Abstract
The susceptibility, but not the magnitude, of long-term depression (LTD) induced by hippocampal CA3-CA1 synaptic activity (synaptic-LTD) increases with advanced age. In contrast, the magnitude of LTD induced by pharmacological activation of CA3-CA1 group I metabotropic glutamate receptors (mGluRs) increases during aging. The present study examined the signaling pathways involved in induction of LTD and the interaction between paired-pulse low frequency stimulation (PP-LFS)-induced synaptic-LTD and group I mGluR selective agonist, (RS)-3,5-dihydroxyphenylglycine (DHPG, 100 µM)-induced DHPG-LTD in hippocampal slices obtained from aged (22–24 mo) male Fischer 344 rats. Prior induction of synaptic-LTD did not affect induction of DHPG-LTD; however, prior induction of the DHPG-LTD occluded synaptic-LTD suggesting that expression of DHPG-LTD may incorporate synaptic-LTD mechanisms. Application of individual antagonist for the group I mGluR (AIDA), the N-methyl-D-aspartate receptor (NMDAR) (AP-5), or L-type voltage-dependent Ca2+ channel (VDCC) (nifedipine) failed to block synaptic-LTD and any two antagonists severely impaired synaptic-LTD induction, indicating that activation of any two mechanisms is sufficient to induce synaptic-LTD in aged animals. For DHPG-LTD, AIDA blocked DHPG-LTD and individually applied NMDAR or VDCC attenuated but did not block DHPG-LTD, indicating that the magnitude of DHPG-LTD depends on all three mechanisms.
Keywords: Aging, calcium, mGluR, NMDA receptors, VDCC, DHPG, synaptic plasticity, long-term depression
1. Introduction
Long-term synaptic depression is a weakening of synaptic transmission and is thought to be involved in organizing neural circuits during development and the establishment and removal of memories in adults (Dumas, 2005). Induction of long-term depression (LTD) depends on a rise in intracellular Ca2+ during synaptic activity (Bear and Malenka, 1994; Christie et al., 1997; Foster, 1999; Foster and Kumar, 2002; Kumar and Foster, 2005; Mulkey and Malenka, 1992; Stanton, 1996). Furthermore, the pattern of synaptic activation can determine the contribution of various Ca2+ sources for LTD induction. Low frequency synaptic activity induces LTD through activation of N-methyl-D-aspartate receptors (NMDARs). Low frequency paired-pulse synaptic activity increases the involvement of non-NMDAR Ca2+ sources, in the induction of synaptic-LTD (Huber et al., 2000; Kemp and Bashir, 1997; Kemp and Bashir, 1999; Kemp et al., 2000). A developmental decline in the propensity for neocortical LTD is associated with a decline in NMDAR function (Dumas, 2005). In contrast, an age-related increase in susceptibility to LTD is observed in the hippocampus, despite decreasing NMDAR function (Bodhinathan et al., 2010a; Burke and Barnes, 2010; Kumar and Foster, 2013), due to increased contributions from L-type voltage-dependent Ca2+ channels (VDCCs) (Norris et al., 1998) and intracellular Ca2+ stores (Kumar and Foster, 2005).
LTD can also be induced pharmacologically through the activation of metabotropic glutamate receptors by the group 1 selective agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) (Huber et al., 2000; Huber et al., 2001; Ireland and Abraham, 2009; Izumi and Zorumski, 2012; Kumar and Foster, 2007b; Mockett et al., 2011; Palmer et al., 1997). The magnitude of DHPG-LTD has been reported to decrease during development (Kemp et al., 2000; Nosyreva and Huber, 2006; Overstreet et al., 1997) and increase with advanced age (Kumar and Foster, 2007b). In young animals, reports indicate that LTD induced by NMDAR or DHPG is mechanistically distinct at CA3-CA1 hippocampal synapses (Connelly et al., 2011; Fitzjohn et al., 1999; Huber et al., 2001; Izumi and Zorumski, 2012; Oliet et al., 1997; Palmer et al., 1997; Pavlov et al., 2004). However, others have employed NMDAR or mGluR antagonists to provide evidence for cross talk between induction mechanisms of these two forms of synaptic depression (Kumar and Foster, 2007b; Norris et al., 1996; Oliet et al., 1997; Palmer et al., 1997).
The current study was designed to specifically investigate the involvement of VDCCs, NMDARs, and mGluRs in the induction of synaptic-LTD induced by using 1 Hz paired-pulse electrical stimulation and LTD induced by bath application of DHPG in slices obtained from senescent animals. Prior induction of synaptic-LTD did not affect induction of DHPG-LTD; however, prior induction of the DHPG-LTD occluded synaptic-LTD suggesting that expression of DHPG-LTD may incorporate synaptic-LTD mechanisms. Application of individual antagonist for the group I mGluR (AIDA), the NMDAR (AP-5), or L-type VDCCs (nifedipine) failed to block synaptic-LTD and any two antagonists severely impaired synaptic-LTD induction, indicating that activation of any two mechanisms is sufficient to induce synaptic-LTD in aged animals. For DHPG-LTD, AIDA blocked DHPG-LTD and individually applied NMDAR or VDCC attenuated but did not block DHPG-LTD, indicating that the magnitude of DHPG-LTD depends on all three mechanisms.
2. Materials and Methods
2.1. Animals
Procedures involving animal subjects have been reviewed and approved by the Institutional Animal Care and Use Committee of University of Florida and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Fischer 344 aged (22–24 months) rats were group housed (2 per cage), maintained on a 12:12 hr light schedule, and provided ad lib access to food and water.
2.2. Hippocampal slice preparation
The methods for hippocampal slice preparation and recording have been published previously (Kumar, 2010; Kumar and Foster, 2007a; Kumar and Foster, 2004; Kumar and Foster, 2005; Kumar and Foster, 2007b; Kumar and Foster, 2013; Kumar et al., 2012; Kumar et al., 2007). Briefly, rats were anesthetized with halothane (Halocarbon Laboratories, River Edge, NJ) and swiftly decapitated. The brains were rapidly removed and the hippocampi were dissected. Hippocampal slices (~ 400 µm) were cut parallel to the alvear fibers using a tissue chopper. The slices were incubated in a holding chamber at room temperature containing artificial cerebrospinal fluid (ACSF) (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. Previous studies indicate that mGluR activation in the hippocampal region CA3 can induce a hyperexcitability of CA3 pyramidal cells (Cuellar et al., 2005; Tan et al., 2003; Young et al., 2004); the CA3 region was surgically removed to control the hyperexcitability. Thirty min before recording, 1–2 slices were transferred to a submersion recording chamber (Harvard Apparatus, Boston, MA) and held between two nylon nets. The chamber was continuously perfused with oxygenated (95% O25% CO2) ACSF at a flow rate of 2–3 ml/min. The pH and temperature were maintained at 7.4 and 30 ± 0.5°C respectively.
