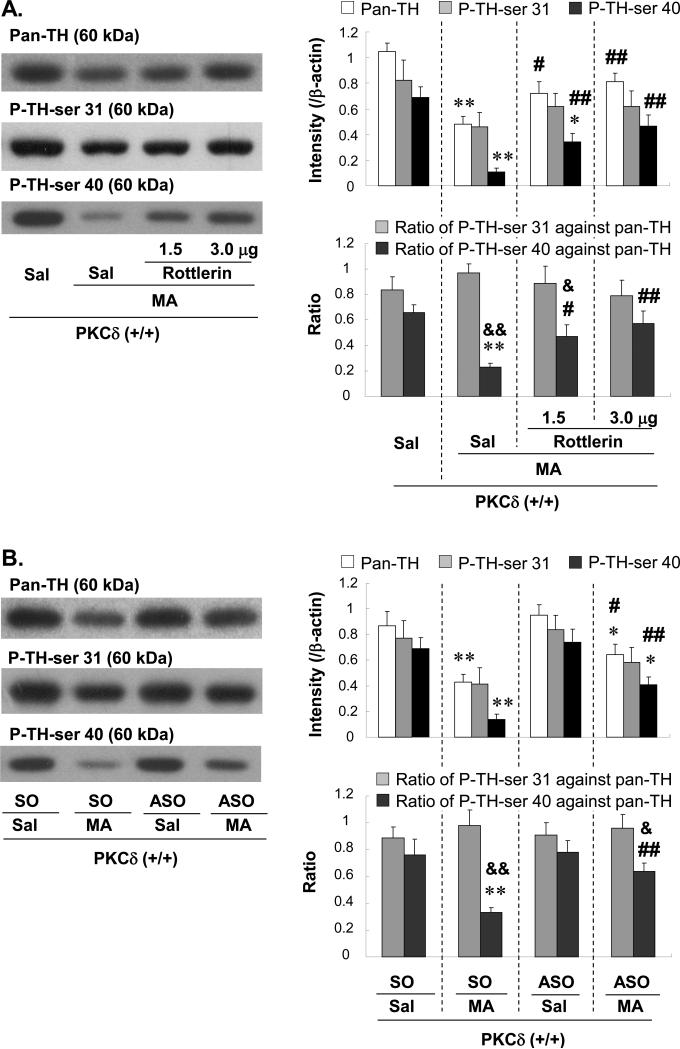
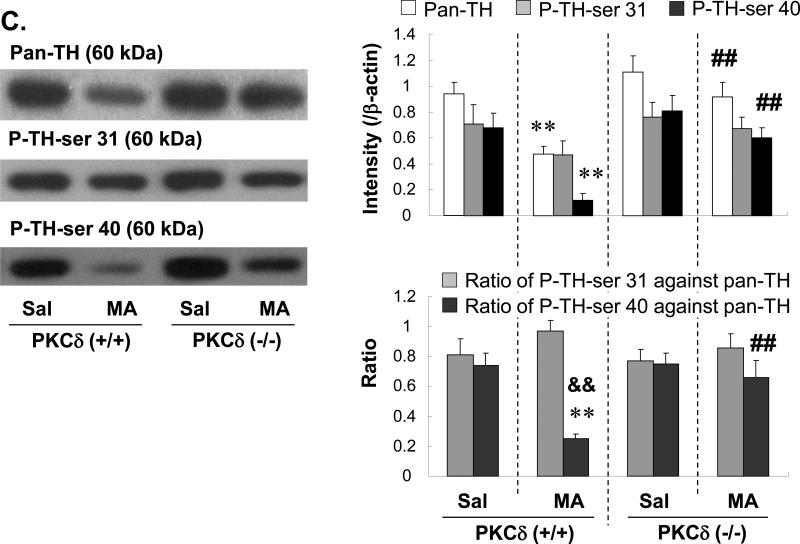
Effects of rottlerin or PKCδ ASO on the MA-induced changes in phospho-TH at ser 40 (PTH-ser 40) in the striatum of PKCδ (+/+) mice: comparison with MA-treated PKCδ (−/−) mice
The current results show that the inhibition of PKCδ can protect against striatal DA depletion. Therefore, using western blot analyses, the effects of MA on pan-TH protein expression in the mouse striatum were evaluated (Fig. 5). A reduction in pan-TH expression caused by MA was attenuated by rottlerin (Fig. 5A), infusion of ASO (Fig. 5B), and in PKCδ (−/−) mice (Fig. 5C).
Fig. 5.
Effect of rottlerin (1.5 or 3.0μg, i.c.v.) (A), ASO (2.5μg, i.c.v.) (B) or PKCδ gene knockout (C) on the changes in Pan-TH, P-TH-ser 31, P-TH-ser 40 3d after the final methamphetamine (MA) administration in the striatum of the mice. Sal = Saline. SO = PKCδ sense oligonucleotide. ASO = PKCδ antisense oligonucleotide. P-TH-ser 31 = phospho-TH at serine 31. P-TH-ser 40 = phospho-TH at serine 40. Each value is the mean ± S.E.M of 6 animals. *P<0.05, **P<0.01 vs. Sal+Sal, SO+Sal, ASO+Sal, Sal-treated PKCδ (+/+) mice or Sal-treated PKCδ (−/−) mice, #P<0.05, ##P<0.01 vs. Sal+MA, SO+MA or MA-treated PKCδ (+/+) mice, &P<0.05, &&P<0.01 vs. respective ratio of P-TH-ser 31 against Pan-TH (one-way ANOVA followed by Fisher's PLSD test).
