Introduction
20-hydroxyeicosatetraenoic acid (20-HETE) is a cytochrome P450 (CYP) metabolite of arachidonic acid (AA) that is a potent endogenous vasoconstrictor of renal [1] and cerebral arteries [2]. 20-HETE activates protein kinase C (PKC) [3, 4], mitogen-activated protein kinases (MAPK) [5], tyrosine kinase [6], and the rho kinase pathway [7] to promote Ca2+ entry through depolarization of vascular smooth muscle (VSM) cells secondary to blockade of the large conductance, calcium sensitive K+ (BK) channel [3, 8]. Previous studies have indicated that elevations in transmural pressure increases the production of 20-HETE in cerebral arteries [9] and that inhibition of the synthesis of 20-HETE impairs the development of active myogenic tone in renal interlobular arteries [10] and the middle cerebral artery of rats [9]. Administration of inhibitors of the synthesis of 20-HETE has been reported to impair autoregulation of renal and cerebral blood flow in rats in vivo [9, 11]. In addition, 20-HETE appears to play a role in modulating tubuloglomerular feedback (TGF) responsiveness in the kidney [12]. In this regard, perfusion of the loop of Henle with AA potentiated, while administration of CYP inhibitors attenuated the TGF response in rats in vivo [12]. While, these studies established a potential role of 20-HETE in the regulation of renal vascular tone, direct evidence is lacking regarding the effects of inhibitors of the production of 20-HETE on myogenic and TGF responses at the level of the isolated perfused Af-Art. Moreover, it remains to be determined whether the modulation of TGF responsiveness seen in previous studies following administration of a 20-HETE inhibitor to the tubular perfusate was due to changes in the synthesis of 20-HETE and sodium transport at the level of the macula densa, or through diffusion of the inhibitor across the macula densa and changes in the formation of 20-HETE and vascular reactivity in the Af-Art. Thus, the present study explored the role of 20-HETE in the regulation of both TGF and the myogenic responses of Af-Art using of N-hydroxy-N-(4-butyl-2-methylphenyl) formamidine (HET0016) a highly selective inhibitor of the synthesis of 20-HETE [13–15] and 20-hydroxyeicosa-6(Z), 15(Z)-dienoic acid (6, 15-20-HEDE) which has been reported to antagonize the vasoconstrictor action of 20-HETE [16, 17]. These studies were performed using Af-Art isolated from the kidneys of both rabbits and mice. The rabbit kidney was used because it is possible to microdissect an Af-Art with an attached macula densa and distal tubule to study both myogenic and TGF responses. We also studied the myogenic response in Af-Art of mice since recent studies have indicated that deletion of CYP4A14 gene can cause hypertension that is associated with increased expression of CYP4A12 and the renal production of 20-HETE, reduced diameter of the Af-Art [18, 19] and elevated vascular reactivity to phenylephrine and angiotensin II [20]. However, the role of endogenously produced 20-HETE in the regulation of vascular tone in isolated perfused Af-Art of the mouse has yet to be directly studied.
Material and methods
Experimental design
Experiments were performed on male New-Zealand white rabbits weighing between 1.5–2.5 kg and 6–9 week old male C57BL/6 mice (18 to 20 g), purchased from Harlan Laboratories. The animals were housed in the Laboratory Animal Facilities at the University of Mississippi Medical Center and received food and water ad libitum. All protocols were approved by the Institutional Animal Care and Use Committee of the University of Mississippi Medical Center and were consistent with the NIH Guide for the Care and Use of Laboratory Animals.
