Abstract
Background
Amniotic fluid embolism (AFE) is a life-threatening obstetric complication that arises in 2 to 8 of every 100 000 deliveries. With a mortality of 11% to 44%, it is among the leading direct causes of maternal death. This entity is an interdisciplinary challenge because of its presentation with sudden cardiac arrest without any immediately obvious cause, the lack of specific diagnostic tests, the difficulty of establishing the diagnosis and excluding competing diagnoses, and the complex treatment required, including cardiopulmonary resuscitation.
Methods
We selectively reviewed pertinent literature published from 2000 to May 2013 that was retrieved by a PubMed search.
Results
The identified risk factors for AFE are maternal age 35 and above (odds ratio [OR] 1.86), Cesarean section (OR 12.4), placenta previa (OR 10.5), and multiple pregnancy (OR 8.5). AFE is diagnosed on clinical grounds after the exclusion of other causes of acute cardiovascular decompensation during delivery, such as pulmonary thromboembolism or myocardial infarction. Its main clinical features are severe hypotension, arrhythmia, cardiac arrest, pulmonary and neurological manifestations, and profuse bleeding because of disseminated intravascular coagulation and/or hyperfibrinolysis. Its treatment requires immediate, optimal interdisciplinary cooperation. Low-level evidence favors treating women suffering from AFE by securing the airway, adequate oxygenation, circulatory support, and correction of hemostatic disturbances. The sudden, unexplained death of a pregnant woman necessitates a forensic autopsy. The histological or immunohistochemical demonstration of formed amniotic fluid components in the pulmonary bloodflow establishes the diagnosis of AFE.
Conclusion
AFE has become more common in recent years, for unclear reasons. Rapid diagnosis and immediate interdisciplinary treatment are essential for a good outcome. Establishing evidence-based recommendations for intervention is an important goal for the near future.
Amniotic fluid embolism (AFE) is an unforeseeable, life-threatening complication of childbirth. It was first described in 1926 by J. R. Meyer (1), and its clinical and morphological features were described by Steiner and Lushbaugh in 1941 (2). Despite an incidence rate that ranges from only 2 to 8 per 100 000 births in different countries (3– 5, e1) (Table 1), AFE is one of the leading causes of death resulting directly from childbirth, accounting for 5% to 15% of cases worldwide (6, e2). According to statistics, it is the most common cause of maternal death in Australia and the second-most common in the USA and the U.K. (6– 8, e3). These are underestimates of the rate of nonfatal and fatal AFE, due to heterogeneous diagnostic criteria and the unreliability of physicians’ death certificates (6, e4).
Table 1. Incidence of amniotic fluid embolism.
| Country | Period | Incidence (n/100000 births) | Case-related mortality | Perinatal mortality |
|---|---|---|---|---|
| Australia*1 | 2001 to 2007 | 3.3 | 35% | 32% |
| USA*1 | 1999 to 2003 | 7.7 | 21.6% | No data |
| Canada*1 | 1991 to 2002 | 6.0 | 13% | No data |
| U.K.*2 | 2005 to 2009 | 2.0 | 20% | 13.5% |
| The Netherlands*2 | 2004 to 2006 | 2.5 | 11% | 38.1% |
*1Retrospective population-based studies
*2Case-related validation from prospective studies
Modified according to (12), Knight M. et al.: BMC Pregnancy & Childbirth 2012; 12: 7
In Germany, cases of maternal death in childbirth are reported by hospitals voluntarily and in anonymized form to the quality assurance agency for the state in question. They are then evaluated annually by a panel of experts (the Maternal Death Working Group of the AQUA Institute). However, only deaths among inpatients are recorded. In 2011 AFE was the leading cause of death resulting directly from childbirth in Germany, accounting for 8 out of 12 cases, but an autopsy was performed in only one case (9). There are no figures on the incidence of AFE in Germany.
In industrialized countries case-related maternal mortality is between 13.5% and 44% (3– 7, 10), and perinatal mortality between 7% and 38% (11– 13). Between 24% and 50% of surviving children manifest persistent neurological deficits (11, 14, e5).
Rapid diagnosis and immediate obstetric and intensive care play a decisive role in maternal prognosis and survival (14, 15).
Varying clinical symptoms, difficult diagnosis (including differential diagnosis), and uncertainty regarding post-mortem evidence mean that AFE poses an interdisciplinary challenge. To our knowledge, the present article is the first to discuss AFE from the point of view of an obstetrician, an intensive care physician, and a forensic pathologist.