2.3. Electrophysiological recordings
Methods for electrophysiological recording of synaptic plasticity have previously been published (Kumar and Foster, 2007b; Norris et al., 1996). Briefly, extracellular field potentials from stratum radiatum of CA1 were recorded with glass micropipettes (4–6 MΩ) filled with recording medium (ACSF) and localized in the middle of stratum radiatum. Stimulating electrodes were located in stratum radiatum of area CA1 at or near the CA3 border or near the subiculum ~1 mm from the recording electrode. For synaptic-LTD experiments, two stimulating electrodes were placed in the stratum radiatum, on either side of the recording electrode. One stimulating electrode was employed to induce synaptic plasticity by pattern stimulation and the other stimulating electrode was used to monitor the stability of the recording conditions and to insure that synaptic changes were not due to changes in slice health or a mechanical shift in electrode position. For synaptic-LTD, only data from slices with a stable recording of the control pathway (<5% change over the entire recording period) were included in the analysis. Diphasic stimulus pulses (100 μsec, SD9 Stimulator, Grass Instrument Co, West Warwick, RI) were delivered to the Schaffer collateral commissural pathway (0.033 Hz) to evoke excitatory postsynaptic potential (EPSP). Aging is associated with a decrease in synaptic strength, which can vary across animals (Foster, 1999; Kumar and Foster, 2013). Furthermore, the induction of synaptic-LTD and metabotropic receptor mediated synaptic plasticity is dependent upon the level of postsynaptic depolarization (Hu et al., 2005; Kerr and Abraham, 1995; Oliet et al., 1997); therefore, in an attempt to control for possible differences in neuronal activity, the baseline stimulus intensity was adjusted to produce an EPSP of ~1 mV. A stable baseline response was collected for at least 10 min prior to experimental manipulations (drug application/pattern stimulation) and for 30–40 min following drug application/pattern stimulation. The signals were amplified, filtered between 1 Hz and 1 kHz and stored on computer disk for off-line analysis. Two cursors were placed around the initial descending phase of the waveform and the maximum slope (mV/ms) of the EPSP was determined by a computer algorithm that found the maximum change across all sets of 20 consecutively recorded points (20 kHz sampling rate) between the two cursors. Changes in transmission properties induced by application of drug or pattern stimulation were calculated as the percent change from the averaged baseline responses collected. For induction of synaptic-LTD, paired-pulse low frequency pattern stimulation (900 pulse pairs delivered at 1 Hz with a 50 ms inter-pulse interval, PP-LFS) was delivered to one stimulating electrode. For most studies of DHPG-LTD, a single stimulating electrode was employed and following collection of baseline data, DHPG (100 µM) was applied for 10 min and EPSP responses were collected for at least 40 min during DHPG washout. For studies examining the effect of synaptic activity on the induction of DHPG-LTD, two stimulating electrodes were placed in the stratum radiatum, on either side (~1 mm) of the recording electrode and the baseline synaptic activation was turned off to one pathway during bath application of DHPG (100 µM) and for 10 min during the initiation of DHPG washout.
2.4. Drugs
All drugs were bath applied by addition to the ACSF. DHPG and (RS)-1-Aminoindan-1,5-dicarboxylic acid (AIDA) were obtained from Tocris (Tocris Bioscience, Ellisville, MO). DHPG (100 µM), and AP-5 (Sigma, 100 µM) were dissolved directly in ACSF; nifedipine (Sigma, 10 µM) was initially dissolved in a small amount of dimethyl sulfoxide (DMSO) and diluted further by ACSF to a final DMSO concentration of 0.01%. AIDA (200 µM) was dissolved in 1.1eq. NaOH and diluted in ACSF. All antagonists were bath applied for 30–60 min before application of DHPG or pattern synaptic stimulation and had no noticeable effect on baseline synaptic transmission.
2.5. Statistical analysis
All statistical analyses were performed using StatView 5.0 (SAS Institute Inc, NC). Data are expressed as mean ± SEM and are expressed as percentage of baseline control responses (set at 100%). Paired Student t-tests were employed to determine whether changes in synaptic strength were different from baseline. Analysis of variance (ANOVA) was used to compare synaptic-LTD or DHPG-LTD across different conditions. Where stated, n represents the number of slices used in each set of experiment.
