Abstract
Disturbed expression of microRNAs (miRNAs) in regulatory T cells (Tregs) leads to development of autoimmunity in experimental mouse models. However, the miRNA expression signature characterizing Tregs of autoimmune diseases, such as rheumatoid arthritis (RA) has not been determined yet. In this study, we have used a microarray approach to comprehensively analyze miRNA expression signatures of both naive Tregs (CD4+CD45RO-CD25++) and memory Tregs (CD4+CD45RO+CD25+++), as well as conventional naive (CD4+CD45RO−CD25−) and memory (CD4+CD45RO+CD25−) T cells (Tconvs) derived from peripheral blood of RA patients and matched healthy controls. Differential expression of selected miRNAs was validated by TaqMan-based quantitative reverse transcription-PCR. We found a positive correlation between increased expression of miR-451 in T cells of RA patients and disease activity score (DAS28), erythrocyte sedimentation rate levels and serum levels of interleukin-6. Moreover, we found characteristic, disease- and treatment-independent, global miRNA expression signatures defining naive Tregs, memory Tregs, naive Tconvs and memory Tconvs. The analysis allowed us to define miRNAs characteristic for a general naive phenotype (for example, miR-92a) and a general memory phenotype (for example, miR-21, miR-155). Importantly, the analysis allowed us to define miRNAs that are specifically expressed in both naive and memory Tregs, defining as such miRNA signature characterizing the Treg phenotype (that is, miR-146a, miR-3162, miR-1202, miR-1246 and miR-4281).
Keywords: Treg, miRNA, naive, memory, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic inflammation of synovial tissue that leads to damage of cartilage and bone, resulting in irreversible joint destruction. A number of genetic factors associated with RA suggest that deviant regulation of T cells might have an important role in the development and course of the disease.1, 2, 3 Moreover, despite the large numbers of regulatory T cells (Tregs) present in the synovial fluid of RA patients4, 5, 6 and despite the treatment, the inflammatory state of the joint persists. This suggests a potential functional defect in the Treg compartment or the resistance of effector T cells to Treg-mediated suppression.7
Tregs maintain immune homeostasis through suppression of excessive lymphocyte proliferation and cytokine production.8 Several studies have indicated that Tregs isolated from RA patients are functionally compromised and fail to suppress production of pro-inflammatory cytokines such as interleukin (IL)-17, interferon-γ and tumor necrosis factor (TNF)-α.9, 10, 11 Defective function of RA Tregs has been associated with exposure to elevated levels of pro-inflammatory cytokines in the synovial fluid of the inflamed joints. TNF-α, by itself, was shown to affect the transcriptional profile of protein-coding genes and the level of Foxp3 phosphorylation, leading to decreased suppressive function of Tregs.12, 13 In addition, increased TNF-α levels detected in synovial fluid of RA patients correlated positively with the deviant expression of microRNAs (miRNAs) in T cells,14 indicating that the pro-inflammatory cytokines affect T cells also at the post-transcriptional level.
MiRNAs are a class of small, 20–22 nucleotide long, noncoding RNA species that regulate gene expression at the post-transcriptional level. After loading into the RNA-induced silencing complex, miRNAs prevent translation or promote degradation of target mRNA by homologous binding to the 3′-untranslated region.15 Thus, miRNAs regulate protein expression and allow intricate fine tuning of cell biological processes. Over the last decade it has become clear that miRNAs regulate the expression of genes in fundamental biological processes related to development,16 proliferation17 and apoptosis18 as well as pathological processes such as cancer and autoimmunity.19, 20, 21 The decisive impact of miRNAs, on the functioning and lineage stability of T-cell subsets, including Tregs, has been reported in a number of mouse studies.21, 22, 23 Indeed, disruption of normal miRNA biogenesis in Tregs leads to breakdown of tolerance and shifts in the lineage specificity of Tregs in experimental model systems.24 A number of studies have recently shown differential expression of specific miRNAs in peripheral blood mononuclear cells, T-cells and tissues of human inflammatory diseases, including systemic lupus erythematosus and RA.14, 25, 26, 27, 28 However, the miRNA expression of peripheral blood Tregs and conventional T cells (Tconvs) of RA patients has not been reported yet. Miyara et al.29 have recently showed that, similar to Tconvs, Tregs can be subclassified into interrelated naive Tregs (that is, resting Tregs) and memory Tregs (that is, activated Tregs), with both overlapping and distinct phenotypic and functional characteristics. Importantly, such classification allows a precise delineation of Treg subpopulations and enables their efficient isolation.30
In this study, we set out to comprehensively analyze miRNA expression signatures of four functionally relevant and distinct CD4+ T-cell subsets (that is, naive Tregs, memory Tregs, naive Tconvs and memory Tconvs) derived from RA patients, before and after anti-TNF-α treatment, and from healthy donors. The global T-cell subset-specific miRNA expression patterns were not altered by either the disease state or the type of treatment received by RA patients. However, the expression of miR-451 correlated positively with the RA disease activity score (DAS28). Furthermore, the analysis of the defined T-cell subsets provided characteristic expression signatures of both naive and memory Treg and Tconv subsets.