Methods
A search of the literature was performed in PubMed using the keywords “amniotic fluid embolism,” “cardiovascular collapse,” “disseminated intravascular coagulation,” “maternal death,” “maternal mortality,” and “forensic pathology,” for the period January 2000 to May 2013. Seminal publications dating from before 2000 were also included.
Pathogenesis
The pathogenesis of AFE is not yet fully clear. Amniotic fluid can enter the maternal circulation via endocervical veins, lesions of the uterus, or the site of placental attachment (16).
Although previously proposed explanations of the development of AFE envisaged a purely mechanical obstruction of the pulmonary vessels by amniotic fluid components (17), today humoral and immunological factors are considered to be responsible (18, 19). This is because in addition to insoluble fetal components (e.g. squames) amniotic fluid also contains numerous vasoactive substances (bradykinin, histamine, and others) and procoagulant substances that can lead to endothelial activation and a massive inflammatory reaction (18, 20). These and other immunological and clinical similarities to anaphylactic shock have led to the anaphylactoid reaction hypothesis (anaphylactoid syndrome of pregnancy [11, 17]). This hypothesis is controversial (19). Another pathophysiological mechanism may be complement activation triggering AFE (18, 19). Why some women tolerate the transfer of amniotic fluid or its components with no problems or clinical symptoms and others do not can currently only be the subject of speculation (13); it is also unclear whether allergic diatheses or previous sensitization to specific fetal antigens are disposing factors for AFE (11, 21).
Pathophysiology and clinical manifestation
AFE occurs during labor and delivery/Cesarean section (55% to 76% antenatally) or up to 48 hours postpartum (3, 4, 11). In rare cases, it also occurs during pregnancy following intrauterine surgery (e.g. abortion) or blunt abdominal trauma (6).
The main risk factors for AFE are as follows (3): maternal age 35 years or above (odds ratio [OR]: 1.86; 95% confidence interval [95% CI]: 0.99 to 3.48), Cesarean delivery (OR: 12.4; 95% CI: 6.5 to 23.6), placenta previa (OR: 10.5; 95% CI: 0.94 to 117.2), and multiple pregnancy (OR: 8.5; 95% CI: 2.92 to 24.6). Despite discrepancies between studies (4, 5, 22), a recent prospective study proposed that induction of labor increases the risk of AFE by 35% (3). The increasing age of pregnant women (e6) and the significantly increased rates of Cesarean sections (e7), placentation disorders (e8), and induced labor (22% of all births in Germany in 2012 [e9]) may therefore be important influencing factors in the increasing incidence of AFE (9).
Pathophysiologically, the first phase of AFE involves pulmonary vasoconstriction with increased pulmonary resistance and pulmonary hypertension. The cardiac consequences of this are acute right heart failure resulting from pressure overload, with dilatation of the right ventricle and severe tricuspid insufficiency (revealed using transesophageal echocardiography [23, 24]). Disturbance of pulmonary perfusion and damage to the gas exchange surfaces caused by inflammation lead to respiratory failure and to hypoxemia. These early pathophysiological changes usually occur within the first 30 to 60 minutes after the onset of clinical symptoms.
Either at full health or following nonspecific prodromal symptoms (e.g. agitation, numbness, feeling cold, lightheadedness), the main initial manifestations of AFE in 30% to 40% of patients are acute dyspnea and cyanosis (50% to 80%), sudden hypotension (56% to 100%), cardiac arrest (30% to 87%), or fetal distress (20% to 36%) detectable by cardiotocography (danger: sudden, unexplainable deterioration in fetal heart rate pattern) (3, 6, 11). Seizures, acute confusion, and in extreme cases unconsciousness/coma occur in 15% to 50% of patients (6, 25, 26). In up to 12% of cases the initial symptom of AFE is life-threatening hemorrhage resulting from coagulopathy (27, e10, e11). These symptoms can vary and may manifest in combination with each other and with differing degrees of severity (3).
The second phase of AFE can also include acute left heart failure with consequent pulmonary edema (51% to 100%) (6). Reactive hypovolemia, cardiodepressive humoral factors from the amniotic fluid, and myocardial ischemia play a major role in this (6, 17, 25, e12).