Isolation and microperfusion of rabbit and mouse afferent arterioles
Male C57BL/6J mice were anesthetized with ketamine (50 mg/Kg) and xylazine (2 mg/Kg), while young, male New Zealand White rabbits were anesthetized with sodium pentobarbital (40 mg/kg, i.v.). After anesthesia, the animals received an iv injection of heparin (500 U) to prevent coagulation. Upon sacrifice, the kidneys were removed, sliced along the corticomedullary axis and placed in ice-cold minimum essential medium (MEM; Gibco, Grand Island, NY) containing 5% bovine serum albumin. Single superficial Af-Art with the attached glomeruli were microdissected using a stereomicroscope (model SMZ 1500; Nikon) and transferred to a temperature-regulated chamber mounted on an inverted microscope (Eclipse Ti; Nikon, Melville, NY). The Af-Art was cannulated with an array of glass pipettes [21] and was perfused with MEM, while the distal tubule was perfused with a NaCl solution (10 or 80 mM). The microdissection and cannulation of the Af-Art preparations were usually completed within 30 minutes and then the samples were then gradually warmed to 37°C. Once the temperature was stable, a 30-min equilibration period was allowed before the baseline diameter of the Af-Art was measured. The imaging system consisted of a microscope (Eclipse Ti; Nikon, Melville, NY), digital charge-coupled device camera (CoolSnap; Photometrics, Tucson, AZ), a xenon light source (LB-LS/30; Sutter Instruments, Novato, CA) and a high resolution monitor. Images of the Af-Art were displayed on the monitor and the diameter of the vessels were measured using NIS-Elements imaging software (Nikon, Melville, NY).
Metabolism of arachidonic acid in rabbit renal microvessels
Previous studies have indicated that renal microvessels isolated from the kidneys of rats [8, 22] produce 20-HETE when incubated with AA. However, similar experiments have yet to be done using renal microvessels isolated from the kidneys of rabbits. In the present study, rabbit kidneys were flushed with a 3% albumin solution containing 1% Evans Blue to stain the renal microvessels. The kidneys were removed and pressed through a 150 μm metal sieve using the barrel of a 30 cc glass syringe to isolate the renal vasculature. Small renal interlobular and afferent arterioles that were trapped on the top of the screen were collected and inspected under a stereomicroscope and any residual contamination by tubules was removed by microdissection. The metabolism of AA was assessed as previously described by our laboratory [23, 24]. Briefly, the microvessels were placed in in 1 ml of a cold physiological salt solution (PSS) containing (in mM) 119 NaCl, 4.7 KCl, 1.17 MgSO4, 1.6 CaCl2, 1.18 NaH2PO4, 24 NaHCO3, 0.03 EDTA, 5.5 dextrose, and 5 HEPES. The vessels were incubated for 60 minutes at 37°C in the presence of 40 μM AA and 1 mM NADPH. The reactions were gently swirled under an atmosphere of 100% O2 to maintain PO2 levels in the incubation media in the range of 100 Torr throughout the experiment. The reactions were terminated by acidification to pH 3.5 with formic acid and the vessels were homogenized in the reaction buffer. An aliquot was collected to determine the protein concentration of the sample and the remainder of the homogenate was extracted with ethyl acetate. The organic layer was dried under nitrogen. Samples were reconstituted in methanol and the metabolites of AA produced were measured using an ABI-Sciex 4000 Q-Trap LC/MS/MS instrument as previously described [23, 24]. Values are expressed as picomoles of product formed per minute per milligram of protein.
Role of 20-HETE in modulating the myogenic response of rabbit and mouse afferent arterioles
These experiments were performed on isolated perfused Af-Art with attached glomeruli isolated from the kidneys of both rabbits and mice. After a 30 min equilibration period, the baseline diameter of the Af-Art was recorded at intraluminal pressures of 60 mmHg. Intraluminal pressure was then increased to 120 mmHg and after a 5 min equilibration period, the diameter of the Af-Art was redetermined. The diameter of the Af-Art was measured at three points along the most active portion within the last 100 μm before entering the glomerulus. In order to determine the role of endogenously produced 20-HETE in modulating vascular tone to changes in transmural pressure, HET0016 (1μM), a selective inhibitor of the synthesis of 20-HETE, was added to the bath for 15 min and the diameters of the Af-Art were again recorded at 60 and 120 mmHg. Then, a stable 20-HETE mimetic, 20-hydroxyeicosa-5(Z), 14(Z)-dienoic acid (5, 14-20-HEDE, 10 μM) or authentic 20-HETE (10 μM) was added to the perfusate, and after a 15 minute equilibration period the pressure-diameter relationships were assessed. To further investigate the role of endogenously formed 20-HETE in the myogenic response additional experiments were performed before and after addition of a chemically and mechanistically different inhibitor of the vasoconstrictor actions of 20-HETE, 6, 15-20-HEDE was added to the vascular perfusate. After a 15-min equilibration period, the diameters of both rabbit and mouse Af-Art were measured at pressures of 60 and 120 mmHg.