Results
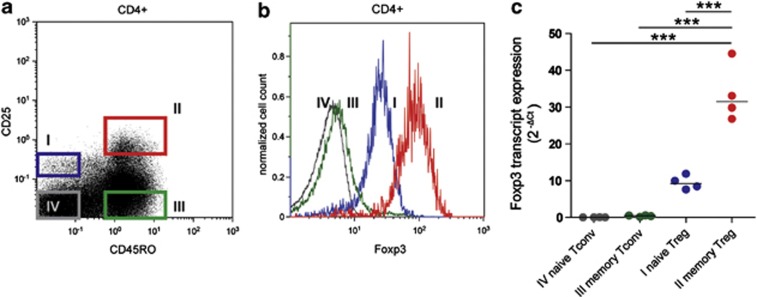
The global T-cell subset-specific miRNA expression patterns are not affected by RA or anti-inflammatory treatment
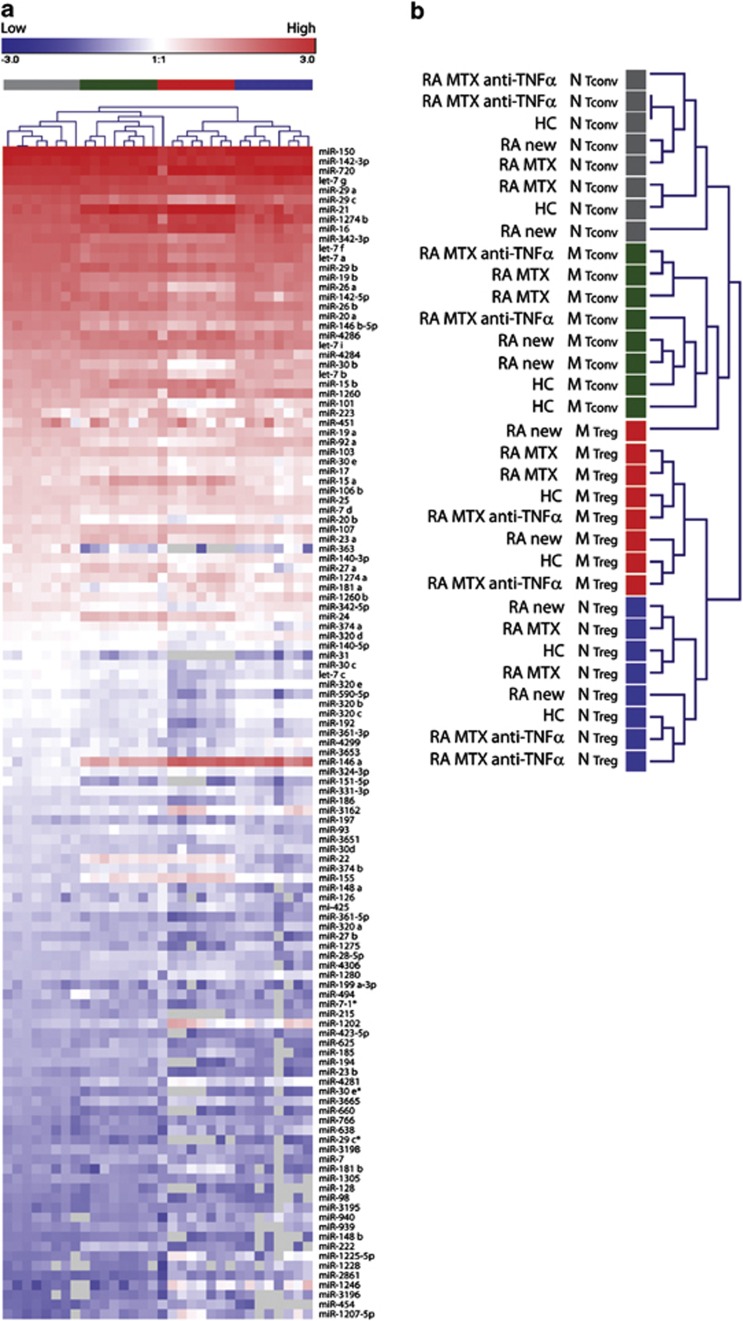
The miRNA signature of Tregs in RA was studied using a cohort of 27 RA patients fulfilling the 1987 ACR criteria for the classification of RA and six healthy controls (HC). The characteristics of all donors are summarized in Table 1. Possible confounding effects of disease modifying anti-rheumatic drugs on T cells were accounted for by including patients before and after the start of treatment with disease modifying anti-rheumatic drugs. Patients were further subdivided according to the type of treatment into two groups, that is, patients treated with methotrexate (MTX) alone, and patients treated with MTX and Adalimumab, an anti-TNF-α monoclonal antibody. CD4+ Tregs and Tconvs were isolated using the gating strategy devised by Miyara et al.,29 which allows subclassification of T cells into naive Tregs (CD45RO−CD25++Foxp3+), memory Tregs (CD45RO+CD25+++Foxp3++), naive Tconvs (CD45RO−CD25−Foxp3−) and memory Tconvs (CD45RO+CD25−Foxp3−), as depicted in Figure 1a. Differential expression of Foxp3 was assessed by fluorescence-activated cell sorting staining (Figure 1b) and confirmed in the sorted T-cell subsets by quantitative reverse transcription (qRT)-PCR (Figure 1c). The percentage of naive and memory Tregs, the expression level of Foxp3 transcript as well as the percentage and numbers of monocytes, natural killer cells, total T and B cells did not differ between HC and RA patient groups (Supplementary Figures 1A and B, and data not shown). The global miRNA expression patterns were determined in 24 subsets of six RA patients and in 8 subsets of two HC (Table 1, array) using the Agilent Human miRNA Microarray (Agilent Technologies, Santa Clara, CA, USA). One hundred and twenty-one human miRNAs passed the detection criteria and were used in the subsequent analysis. To determine whether or not the global miRNA expression patterns were affected by the disease or the type of treatment, we performed unsupervised hierarchical clustering of all samples. Clustering separated the samples, with one exception, into four phenotypic groups (naive Tconv, memory Tconv, memory Treg and naive Treg), irrespective of the disease or type of treatment, indicating that each T-cell subset is characterized by a very specific miRNA signature. No further biologically meaningful clustering pattern was observed. This indicates that the global miRNA expression patterns characterizing T-cell subsets are not affected by the disease or the anti-inflammatory treatment (Figures 2a and b).
Table 1. Donors' characteristics.
| Donors | Gender | Age (year) | DAS28 score | ESR (mm h−1) | ACPA (U ml−1) | RF (U ml−1) | Non steroid anti-inflammatory drugs | MTX (mg per week) | Anti-TNFα (mg per 2 weeks) | miRNA analysis type |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy controls | ||||||||||
| HC-2 | F | 44 | ND | ND | ND | ND | — | — | — | Array |
| HC-6b | F | 50 | ND | ND | ND | ND | — | — | — | Array |
| HC-1 | M | 45 | ND | ND | ND | ND | — | — | — | qRT–PCR validation |
| HC-3 | F | 52 | ND | ND | ND | ND | — | — | — | qRT–PCR validation |
| HC-5 | F | 53 | ND | ND | ND | ND | — | — | — | qRT–PCR validation |
| HC-7 | F | 48 | ND | ND | ND | ND | — | — | — | qRT–PCR validation |
| Newly diagnosed patients | ||||||||||
| RA new | ||||||||||
| RA-10 | M | 42 | 4.2 | 9 | 39 | 15 | Yes | — | — | Array |
| RA-27 | M | 50 | 2.9 | 15 | >340 | 650 | Yes | — | — | Array |
| RA-4 | M | 46 | 5.4 | 67 | 91 | 21 | Yes | — | — | qRT–PCR validation |
| RA-14 | F | 42 | 6.0 | 30 | 468 | 71 | Yes | — | — | qRT–PCR validation |
| RA-33 | F | 29 | 4.5 | 51 | >340 | 15 | Yes | — | — | qRT–PCR validation |
| RA-7 | M | 68 | 4.2 | 22 | >340 | 78 | Yes | — | — | qRT–PCR 2 |
| RA-12 | F | 43 | 3.8 | 11 | 43 | 196 | Yes | — | — | qRT–PCR 2 |
| RA-32 | F | 51 | 5.4 | 37 | 328 | 69 | Yes | — | — | qRT–PCR 2 |
| Treated patients | ||||||||||
| RA MTX | ||||||||||
| RA-15 | M | 45 | 1.3 | 3 | >340 | 53 | Yes | 25 | — | Array |
| RA-22 | F | 56 | 5.2 | 15 | >340 | 20 | Yes | 15 | — | Array |
| RA-1 | F | 60 | 5.3 | 20 | 0 | 0 | No | 15 | — | qRT–PCR validation |
| RA-9 | F | 80 | 4.0 | 33 | 0 | 0 | Yes | 20 | — | qRT–PCR validation |
| RA-21 | F | 54 | 2.8 | 38 | 107 | 71 | Yes | 15 | — | qRT–PCR validation |
| RA-26 | M | 67 | 3.4 | 11 | 0 | 16 | Yes | 15 | — | qRT–PCR validation |
| RA-13 | M | 50 | 3.9 | 6 | 0 | 0 | Yes | 25 | — | qRT–PCR 2 |
| RA-16 | F | 30 | 7.8 | 70 | 3 | 436 | Yes | 25 | — | qRT–PCR 2 |
| RA-18 | M | 50 | 3.7 | 3 | 1 | 13 | Yes | 25a | — | qRT–PCR 2 |
| RA-20 | F | 30 | 5.9 | 27 | 3 | 436 | Yes | 25a | — | qRT–PCR 2 |
| RA-23 | F | 48 | 3.1 | 13 | 34 | 31 | Yes | 25 | — | qRT–PCR 2 |
| RA MTX+anti-TNF-α | ||||||||||
| RA-8 | F | 41 | 2.7 | 5 | 15 | 224 | Yes | 15 | 40 | Array |
| RA-28 | F | 30 | 2.7 | 18 | 4 | 29 | Yes | 25 | 40 | Array |
| RA-2 | F | 62 | 2.7 | 12 | 42 | 33 | Yes | 15 | 40 | qRT–PCR validation |
| RA-29 | M | 59 | 2.7 | 6 | >340 | 590 | Yes | 10 | 40 | qRT–PCR validation |
| RA-30 | F | 55 | 2.8 | 24 | >340 | 593 | No | 20 | 40 | qRT–PCR validation |
| RA-3 | F | 61 | 2.6 | 18 | 1 | 54 | No | 15 | 40b | qRT–PCR 2 |
| RA-11 | M | 60 | 2.5 | 16 | >1000 | 331 | Yes | 12.5 | 40 | qRT–PCR 2 |
| RA-31 | F | 51 | 1.8 | 13 | >340 | 365 | No | — | 40 | qRT–PCR 2 |
Abbreviations: ACPA, anti-cyclic citrullinated peptide antibodies; DAS28, disease activity score; ESR, erythrocyte sedimentation rate; miRNA, microRNA; MTX, methotrexate; ND, not determined; RA, rheumatoid arthritis; RF, rheumatoid factor; TNF, tumor necrosis factor.