According to the US registry, 56% of women do not survive the first two hours following the acute event (11). In the UKOSS study (3), maternal death occurred at a median of 1 hour, 40 minutes (range: 0 to 23 hours) after manifestation of AFE. Causes of death following survival of the initial phase are sudden cardiac arrest, hemorrhage resulting from coagulopathy or acute respiratory distress syndrome (ARDS), and/or multiple organ failure (6). In 30% to 45% of patients surviving the initial phase, coagulopathy develops with severe bleeding resulting from disseminated intravascular coagulation (DIC), which can occur as early as the first 10 to 30 minutes (within 4 hours in 50% of cases) or up to 9 hours after initial clinical manifestation (11, 26, 28, 29). The cause of DIC is as yet unclear. Amniotic fluid contains many procoagulant substances (including tissue factor and phosphatidylserine), which can lead directly or indirectly (via cytokines or complement activation) to DIC with consumptive coagulopathy and secondary hyperfibrinolysis via activation of the extrinsic coagulation cascade (18, 21, 30, 31). There is also a controversial hypothesis that coagulopathy may be the result of massive hyperfibrinolysis, as amniotic fluid also contains increased concentrations of urokinase-like plasminogen activator and plasminogen activator 1, among other substances (32, e13, e14). Current coagulation studies using rotational thromboelastometry show signs of hyperfibrinolysis and massive hypofibrinogenemia as early as in the initial phase of AFE (33, e15). Nine cases of uneventful subsequent pregnancy have been reported in women who had AFE in previous pregnancies (6).
Diagnosis
Diagnosis of AFE is based on clinical symptoms after other causes/diagnoses have been excluded. AFE should be considered in every case of sudden maternal cardiovascular collapse and/or maternal death in childbirth with unexplained etiology. There are currently no uniform clinical diagnostic criteria for AFE; the criteria most frequently cited in the current literature are those of the UKOSS (UK Obstetric Surveillance System [3]) and those of Benson (18) (Table 2). The US AFE registry restricts the time of main symptom onset to 30 minutes after birth (11).
Table 2. Amniotic fluid embolism diagnosis criteria.
| UK Obstetric Surveillance System (UKOSS) 2010 (3) | Benson M. et al. 2007 (18) |
|---|---|
No other clear cause: acute cardiovascular collapse with one or more of the following signs:
|
Pregnant women up to 48 hours after birth with one or more of the following symptoms and requiring treatment:
|
To date there are no specific laboratory tests to diagnose AFE.
Evidence of fetal cells in the pulmonary vessels is not a reliable diagnostic criterion and is not pathognomonic for AFE, as fetal cells can be detected in 21% to 100% of pregnant woman without AFE (6). Promising diagnostic markers of AFE such as zinc coproporphyrin, sialyl-Tn antigen, tryptase, or C3 and C4 complement and detection of insulin-like growth factor binding protein-1 (e16) have not been established in routine clinical diagnosis (6, 16). Hemodynamic parameters, ECG, blood gas analysis, chest X-ray, and laboratory tests (including blood count, cardiac enzymes, and coagulation tests) and specific tests such as transesophageal echocardiography (TEE) and rotational thromboelastometry are less useful for confirming diagnosis but much more so for monitoring and treatment optimization.
Differential diagnosis
The symptom mimicry of AFE and the similarities of its main clinical symptoms to those of other diseases (box) often lead to delays in diagnosis and treatment. Symptom-related clinical pictures can only be defined through careful evaluation of clinical, apparative, and laboratory findings concerning AFE. The most common differential diagnosis is AFE versus pulmonary embolism; the latter differs most markedly from AFE in its typical risk factors, chest pain, rarer initial hypotension, and usually the absence of coagulopathy. Differential diagnoses according to clinical symptoms for AFE versus pulmonary embolism, myocardial infarction, and peripartal cardiomyopathy are shown in Table 3 (e17).
Box. Differential diagnosis of amniotic fluid embolism.
Pulmonary embolism
Air embolism
Acute myocardial infarction
Septic shock
Peripartal cardiomyopathy
Anaphylactic shock
Anesthesiological complications: high spinal/epidural block, reaction to local anesthetic drugs, aspiration
-
Obstetric complications:
-
Placental abruption:
Abdominal pain, uterine tetanus, ultrasound evidence of retroplacental hematoma
-
Eclampsia:
Tonic-clonic seizures in pre-eclampsia
-
Uterine rupture:
Previous Cesarean section, major suprasymphysary pain, sudden cessation of labor
-
Postpartum hemorrhage:
e.g. erratic, intermittent bleeding in uterine atony
-
In view of the increasing number of pregnant women with heart diseases, women with cardiovascular risk factors should, wherever possible, receive interdisciplinary counselling prior to a planned pregnancy (e18).