Role of 20-HETE in modulating tubuloglomerular feedback responses in double perfused, rabbit afferent arteriole and macula densa preparations
Af-Art with an attached glomeruli, distal tubule and intact macula densa were microdissected from the kidneys of rabbits. Both the Af-Art and distal tubule were cannulated and simultaneously microperfused [25]. The pressure in the Af-Art was set to 60 mmHg and the distal tubule was perfused with a solution containing 10 mM NaCl at a rate of 5–10 nl/min [26]. After a 30 minute equilibration period, the diameter of the Af-Art was measured. The NaCl concentration in the distal tubular perfusate was then increased to 80 mM and the change in the diameter of the Af-Art determined. Following these baseline measurements, a stable 20-HETE mimetic (10 μM) was added to the tubular perfusate and the sodium concentration of the distal tubular perfusate was increased from 10 to 80 mM, and the change in the diameter of the Af-Art was determined. In other experiments, 6, 15-20-HEDE, a 20-HETE antagonist, was also added to vascular perfusate to determine if it could block the ability of the 20-HETE agonist to potentiate the Af-Art response to an elevation in distal tubular sodium concentration.
Statistical analysis
Mean values ± SEM are presented. The significance of differences in control and experimental values within the same animal were determined by a paired t-test. The significance of differences in corresponding mean values between groups was determined by ANOVA followed by Holm-Sidak test. A value of P<0.05 was considered to be significant.
Results
Effect of HET0016 on renal metabolism of AA in rabbit renal microvessels
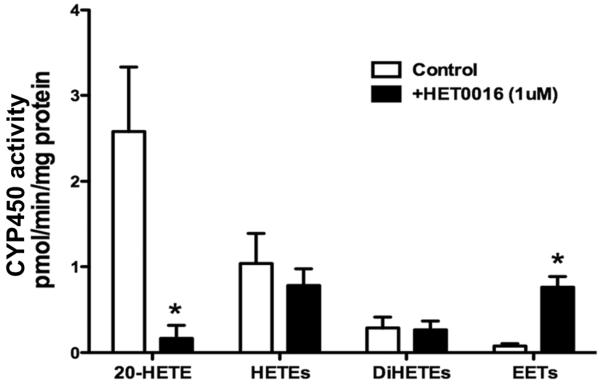
Figure 1 presents representative LC-MS/MS chromatograms of the CYP450 metabolites of AA produced from rabbit preglomerular microvessels treated with vehicle (Figure 1A) or HET0016 (1 μM) (Figure 1B). Rabbit renal microvessels avidly produced 20-HETE, 15-HETE, 12-HETE and 5-HETE, as well as 14, 15-DiHETE and 11, 12-DiHETE when incubated with AA. Administration of HET0016 significantly reduced the production of 20-HETE, while increasing the production of epoxyeicosatrienoic acids (EETs). A summary of the effects of HET0016 on the production of 20-HETE, EETs, DiHETEs, and HETEs by rabbit preglomerular vessels are presented in Figure 2. HET0016 reduced the synthesis of 20-HETE by >80%, while the production of EETs increased. HET0016 had no significant effect on the formation of any of the other HETEs or DiHETEs produced by rabbit preglomerular arterioles.
Figure 1.

Representative liquid chromatography/mass spectroscopy profiles of cytochrome P450 metabolites of arachidonic acid produced by rabbit renal microvessels. The vessels were incubated for 30 minutes with arachidonic acid (40 μM) and 1 mM NADPH under control conditions (Figure 1A) and in the presence of HET0016 (1 μM) (Figure 1B). The vessels produced 5-, 8-, 12-, 15-, 19, 20-hydroxyeicosatetraeoic acids (HETEs) along with several dihydroxyeicosatrienoic acids (DiHETEs) and epoxyeicosatrenoic acids (EETs) in control vessels. The production of 20-HETE was selectively reduced by incubation of the vessels with HET0016.
Figure 2.
Summary of the effects of HET0016 (1 μM) on the CYP450-dependent metabolism of arachidonic acid on 20-HETE, HETEs, DiHETEs and EETs by rabbit renal microvessels. Mean values ± SE from 4 experiments are presented. * P<0.05 from the corresponding control value.