Per 6 months.
Per month.
Figure 1.
Purification of naive and memory Tconv and Treg populations. (a) Representative fluorescence-activated cell sorting (FACS) dot plot depicting the gating strategy for purification of CD4+, naive and memory Treg and Tconv subsets, based on the expression levels of CD45RO and CD25: I naive Tregs (CD45RO−CD25++), II memory Tregs (CD45RO+CD25+++), III memory Tconvs (CD45RO+CD25−) and IV naive Tconvs (CD45RO−CD25−). (b) Representative histogram depicting FACS analysis of Foxp3 protein expression in four CD4+ T-cell subsets. (c) Naive Tregs, memory Tregs, memory Tconvs and naive Tconvs were FACS purified from peripheral blood mononuclear cell (PBMC) of healthy donors, and Fosxp3 transcript levels were analyzed by qRT-PCR. Relative expression, normalized to the average of TATA box binding protein and RPII reference genes is shown. Lines indicate median expression values. Each dot represents a separate donor (n=4). Statistical analysis: repeated measures analysis of variance test with Bonferroni's multiple comparison post-test. ***P<0.0001.
Figure 2.
Unsupervised hierarchical clustering of globally expressed miRNAs. (a) MiRNA expression signatures characterizing Tconvs and Tregs were assessed in 32 samples derived from six RA patients and two HC (Table 1, array) using miRNA microarray. A heatmap of the 121 human miRNAs detected in at least 50% of samples, ranked according to their expression in naive Tconvs is shown. Top legend (red to blue) depicts range of log expression values. Gray coloring indicates expression below the detection limit. Similarities between samples were assessed by one-way unsupervised hierarchical clustering (Pearson's correlation, complete linkage) using the Genesis software. (b) Enlargement of the hierarchical tree from a. Color coding corresponds to the four T-cell phenotypes. HC, healthy control; RA new, RA patients before the start of treatment with disease modifying anti-rheumatic drugs; RA MTX, RA patients treated for 3 months with MTX alone; RA MTX anti-TNF-α, RA patients treated with MTX and anti-TNFα monoclonal antibody; N, naive; M, memory.
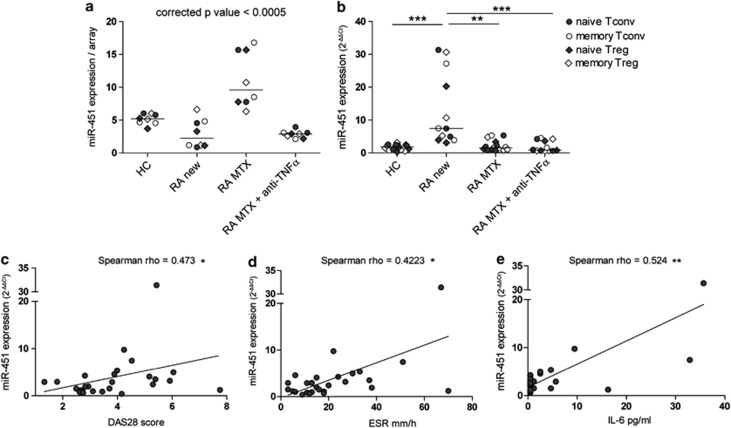
Expression of miR-451 in T cells positively correlates with the DAS28
To identify specific miRNAs that were differentially expressed as a result of disease characteristics or treatment, we performed an intergroup statistical analysis. From this analysis, miR-451 was identified to be significantly higher expressed in patients treated with MTX (RA MTX, fold difference ⩾2, corrected P-value <0.05), irrespective of the T-cell subset (Figure 3a). Surprisingly, qRT–PCR analysis performed in a new cohort of 10 RA patients and 4 HC (Table 1, qRT–PCR validation) identified miR-451 to be significantly higher expressed in a group of newly diagnosed, disease modifying anti-rheumatic drug-free RA patients (RA new; Figure 3b). This discrepancy prompted us to investigate whether the expression of miR-451 in T cells is related to the disease characteristics of individual patients in the cohorts included in the study. Indeed, analysis of 27 patients revealed that the expression of miR-451 correlated positively with DAS28 and, more specifically, with the erythrocyte sedimentation rate (ESR; Figures 3c and d). Correlations with anti-cyclic citrullinated peptide antibodies, rheumatoid factor level or age were not found (data not shown). Both DAS28 score and ESR have been previously correlated with the serum levels of pro-inflammatory cytokine IL-6.31 Moreover, IL-6 has been shown to induce expression of miR-451 in dendritic cells.32 To test whether an increased expression of miR-451 in T cells could be related to an increased presence of IL-6, we measured IL-6 levels in the sera of RA patients. Indeed, we found a positive correlation between the miR-451 expression in T cells and serum levels of IL-6 (Figure 3e), indicating that the inflammatory status of RA patients, resulting from an active disease, may be responsible for the observed increased expression of miR-451.
Figure 3.
Expression of miR-451 correlates with DAS28. (a) MiR-451 expression was assessed in Tconvs and Tregs, of six RA patients receiving different treatments, and two HC by miRNA microarray (Table 1, array). MiR-451 levels are higher in all T-cell subsets of patients treated with MTX. The horizontal lines indicate the median values. Statistical analysis: analysis of variance test with the Benjamini–Hochberg correction for multiple testing. (b) MiR-451 expression was assessed in Tconvs and Tregs in a new cohort of ten RA patients receiving different treatment and four HC by qRT-PCR (Table 1, qRT–PCR validation). Expression was normalized to the average of RNU44 and RNU48 reference genes and to a calibrator sample (RA-29 naive Tconv), which was set to 1. The horizontal lines indicate the median values. Statistical analysis: the Kruskal–Wallis test with Dunn's multiple comparison post-test. HC, healthy control; RA new, RA patients before the start of treatment with disease modifying anti-rheumatic drugs; RA MTX, RA patients treated for 3 months with MTX alone; RA MTX anti-TNFα, RA patients treated with MTX and anti-TNFα monoclonal antibody. (c–e) MiR-451 expression was assessed in naive Tconvs of 27 RA patients (Table 1, all patients) by qRT–PCR. Expression was normalized to the average of RNU44 and RNU48 reference genes and a calibrator sample (RA-29 naive Tconv), which was set to 1. Each dot represents one RA patient. (c) MiR-451 expression correlates positively with DAS28 in RA patients. (d) MiR-451 expression correlates positively with the erythrocytes sedimentation rate (ESR) in RA patients. (e) MiR-451 expression correlates positively with IL-6 levels detected in the serum of RA patients. *P<0.05, **P<0.01, ***P<0.0001.