3. Results
3.1. Interaction between synaptic-LTD and DHPG-LTD during senescence
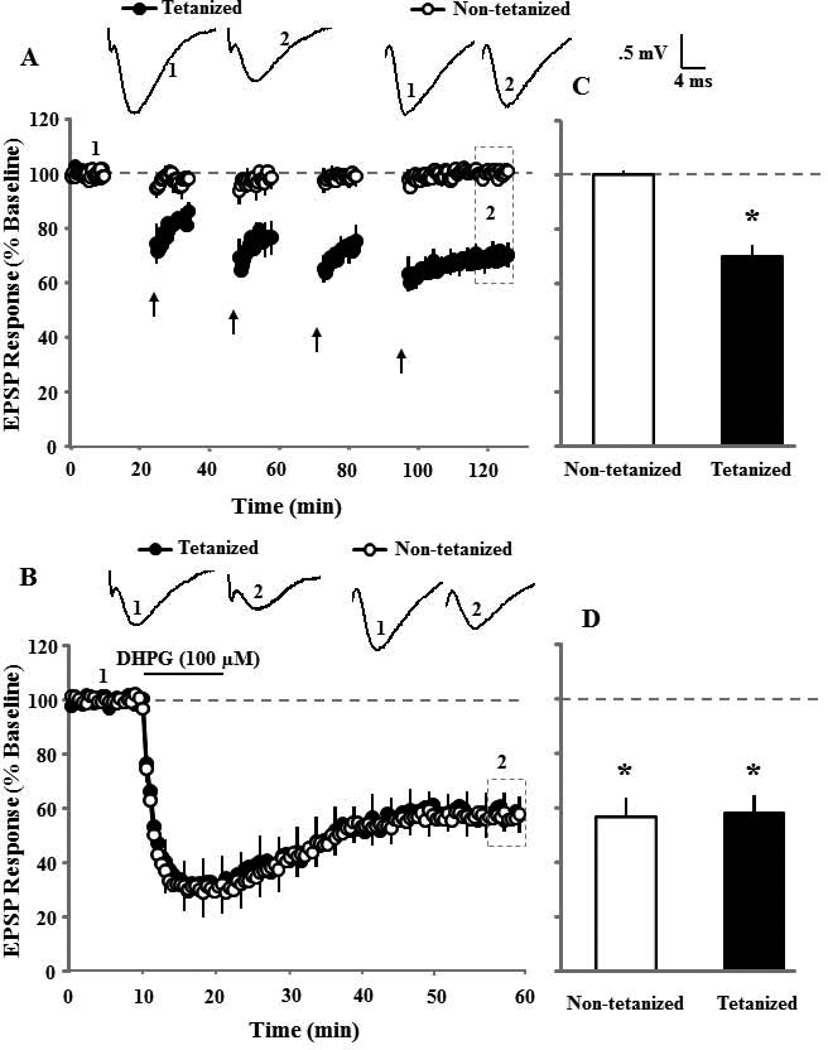
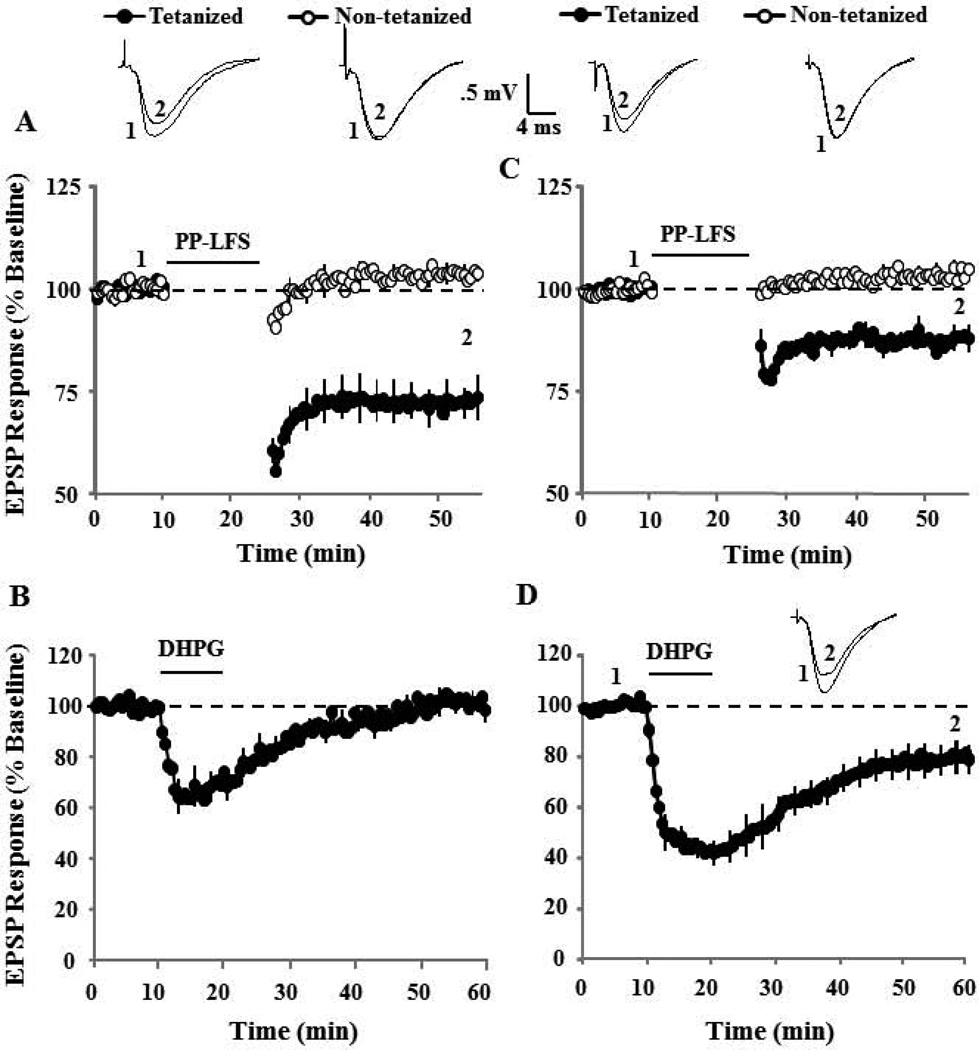
To test whether induction of synaptic-LTD interferes with subsequent induction of DHPG-LTD in aged animals, four 15 min episodes of 1 Hz paired-pulse low frequency stimulation (PP-LFS) (10 min between episodes) were delivered to induce asymptotic synaptic-LTD. Synaptic responses were recorded for 30 min following the last episode of PP-LFS. In addition, a control pathway that did not receive pattern stimulation was also recorded to insure specificity of synaptic changes (Fig 1A). The last 10 min of recording was used as a new baseline to examine the ability of bath application of DHPG (100 µM, 10 min) to induce DHPG-LTD. Figure 1B illustrates that DHPG application resulted in a similar pattern of synaptic change in the non-tetanized and previously tetanized pathways, starting with a transient decrease in the synaptic responses, which stabilized approximately 15 min after the start of DHPG washout (Fig 1B). The mean response, 30 min after induction of synaptic-LTD (Fig 1C) and DHPG-LTD (Fig 1D), were compared to the appropriate baseline. Pattern stimulation resulted in a significant (p < 0.005, n = 5) decrease in the synaptic response relative to the baseline only for the pathway that received PP-LFS (Fig 1C). The subsequent application of DHPG decreased the synaptic response in the pathway that received PP-LFS (p < 0.01, n = 5) and the control pathway (p < 0.005, n = 5). An ANOVA on the percent change in the synaptic response, 30 min following DHPG wash out, indicated no difference between the previously tetanized (54.6±9.0, n = 5) and non-tetanized (50.9±8.0, n = 5) pathways (Fig 1D).
Figure 1.
Synaptic-LTD induced by low frequency electrical stimulation does not inhibit subsequent induction of DHPG-LTD. A) Time course of synaptic responses during the 10 min baseline and 30 minutes following last episode of pattern stimulation for tetanized (filled circles, n = 5) and non-tetanized (open circles, n = 5) pathways. Four episodes of PP-LFS (arrows), with 10 min baseline recording between each episode, were employed to induce maximal synaptic-LTD. The inserts are representative traces of the EPSP responses from tetanized (left) and non-tetanized (right) pathway for the indicated time points. B) The synaptic response for the last 10 min of the 30 min synaptic-LTD recording was renormalized to obtain a new baseline (dashed line). Time course of synaptic responses are shown and illustrate the effect of DHPG (100 µM, for 10 min) application (solid line), on the previously tetanized (filled circles, n = 5) and non-tetanized (open circles, n = 5) pathways. Error bars equal SEM and alternate every five sweeps in this and in subsequent figures. The inserts are representative traces of the EPSP responses from tetanized (left) and non-tetanized (right) pathway for the indicated time points. Bar diagram showing the average magnitude of synaptic-LTD (C) and DHPG-LTD (D) during last 5 min of recording (dotted area in time course) for tetanized (filled bar) and non-tetanized (open bar) pathways. Asterisk indicates significant (p < 0.05) decrease in the response relative to the baseline (dashed line).
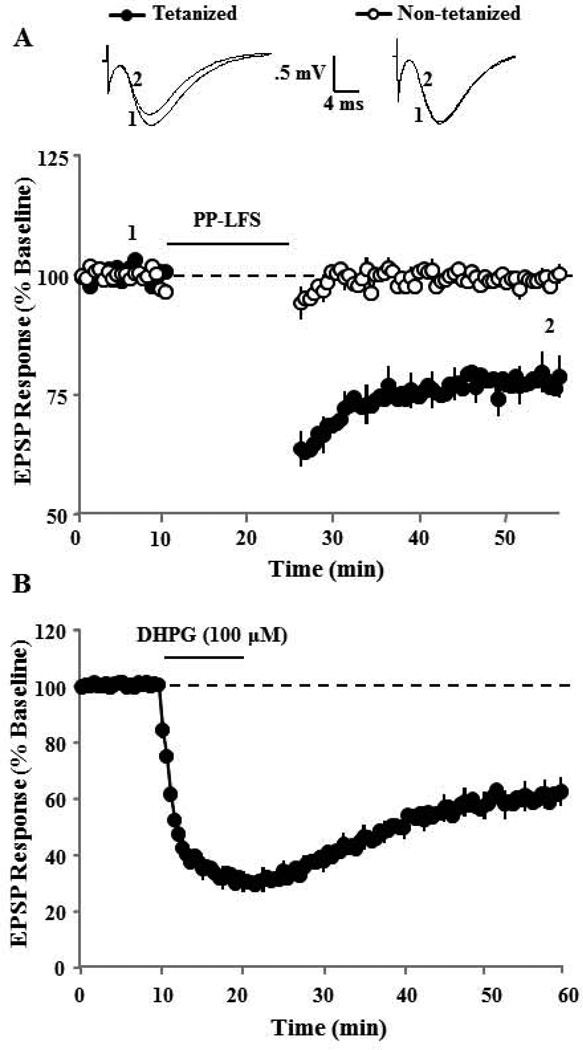
To determine if DHPG-LTD could occlude synaptic-LTD, the order of LTD-induction was reversed and synaptic-LTD was induced 30–40 min following bath application of DHPG (100 µM, 10 min). Application of DHPG (100 µM, 10 min) resulted in a significant (p < 0.01) decrease (63.3±9.2, n = 6) in the synaptic response recorded 30 min following DHPG washout (Fig 2A & C). The last 10 min of the DHPG-LTD recording was used as new baseline for examination of synaptic-LTD. A single PP-LFS episode was applied to induce synaptic-LTD. The PP-LFS failed to decrease the synaptic response (104.0±10.7, n = 6), indicating that prior induction of DHPG-LTD occluded induction of subsequent synaptic-LTD (Fig 2B & C). Together, these results indicate an interaction in the expression of these two forms of synaptic depression such that DHPG-LTD occludes synaptic-LTD, but synaptic-LTD does not necessarily obstruct DHPG-LTD.
Figure 2.