The expression of TH phosphorylation was also examined. Treatment with MA resulted in a significant decrease in P-TH-ser 40 expression in PKCδ (+/+) mice (Sal+Sal vs. Sal+MA; P<0.01) whereas MA did not significantly alter P-TH-ser 31 expression (Fig. 5A). The decrease in the ratio of P-TH-ser 40 versus pan-TH was significantly more pronounced, as compared to the ratio of P-TH-ser 31 versus pan-TH (P<0.01), although this difference was not observed in the absence of MA. Rottlerin treatment (1.5 or 3.0μg, i.c.v.) significantly attenuated the MA-induced decrease in P-TH-ser 40 in a dose-dependent manner (Sal+MA vs. rottlerin 1.5 or 3.0μg+MA; P<0.01). The MA-induced decrease in the ratio of P-TH-ser 40 against pan-TH was also significantly attenuated in the presence of rottlerin (Sal+MA vs. rottlerin 1.5 or 3.0μg+MA; P<0.05 or P<0.01; Fig. 5A).
Additionally, SO-infused subjects receiving MA exhibited a significant reduction in P-TH-ser 40 expression (P<0.01 vs. SO+Sal). The ratio of P-TH-ser 40 versus pan-TH was significantly lower (P<0.01) than the ratio of P-TH-ser 31 versus pan-TH in SO-infused MA-treated PKCδ (+/+) mice. ASO (2.5μg, i.c.v.)-infused MA-treated PKCδ (+/+) mice showed significantly higher P-TH-ser 40 expression than did SO-infused MA-treated PKCδ (+/+) mice (ASO+MA vs. SO+MA; P<0.01). The ratio of P-TH-ser 40 versus pan-TH in ASO-infused MA-treated PKCδ (+/+) mice was consistently and significantly higher (P<0.01) than that in SO-infused MA-treated PKCδ (+/+) mice (Fig. 5B). The expression and ratio of P-TH-ser 40 in the MA-treated PKCδ (+/+) mice infused with rottlerin or ASO were comparable to those in MA-treated PKCδ (−/−) mice (Fig. 5C).
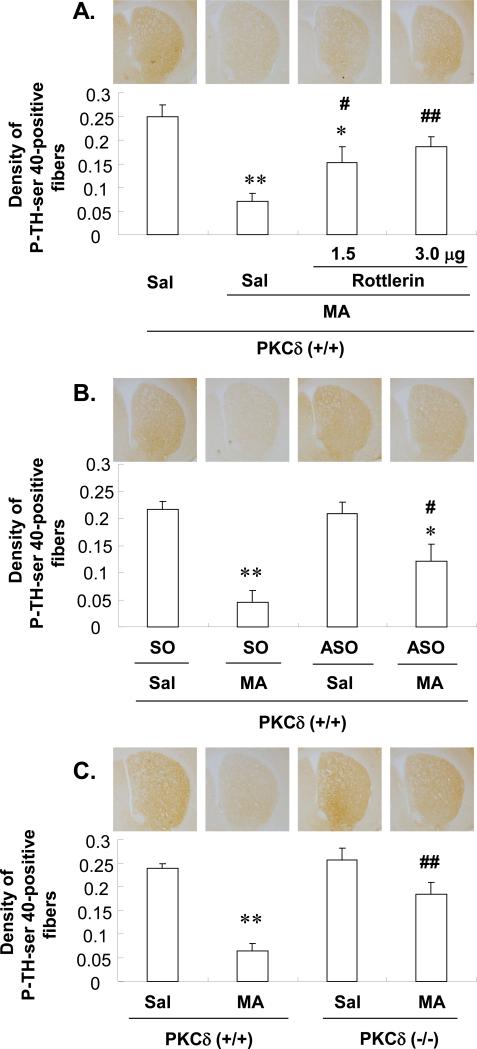
Immunocytochemical analysis showed significant MA-induced reductions in the striatal immunoreactivity of P-TH-ser 40 of PKCδ (+/+) mice (Sal+Sal vs. Sal+MA; P<0.01; SO+Sal vs. SO+MA; P<0.01). Pre-treatment with rottlerin (1.5 or 3.0μg, i.c.v.) significantly attenuated the MA-induced reduction of this immunoreactivity in a dose-dependent manner (Sal+MA vs. rottlerin 1.5 or 3.0μg+MA, P<0.05 or P<0.01; Fig. 6A). ASO infusion (2.5μg, i.c.v.) significantly attenuated the MA-induced reduction of the immunoreactivity of P-TH-ser 40 (P<0.05 vs. SO+MA; Fig. 6B). MA-induced reductions in this immunoreactivity were consistently less pronounced in PKCδ (−/−) mice (MA-treated PKCδ (+/+) vs. MA-treated PKCδ (−/−); P<0.01; Fig. 6C).
Fig. 6.
Effect of rottlerin (1.5 or 3.0μg, i.c.v.) (A), ASO (2.5μg, i.c.v.) (B) or PKCδ gene knockout (C) on the decrease in the immunoreactivity of phospho-TH at ser 40 (P-TH-ser 40-IR) 3d after the final methamphetamine (MA) administration in the striatum of the mice. Sal = Saline. SO = PKCδ sense oligonucleotide. ASO = PKCδ antisense oligonucleotide. Each value is the mean ± S.E.M of 6 animals. *P<0.05, **P<0.01 vs. Sal+Sal, SO+Sal, ASO+Sal, Sal-treated PKCδ (+/+) mice or Sal-treated PKCδ (−/−) mice, #P<0.05, ##P<0.01 vs. Sal+MA, SO+MA or MA-treated PKCδ (+/+) mice (one-way ANOVA followed by Fisher's PLSD test).