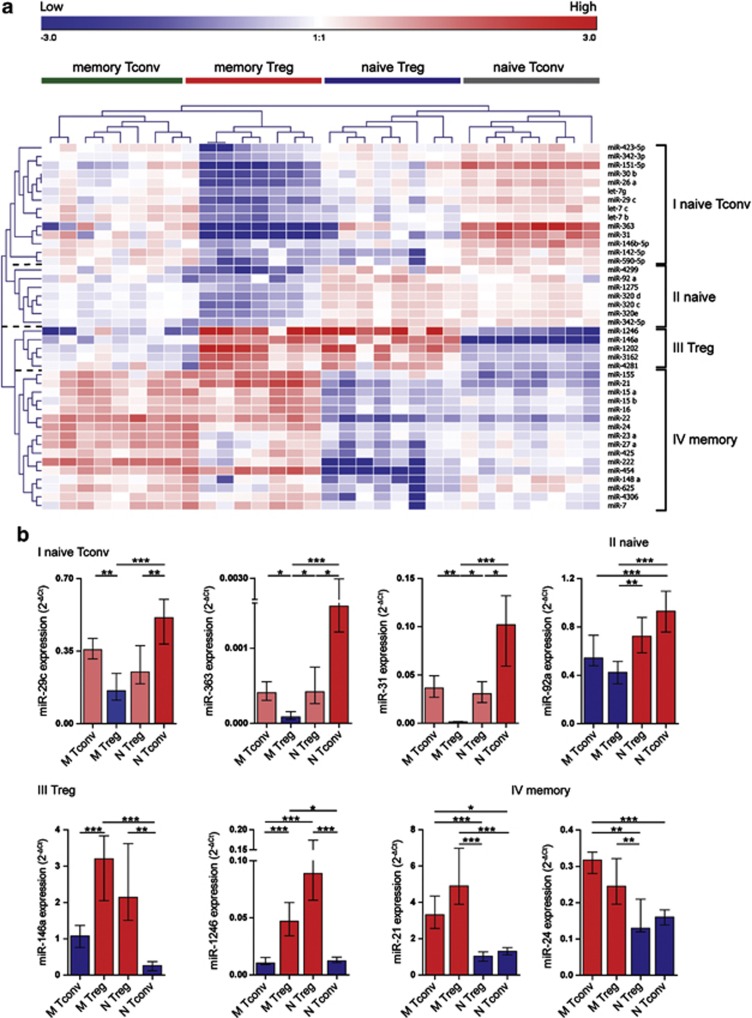
Naive and memory Treg subsets are characterized by distinct miRNA signatures
Unsupervised clustering separated both Treg and Tconv samples into either a naive or a memory phenotype, which was irrespective of disease characteristics or the type of treatment (Figure 2). To determine which miRNAs in particular were responsible for this clustering pattern, we performed statistical analysis between the four T-cell subsets across all eight donors. This resulted in the identification of 42 significantly differently expressed human miRNAs (Table 2, fold difference ⩾2, corrected P-value <0.005). Two-way unsupervised hierarchical clustering revealed four distinct miRNA clusters (I–IV) separating the samples into four cellular phenotypes (Figure 4a, and Table 2). MiRNAs defined in cluster I were overrepresented in naive Tconvs and under-represented in memory Tregs. MiRNAs defined in cluster II were overrepresented in naive T cells irrespective of CD25 expression. MiRNAs defined in cluster III were overrepresented in Tregs irrespective of naive or memory phenotype. MiRNAs defined in cluster IV were overrepresented in memory T cells irrespective of CD25 expression (Figure 4a). To validate these findings, we selected nine miRNAs, two to three from each cluster, based on their high expression and/or high-fold difference between T-cell subsets, and based on the literature reporting involvement of a given miRNA in the T-cell biology23, 33, 34, 35 (Table 2, in bold), and we assessed their expression in a new group of 14 donors (Table 1, qRT–PCR validation). Expression patterns of miR-29c, miR-363, miR-31, miR-92a, miR-146a, miR-1246, miR-21 and miR-24 were consistent with the array data (Figure 4b), whereas the expression of miR-320d could not be determined owing to the lack of specificity of the primers/probe pair.
Table 2. MiRNAs with significant differential expression levels between T-cell subsets.
|
Cluster I: naive Tconv | ||||||
|---|---|---|---|---|---|---|
|
Geomean expression array data |
Min–max |
Change vs naive Tconv (%) |
||||
| miRNA | naive Tconv | Memory Tconv | Memory Treg | Naive Treg | Naive Tconv | |
| miR-7g | 66 | 54–78 | 87% | 46% | 78% | 100% |
| miR-29c | 32 | 28–38 | 65% | 27% | 61% | 100% |
| miR-342-3p | 26 | 24–27 | 72% | 45% | 78% | 100% |
| miR-26a | 18 | 13–21 | 64% | 24% | 66% | 100% |
| miR-142-5p | 17 | 14–22 | 95% | 49% | 46% | 100% |
| miR-146b-5p | 13 | 10–19 | 59% | 36% | 50% | 100% |
| miR-30b | 7.3 | 6.0–7.9 | 70% | 28% | 72% | 100% |
| let-7b | 6.9 | 6.2–7.7 | 112% | 50% | 81% | 100% |
| miR-363 | 2.4 | 1.5–4.3 | 20% | 0.3% | 23% | 100% |
| let-7c | 1.3 | 1.1–1.8 | 94% | 43% | 61% | 100% |
| miR-31 | 1.1 | 0.8–1.5 | 44% | 0.3% | 26% | 100% |
| miR-151-5p | 1.0 | 0.8–1.3 | 31% | 2% | 37% | 100% |
| miR-590-5p | 1.0 | 0.8–1.2 | 79% | 39% | 48% | 100% |
| miR-423-5p | 0.3 | 0.2–0.4 | 89% | 17% | 82% | 100% |
|
Cluster II: naive | ||||||
|---|---|---|---|---|---|---|
|
Geomean expression array data |
Min–max |
Change vs naive Treg (%) |
||||
| miRNA | Naive Treg | Memory Tconv | Memory Treg | Naive Treg | Naive Tconv | |
| miR-92a | 4.7 | 3.5–5.7 | 63% | 46% | 100% | 87% |
| miR-342-5p | 2.2 | 1.9–2.7 | 79% | 50% | 100% | 107% |
| miR-320d | 2.1 | 1.7–2.3 | 65% | 48% | 100% | 81% |
| miR-320e | 1.4 | 1.1–1.7 | 70% | 42% | 100% | 87% |
| miR-4299 | 1.4 | 1.0–1.4 | 66% | 40% | 100% | 74% |
| miR-320c | 1.3 | 1.0–2.0 | 71% | 44% | 100% | 91% |
| miR-1275 | 0.6 | 0.3–0.8 | 72% | 16% | 100% | 86% |
|
Cluster III: Treg | ||||||
|---|---|---|---|---|---|---|
|
Geomean expression array data |
Min–max |
Change vs naive Treg (%) |
||||
| miRNA | Naive Treg | Memory Tconv | Memory Treg | Naive Treg | Naive Tconv | |
| miR-146a | 20 | 9–34 | 35% | 100% | 91% | 5% |
| miR-3162 | 2.1 | 0.6–4.2 | 50% | 100% | 88% | 37% |
| miR-1202 | 1.9 | 0.4–5.9 | 32% | 100% | 97% | 23% |
| miR-1246 | 0.9 | 0.1–3.1 | 9% | 100% | 135% | 6% |
| miR-4281 | 0.8 | 0.2–1.7 | 68% | 100% | 107% | 40% |
|
Cluster IV: memory | ||||||
|---|---|---|---|---|---|---|