DHPG-LTD induced by bath application of DHPG occludes subsequent induction of synaptic-LTD. A) Time course of synaptic responses showing the effect of DHPG application (solid line, n = 6). B) Synaptic-LTD was induced by one episode of PP-LFS and responses were recorded for 30 min post tetanus. Prior induction of DHPG-LTD completely blocks the induction of synaptic-LTD. C) Bar diagram showing the average magnitude of DHPG-LTD and synaptic-LTD during last 5 min of recording (dotted area in time course A & B).
3.2. Role of Ca2+ sources in synaptic-LTD and DHPG-LTD during senescence
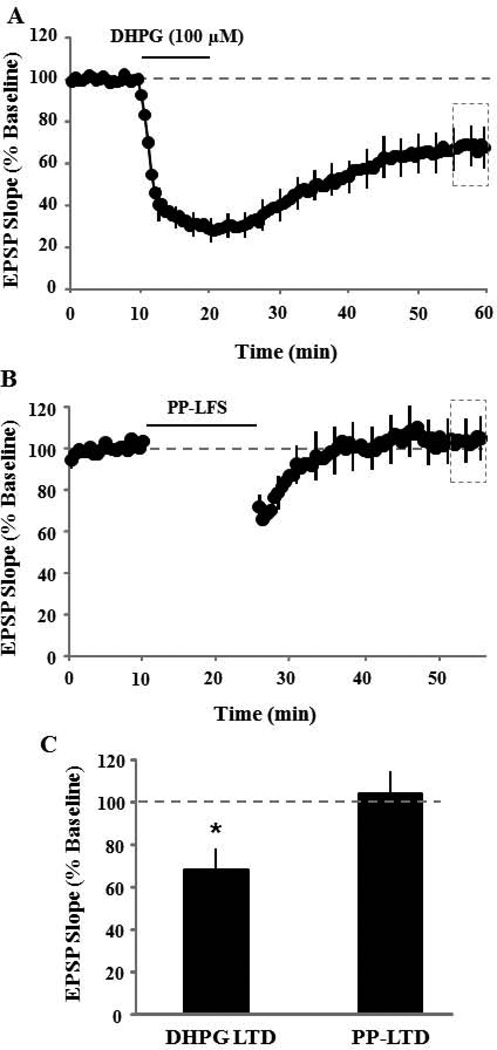
To determine the contribution of different Ca2+ sources to synaptic-LTD and DHPG-LTD in aged animals, mGluRs, NMDARs, or L-channels were blocked with AIDA (200 µM), AP-5 (100 µM), or nifedipine (10 µM), respectively. Following blockade, a single episode of PP-LFS was employed to induce synaptic-LTD or DHPG (100 µM, 10 min) was bath applied to induce DHPG-LTD. For comparison of drug effects with control conditions (Fig 7), synaptic-LTD or DHPG-LTD was examined in control slices, which were interleaved with drug treated slices. Pattern stimulation to induce synaptic-LTD in control slices (n = 18) resulted in a significant reduction in the synaptic response compared to baseline (77.8±3.8, p < 0.0001) (Fig 3A). Similarly, application of DHPG to induce DHPG-LTD in interleaved control slices (n = 17) resulted in a significant reduction in the synaptic response compared to baseline (61.3±4.9, p < 0.0001) (Fig 3B).
Figure 7.
Bar diagram showing mean summary of EPSP responses following induction of either synaptic-LTD (A) by 1 Hz PP pattern stimulation (n = 18) or DHPG-LTD (B) by bath application of DHPG (n = 17). Synaptic-LTD was recorded under control, AIDA, AP-5, nifedipine, AP-5 + nifedipine, AIDA + AP-5, and AIDA + nifedipine. Relative to control, synaptic-LTD was blocked by AP-5 + nifedipine (n = 6) and AIDA + nifedipine (n = 6). While relative to control, DHPG-LTD was blocked by pre-incubation with AIDA (n = 8); AP-5 (n = 6), nifedipine (n = 8), and AP-5 + nifedipine (n = 8) did not completely block but significantly reduced the DHPG-LTD. Asterisk indicates significant depression from baseline and pound sign indicates significant difference from the control level.
Figure 3.
Synaptic-LTD and DHPG-LTD in control slices. Control slices were interleaved with drug treated slices. A) Time course of changes in the slope of population EPSPs for the tetanized (filled symbol, n = 18) and non-tetanized (open symbols, n = 18) pathways after delivery of a single episode of PP-LFS (solid line). The inserts are representative traces of the EPSP responses from tetanized (left) and non-tetanized (right) pathway for the indicated time points. B) Time course of changes in the slope of population EPSPs for controls slices (n = 17) treated with DHPG (100 µM, solid line).
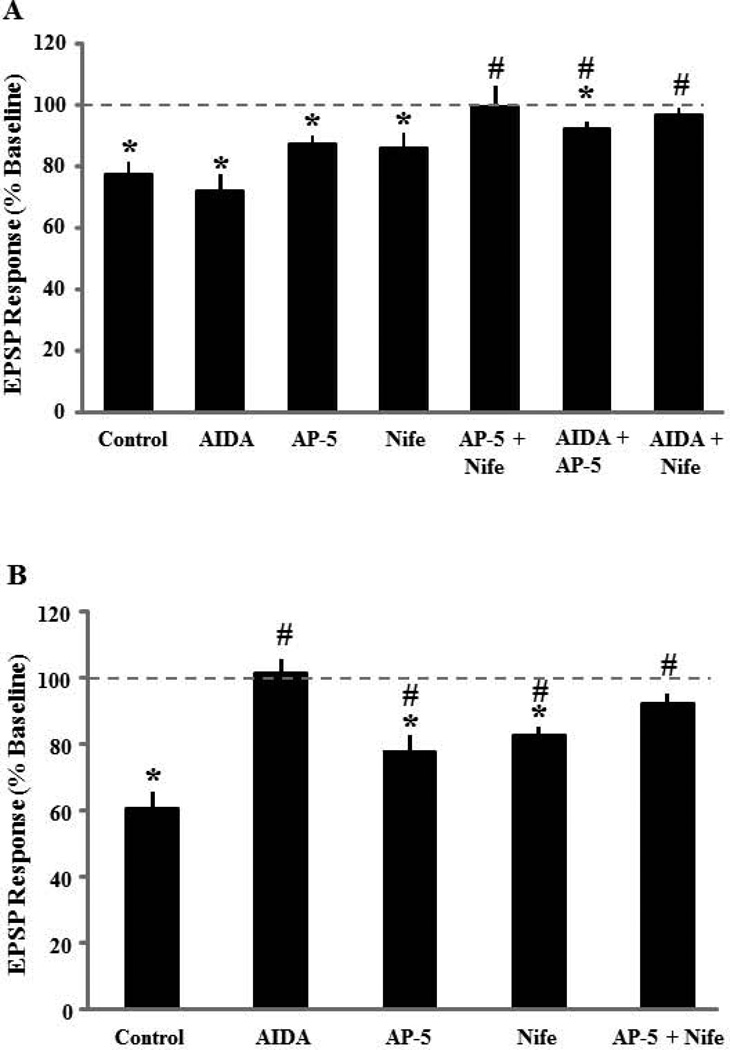
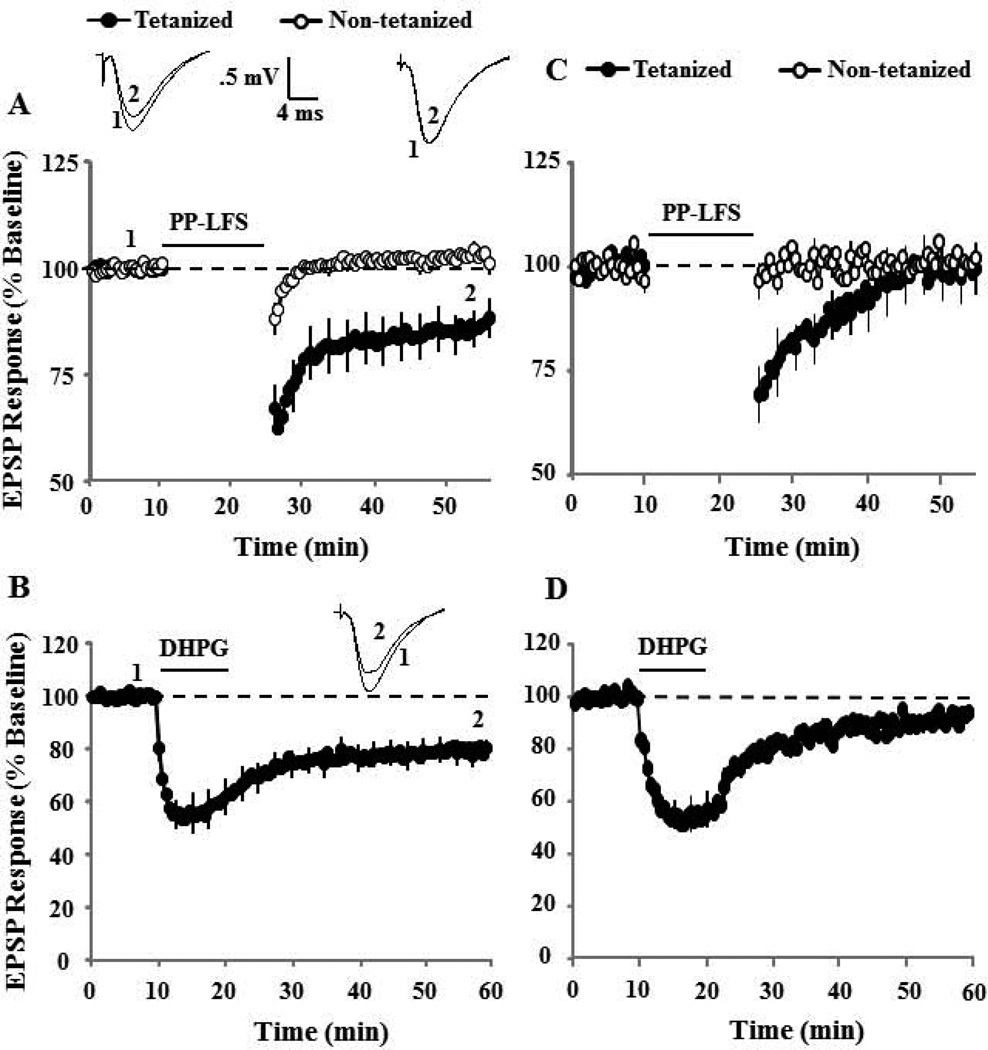
Robust synaptic-LTD could be induced in the presence of the group I mGluR antagonist, AIDA (p < 0.01 compared to baseline, n = 5) (Fig 4A). In contrast, prior application of AIDA blocked DHPG-LTD (101.3±4.1, n = 9) (Fig 4B). Next, we determine the effect of the NMDAR antagonist, AP-5 (100 µM) on synaptic-LTD and DHPG-LTD. While synaptic-LTD induced by pattern stimulation in presence of AP-5 was intermediate between baseline and controls (87.6±2.7, n = 5), the decrease in the synaptic response was different from the baseline (p < 0.01, Fig 4C). In the presence of AP-5, DHPG application (100 µM, 10 min) also induced significant DHPG-LTD (79.7±7.0, p < 