Effects of 20-HETE on the myogenic response of rabbit and mouse afferent arteriole
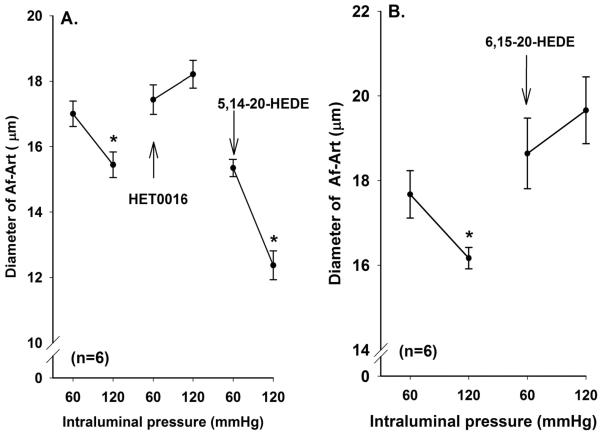
The effects of HET0016 and 6, 15-20-HEDE on the myogenic response of rabbit Af-Art are presented in Figure 3. The diameter of the Af-Art is decreased by 9 ± 1% when perfusion pressure was increased from 60 to 120 mmHg. Administration of a 20-HETE synthesis inhibitor, HET0016 (1 μM) completely blocked the myogenic response in the rabbit Af-Art (Figure 3A). Similar results were obtained when a chemically and mechanistically different inhibitor of the actions of 20-HETE was added to the fluid perfusing the Af-Art (Figure 3B). Addition of a 20-HETE mimetic (5, 14-20-HEDE) or authentic 20-HETE to the vascular perfusate restored the vasoconstrictor response in vessels treated with HET0016 and diameter of the Af-Art fell by 26 ± 0.5% and 7 ± 0.02% , respectively, when renal perfusion pressure was elevated (Figure 3A).
Figure 3.
Effects of HET0016 (1 μM), authentic 20-HETE (10 μM) and the 20-HETE mimetic 5, 14-20-HEDE (10 μM) on the myogenic response of rabbit Af-Art (Figure 3A). Figure 3B presents the effects of the 20-HETE antagonist, 6, 15-20-HEDE (10 μM) on the myogenic response of rabbit Af-Art. HET0016 (Figure 3A) and 6, 15-20-HEDE (Figure 3B) completely blocked the myogenic response in rabbit Af-Art. Administration of the mimetic, 5, 14-20-HEDE reduced the basal diameter and restored the myogenic response in Af-Art pretreated with HET0016. Administration of the 20-HETE had a similar effect and restored the myogenic response in Af-Art pretreated with HET0016. Numbers in parentheses indicate the number of vessels studied. * indicates a significance difference of P< 0.05 from the corresponding control value measured at 60 mmHg.
The effects of HET0016 and 6, 15-20-HEDE on the myogenic response of mouse Af-Art are presented in Figure 4. Under control conditions, the diameter of the Af-Art decreased by 9 ± 0.5% when the perfusion pressure was increased from 60 to 120 mmHg (Figure 4A). Blockade of the synthesis of 20-HETE with HET0016 or its vasoconstrictor actions with 6, 15-20-HEDE, increased the basal diameter of the Af-Art and completely blocked the myogenic constriction in response to elevations in perfusion pressure. Addition of a 20-HETE mimetic (5, 14-20-HEDE) restored the vasoconstrictor response in vessels treated with HET0016 and vessel diameter fell by 19 ± 2% in response to an elevation in perfusion pressure from 60 to 120 mmHg (Figure 4A).
Figure 4.
Effect of HET0016 (1 μM) and the 20-HETE mimetic 5, 14 20-HEDE (10 μM) on the myogenic response of the Af-Art of the mouse (Figure 4A). Figure 4B presents the effects of the 20-HETE antagonist 6, 15-20-HEDE on the myogenic response of mouse Af-Art. Both HET0016 and 6, 15-20-HEDE (10 μM) completely blocked the myogenic response mouse Af-Art. Addition of the 20-HETE mimetic 5, 14 20-HEDE (10 μM) restored the myogenic response of mouse Af-Art treated with HET0016. Numbers in parentheses indicate the number of vessels studied. * indicates a significance difference of P< 0.05 from the control diameter measured at 60 mmHg. # indicates a significant difference from the baseline measurement at 120 mmHg.