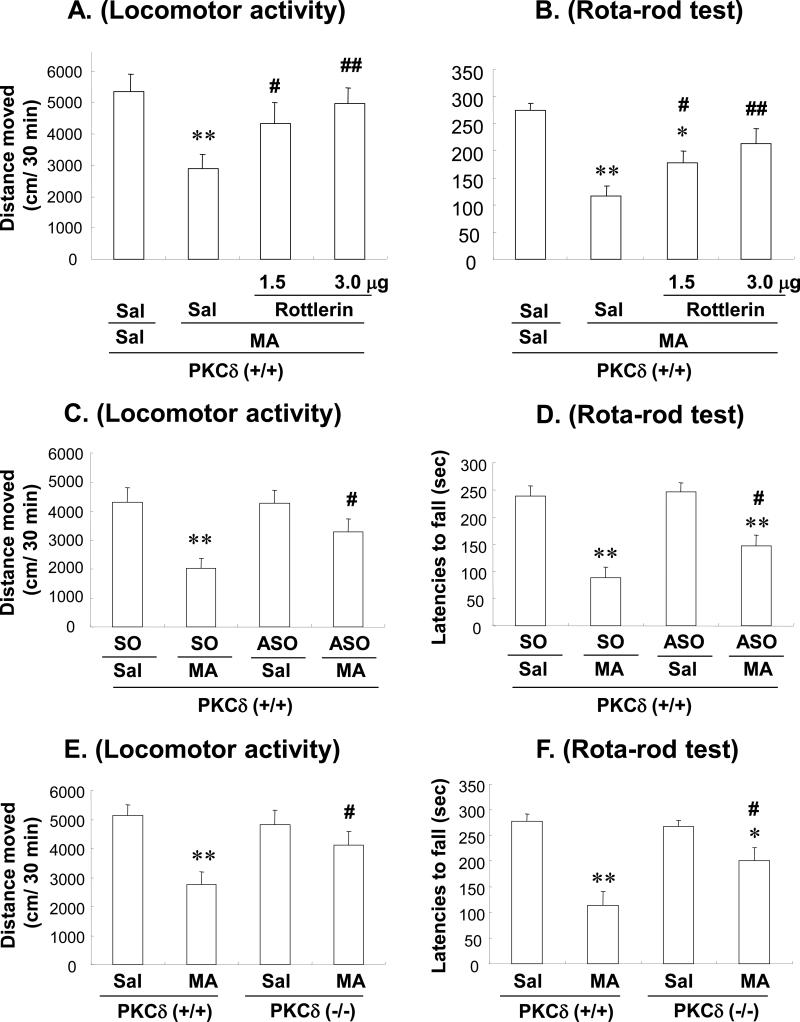
Effects of rottlerin or PKCδ ASO on MA-induced changes in locomotor activity and rotarod performance in PKCδ (+/+) mice: comparison with MA-treated PKCδ (−/−) mice
Treatment with MA resulted in a significant decrease of locomotor activity (Sal+Sal vs. Sal+MA; P<0.01) and rota-rod performance (Sal+Sal vs. Sal+MA; P<0.01) in PKCδ (+/+) mice. Rottlerin treatment (1.5 or 3.0μg, i.c.v.) significantly attenuated the MA-induced reductions in the locomotor activity and rota-rod performance in a dose-dependent manner (Sal+MA vs. rottlerin 1.5 or 3.0μg+MA; P<0.01; Figs. 7A and B). Additionally, SO-infused mice treated with MA exhibited a significant reduction in locomotor activity (P<0.01 vs. SO+Sal) and rota-rod performance (P<0.01 vs. SO+Sal). The reduction in locomotor activity and rota-rod performance was significantly attenuated by ASO (2.5μg, i.c.v.) infusion (Sal+ASO with MA vs. Sal+SO with MA; P<0.05; Fig. 7C and D). The locomotor activity and rota-rod performance of MA-treated PKCδ (+/+) mice infused with rottlerin or ASO were consistently comparable to that of MA-treated PKCδ (−/−) mice (Fig. 7E and F).
Fig. 7.
Effect of rottlerin (1.5 or 3.0 μg, i.c.v.) (A, B), ASO (2.5 μg, i.c.v.) (C, D) or PKCδ gene deletion (E, F) on methamphetamine (MA)-induced behavioural impairments [locomotor activity (A, C, E) and rota-rod performance (B, D, F)] in mice. Sal = Saline. SO = PKCδ sense oligonucleotide. ASO = PKCδ antisense oligonucleotide. Each value is the mean ± S.E.M of 12 animals. *P<0.05, **P<0.01 vs. Sal+Sal with Sal, Sal+SO with Sal, Sal+ASO with Sal, Sal+Sal-treated PKCδ (+/+) mice or Sal+Sal-treated PKCδ (−/−) mice, #P<0.05, ##P<0.01 vs. Sal+MA, SO+MA or MA-treated PKCδ (+/+) mice (one-way ANOVA followed by Fisher's PLSD test).
Discussion
The present findings demonstrate that treatment with high doses of MA results in a significant increase of PKCδ expression in the striatum of mice. Genetic inhibition of PKCδ or use of the pharmacological PKCδ inhibitor rottlerin significantly attenuates MA-induced hyperthermia as well as decreases in TH expression, DA content, and behavioural activity. Furthermore, MA significantly inhibits the phosphorylation of TH at ser 40 but not at ser 31. Importantly, the genetic and pharmacological inhibition of PKCδ significantly attenuates a MA-induced selective reduction in the phosphorylation of TH at ser 40. Thus, the phosphorylation of TH at ser 40, mediated by PKCδ inhibition, may be protective against MA-induced dopaminergic toxicity. To our knowledge, this is the first demonstration concerning impairments in the phosphorylation of TH during MA-induced dopaminergic toxicity in vivo.