|
Geomean expression array data |
Min–max |
Change vs naive Treg (%) |
||||
| miRNA | Naive Tconv | Memory Tconv | Memory Treg | Naive Treg | Naive Tconv | |
| miR-21 | 104 | 73–150 | 100% | 179% | 33% | 29% |
| miR-16 | 44 | 32–58 | 100% | 118% | 48% | 55% |
| miR-15b | 10 | 6–14 | 100% | 130% | 45% | 63% |
| miR-15a | 6.7 | 4.0–9.2 | 100% | 103% | 35% | 43% |
| miR-23a | 5.0 | 4.0–6.2 | 100% | 63% | 44% | 53% |
| miR-24 | 4.9 | 4.0–6.8 | 100% | 81% | 38% | 39% |
| miR-27a | 4.1 | 3.2–5.5 | 100% | 61% | 30% | 50% |
| miR-22 | 2.7 | 1.2–3.2 | 100% | 86% | 15% | 22% |
| miR-155 | 2.2 | 1.9–4.4 | 100% | 157% | 53% | 29% |
| miR-425 | 0.7 | 0.6–0.9 | 100% | 95% | 49% | 74% |
| miR-4306 | 0.6 | 0.5–0.7 | 100% | 103% | 30% | 71% |
| miR-148a | 0.5 | 0.4–0.7 | 100% | 87% | 26% | 109% |
| miR-222 | 0.5 | 0.2–0.4 | 100% | 43% | 4% | 34% |
| miR-625 | 0.4 | 0.3–0.6 | 100% | 73% | 29% | 82% |
| miR-7 | 0.3 | 0.3–0.5 | 100% | 112% | 37% | 65% |
| miR-454 | 0.2 | 0.1–0.3 | 100% | 166% | 3% | 47% |
Abbreviations: miRNA, microRNA; Tconv, conventional T cell; Tregs, regulatory T cell. Bold values indicate P<0.05.
Figure 4.
MiRNA signatures characterizing T-cell subsets. (a) Heatmap (Pearson's correlation, median centering miRNAs, complete linkage) showing the miRNA expression signatures that characterize Tconvs and Tregs in 32 samples derived from six RA patients and two healthy controls, using miRNA microarray. Statistical analysis of 121 detected miRNAs revealed 42 miRNAs significantly differently expressed between T-cell subsets of eight donors (n=32 samples, Friedman's test, P<0.005, fold difference ⩾2 in at least one of six comparisons). Top legend (red to blue) depicts range of expression values (log values). Each T-cell subset is characterized by specific miRNA signature. (b) Eight miRNAs, differentially expressed between the four clusters as identified in a, were selected for qRT–PCR validation in a new cohort of donors (n=14, Table 1, qRT–PCR validation). Relative expression, normalized to the average of RNU44 and RNU48 reference genes is shown. Data are depicted as median values with interquartile range. Statistical analysis: Friedman's test with Dunn's multiple comparison post-test. *P<0.05, **P<0.01, ***P<0.0001.
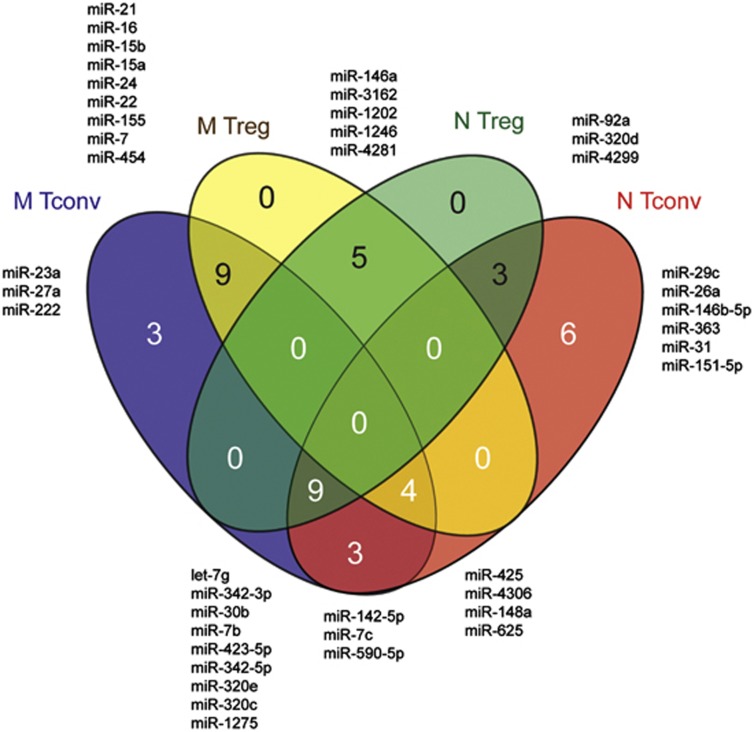
To better visualize the overlap between miRNA signatures defining each of the four T-cell subsets, we made a Venn diagram based on high expression levels of the significantly differentially expressed miRNAs in each of the subsets, as defined by the percentages given in Table 2 (Figure 5). In the Tconv subset, 16 miRNAs showed a consistent high expression in both naive and memory phenotypes, of which 9 were also high in naive Tregs and 4 were also high in memory Tregs. Three miRNAs were highly expressed specifically in the memory Tconvs and six miRNAs were highly expressed specifically in naive Tconvs. In the Treg subset, five miRNAs, that is, miR-146a, miR-3162, miR-1202, miR-1246 and miR-4281, showed a consistent high expression irrespective of the naive or memory phenotype. Nine memory Treg-specific miRNAs were also highly expressed in the memory Tconvs, and therefore should be regarded as miRNAs that define a general memory phenotype. Similarly, the three naive Treg-specific miRNAs were also highly expressed in the naive Tconvs, and as such should be regarded as miRNAs defining a general naive phenotype. Thus, the differential expression of miRNAs between naive and memory Treg subsets is partly a consequence of their respective naive and memory phenotypes. Together, these data show that some miRNAs show an abundant expression confined to Tregs irrespective of their maturation stage (for example, miR-146a and miR-1246), whereas several miRNAs previously linked to the Treg phenotype (for example, miR-21 and miR-155) are in fact specific for a memory phenotype.
Figure 5.
Venn diagram depicting subset specificity of miRNAs. MiRNAs specific for each T-cell subset were selected based on an expression level of ⩾70% as depicted in Table 2.