Effects of 20-HETE on the tubuloglomerular feedback response in double perfused rabbit Af-Art/macula densa preparations
The effects of administration of an inhibitor of the vasoconstrictor actions of 20-HETE on TGF response of the rabbit Af-Art to elevations in sodium delivery to the macula densa is presented in Figure 5. Increasing the sodium concentration of the distal tubular perfusate from 10 to 80 mM produced a marked constriction of the rabbit Af-Art. Addition of 6, 15-20-HEDE to the vascular perfusate completely blocked the TGF-mediated constriction of the Af-Art (Figure 5B). In other experiments, addition of the 20-HETE mimetic, 5, 14-20-HEDE to the distal tubular perfusate enhanced the vasoconstrictor response of the Af-Art to an elevation in the NaCl concentration at the macula densa (Figure 6A). However, this response was significantly attenuated when the 20-HETE antagonist, 6, 15-20-HEDE, was added to the vascular perfusate (Figure 6B).
Figure 5.
Effect of perfusion of the macula densa with the 20-HETE antagonist 6, 15-20-HETE (10 μM) on tubuloglomerular feedback response of rabbit Af-Art. A representative picture of a double perfused rabbit macula densa preparation is presented in Figure 5A. The change in diameter in response to elevations in Na concentration of the fluid perfusing the macula densa from 10–80 mM is presented before and after addition of the 20-HETE antagonist 6, 15-20-HEDE to the fluid perfusing the Af-Art (Figure 5B). Numbers in parentheses indicate the number of vessels studied. * indicates a significance difference of P< 0.05 from the control diameter measured at 60 mmHg.
Figure 6.
Effect of perfusion of the macula densa with the 20-HETE mimetic 5, 14-20-HEDE (10 μM) on tubuloglomerular feedback response of rabbit Af-Art (Figure 6B). The change in diameter in response to elevations in Na concentration of the fluid perfusing the macula densa from 10–80 mM is presented before and after addition of the 20-HETE mimetic 5, 14-20-HEDE (10 μM) to the tubular perfusate. Figure 6B presents the effects of addition of the 20-HETE antagonist 6, 15-20-HEDE to the vascular perfusate on the TGF response to addition of the 20-HETE mimetic 5, 14-20-HEDE (10 μM) to the tubular perfusate. Numbers in parentheses indicate the number of vessels studied. * indicates a significant difference of P < 0.05, from the corresponding control value within each group. # indicates a significant difference from the baseline measurement at 80 mM.
Discussion
The present study examined the role of endogenously formed 20-HETE on myogenic and TGF responses in isolated perfused Af-Art in the absence of confounding systemic hemodynamic, neural and hormonal influences. Studies were done using Af-Art isolated from two different species to ensure that the response was not species dependent. Rabbits were used since it is possible to microdissect Af-Art with an attached glomeruli, macula densa, and distal tubule to directly study TGF responses. Additional studies were performed using Af-Art isolated from the kidney of mice since recent studies have suggested that the development of hypertension in CYP4A14 KO mice may be due to 20-HETE dependent increase in renal vascular tone [18, 20]. However, no direct studies have been performed to determine the role of endogenously-formed 20-HETE in the regulation of myogenic tone in isolated perfused mouse Af-Art. Similar to what has been reported in renal microvessels isolated from the kidneys of rats [8], we demonstrated that 20-HETE is produced by rabbit renal microvessels and it can be selectively reduced by administration of HET0016.
In the present study, blockade of the synthesis of 20-HETE with HET0016 completely eliminated the myogenic response of isolated perfused Af-Art of both mice and rabbits. Similar results were obtained using 6, 15-20-HEDE, a 20-HETE antagonist that has been reported to block the vasoconstrictor actions of 20-HETE. The effects of HET0016 were reversed by exogenous administration of a 20-HETE mimetic, 5, 14-20-HEDE in the Af-Art of rabbits and mice, suggesting that endogenously formed 20-HETE plays a role in mediating the myogenic response in both of these species. However, there is some evidence that 20-HETE can be converted by cyclooxygenase to a vasodilator in the isolated perfused rabbit kidney [27]. Since, 5, 14-20-HEDE is not a substrate for cyclooxygenase, we also studied the ability of authentic 20-HETE to alter the myogenic response of the Af-Art of the rabbit. We found that 20-HETE added to the vascular perfusate had a similar effect as 5, 14-20-HEDE to restore pressure-dependent constriction in rabbit Af-Art treated with HET0016. These results further support our conclusion that endogenously formed 20-HETE plays an important role in the regulation of myogenic tone in the Af-Art of both rabbits and mice, and that blockade of the myogenic response following administration of HET0016 is due to a reduction in the production of 20-HETE. These findings are entirely consistent with previous results indicating that blockade of the synthesis of 20-HETE and EETs with 17-ODYA attenuated pressure induced constriction of the Af-Art of rats using the isolated perfused juxtamedullary preparation [10]. However, it is difficult to determine whether the changes in the response seen using this preparation are due to blockade of endogenous 20-HETE formation in the Af-Art or due to some paracrine effect secondary to reduced formation of 20-HETE in the surrounding tubules.