Phosphorylation is a key post-translational mechanism that regulates TH activity. Several kinases, including PKC, PKA, CaMK II, and MAPKs (i.e., ERK, JNK, P38), have been shown to phosphorylate one or more of these sites to increase TH activity, depending on the experimental circumstance (Dunkley et al., 2004). Several phosphorylation sites have been identified on TH and the phosphorylation of TH influences its activity (Lee et al., 1989). The phosphorylation of TH at the N-terminal serine (ser) amino sites ser 8, ser 19, ser 31, and ser 40 leads to activation of TH. Various protein kinases have been shown to phosphorylate these serine residues to varying degrees. Among the serine phosphorylation sites, TH-ser 40 is a major residue that positively regulates TH activity in vivo (Campbell et al., 1986; Wu et al., 1992).
Zhang et al. (2007a) found a high expression of PKCδ in dopaminergic neurons and initially hypothesized that PKCδ might phosphorylate TH to increase its activity. To test this, they used the PKCδ inhibitor rottlerin to inhibit the kinase and anticipated that inhibition of PKCδ would result in inhibition of TH activity. Unexpectedly, a dose-dependent increase in TH activity and DA levels was observed in cells treated with rottlerin.
Similar to the current data, Zhang et al. (2007b) provided evidence that rottlerin treatment can rescue TH-positive neurons from MPP+-induced neurotoxicity in vitro. The extension of such studies using animal models revealed that rottlerin effectively attenuates MPTP-induced increases in PKCδ kinase activity in mice. Furthermore, rottlerin administration (20mg/kg, p.o.) attenuates neurochemical depletion and locomotor activity in MPTP-treated animals, which demonstrates a protective effect against neurochemical and behavioural deficits. Rottlerin treatment (20mg/kg, p.o.) also protected against MPTP-induced loss of TH-positive neurons in the substantia nigra. These results are, at least in part, consistent with the current findings and indicate a role for PKCδ in MA-induced dopaminergic toxicity. However, it is accepted that there may be pharmacological differences in dopaminergic toxicity when using MPTP versus MA (Gerlach and Riederer, 1996; Bourque et al., 2009; Kim et al., 2000).
The regulation of TH activity and DA content is critical for normal dopaminergic neurotransmission in the central nervous system (CNS). Excessive DA production may not only alter neurotransmission but can contribute to neuronal death through increases in oxidative stress (Hoyt et al., 1997; Luo et al., 1998). PKCδ has been recognized as an oxidative stress kinase in the CNS (Kanthasamy et al., 2003; Kaul et al., 2005). Oxidative stress and apoptotic cell death have been implicated in several neurodegenerative disorders, including PD (Jung et al., 2010; West et al., 2005; Smith et al., 2006).
Temperature dysregulation also appears to be an important factor in the mediation of toxic responses to MA (Cadet et al., 2003; Krasnova and Cadet, 2009). Evidence suggests that the degree of MA-induced neurodegeneration is correlated with the degree of hyperthermia. Bowyer et al. (1992) have specifically examined the effects of ambient temperature on MA-induced neuroadaptation/toxicity and their interrelationships. Conditions that cause hypothermia or prevent increases in core body temperature are, at least in part, protective against MA toxicity. This protection is probably related to hypothermia-induced inhibitory effects on oxidative insults (Hsu et al., 2006; Zhao et al., 2007). Several recent studies have demonstrated that oxidative stress in dopaminergic neurons persistently activates PKCδ. (Kanthasamy et al., 2010; Miller et al., 2007) Thus, the possibility arises that the hypothermic activity mediated by PKCδ inhibition may be implicated with antioxidant properties (data not shown). This said, it is known that some genetic and pharmacological manipulations can block MA toxicity without influencing drug-induced thermal responses.
Interestingly, the neurotoxic effects of MA in mice appear to be specific to the nigrostriatal dopaminergic system (Kim et al., 2000; Gerlach and Riederer, 1996). The influence of MA-induced neurochemical and histopathological changes on the motor behaviour of animals has not yet been systemically studied, although recent evidence demonstrates MA-induced behavioural deficits in mice (Jung et al., 2010). Due to the similarities between the neuropathological profiles of high-dose MA treatment in rodents and PD in humans, it was hypothesized that neurotoxic doses of MA may lead to behavioural deficits in rodents. The present findings show that MA treatment resulted in a significant reduction of behavioural activity in PKCδ (+/+)-mice but not in rottlerin-treated, ASO-treated, or PKCδ (−/−)-mice. Several investigators have demonstrated a significant correlation between a reduction in behavioural activity and the degree of striatal DA loss (Neill et al., 1974; Lemard and Beer, 1975; Jung et al., 2010). Typically, these studies illustrate that pronounced impairments in behavioural responding occur after reductions of 85% or more of striatal DA contents. In these findings, however, an overt impairment of behavioural activity was noted, although MA treatment resulted in an approximately 65 % reduction of striatal DA in PKCδ (+/+)-mice. These data suggest that the MA treatment produced a moderate behavioural deficit relative to that of the MPTP case (Kim et al., 2000; Gerlach and Riederer, 1996). Thus, these results suggest that MA may be useful as a laboratory tool for modeling basal ganglia dysfunction in the rodent and that over-expression of PKCδ may contribute to MA-induced behavioural deficits through an unknown mechanism.