0.01 relative to baseline, n = 8), which was intermediate between baseline and control DHPG-LTD (Fig 4D). Finally, the effect of L-channel blockade on synaptic and DHPG-LTD was similar to that observed for NMDAR blockade. Synaptic-LTD was induced in the presence of nifedipine (86.3±4.6, p < 0.05 relative to baseline, n = 9) (Fig 5A). Again, the response was intermediate between baseline and synaptic-LTD induced under control conditions. Bath application of DHPG also induced significant LTD in presence of nifedipine (79.8±4.2, p < 0.001 relative to baseline, n = 9), which was intermediate between baseline and control DHPG-LTD (Fig 5B).
Figure 4.
Effect of the group I mGluR antagonist, AIDA, and NMDAR antagonist, AP-5, on synaptic-LTD and DHPG-LTD. A) Time course of changes in the slope of population EPSPs for the tetanized (filled symbol) and non-tetanized (open symbols) pathways after delivery of PP-LFS (solid line) in presence of AIDA (200 µM, n = 5). The inserts are representative traces of the EPSP responses from tetanized (left) and non-tetanized (right) pathway for the indicated time points. B) Time course of changes in the synaptic response following bath application of DHPG (solid line) in the presence of AIDA (n = 9). The NMDAR antagonist, AP-5, does not block synaptic-LTD or DHPG-LTD. C) Time course of changes in the slope of population EPSPs for the tetanized (filled symbol) and control (open symbols) pathways in the presence of AP-5 (100 µM, n = 5). The synaptic response examined 30 min following PP-LFS (solid line) was different from the baseline (dashed line). The inserts are representative traces of the EPSP responses from tetanized (left) and control (right) pathway for the indicated time points. D) Time course of synaptic responses showing the effect of DHPG application (solid line), on the synaptic response in the presence of AP-5 (n = 8). AP-5 did not block DHPG-LTD, such that the synaptic response was decreased below baseline (dashed line) 30 min following the initiation of DHPG washout. The inserts are representative traces of the EPSP responses before (1) and after (2) DHPG application.
Figure 5.
The combination of NMDAR and L-type voltage gated Ca2+ channel antagonism blocks synaptic-LTD and DHPG-LTD. A) Time course of changes in the EPSP responses for the tetanized (filled symbol) and control (open symbols) pathways in the presence of nifedipine (10 µM, n = 9). The inserts are representative traces of the EPSP responses from tetanized (left) and control (right) pathway for the indicated time points. B) Time course of synaptic responses showing the effect of DHPG application (solid line), in the presence of nifedipine (n = 9). The inserts are representative traces of the EPSP responses before (1) and after (2) DHPG application. Blockade of NMDAR and L-type Ca2+ channels significantly attenuated synaptic and DHPG-LTD. C) Time course of changes in the EPSP responses for the tetanized (filled symbol) and control (open symbols) pathways in the presence of AP-5 + nifedipine (n = 6). D) Time course of synaptic responses showing the effect of DHPG application (solid line) in the presence of AP-5 + nifedipine (n = 8).
It appears that blockade of NMDAR or L-type channels may reduce, but does not block synaptic-LTD and DHPG-LTD. Therefore, we combined bath application of AP-5 (100 µM) and nifedipine (10 µM) in an attempt to completely block synaptic changes. AP-5 + nifedipine blocked synaptic-LTD (99.4±6.6, n = 6) and DHPG-LTD (91.6±4.2, n = 8) such that the synaptic response following pattern stimulation or bath application of DHPG was not different from the baseline (Fig 5C&D).