We also examined whether 20-HETE plays a role in mediating TGF using dual perfusion of the distal nephron and Af-Art of the rabbit. A role of 20-HETE in TGF was first suggested by Zou and colleagues [12] in which they reported that perfusion of the loop of Henle of rats in vivo with AA potentiated, while CYP inhibitors attenuated the TGF response. However, it was not possible in these studies to determine whether these effects were due to an inhibitory action of 20-HETE on sodium reabsorption in the macula densa and changes in the release of the vasoconstrictor mediator of TGF or whether AA and the cytochrome P450 inhibitors diffused across the macula densa to the Af-Art to alter 20-HETE production and vascular tone. The results of the present study confirm that administration of a 20-HETE agonist to the tubular perfusate potentiates the TGF-mediated constriction of the Af-Art and that this effect can be blocked by perfusion of the lumen of the Af-Art with a 20-HETE antagonist. Moreover, administration of the 20-HETE antagonist to the Af-Art alone, blocked TGF-mediated constriction. These findings suggest that the potentiation of the TGF response seen after addition of the 20-HETE agonist to the tubular perfusate is likely is due to diffusion across the macula densa to the Af-Art where it enhances the vasoconstrictor response to an elevation in sodium concentration delivered to the macula densa.
The mechanism by which 20-HETE enhances myogenic and TGF responsiveness of the Af-Art remains to be determined. Previous studies have indicated that increases in transmural pressure can enhance the endogenous production of 20-HETE in rat middle cerebral arteries and that blockade of the formation of 20-HETE attenuates the myogenic response in these vessels [9]. 20-HETE is a potent constrictor of renal and cerebral arteries including the Af-Art [1, 2, 28]. It blocks the BK channels which depolarizes vascular smooth muscle cells and enhances Ca2+ entry through voltage sensitive Ca2+ channels [29–32]. In this way it plays a permissive role in the myogenic response in vessels that produce 20-HETE. It also is thought to potentiate the effects of other vasoconstrictors, such as angiotensin [33], serotonin [34], vasopressin [35] and adenosine that activate G-protein coupled receptors to promote release of intracellular Ca2+. The rise in intracellular Ca2+ then activates Ca2+ sensitive phospholipases in vascular smooth muscle cells to release AA and increase the formation of 20-HETE. 20-HETE blocks activation of BK channels, which normally hyperpolarize VSM and limit Ca2+ entry through voltage sensitive Ca2+ channels [29, 31, 36].
The significance of the present study is that it now establishes in 2 additional species that the endogenous formation of 20-HETE in the renal microcirculation plays a key role in the modulation of both myogenic and TGF mediated regulation of Af-Art tone. Up-regulation of the formation of 20-HETE in the renal microcirculation will enhance the resistance of the Af-Art and myogenic and TGF responsiveness, leading to reductions in glomerular capillary pressure and GFR. Furthermore, it will also lower pressures in the post-glomerular circulation (peritubular capillaries and vasa recta) that determine renal interstitial pressure which determines the pressure natriuresis relationship [13]. Thus, upregulation of 20-HETE production in the Af-Art should promote sodium retention and the development of hypertension by decreasing GFR and resetting the pressure natriuresis relationship. On the other hand, 20-HETE mediated elevations in renal vascular resistance would be expected to oppose transmission of systemic pressure to the glomerular circulation and the development of hypertension-induced glomerular injury. This view is consistent with previous results using the SHR model of hypertension which is associated with elevations in the production of 20-HETE in the renal microcirculation [37], elevated preglomerular vascular resistance [38] that is 20-HETE dependent [39], but is largely protected from hypertension-induced renal damage [40]. On the other hand, the production of 20-HETE in the renal microcirculation is reduced in Dahl salt-sensitive rats [41, 42], and they exhibit elevations in glomerular capillary pressures during the development of hypertension and rapidly develop severe glomerular injury. The formation of 20-HETE in the renal circulation is also reduced in both type I [43] and type II [44] diabetic animal models that exhibit hyperfiltration and develop glomerular disease. Overall, these findings suggest that genetic and/or dietary induced modulation of the expression of CYP enzymes in the renal microcirculation likely influences Af-Art tone, transmission of pressure to the glomerular and post-glomerular capillaries, and the development of hypertension and renal end organ damage.