Although most earlier studies used rottlerin as a PKCδ-selective inhibitor (Gschwendt, 1999; Gschwendt et al., 1994; Li et al., 2004; Yokoyama et al., 2005; Zhang et al., 2007a; Zhang et al., 2007b), recent evidence has shown that rottlerin might not act directly on PKCδ but produces cellular changes that mimic those produced by the direct inhibition of PKCδ (Soltoff, 2007; Susarla et al., 2003; Tapia et al., 2006). Regardless, the effects of rottlerin were comparable with those of PKCδ-ASO infusion and PKCδ-gene knockouts in this study. Thus, it is possible that rottlerin mimics the inhibition of PKCδ activity in the current experimental circumstances, although further cellular and molecular investigation is required to address this matter.
It is recognized that the SH-SY5Y cell line possesses many of the same qualities as substantia nigra neurons and is thus suitable for use as an in vitro model to study the death of dopaminergic neurons (Takahashi et al., 1994; Tian et al., 2007; Wang et al., 2008; Suwanjang et al., 2010; Tiong et al., 2010). The PKC family consists of at least 12 isozymes of which PKCδ and PKCε are expressed in SH-SY5Y dopaminergic neuroblastoma cells (Zeidman et al., 1999; Mackay and Mochly-Rosen, 2001; Pan et al., 2008). However, inhibition of PKCδ with rottlerin did not reverse the cell injury caused by 6-OHDA in SHSY5Y cells (Tiong et al., 2010). Although MA treatment significantly reduced the viability of SH-SY5Y cells in a concentration-related manner in our pilot study, rottlerin (at a level of 5μM) did not significantly affect MA-induced reduced viability of SH-SY5Y cells (data not shown).
Similarly, PC12 pheochromocytoma cells have been widely used to study the molecular mechanisms of neuronal cell death (Ohmichi et al., 1993; Xia et al., 1995; Wei et al., 1997; MacDonald et al., 1999). Uemura et al. (2003) demonstrated that PKC is protective against MA-induced death. In addition, MA-induced cell death was inhibited by a PKC activator, 12, 13-phorbol myristate acetate and enhanced by PKC inhibitors, bisindolylmaleimide and calphostin C. However, cell death was not influenced by rottlerin (Reyland et al., 1999). Uemura et al. (2003) also showed that PKCδ and atypical PKC isoforms are not likely to be involved in the MA-induced death of PC12 cells. Although an in vitro system can easily provide advantages for understanding signaling transduction the above in vitro findings may not support the current in vivo data. Therefore, a precise role for PKCδ in an in vitro model of MA-induced dopaminergic neurotoxicity remains to be fully determined.
In conclusion, it is suggested that MA-induced PKCδ over-expression impairs the striatal dopaminergic system through a significant inhibition of TH phosphorylation at ser 40. In addition, the genetic or pharmacological inhibition of PKCδ requires an enhancement of TH phosphorylation at ser 40 in response to MA insult. Finally, it is proposed that gene depletion or pharmacological inhibition of PKCδ may offer novel therapeutic strategies against striatal dopaminergic toxicity in vivo, induced by MA.
Acknowledgements
This study was supported by a grant from the Brain Research Center from 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea, and by a grant (#E00025) of the Korea-Japan Joint Research Program, National Research Foundation of Korea, Republic of Korea. This work was, in part, supported by grants from Ministry of Health Labour and Welfare (MHLW):Research on Risk of Chemical Substances, and Ministry of Education, Culture, Sports, Science and Technology(MEXT):Academic Frontier Project. Xuan-Khanh Thi Nguyen and Jae-Hyung Bach were supported by BK 21 program.
Abbreviations
- MA
methamphetamine
- PKC
protein kinase C
- ASO
PKCδ antisense oligonucleotide
- TH
tyrosine hydroxylase
- PKA
protein kinase Ai.c.v., intracerebroventricular
- CaMK
Ca2+/calmodulin-dependent protein kinases
- DA
dopamine
- IR
immunoreactivity
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinases
- JNK
c-Jun N-terminal kinase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MPP+
1-methyl-4-phenylpyridinium
- PD
Parkinson's disease
Footnotes
Chu Xuan Duong's present address; Cantho University of Medicine and Pharmacy, Cantho City, Vietnam
References
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Front. Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr., Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J. Pharmacol. Exp. Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Speed kills: cellular and molecular bases of methamphetamine-induced nerve terminal degeneration and neuronal apoptosis. FASEB J. 2003;17:1775–1788. doi: 10.1096/fj.03-0073rev. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J. Biol. Chem. 1986;261:10489–10492. [PubMed] [Google Scholar]
- Choi DY, Liu M, Hunter RL, Cass WA, Pandya JD, Sullivan PG, Shin EJ, Kim HC, Gash DM, Bing G. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS One. 2009;4:e5482. doi: 10.1371/journal.pone.0005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey EC, Newton AC, Mochly-Rosen D, Fields AP, Reyland ME, Insel PA, Messing RO. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L429–438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J. Neurochem. 2004;91:1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd ed. Academic Press; San Diego: 2008. [Google Scholar]