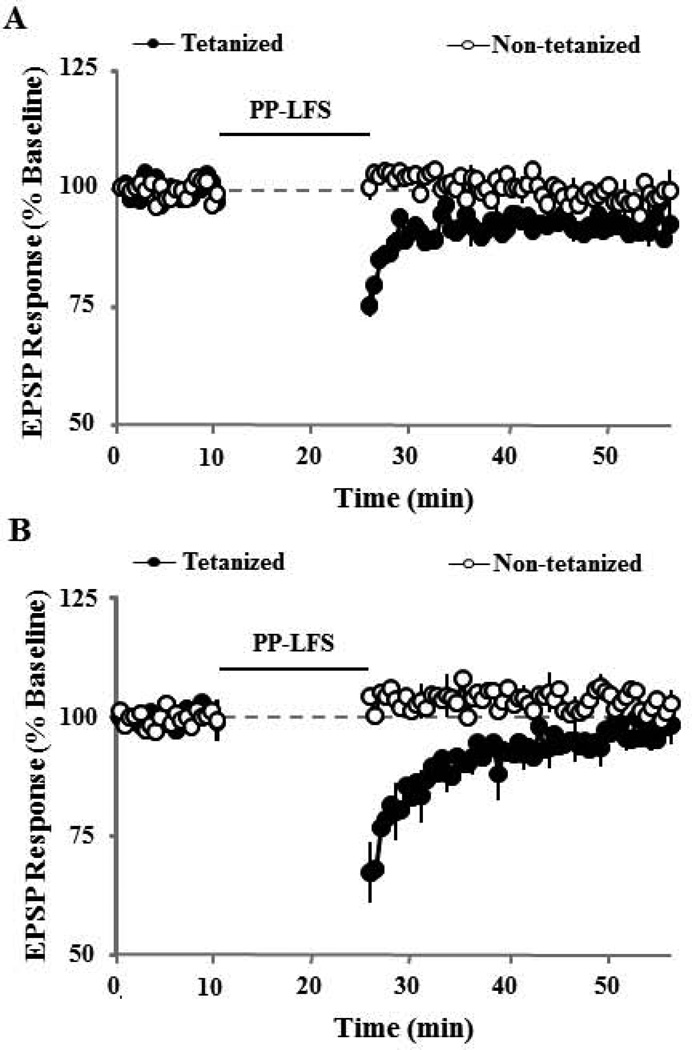
To investigate whether group I mGluR interacted with NMDAR and L-type channel activity to contribute to synaptic-LTD, we combined bath application of AIDA (200 µM) and AP-5 (100 µM) or nifedipine (10 µM). While the magnitude of synaptic-LTD induced by pattern stimulation in presence of AIDA+AP-5 exhibited a marked reduction (92.3±2.4, n = 5), the synaptic response was significantly different from the baseline (p < 0.03, Fig 6A). Interestingly, bath application of AIDA and nifedipine, blocked synaptic-LTD such that the synaptic response (97.0±2.2, n = 6) was not different from the baseline (Fig 6B). Because AIDA completely blocked DHPG-LTD, we did not examine the combined effects of AIDA and other antagonists on DHPG-LTD. The results indicate that the activity of group I mGluRs is required for DHPG induced LTD, but not for LTD induced by PP-LFS. Furthermore, NMDAR and L-type channel activity is not required for induction of synaptic-LTD or DHPG-LTD; however, these channels may contribute to the magnitude of LTD in aged animals, such that any two antagonists will attenuate either form of the LTD.
Figure 6.
Synaptic-LTD is blocked by co application group I mGluR antagonist, AIDA and the L-type voltage gated Ca2+ channel antagonist, nifedipine. Time course of changes in the slope of EPSPs for the tetanized (filled symbol) and control (open symbols) pathways in the presence of A) AIDA + AP-5 (n = 5) or B) AIDA + nifedipine (n = 6). The synaptic response examined 30 min following PP-LFS (solid line) in slices treated with AIDA and nifedipine was not different from the baseline (dashed line).
To examine whether drug treatments reduced the magnitude of LTD, an ANOVA was used to compare the level of LTD for the various drug treatments compared to LTD under control conditions. For synaptic-LTD, a significant effect of drug condition was observed [F(6,47) = 4.0, p < 0.005] and post hoc tests (p < 0.05) indicated that, compared to the control condition, synaptic-LTD was not affected by any single drug, but was reduced by the combination of AP5 + nifedipine, AIDA + AP5, or AIDA + nifedipine (Fig 7A). For DHPG-LTD, an ANOVA indicated a significant effect of drug treatment [F(4,45) = 13.5, p < 0.0001] and post hoc tests (p < 0.05) indicated that, compared to the control condition, AIDA, AP5, nifedipine, and the combination of AP5 + nifedipine reduced the level of DHPG-LTD (Fig 7B). Together, these results suggest that Ca2+ from multiple sources are involved in PP-LFS induced synaptic-LTD and Ca2+ from any two sources is sufficient to achieve a level of synaptic-LTD, which is similar to controls slices. In contrast, different Ca2+ sources contribute to the magnitude of DHPG-LTD, such that blockade of any source reduces DHPG-LTD and antagonism of any two sources completely blocked DHPG-LTD.
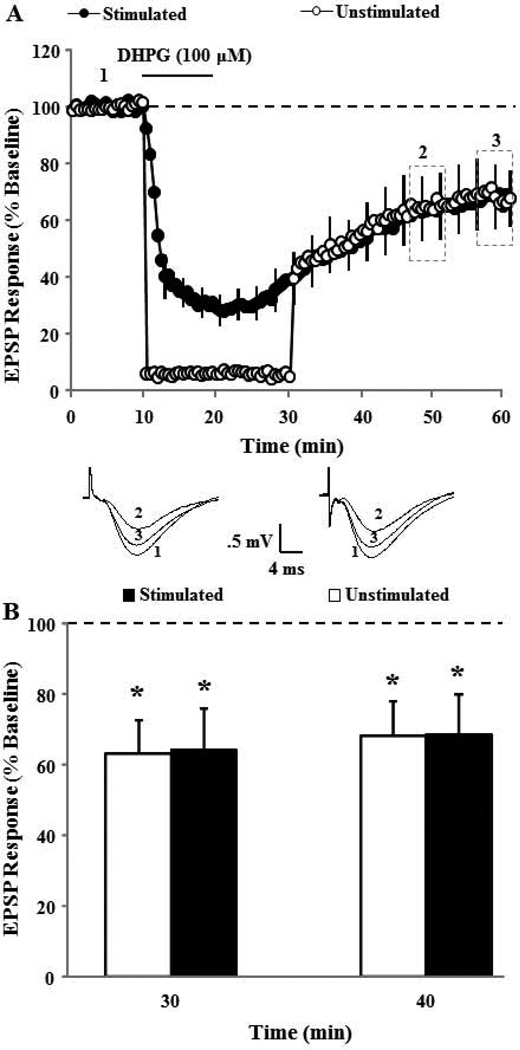
The ability of AP-5 and nifedipine to reduce DHPG-LTD in aged animals suggests a role for neural activity in determining the magnitude of DHPG-LTD. For all studies, region CA3 was surgically removed to control for synaptic activity induced by DHPG mediated hyperexcitability of CA3 pyramidal cells (Cuellar et al., 2005; Tan et al., 2003; Young et al., 2004). Nevertheless, to examine the possibility that neural activity due to the 0.033 Hz stimulation used to evoke field potentials for data collection contributed to DHPG-LTD, we examined the extent of DHPG-LTD induced by bath application of DHPG in the absence of synaptic stimulation. For this study, two pathways were recorded and the baseline synaptic activation was turned off to one pathway during bath application of DHPG (100 µM) and for 10 min during the initiation of DHPG washout. DHPG application induced significant DHPG-LTD in stimulated (70.9±10.1, p < 0.03, n = 6) and unstimulated (68.6±10.6, p < 0.03, n = 6) pathways when compared to baseline and no difference was observed in the magnitude of DHPG-LTD induced by DHPG between stimulated and unstimulated pathways (Fig 8A & B).
Figure 8.
DHPG-LTD does not require synaptic activation. A) Time course of changes in synaptic responses due to DHPG application for two pathways. DHPG induced a similar level of DHPG-LTD in the pathway that received continuous 0.033 Hz stimulation (Stimulated: filled circles, n = 6) during DHPG application and for the other pathway in which the basal stimulation was turned off during the 10 min of DHPG application and for the initial 10 min during drug washout (Unstimulated: open circles, n = 6). Inserts are representative traces of EPSP responses for the time points indicated in the figure for Stimulated (right) and Unstimulated (left) pathways. B) Bars show the mean+SEM DHPG-LTD at 30 and 40 min (dotted area in time course) after the start of DHPG washout for Stimulated (filled) and Unstimulated (open) pathways.