Discussion
Many studies, mostly in experimental models, have shown the critical involvement of miRNAs in the maintenance of Tregs lineage.21, 24 Recently, Miyara et al.29 fueled the notion of further complexity in the Treg compartment and underlined the necessity to consider naive and memory Tregs as separate regulatory entities. In this study, we performed a comprehensive analysis of the miRNA expression profiles of naive Tregs, memory Tregs, naive Tconvs and memory Tconvs in RA patients and HC. We found characteristic, disease- and treatment-independent, global miRNA expression signatures allowing a clear, miRNA-based subclassification of T-cell subsets into naive Tconv, naive Treg and memory-like phenotypes. In addition, we found that the expression of miR-451 in T cells is associated with DAS28, ESR and serum levels of IL-6.
Several reports have shown the critical involvement of miRNAs in the maintenance of Treg lineage stability and function. The work of Liston et al.,21 Zhou et al.24 and Cobb et al.36 clearly demonstrates that disturbed expression of miRNAs leads to serious and complex alterations in Treg function, resulting in the development of systemic autoimmune-like conditions in mice. MiRNA expression signatures characterizing Tregs of both mice and man have been described.33, 36, 37, 38 However, the gating strategies employed so far, based on the CD25high expression, did not include a memory marker and as such did not separate naive and memory Treg populations. In this study we used a recently described gating strategy,29 and we show that human naive and memory Tregs are characterized by distinct miRNA expression signatures. Our data clearly show that several miRNAs previously linked to the Treg phenotype (that is, miR-21, miR-22, miR-24 and miR-155)23, 33, 38 are differentially expressed between naive and memory Tregs, and are specifically confined to a memory T-cell phenotype. Especially intriguing, in this respect, is the role of miR-155, which has been shown to provide a ‘competitive fitness' to Tregs in mice.22 Differential expression of miR-155 between naive and memory Tregs has recently been reported in a study by Seddiki et al.,39 which is consistent with our data. Importantly, we found that five miRNAs (miR-146a, miR-3162, miR-1202, miR-1246 and miR-4281) were significantly enriched in both naive and memory Tregs, indicating their involvement in the maintenance of a specific Treg phenotype, whereas three miRNAs were significantly under-represented in both Treg subsets (miR-142-5p, let-7c and miR-590-5p). Of the miRNAs enriched in Tregs, miR-146a stands out because of its abundant expression level. MiR-146a is known for its involvement in innate immunity where it has been shown to inhibit myeloid cell proliferation and oncogenesis.40, 41 In adaptive immunity, miR-146a has been reported to control TCR signaling via negative regulation of NF-κB42 as well as to impair IL-2 production and to protect T cells from antigen-induced cell death.43 Interestingly, miR-146a is induced upon T-cell activation and its elevated expression has been previously linked to the memory T-cell phenotype.43 Indeed, we found increased miR-146a levels in memory Tconvs compared with naive Tconvs. Notably, miR-146a expression has been studied extensively in the context of Treg cell function in mice. It was shown that miR-146a positively regulates the suppressive capacity of Tregs by targeting STAT1, a transcription factor involved in the interferon-γ signaling.23 Importantly, no differential expression of miR-146a was noted in our study between patients and controls, or in RA patients between different arms of treatment (MTX and MTX+anti-TNF-α), suggesting no overt, miR-146a-mediated deregulation of Treg function in RA. In a recent study, Rossi et al.38 described the miRNA signature of 17 defined lymphocyte subsets and focused on naive T-cells. In our analysis, in which we used a slightly different sorting strategy to purify naive CD4+ T cells, we could confirm the naive Tconv-specific expression of miR-146b-5p and miR-26a. The pattern of miR-125b expression in our data was also consistent with the one described by Rossi et al.,38 that is, relatively high levels in naive Tconvs and virtually absent in memory T cells. However, because of the very low absolute expression level, even in the naive Tconvs, miR-125b did not pass the filtering criteria and was not included in our analysis.
Memory Tregs are composed of natural Tregs, which arise in the thymus, and inducible Tregs, which arise in the periphery in response to antigen exposure.44 The recently described possibility to separate natural Tregs and inducible Tregs based on their differential expression of neutropilin-145 could be used in future studies to determine whether natural Tregs and inducible Tregs share a common miRNA expression signature.
Our profiling did not reveal global RA-specific changes in the miRNA signatures of the four T-cell subsets. However, we found a positive correlation between the expression of miR-451 and DAS28, and more specifically ESR. Furthermore, elevated miR-451 expression correlated with increased serum levels of IL-6 in RA patients with active disease. A negative feedback loop between IL-6 and miR-451 has been described in dendritic cells. IL-6 was shown to induce miR-451, which in turn led to a decreased expression of pro-inflammatory cytokines, such as IL-6, by direct repression of the YWHAZ adapter protein.32 Further studies are warranted to determine whether this proposed relationship between IL-6 and miR-451 exists in T cells.
Our study clearly shows that defined T-cell subsets are characterized by specific miRNA expression signatures. As such, subtle changes in the CD4+ T-cell composition (for example, the ratio of naive to memory T cells) between HC and RA patients may readily influence the outcome of the analysis, especially for miRNAs that show significantly different expression between the naive and memory subsets (for example, miR-21, miR-155 or miR-146a). Recently, Li et al.14 reported increased miR-146a levels in T cells from synovial fluid of RA patients, as well as in T cells treated with TNF-α. In our analysis of naive and memory T cells isolated from peripheral blood, expression of miR-146a did not differ between HC and RA patients. Consistently, we did not detect increased TNF-α level in the sera of RA patients (data not shown). The synovial fluid T-cell compartment is almost entirely composed of memory T cells46 that are characterized by higher levels of miR-146a than naive T cells (this study and Curtale et al.43). Moreover, it has been shown that T-cell activation, which takes place in the synovial fluid, significantly induces miR-146a expression.43 Thus, a shift in the composition of synovial fluid T cells together with an ongoing inflammatory state of the joint might be responsible for the relatively high expression of miR-146a observed in synovial fluid T cells. Fulci et al.47 have recently reported increased levels of miR-223 in peripheral blood CD4+ T cells of RA patients. We did not observe such increase in our analysis. This discrepancy could be a result of an interdonor variation.
Overall, our study provides important insight into the expression of specific miRNAs in defined T-cell subsets, especially in naive and memory Tregs. Furthermore, our data underline the necessity to analyze isolated T-cell populations in the search for disease-specific molecular cues such as miRNAs.
Materials and methods
Patient's characteristics
Peripheral blood was obtained from 27 RA patients and 6 HC. All RA patients fulfilled the 1987 ACR criteria for the classification of RA. Twenty-four patients had active disease as defined by the EULAR criteria for active disease (DAS28 score >2.6). For 26 patients, the DAS28 score was calculated at the time of recruitment (for 1 patient, DAS3 score was calculated). IgM rheumatoid factor and anti-cyclic citrullinated peptide antibodies were routinely tested, respectively, by turbidimetrics (Roche, Mannheim, Germany) and fluorescent enzyme immune assay (Phadia, Thermo Fisher Scientific, Uppsala, Sweden). Clinical characteristics of patients, including medication are summarized in Table 1. None of the patients received steroid treatment. The study was approved by the UMCG institutional medical ethical committee (2009.118) in accordance with the standards laid down in the 1964 Declaration of Helsinki.