- Gerlach M, Riederer P. Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man. J. Neural Transm. 1996;103:987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT. Protein kinase C and dopamine release--II. Effect of dopamine acting drugs in vivo. Biochem. Pharmacol. 1988;37:4009–4017. doi: 10.1016/0006-2952(88)90087-1. [DOI] [PubMed] [Google Scholar]
- Giambalvo CT. Protein kinase C and dopamine release--III. Effect of dopamine depleting drugs. Biochem. Pharmacol. 1989;38:4445–4454. doi: 10.1016/0006-2952(89)90655-2. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. Protein kinase C delta. Eur. J. Biochem. 1999;259:555–564. doi: 10.1046/j.1432-1327.1999.00120.x. [DOI] [PubMed] [Google Scholar]
- Gschwendt M, Muller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Wakade AR. Activation and multiple-site phosphorylation of tyrosine hydroxylase in perfused rat adrenal glands. J. Neurochem. 1992;58:57–64. doi: 10.1111/j.1471-4159.1992.tb09276.x. [DOI] [PubMed] [Google Scholar]
- Hoyt KR, Reynolds IJ, Hastings TG. Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp. Neurol. 1997;143:269–281. doi: 10.1006/exnr.1996.6374. [DOI] [PubMed] [Google Scholar]
- Hsu SF, Niu KC, Lin CL, Lin MT. Brain cooling causes attenuation of cerebral oxidative stress, systemic inflammation, activated coagulation, and tissue ischemia/injury during heatstroke. Shock. 2006;26:210–220. doi: 10.1097/01.shk.0000223124.49265.10. [DOI] [PubMed] [Google Scholar]
- Hufton SE, Jennings IG, Cotton RG. Structure and function of the aromatic amino acid hydroxylases. Biochem. J. 1995;311(Pt 2):353–366. doi: 10.1042/bj3110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Ichinose H, Ohye T, Fujita K, Pantucek F, Lange K, Riederer P, Nagatsu T. Quantification of mRNA of tyrosine hydroxylase and aromatic L-amino acid decarboxylase in the substantia nigra in Parkinson's disease and schizophrenia. J. Neural Transm. Park. Dis. Dement. Sect. 1994;8:149–158. doi: 10.1007/BF02250926. [DOI] [PubMed] [Google Scholar]
- Jung BD, Shin EJ, Nguyen XK, Jin CH, Bach JH, Park SJ, Nah SY, Wie MB, Bing G, Kim HC. Potentiation of methamphetamine neurotoxicity by intrastriatal lipopolysaccharide administration. Neurochem. Int. 2010;56:229–244. doi: 10.1016/j.neuint.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A, Jin H, Mehrotra S, Mishra R, Kanthasamy A, Rana A. Novel cell death signaling pathways in neurotoxicity models of dopaminergic degeneration: Relevance to oxidative stress and neuroinflammation in Parkinson's disease. NeuroToxicology. 2010;31:555–561. doi: 10.1016/j.neuro.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanthasamy AG, Anantharam V, Zhang D, Latchoumycandane C, Jin H, Kaul S, Kanthasamy A. A novel peptide inhibitor targeted to caspase-3 cleavage site of a proapoptotic kinase protein kinase C delta (PKCdelta) protects against dopaminergic neuronal degeneration in Parkinson's disease models. Free Radic. Biol. Med. 2006;41:1578–1589. doi: 10.1016/j.freeradbiomed.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid. Redox. Signal. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- Kaul S, Anantharam V, Yang Y, Choi CJ, Kanthasamy A, Kanthasamy AG. Tyrosine phosphorylation regulates the proteolytic activation of protein kinase Cdelta in dopaminergic neuronal cells. J. Biol. Chem. 2005;280:28721–28730. doi: 10.1074/jbc.M501092200. [DOI] [PubMed] [Google Scholar]
- Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur. J. Neurosci. 2003;18:1387–1401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- Kim HC, Jhoo WK, Shin EJ, Bing G. Selenium deficiency potentiates methamphetamine-induced nigral neuronal loss; comparison with MPTP model. Brain Res. 2000;862:247–252. doi: 10.1016/s0006-8993(00)02085-0. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: relevance to oxidative stress and dopaminergic degeneration. Neuroscience. 2003;119:945–964. doi: 10.1016/s0306-4522(03)00226-4. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc .Natl. Acad. Sci. U. S. A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res. Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchoumycandane C, Anantharam V, Kitazawa M, Yang Y, Kanthasamy A, Kanthasamy AG. Protein kinase Cdelta is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 2005;313:46–55. doi: 10.1124/jpet.104.078469. [DOI] [PubMed] [Google Scholar]
- Lee KY, Lew JY, Tang D, Schlesinger DH, Deutch AY, Goldstein M. Antibodies to a synthetic peptide corresponding to a Ser-40-containing segment of tyrosine hydroxylase: activation and immunohistochemical localization of tyrosine hydroxylase. J. Neurochem. 1989;53:1238–1244. doi: 10.1111/j.1471-4159.1989.tb07420.x. [DOI] [PubMed] [Google Scholar]
- Lenard LG, Beer B. Relationship of brain levels of norepinephrine and dopamine to avoidance behavior in rats after intraventricular administration of 6-hydoxydopamine. Pharmacol. Biochem. Behav. 1975;3:895–899. doi: 10.1016/0091-3057(75)90123-9. [DOI] [PubMed] [Google Scholar]