4. Discussion
Consistent with our previous research, maximal synaptic-LTD was ~75–80% of baseline (Foster and Kumar, 2007) and the magnitude of DHPG-LTD was greater, ~50–60% of baseline (Kumar and Foster, 2007b). The reduced magnitude for synaptic-LTD relative to DHPG-LTD is likely due to induction of synaptic-LTD of a subpopulation of synapses that experience suprathreshold activity during induction stimulation. Thus, we might expect synaptic-LTD to reduce rather than occlude DHPG-LTD, which likely effects a greater proportion of the population of synapses examined. However, prior induction of synaptic-LTD did not reduce subsequent DHPG-LTD in aged animals (Fig 1). Previously, researchers have suggested that the inability of synaptic-LTD to reduce DHPG-LTD in young animals may result from differences in expression mechanisms (Huber et al., 2001). Alternatively, if synaptic-LTD results in the complete silencing or elimination of a subpopulation of synapses then this subpopulation would not contribute to subsequent LTD induced by DHPG.
Induction of the DHPG-LTD occluded synaptic-LTD at senescent CA3-CA1 hippocampal synapses (Fig 2). The occlusion of synaptic-LTD may be related to the larger magnitude of DHPG-LTD. Synaptic-LTD can be observed when metabotropic receptor activation induces LTD that is only 80–90% of baseline (McCutchen et al., 2006; Wang et al., 2008). Together, these findings suggest that the induction of maximal DHPG-LTD may incorporate synaptic-LTD mechanisms, thus occluding subsequent induction of synaptic-LTD.
For synaptic-LTD, antagonism of any single Ca2+ source failed to impair induction of LTD. This result is different from previous work using 1 Hz LFS pattern stimulation, which demonstrated a diminished role of NMDARs with age (Norris et al., 1996), and a requirement for Ca2+ influx through VDCCs (Norris et al., 1998) and Ca2+ induced Ca2+ release from intracellular stores (Kumar and Foster, 2005). The PP-LFS paradigm employed in the current study is likely to have overcome the effects of blockade of any single Ca2+ source. For PP-LFS, blockade by any two antagonists prevented or reduced synaptic-LTD. The results provide some of the first evidence, in aged animals, that group I mGluRs contribute to VDCC and NMDAR mechanisms involved in synaptic-LTD. The role for group I mGluRs in synaptic-LTD is unknown; however, activation of group I mGluRs increases Ca2+ influx through NMDARs (Doherty et al., 2000; Fitzjohn et al., 1996; Harris et al., 2003; Mannaioni et al., 2001) and L-type Ca2+ channels (Bonsi et al., 2005; Derjean et al., 2005; Endoh, 2004; Heinke and Sandkuhler, 2005; Kreitzer and Malenka, 2005; Mao and Wang, 2002; Mao and Wang, 2003). In this way, group I mGluR activity during pattern stimulation may promote induction of synaptic-LTD by enhancing multiple Ca2+ influx pathways. Finally, it may be important that when AIDA and AP5 were applied, a small synaptic depression could still be induced, presumably due to the increased contribution of Ca2+ from VDCCs and intracellular stores in aged animals (Bodhinathan et al., 2010b; Kumar and Foster, 2005; Thibault and Landfield, 1996).
In contrast to the permissive nature of group I mGluR activation in synaptic-LTD, mGluRs are critical for DHPG-LTD. Antagonists of VDCCs or NMDARs, which individually had no effect on synaptic-LTD, reduced the magnitude of DHPG-LTD (Kumar and Foster, 2007b). Moreover, the combination of VDCC and NMDAR blockade, which prevented synaptic-LTD, diminished but did not block DHPG-LTD. It is possible that DHPG-LTD under VDCC and NMDAR blockade was due to Ca2+ from internal stores, since DHPG-mediated Ca2+ release from this sources tends to increase in aged animals (McQuail et al., 2013). Together, the results indicate that the magnitude of DHPG-LTD in aged animals is a function of the combination of Ca2+ sources.
Previous research indicates that an age-related dysregulation of Ca2+ homeostasis involving an increase in Ca2+ from VDCCs and intracellular Ca2+ stores mediates the increased susceptibility for induction of synaptic-LTD. Furthermore, Ca2+ dysregulation and susceptibility to synaptic-LTD are associated with impaired memory (Foster, 2012; Foster and Kumar, 2007). The current study indicates that various Ca2+ sources contribute to the magnitude of DHPG-LTD, such that blockade of any single source reduces, but does not block DHPG-LTD. The results suggest that the increased magnitude of DHPG-LTD observed during aging may also be related to the extent of Ca2+ dysregulation (Kumar and Foster, 2007b). Thus, we might expect that the magnitude of DHPG-LTD under NMDAR blockade will be related to the increase in Ca2+ from VDCCs and intracellular Ca2+ stores, and possibly to impaired memory.
Acknowledgements
This work was supported by, National Institutes of Health Grants, AG037984 and AG036800, and the Evelyn F. McKnight Brain Research Grant.
References
- Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4(3):389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular Redox State Alters NMDA Receptor Response during Aging through Ca2+/Calmodulin-Dependent Protein Kinase II. J Neurosci. 2010a;30(5):1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: role for ryanodine receptor mediated calcium signaling. J Neurophysiol. 2010b;104(5):2586–2593. doi: 10.1152/jn.00577.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsi P, Cuomo D, De Persis C, Centonze D, Bernardi G, Calabresi P, Pisani A. Modulatory action of metabotropic glutamate receptor (mGluR) 5 on mGluR1 function in striatal cholinergic interneurons. Neuropharmacology. 2005;49(Suppl 1):104–113. doi: 10.1016/j.neuropharm.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33(3):153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Schexnayder LK, Johnston D. Contribution of voltage-gated Ca2+ channels to homosynaptic long-term depression in the CA1 region in vitro. J Neurophysiol. 1997;77(3):1651–1655. doi: 10.1152/jn.1997.77.3.1651. [DOI] [PubMed] [Google Scholar]
- Connelly T, Fan Y, Schulz PE. Distinct mechanisms contribute to agonist and synaptically induced metabotropic glutamate receptor long-term depression. Eur J Pharmacol. 2011;667(1–3):160–168. doi: 10.1016/j.ejphar.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Cuellar JC, Griffith EL, Merlin LR. Contrasting roles of protein kinase C in induction versus suppression of group I mGluR-mediated epileptogenesis in vitro. J Neurophysiol. 2005;94(5):3643–3647. doi: 10.1152/jn.00548.2005. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Plateau potentials and membrane oscillations in parasympathetic preganglionic neurones and intermediolateral neurones in the rat lumbosacral spinal cord. J Physiol. 2005;563(Pt 2):583–596. doi: 10.1113/jphysiol.2004.076802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty AJ, Palmer MJ, Bortolotto ZA, Hargreaves A, Kingston AE, Ornstein PL, Schoepp DD, Lodge D, Collingridge GL. A novel, competitive mGlu(5) receptor antagonist (LY344545) blocks DHPG-induced potentiation of NMDA responses but not the induction of LTP in rat hippocampal slices. Br J Pharmacol. 2000;131(2):239–244. doi: 10.1038/sj.bjp.0703574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Prog Neurobiol. 2005;76(3):189–211. doi: 10.1016/j.pneurobio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Endoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 2004;1024(1–2):212–224. doi: 10.1016/j.brainres.2004.07.074. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Irving AJ, Palmer MJ, Harvey J, Lodge D, Collingridge GL. Activation of group I mGluRs potentiates NMDA responses in rat hippocampal slices. Neurosci Lett. 1996;203(3):211–213. doi: 10.1016/0304-3940(96)12301-6. [DOI] [PubMed] [Google Scholar]
- Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacology. 1999;38(10):1577–1583. doi: 10.1016/s0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30(3):236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca(2)(+) channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96(3):283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Calcium dysregulation in the aging brain. Neuroscientist. 2002;8(4):297–301. doi: 10.1177/107385840200800404. [DOI] [PubMed] [Google Scholar]