Isolation of Treg and Tconv cells
Peripheral blood mononuclear cells from RA patients and HC were isolated by density gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway) immediately after blood withdrawal into heparin-supplemented vacutainer tubes (Becton-Dickinson Biosciences, Franklin Lakes, NJ, USA). CD3+CD8−CD45RO−CD25−, CD3+CD8−CD45RO−CD25++, CD3+CD8−CD45RO+CD25− and CD3+CD8−CD45RO+CD25+++ T cells were isolated from peripheral blood mononuclear cells by fluorescence-activated cell sorting (MoFlo, Beckman Coulter, Brea, CA, USA) using anti-human-CD3 (OKT3, eBioscience, San Diego, CA, USA), anti-human-CD8 (MCD8, IQ Products, Groningen, The Netherlands) anti-human-CD45RO (UCHL1, eBioscience) and anti-human-CD25 (2A3, Becton-Dickinson Biosciences). Cells were immediately lysed with Qiazol reagent (Qiagen, Venlo, The Netherlands).
Intracellular Foxp3 staining
Peripheral blood mononuclear cells were fixed, permeabilized and washed using the Foxp3-staining Buffer Set (eBioscience). Cells were stained with anti-human-CD3 (OKT3, eBioscience), anti-human-CD8 (MCD8, IQ Products) anti-human-CD45RO (UCHL1, eBioscience), anti-human-CD25 (2A3, Becton-Dickinson Biosciences) and anti-human-Foxp3 (clone 206D, BioLegend, San Diego, CA, USA) monoclonal antibodies. Flow cytometry analysis was performed using an LSR-II Flow Cytometer and FACSDiva software (Becton-Dickinson Biosciences). Data were analyzed using Kaluza software (version 1.2, Beckman Coulter).
RNA isolation
Total cellular RNA was extracted using the miRNeasy Mini Kit (Qiagen), following the manufacturer's instructions, with an additional chloroform extraction step and a triple washing step. RNA concentrations were quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and RNA quality was assessed using the Experion HighSens RNA analysis Kit (Bio-Rad, Hercules, CA, USA). RNA samples with an RNA quality indicator value above 8.8 were used for downstream applications.
Microarray analysis and statistics
Approximately 100 ng of total RNA was dephosphorylated, desalted and labeled with Cy-3 and hybridized to the Agilent Human miRNA Microarray (catelog number G4870A), which contained probes for 1205 human and 144 human viral miRNAs. Arrays were scanned using an Agilent scanner according to the manufacturer's instructions (Agilent Technologies). Array images were analyzed using Agilent feature extraction software (v10.7.3.1). All further analyses were performed using GeneSpring GX version 12.5 software (Agilent Technologies). TXT files for each array were preprocessed using default settings as follows: threshold raw signals to 1.0, followed by 95 percentile shift normalization. We excluded control probes, miRNAs detected in less than 50% of samples (16 out of 32 samples), and miRNAs with an expression value below −3.5 in more than 25% of samples (8 out of 32 samples), leaving 121 human miRNAs and 4 human viral miRNAs. Hierarchical clustering was performed using Genesis software (Graz University of Technology) and Pearson's correlation as the distance metric. Differential expression analysis between four treatment groups, irrespective of T-cell subset, was performed using an analysis of variance test with the Benjamini–Hochberg correction for multiple testing. Probes with corrected P-value <0.05 and fold difference ⩾2 in at least one of the comparisons between the groups were considered for further analysis. Differential expression analysis across all subjects (n=8) was performed using a Friedman test between four T-cell subsets, with the Benjamini–Hochberg correction for multiple testing. Probes with corrected P-value <0.005 and a fold difference ⩾2 in at least one of the comparisons between the subsets were considered for further analysis. Venn diagrams were constructed using an online tool http://www.bioinfogp.cnb.csic.es/tools/venny/.
Microarray data
The microarray data have been deposited into Gene Expression Omnibus and are accessible with the following series record number: GSE50646.
qRT–PCR
Gene expression levels were analyzed by qRT–PCR. For miRNA-specific complementary DNA synthesis, RNA was reverse transcribed using the Taqman MicroRNA Reverse Transcription kit in combination with multiplexed reverse transcription primers of TaqMan microRNA Assays (Life Technologies, Carlsbad, CA, USA) for miR-363-3p (ID: 001271), miR-31-5p (ID: 002279), miR-29c-3p (ID: 000587), miR-92a-3p (ID: 000431), miR-320d (ID: 241066_mat), miR-146a-5p (ID: 000468), miR-1246 (ID: 462575_mat), miR-21-5p (ID: 000397), miR-24-3p (ID: 000402), miR-451a (ID: 001141), RNU44 (ID: 001094) and RNU48 (ID: 0010060). Complementary DNA synthesis for mRNA was performed using Superscript III RTase (Life Technologies).
The qPCR reaction was performed using qPCR MasterMix Plus (Eurogentec, Liege, Belgium) and Taqman Gene expression assays for miRNAs and for Foxp3 (ID: Hs 01085834_m1) (Life Technologies). Gene-specific primers and probe (Integrated DNA Technologies, Coralville, IA, USA) were used for detection of TATA box binding protein: forward 5′-GCCCGAAACGCCGAATAT-3′, reverse 5′-CCGTGGTTCGTGGCTCTCT-3′ and probe 5′-6-FAM-ATCCCAAGCGGTTTGCTGCGG-BHQ-1-3′ and for the detection of RPII: forward 5′-CGTACGCACCACGTCCAAT-3′, reverse 5′-CAAGAGAGCCAAGTGTCGGTAA-3′, and probe 5′-6-FAM-TACCACGTCATCTCCTTTGATGGCTCCTAT-BHQ-1-3′.
All reactions were run in triplicate. Mean cycle threshold (Ct) values for all genes were quantified with the Sequence Detection software (version 2.3, Life Technologies) using ABI7900HT thermo cycler (Life Technologies). RNU48 and RNU44 served as endogenous controls for the selected miRNAs, whereas TATA box binding protein and RPII served as endogenous controls for Foxp3, resulting in ΔCt values. Relative expression levels were determined using the 2−ΔCt formula. For the detection of miR-451 (Figures 3b–e), relative expression levels were calculated with the 2−ΔΔCt formula, where RA-29-naive Tconv sample served as a calibrator.
Quantification of circulating cytokines
Peripheral blood was collected in anticoagulant-free tubes and incubated to coagulation for 1 h at RT. Serum was collected by centrifugation (1400 g for 10 min) and stored at −80 °C. Sera samples were thawed to quantify levels of cytokines using Human Th1/Th2 Essential 6-plex (Affymetrix, eBioscience) according to the manufacturer's instruction. Data analyses were performed using StarStation software (Applied Cytometry, Birmingham, UK).
Statistical analysis
Statistical analysis of qRT–PCR data was performed with the GraphPad Prism software (La Jolla, CA, USA). Comparisons of multiple unpaired samples were performed using the Kruskal–Wallis test with Dunn's multiple comparison post-test; comparisons of more than two paired samples were performed using the Friedman test with Dunn's multiple comparison post-test. Comparisons of more than two paired samples with less than six replicates were performed using the repeated measures analysis of variance test with Bonferroni's multiple comparison post-test. For correlation comparisons, the Spearman test was used. All statistical analyses were two sided, and the significance level used was P<0.05.