- Li C, Chen X, Williams JA. Regulation of CCK-induced amylase release by PKC-delta in rat pancreatic acinar cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G764–771. doi: 10.1152/ajpgi.00111.2004. [DOI] [PubMed] [Google Scholar]
- Li Z, Choi DY, Shin EJ, Hunter RL, Jin CH, Wie MB, Kim MS, Park SJ, Bing G, Kim HC. Phenidone protects the nigral dopaminergic neurons from LPS-induced neurotoxicity. Neurosci. Lett. 2008;445:1–6. doi: 10.1016/j.neulet.2008.08.053. [DOI] [PubMed] [Google Scholar]
- Liedtke CM, Cole T. Antisense oligodeoxynucleotide to PKC-delta blocks alpha 1-adrenergic activation of Na-K-2Cl cotransport. Am. J. Physiol. 1997;273:C1632–1640. doi: 10.1152/ajpcell.1997.273.5.C1632. [DOI] [PubMed] [Google Scholar]
- Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J. Neurochem. 2010;112:773–783. doi: 10.1111/j.1471-4159.2009.06497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hunter R, Nguyen XV, Kim HC, Bing G. Microsomal epoxide hydrolase deletion enhances tyrosine hydroxylase phosphorylation in mice after MPTP treatment. J. Neurosci. Res. 2008;86:2792–2801. doi: 10.1002/jnr.21725. [DOI] [PubMed] [Google Scholar]
- Luo Y, Umegaki H, Wang X, Abe R, Roth GS. Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J. Biol. Chem. 1998;273:3756–3764. doi: 10.1074/jbc.273.6.3756. [DOI] [PubMed] [Google Scholar]
- Macdonald NJ, Perez-Polo JR, Bennett AD, Taglialatela G. NGF-resistant PC12 cell death induced by arachidonic acid is accompanied by a decrease of active PKC zeta and nuclear factor kappa B. J. Neurosci. Res. 1999;57:219–226. doi: 10.1002/(SICI)1097-4547(19990715)57:2<219::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mackay K, Mochly-Rosen D. Localization, anchoring, and functions of protein kinase C isozymes in the heart. J. Mol. Cell Cardiol. 2001;33:1301–1307. doi: 10.1006/jmcc.2001.1400. [DOI] [PubMed] [Google Scholar]
- Meloni R, Leboyer M, Bellivier F, Barbe B, Samolyk D, Allilaire JF, Mallet J. Association of manic-depressive illness with tyrosine hydroxylase microsatellite marker. Lancet. 1995;345:932. doi: 10.1016/s0140-6736(95)90053-5. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain Res. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- Nagatsu T. Biochemical aspects of Parkinson's disease. Adv. Neurol. 1993;60:165–174. [PubMed] [Google Scholar]
- Nagatsu T, Levitt M, Udenfriend S. Tyrosine Hydroxylase. The Initial Step in Norepinephrine Biosynthesis. J. Biol. Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J. Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Hayashi N, Kaneko YS, Mori K, Sabban EL, Nagatsu T, Ota A. Role of N-terminus of tyrosine hydroxylase in the biosynthesis of catecholamines. J. Neural Transm. 2009;116:1355–1362. doi: 10.1007/s00702-009-0227-8. [DOI] [PubMed] [Google Scholar]
- Neill DB, Boggan WO, Grossman SP. Impairment of avoidance performance by intrastriatal administration of 6-hydroxydopamine. Pharmacol. Biochem. Behav. 1974;2:97–103. doi: 10.1016/0091-3057(74)90140-3. [DOI] [PubMed] [Google Scholar]
- Nguyen XV, Liu M, Kim HC, Bing G. Effects of prodynorphin deletion on striatal dopamine in mice during normal aging and in response to MPTP. Exp. Neurol. 2009;219:228–238. doi: 10.1016/j.expneurol.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Ohmichi M, Zhu G, Saltiel AR. Nerve growth factor activates calcium-insensitive protein kinase C-epsilon in PC-12 rat pheochromocytoma cells. Biochem. J. 1993;295(Pt 3):767–772. doi: 10.1042/bj2950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TT, Neo KL, Hu LF, Yong QC, Bian JS. H2S preconditioning-induced PKC activation regulates intracellular calcium handling in rat cardiomyocytes. Am. J. Physiol. Cell Physiol. 2008;294:C169–177. doi: 10.1152/ajpcell.00282.2007. [DOI] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J. Biol. Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Chae JS, Park SJ, Kim SC, Koo KH, Yamada K, Nabeshima T, Kim HC. Growth hormone-releaser diet attenuates beta-amyloid(1-42)-induced cognitive impairment via stimulation of the insulin-like growth factor (IGF)-1 receptor in mice. J. Pharmacol. Sci. 2009;109:139–143. doi: 10.1254/jphs.08145sc. [DOI] [PubMed] [Google Scholar]
- Shin EJ, Nguyen TXK, Nguyen TPK, Mai AT, Park SJ, Bach JH, Park DH, Kim HR. Attenuation of Methamphetamine-Induced Nigrostriatal Dopaminergic Toxicity by Pharmacological and Genetic Inhibition of Protein Kinase Cδ, Spring International Convention of the Pharmaceutical Society of Korea. Pharmaceutical Society of Korea, Daegu EXCO. 2010;P144:P1–96. [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, Dewey WL. Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain. 2007;127:129–139. doi: 10.1016/j.pain.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol. Res. 2006;54:474–480. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends. Pharmacol. Sci. 2007;28:453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Steinberg SF. Distinctive activation mechanisms and functions for protein kinase Cdelta. Biochem. J. 2004;384:449–459. doi: 10.1042/BJ20040704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susarla BT, Robinson MB. Rottlerin, an inhibitor of protein kinase Cdelta (PKCdelta), inhibits astrocytic glutamate transport activity and reduces GLAST immunoreactivity by a mechanism that appears to be PKCdelta-independent. J. Neurochem. 2003;86:635–645. doi: 10.1046/j.1471-4159.2003.01886.x. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Alterio J, Campbell DG, Le Bourdelles B, Mallet J, Haavik J, Cohen P. Phosphorylation and activation of human tyrosine hydroxylase in vitro by mitogen-activated protein (MAP) kinase and MAP-kinase-activated kinases 1 and 2. Eur. J. Biochem. 1993;217:715–722. doi: 10.1111/j.1432-1033.1993.tb18297.x. [DOI] [PubMed] [Google Scholar]