Conclusion
These studies establish an important role of 20-HETE in modulating myogenic and TGF responses of the Af-Art. This may help explain how deficiencies in the renal formation of 20-HETE promote the development of glomerular disease in salt sensitive models of hypertension but protects against the development of renal injury in SHR and androgen induced forms of hypertension in which the formation of 20-HETE in the renal microcirculation is elevated.
Acknowledgements
The authors wish to thank Chris Purser for technical assistance in the LC/MS analysis of the metabolites of AA. This study was supported in part by grants H36279, 29587 86767, GM31278 from the NIH.
References
- 1.Ma YH, et al. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72(1):126–36. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 2.Harder DR, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266(5 Pt 2):H2098–107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 3.Lange A, et al. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272(43):27345–52. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 4.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol. 2002;137(8):1362–70. doi: 10.1038/sj.bjp.0704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muthalif MM, et al. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95(21):12701–6. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun CW, et al. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33(1 Pt 2):414–8. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 7.Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41(3 Pt 2):801–6. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- 8.Imig JD, et al. Formation and actions of 20-hydroxyeicosatetraenoic acid in rat renal arterioles. Am J Physiol. 1996;270(1 Pt 2):R217–27. doi: 10.1152/ajpregu.1996.270.1.R217. [DOI] [PubMed] [Google Scholar]
- 9.Gebremedhin D, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87(1):60–5. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 10.Imig JD, et al. Cytochrome P-450 inhibitors alter afferent arteriolar responses to elevations in pressure. Am J Physiol. 1994;266(5 Pt 2):H1879–85. doi: 10.1152/ajpheart.1994.266.5.H1879. [DOI] [PubMed] [Google Scholar]
- 11.Zou AP, et al. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol. 1994;266(2 Pt 2):F275–82. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 12.Zou AP, et al. Effect of P-450 omega-hydroxylase metabolites of arachidonic acid on tubuloglomerular feedback. Am J Physiol. 1994;266(6 Pt 2):F934–41. doi: 10.1152/ajprenal.1994.266.6.F934. [DOI] [PubMed] [Google Scholar]
- 13.Williams JM, et al. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49(3):687–94. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 14.Seki T, et al. Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull. 2005;28(9):1651–4. doi: 10.1248/bpb.28.1651. [DOI] [PubMed] [Google Scholar]
- 15.Sato M, et al. Discovery of a N'-hydroxyphenylformamidine derivative HET0016 as a potent and selective 20-HETE synthase inhibitor. Bioorg Med Chem Lett. 2001;11(23):2993–5. doi: 10.1016/s0960-894x(01)00614-x. [DOI] [PubMed] [Google Scholar]
- 16.Alonso-Galicia M, et al. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277(5 Pt 2):F790–6. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, et al. 20-hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem. 2003;11(13):2803–21. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 18.Holla VR, et al. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci USA. 2001;98(9):5211–6. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stec DE, et al. Distribution of cytochrome P-450 4A and 4F isoforms along the nephron in mice. Am J Physiol Renal Physiol. 2003;284(1):F95–102. doi: 10.1152/ajprenal.00132.2002. [DOI] [PubMed] [Google Scholar]
- 20.Fidelis P, et al. Renal function and vasomotor activity in mice lacking the Cyp4a14 gene. Exp Biol Med (Maywood) 2010;235(11):1365–74. doi: 10.1258/ebm.2010.009233. [DOI] [PubMed] [Google Scholar]
- 21.Ge Y, et al. Impaired myogenic constriction of the renal afferent arteriole in a mouse model of reduced betaENaC expression. Am J Physiol Renal Physiol. 2012;302(11):F1486–93. doi: 10.1152/ajprenal.00638.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harder DR, et al. Identification of a putative microvascular oxygen sensor. Circ Res. 1996;79(1):54–61. doi: 10.1161/01.res.79.1.54. [DOI] [PubMed] [Google Scholar]