Acknowledgments
We gratefully acknowledge Geert Mesander and Henk Moes for excellent flow cytometrical technical assistance. This study was supported by a grant from the Jan Kornelis de Cock Foundation, an Ubbo Emius Fellowship, and an unrestricted research grant from Abbott.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Genes and Immunity website (http://www.nature.com/gene)
Supplementary Material
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- Lundy SK, Sarkar S, Tesmer LA, Fox DA. Cells of the synovium in rheumatoid arthritis. T lymphocytes. Arthritis Res Ther. 2007;9:202. doi: 10.1186/ar2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Developments in the scientific understanding of rheumatoid arthritis. Arthritis Res Ther. 2009;11:249. doi: 10.1186/ar2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–2785. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- Mottonen M, Heikkinen J, Mustonen L, Isomaki P, Luukkainen R, Lassila O. CD4+ CD25+ T cells with the phenotypic and functional characteristics of regulatory T cells are enriched in the synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol. 2005;140:360–367. doi: 10.1111/j.1365-2249.2005.02754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Cao D, Widhe M, Roth K, Herrath J, Engstrom M, et al. FOXP3 expression in blood, synovial fluid and synovial tissue during inflammatory arthritis and intra-articular corticosteroid treatment. Ann Rheum Dis. 2009;68:1908–1915. doi: 10.1136/ard.2008.100768. [DOI] [PubMed] [Google Scholar]
- Wehrens EJ, Prakken BJ, van Wijk F. T cells out of control—impaired immune regulation in the inflamed joint. Nat Rev Rheumatol. 2013;9:34–42. doi: 10.1038/nrrheum.2012.149. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Borja F, Jury EC, Mauri C, Ehrenstein MR. Defects in CTLA-4 are associated with abnormal regulatory T cell function in rheumatoid arthritis. Proc Natl Acad Sci USA. 2008;105:19396–19401. doi: 10.1073/pnas.0806855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notley CA, Ehrenstein MR. The yin and yang of regulatory T cells and inflammation in RA. Nat Rev Rheumatol. 2010;6:572–577. doi: 10.1038/nrrheum.2010.143. [DOI] [PubMed] [Google Scholar]
- Nagar M, Jacob-Hirsch J, Vernitsky H, Berkun Y, Ben-Horin S, Amariglio N, et al. TNF activates a NF-kappaB-regulated cellular program in human CD45RA- regulatory T cells that modulates their suppressive function. J Immunol. 2010;184:3570–3581. doi: 10.4049/jimmunol.0902070. [DOI] [PubMed] [Google Scholar]
- Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- Li J, Wan Y, Guo Q, Zou L, Zhang J, Fang Y, et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12:R81. doi: 10.1186/ar3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- Lee KH, Chen YL, Yeh SD, Hsiao M, Lin JT, Goan YG, et al. MicroRNA-330 acts as tumor suppressor and induces apoptosis of prostate cancer cells through E2F1-mediated suppression of Akt phosphorylation. Oncogene. 2009;28:3360–3370. doi: 10.1038/onc.2009.192. [DOI] [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lu LF, O'Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley KM, Satoh M, Chan AL, Bubb MR, Reeves WH, Chan EK. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther. 2008;10:R101. doi: 10.1186/ar2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, et al. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- Stagakis E, Bertsias G, Verginis P, Nakou M, Hatziapostolou M, Kritikos H, et al. Identification of novel microRNA signatures linked to human lupus disease activity and pathogenesis: miR-21 regulates aberrant T cell responses through regulation of PDCD4 expression. Ann Rheum Dis. 2011;70:1496–1506. doi: 10.1136/ard.2010.139857. [DOI] [PubMed] [Google Scholar]
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Miyara M, Gorochov G, Ehrenstein M, Musset L, Sakaguchi S, Amoura Z. Human FoxP3+ regulatory T cells in systemic autoimmune diseases. Autoimmun Rev. 2011;10:744–755. doi: 10.1016/j.autrev.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Milman N, Karsh J, Booth RA. Correlation of a multi-cytokine panel with clinical disease activity in patients with rheumatoid arthritis. Clin Biochem. 2010;43:1309–1314. doi: 10.1016/j.clinbiochem.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Rosenberger CM, Podyminogin RL, Navarro G, Zhao GW, Askovich PS, Weiss MJ, et al. miR-451 regulates dendritic cell cytokine responses to influenza infection. J Immunol. 2012;189:5965–5975. doi: 10.4049/jimmunol.1201437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, et al. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol. 2009;39:1608–1618. doi: 10.1002/eji.200838509. [DOI] [PubMed] [Google Scholar]
- Smigielska-Czepiel K, van den Berg A, Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, et al. Dual role of miR-21 in CD4+ T-cells: activation-induced miR-21 supports survival of memory T-cells and regulates CCR7 expression in naive T-cells. PLoS One. 2013;8:e76217. doi: 10.1371/journal.pone.0076217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner S, Herndler-Brandstetter D, Arnold CR, Wiegers GJ, Villunger A, Hackl M, et al. Upregulation of miR-24 is associated with a decreased DNA damage response upon etoposide treatment in highly differentiated CD8(+) T cells sensitizing them to apoptotic cell death. Aging Cell. 2012;11:579–587. doi: 10.1111/j.1474-9726.2012.00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BS, Hertweck A, Smith J, O'Connor E, Graf D, Cook T, et al. A role for Dicer in immune regulation. J Exp Med. 2006;203:2519–2527. doi: 10.1084/jem.20061692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezova R, Slaby O, Faltejskova P, Mikulkova Z, Buresova I, Raja KR, et al. microRNA-342, microRNA-191 and microRNA-510 are differentially expressed in T regulatory cells of type 1 diabetic patients. Cell Immunol. 2010;260:70–74. doi: 10.1016/j.cellimm.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Rossi RL, Rossetti G, Wenandy L, Curti S, Ripamonti A, Bonnal RJ, et al. Distinct microRNA signatures in human lymphocyte subsets and enforcement of the naive state in CD4(+) T cells by the microRNA miR-125b. Nat Immunol. 2011;12:796–803. doi: 10.1038/ni.2057. [DOI] [PubMed] [Google Scholar]
- Seddiki N, Swaminathan S, Phetsouphanh C, Kelleher AD. miR-155 is differentially expressed in Treg subsets, which may explain expression level differences of miR-155 in HIV-1 infected patients. Blood. 2012;119:6396–6397. doi: 10.1182/blood-2012-02-412874. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Taganov KD, Rao DS, Yang L, Zhao JL, Kalwani M, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208:1189–1201. doi: 10.1084/jem.20101823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-kappaB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108:9184–9189. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Boldin MP, Yu Y, Liu CS, Ea CK, Ramakrishnan P, et al. miR-146a controls the resolution of T cell responses in mice. J Exp Med. 2012;209:1655–1670. doi: 10.1084/jem.20112218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtale G, Citarella F, Carissimi C, Goldoni M, Carucci N, Fulci V, et al. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood. 2010;115:265–273. doi: 10.1182/blood-2009-06-225987. [DOI] [PubMed] [Google Scholar]
- Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003 Mar;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1–19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejaco C, Duftner C, Klauser A, Schirmer M. Altered T-cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatol Int. 2010;30:297–303. doi: 10.1007/s00296-009-0949-9. [DOI] [PubMed] [Google Scholar]
- Fulci V, Scappucci G, Sebastiani GD, Giannitti C, Franceschini D, Meloni F, et al. miR-223 is overexpressed in T-lymphocytes of patients affected by rheumatoid arthritis. Hum Immunol. 2010;71:206–211. doi: 10.1016/j.humimm.2009.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.