- Suwanjang W, Phansuwan-Pujito P, Govitrapong P, Chetsawang B. The protective effect of melatonin on methamphetamine-induced calpain-dependent death pathway in human neuroblastoma SH-SY5Y cultured cells. J. Pineal. Res. 2010;48:94–101. doi: 10.1111/j.1600-079X.2009.00731.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Deng Y, Maruyama W, Dostert P, Kawai M, Naoi M. Uptake of a neurotoxin-candidate, (R)-1,2-dimethyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline into human dopaminergic neuroblastoma SH-SY5Y cells by dopamine transport system. J. Neural. Transm. Gen. Sect. 1994;98:107–118. doi: 10.1007/BF01277014. [DOI] [PubMed] [Google Scholar]
- Tapia JA, Jensen RT, Garcia-Marin LJ. Rottlerin inhibits stimulated enzymatic secretion and several intracellular signaling transduction pathways in pancreatic acinar cells by a non-PKC-delta-dependent mechanism. Biochim. Biophys. Acta. 2006;1763:25–38. doi: 10.1016/j.bbamcr.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Thibaut F, Ribeyre JM, Dourmap N, Meloni R, Laurent C, Campion D, Menard JF, Dollfus S, Mallet J, Petit M. Association of DNA polymorphism in the first intron of the tyrosine hydroxylase gene with disturbances of the catecholaminergic system in schizophrenia. Schizophr. Res. 1997;23:259–264. doi: 10.1016/s0920-9964(96)00118-1. [DOI] [PubMed] [Google Scholar]
- Tian LL, Zhou Z, Zhang Q, Sun YN, Li CR, Cheng CH, Zhong ZY, Wang SQ. Protective effect of (+/−) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells. Cell. Physiol. Biochem. 2007;20:1019–1032. doi: 10.1159/000110682. [DOI] [PubMed] [Google Scholar]
- Tiong CX, Lu M, Bian JS. Protective effect of hydrogen sulphide against 6-OHDA-induced cell injury in SH-SY5Y cells involves PKC/PI3K/Akt pathway. Br. J. Pharmacol. 2010;161:467–480. doi: 10.1111/j.1476-5381.2010.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K, Aki T, Yamaguchi K, Yoshida K. Protein kinase C-epsilon protects PC12 cells against methamphetamine-induced death: possible involvement of suppression of glutamate receptor. Life Sci. 2003;72:1595–1607. doi: 10.1016/s0024-3205(02)02450-5. [DOI] [PubMed] [Google Scholar]
- Wang SF, Yen JC, Yin PH, Chi CW, Lee HC. Involvement of oxidative stress-activated JNK signaling in the methamphetamine-induced cell death of human SH-SY5Y cells. Toxicology. 2008;246:234–241. doi: 10.1016/j.tox.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Wei Q, Jurma OP, Andersen JK. Increased expression of monoamine oxidase-B results in enhanced neurite degeneration in methamphetamine-treated PC12 cells. J. Neurosci. Res. 1997;50:618–626. doi: 10.1002/(SICI)1097-4547(19971115)50:4<618::AID-JNR12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- West JD, Marnett LJ. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- Wu J, Filer D, Friedhoff AJ, Goldstein M. Site-directed mutagenesis of tyrosine hydroxylase. Role of serine 40 in catalysis. J. Biol. Chem. 1992;267:25754–25758. [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kaul S, Zhang D, Anantharam V, Kanthasamy AG. Suppression of caspase-3-dependent proteolytic activation of protein kinase C delta by small interfering RNA prevents MPP+-induced dopaminergic degeneration. Mol. Cell Neurosci. 2004;25:406–421. doi: 10.1016/j.mcn.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Yokoyama G, Fujii T, Tayama K, Yamana H, Kuwano M, Shirouzu K. PKCdelta and MAPK mediate G(1) arrest induced by PMA in SKBR-3 breast cancer cells. Biochem. Biophys. Res. Commun. 2005;327:720–726. doi: 10.1016/j.bbrc.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Zeidman R, Pettersson L, Sailaja PR, Truedsson E, Fagerstrom S, Pahlman S, Larsson C. Novel and classical protein kinase C isoforms have different functions in proliferation, survival and differentiation of neuroblastoma cells. Int. J. Cancer. 1999;81:494–501. doi: 10.1002/(sici)1097-0215(19990505)81:3<494::aid-ijc26>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Zhang D, Kanthasamy A, Yang Y, Anantharam V. Protein kinase C delta negatively regulates tyrosine hydroxylase activity and dopamine synthesis by enhancing protein phosphatase-2A activity in dopaminergic neurons. J. Neurosci. 2007a;27:5349–5362. doi: 10.1523/JNEUROSCI.4107-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Anantharam V, Kanthasamy A, Kanthasamy AG. Neuroprotective effect of protein kinase C delta inhibitor rottlerin in cell culture and animal models of Parkinson's disease. J. Pharmacol. Exp. Ther. 2007b;322:913–922. doi: 10.1124/jpet.107.124669. [DOI] [PubMed] [Google Scholar]
- Zhao H, Steinberg GK, Sapolsky RM. General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J. Cereb. Blood Flow. Metab. 2007;27:1879–1894. doi: 10.1038/sj.jcbfm.9600540. [DOI] [PubMed] [Google Scholar]