- 23.Dunn KM, et al. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008;295(6):H2455–65. doi: 10.1152/ajpheart.00512.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JM, et al. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2012;302(10):R1209–18. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993;92(2):1093–8. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R, Pittner J, Persson AE. Changes of cell volume and nitric oxide concentration in macula densa cells caused by changes in luminal NaCl concentration. J Am Soc Nephrol. 2002;13(11):2688–96. doi: 10.1097/01.asn.0000033275.17169.67. [DOI] [PubMed] [Google Scholar]
- 27.Carroll MA, et al. Cyclooxygenase dependency of the renovascular actions of cytochrome P450-derived arachidonate metabolites. J Pharmacol Exp Ther. 1992;260(1):104–9. [PubMed] [Google Scholar]
- 28.Arima S, et al. 20-HETE requires increased vascular tone to constrict rabbit afferent arterioles. Hypertension. 1996;27(3 Pt 2):781–5. doi: 10.1161/01.hyp.27.3.781. [DOI] [PubMed] [Google Scholar]
- 29.Zou AP, et al. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270(5 Pt 2):F822–32. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 30.Roman RJ, Alonso-Galicia M. P-450 Eicosanoids: A Novel Signaling Pathway Regulating Renal Function. News Physiol Sci. 1999;14:238–242. doi: 10.1152/physiologyonline.1999.14.6.238. [DOI] [PubMed] [Google Scholar]
- 31.Harder DR, et al. Transduction of physical force by the vascular wall Role of phospholipase C and cytochrome P450 metabolites of arachidonic acid. Trends Cardiovasc Med. 1995;5(1):7–14. doi: 10.1016/1050-1738(94)00026-R. [DOI] [PubMed] [Google Scholar]
- 32.Zou AP, et al. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270(1 Pt 2):R228–37. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 33.Alonso-Galicia M, et al. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2002;283(1):R60–8. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 34.Alonso-Galicia M, et al. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke. 1999;30(12):2727–34. doi: 10.1161/01.str.30.12.2727. discussion 2734. [DOI] [PubMed] [Google Scholar]
- 35.Zhang F, et al. Modulation by 20-HETE of phenylephrine-induced mesenteric artery contraction in spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;38(6):1311–5. doi: 10.1161/hy1201.096116. [DOI] [PubMed] [Google Scholar]
- 36.Gebremedhin D, et al. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507(Pt 3):771–81. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sacerdoti D, et al. Renal cytochrome P-450-dependent metabolism of arachidonic acid in spontaneously hypertensive rats. Biochem Pharmacol. 1988;37(3):521–7. doi: 10.1016/0006-2952(88)90223-7. [DOI] [PubMed] [Google Scholar]
- 38.Gebremedhin D, et al. Role of cytochrome P-450 in elevating renal vascular tone in spontaneously hypertensive rats. J Vasc Res. 1993;30(1):53–60. doi: 10.1159/000158975. [DOI] [PubMed] [Google Scholar]
- 39.Imig JD, et al. Elevated renovascular tone in young spontaneously hypertensive rats. Role of cytochrome P-450. Hypertension. 1993;22(3):357–64. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 40.Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol. 1998;275(2 Pt 2):R426–38. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- 41.Williams JM, et al. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol Renal Physiol. 2008;295(6):F1764–77. doi: 10.1152/ajprenal.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33(1 Pt 2):419–23. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 43.Benter IF, et al. Inhibition of Ca2+/calmodulin-dependent protein kinase II, RAS-GTPase and 20-hydroxyeicosatetraenoic acid attenuates the development of diabetes-induced vascular dysfunction in the rat carotid artery. Pharmacol Res. 2005;52(3):252–7. doi: 10.1016/j.phrs.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Luo P, et al. Glomerular 20-HETE, EETs, and TGF-beta1 in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;296(3):F556–63. doi: 10.1152/ajprenal.90613.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]