- Foster TC, Kumar A. Susceptibility to induction of long-term depression is associated with impaired memory in aged Fischer 344 rats. Neurobiol Learn Mem. 2007;87(4):522–535. doi: 10.1016/j.nlm.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BR, Gibson DA, Prendergast MA, Blanchard JA, Holley RC, Hart SR, Scotland RL, Foster TC, Pedigo NW, Littleton JM. The neurotoxicity induced by ethanol withdrawal in mature organotypic hippocampal slices might involve cross-talk between metabotropic glutamate type 5 receptors and N-methyl-D-aspartate receptors. Alcohol Clin Exp Res. 2003;27(11):1724–1735. doi: 10.1097/01.ALC.0000093601.33119.E3. [DOI] [PubMed] [Google Scholar]
- Heinke B, Sandkuhler J. Signal transduction pathways of group I metabotropic glutamate receptor-induced long-term depression at sensory spinal synapses. Pain. 2005;118(1–2):145–154. doi: 10.1016/j.pain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Hu B, Karnup S, Zhou L, Stelzer A. Reversal of hippocampal LTP by spontaneous seizure-like activity: role of group I mGluR and cell depolarization. J Neurophysiol. 2005;93(1):316–336. doi: 10.1152/jn.00172.2004. [DOI] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288(5469):1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86(1):321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Ireland DR, Abraham WC. Mechanisms of group I mGluR-dependent long-term depression of NMDA receptor-mediated transmission at Schaffer collateral-CA1 synapses. J Neurophysiol. 2009;101(3):1375–1385. doi: 10.1152/jn.90643.2008. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. NMDA receptors, mGluR5, and endocannabinoids are involved in a cascade leading to hippocampal long-term depression. Neuropsychopharmacology. 2012;37(3):609–617. doi: 10.1038/npp.2011.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. NMDA receptor-dependent and -independent long-term depression in the CA1 region of the adult rat hippocampus in vitro. Neuropharmacology. 1997;36(3):397–399. doi: 10.1016/s0028-3908(96)90015-5. [DOI] [PubMed] [Google Scholar]
- Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38(4):495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- Kemp N, McQueen J, Faulkes S, Bashir ZI. Different forms of LTD in the CA1 region of the hippocampus: role of age and stimulus protocol. Eur J Neurosci. 2000;12(1):360–366. doi: 10.1046/j.1460-9568.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- Kerr DS, Abraham WC. Cooperative interactions among afferents govern the induction of homosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci U S A. 1995;92(25):11637–11641. doi: 10.1073/pnas.92.25.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Dopamine modulation of state-dependent endocannabinoid release and long-term depression in the striatum. J Neurosci. 2005;25(45):10537–10545. doi: 10.1523/JNEUROSCI.2959-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. Carbachol-Induced Long-Term Synaptic Depression Is Enhanced During Senescence at Hippocampal CA3-CA1 Synapses. J Neurophysiol. 2010;104(2):607–616. doi: 10.1152/jn.00278.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster T. Environmental enrichment decreases the afterhyperpolarization in senescent rats. Brain Res. 2007a;1130(1):103–107. doi: 10.1016/j.brainres.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol. 2004;91(6):2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Intracellular calcium stores contribute to increased susceptibility to LTD induction during aging. Brain Res. 2005;1031(1):125–128. doi: 10.1016/j.brainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Shift in induction mechanisms underlies an age-dependent increase in DHPG-induced synaptic depression at CA3 CA1 cynapses. J Neurophysiol. 2007b;98(5):2729–2736. doi: 10.1152/jn.00514.2007. [DOI] [PubMed] [Google Scholar]
- Kumar A, Foster TC. Linking Redox Regulation of NMDAR Synaptic Function to Cognitive Decline during Aging. J Neurosci. 2013;33(40):15710–15715. doi: 10.1523/JNEUROSCI.2176-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, Foster TC. Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiol Aging. 2012;33(4):828, e1–e17. doi: 10.1016/j.neurobiolaging.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging Effects on the Limits and Stability of Long-Term Synaptic Potentiation and Depression in Rat Hippocampal Area CA1. J Neurophysiol. 2007;98(2):594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21(16):5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Glutamate cascade to cAMP response element-binding protein phosphorylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors. Mol Pharmacol. 2002;62(3):473–484. doi: 10.1124/mol.62.3.473. [DOI] [PubMed] [Google Scholar]
- Mao L, Wang JQ. Group I metabotropic glutamate receptor-mediated calcium signalling and immediate early gene expression in cultured rat striatal neurons. Eur J Neurosci. 2003;17(4):741–750. doi: 10.1046/j.1460-9568.2003.02495.x. [DOI] [PubMed] [Google Scholar]
- McCutchen E, Scheiderer CL, Dobrunz LE, McMahon LL. Coexistence of muscarinic long-term depression with electrically induced long-term potentiation and depression at CA3-CA1 synapses. J Neurophysiol. 2006;96(6):3114–3121. doi: 10.1152/jn.00144.2006. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Davis KN, Miller F, Hampson RE, Deadwyler SA, Howlett AC, Nicolle MM. Hippocampal Galphaq/(1)(1) but not Galphao-coupled receptors are altered in aging. Neuropharmacology. 2013;70:63–73. doi: 10.1016/j.neuropharm.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockett BG, Guevremont D, Wutte M, Hulme SR, Williams JM, Abraham WC. Calcium/Calmodulin-Dependent Protein Kinase II Mediates Group I Metabotropic Glutamate Receptor-Dependent Protein Synthesis and Long-Term Depression in Rat Hippocampus. J Neurosci. 2011;31(20):7380–7391. doi: 10.1523/JNEUROSCI.6656-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18(9):3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16(17):5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile X syndrome. J Neurophysiol. 2006;95(5):3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18(6):969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Overstreet LS, Pasternak JF, Colley PA, Slater NT, Trommer BL. Metabotropic glutamate receptor mediated long-term depression in developing hippocampus. Neuropharmacology. 1997;36(6):831–844. doi: 10.1016/s0028-3908(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Irving AJ, Seabrook GR, Jane DE, Collingridge GL. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36(11–12):1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Pavlov I, Riekki R, Taira T. Synergistic action of GABA-A and NMDA receptors in the induction of long-term depression in glutamatergic synapses in the newborn rat hippocampus. Eur J Neurosci. 2004;20(11):3019–3026. doi: 10.1111/j.1460-9568.2004.03806.x. [DOI] [PubMed] [Google Scholar]
- Stanton PK. LTD, LTP, the sliding threshold for long-term synaptic plasticity. Hippocampus. 1996;6(1):35–42. doi: 10.1002/(SICI)1098-1063(1996)6:1<35::AID-HIPO7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tan Y, Hori N, Carpenter DO. The mechanism of presynaptic long-term depression mediated by group I metabotropic glutamate receptors. Cell Mol Neurobiol. 2003;23(2):187–203. doi: 10.1023/A:1022949922364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272(5264):1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang Z, Shang J, Jiang ZZ, Wang S, Liu Y, Zhang LY. Activation of group I metabotropic glutamate receptors induces long-term depression in the hippocampal CA1 region of adult rats in vitro. Neurosci Res. 2008;62(1):43–50. doi: 10.1016/j.neures.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Young SR, Chuang SC, Wong RK. Modulation of afterpotentials and firing pattern in guinea pig CA3 neurones by group I metabotropic glutamate receptors. J Physiol. 2004;554(Pt 2):371–385. doi: 10.1113/jphysiol.2003.051847. [DOI] [PMC free article] [PubMed] [Google Scholar]