Treatment
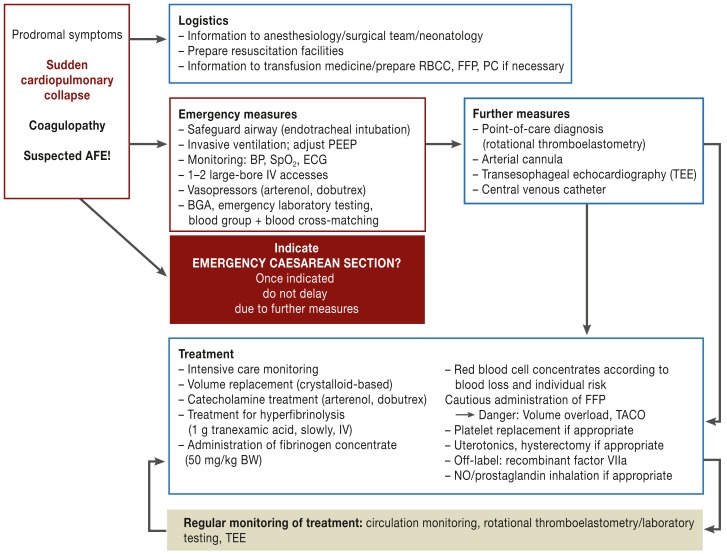
One possible treatment procedure is shown in Figure 1. Symptom-related acute therapy is based on clinical experience and has little supporting evidence. The highest priority in cases of suspected AFE is to safeguard the airways using endotracheal intubation and early, sufficient oxygenation using an optimized Fi02:PEEP (positive end-expiratory pressure) ratio. Reliable prevention against aspiration is essential. Depending on hemodynamic status, early use of vasopressors (e.g. noradrenaline, dobutamine) may be indicated in addition to crystalloid-based volume replacement (6, 24). Blood should immediately be taken for laboratory diagnosis including coagulation tests, cross-matching, blood gas analysis, and—if available—rotational thromboelastometry. Rotational thromboelastometry is a point-of-care test to distinguish between hemostasis disorders and to assess their severity. Further measures such as fitting an arterial cannula or central venous catheter should not delay any emergency Cesarean section. In the event of cardiac arrest or life-threatening cardiac arrhythmia, emergency Cesarean section should be performed with resuscitation facilities available, if possible within 3 to 5 minutes (25, 27). This increases the chance of survival of the neonate without neurological disabilities (11) and also improves venous backflow to the right heart by emptying the uterus (21). Also, following successful resuscitation, both mother and child benefit from immediate delivery, and care of the neonate should be optimized by the involvement of a neonatology team. Postpartally, uterotonics should be administered immediately to prevent uterine atony; hysterectomy should be performed promptly in case of treatment-refractory uterine atony or persistent bleeding (13). Differentiated use of catecholamines can be optimized using transesophageal echography (26). Alpha stimulation can if necessary be enhanced using additional inotropy support. Both diagnostically and therapeutically, it is important to monitor cardiac pump function (26).
Figure 1.
Interdisciplinary action for suspected amniotic fluid embolism (AFE)
RBCC: red blood cell concentrate; FFP: fresh-frozen plasma; PC: platelet concentrate; PEEP: positive end-expiratory pressure; BP: blood pressure; SpO2: partial oxygen saturation; ECG, electrocardiography; BGA: blood gas analysis; BW, body weight; TACO: transfusion-related acute cardiac overload; NO: nitric oxide
For subsequent treatment, prompt optimization of coagulation status is the most important measure. In addition to causative therapy, initial administration of transexamic acid to treat hyperfibrinolysis and the use of fibrinogen concentrate (for fibrinogen levels below 2 g/L) are essential and if possible should be performed using rotational thromboelastometry (33). Replacement of red blood cell concentrates and fresh-frozen plasma (FFP) should be performed according to blood loss/severity of bleeding and in line with the risk profile of the patient; care must be taken to avoid volume overload (danger: pulmonary edema) in these pregnant women. FFP should be administered cautiously and preferably while monitoring volume status with TEE (26). The use of recombinant factor VIIa should only be considered if even massive coagulation factor replacement is insufficient to improve hemastosis and stop bleeding (34). Training programs with an interdisciplinary focus for acute treatment of obstetric emergencies can contribute to improved clinical outcomes (e19, e20). This has begun in individual facilities in Germany.
Forensic post-mortem evidence of AFE
The unexpected death of a pregnant woman during childbirth can lead to accusations against a physician if relatives suspect that the cause of death was a treatment error (35, 36). Even in the event of death from extensive hemorrhage, evidence of AFE can explain what has occurred and can relieve the treating physician of the accusation of violation of the regulations of medical practice (28, 37). For example, evaluation of cause of death information showed that in 30% to 40% of cases of histologically confirmed AFE the clinical conclusion had been hemorrhagic shock, and AFE had not been considered as the indirect cause (38).
As the sudden, unexpected death of a pregnant woman of unknown cause must be classified as “unexplained death,” an autopsy must be requested.
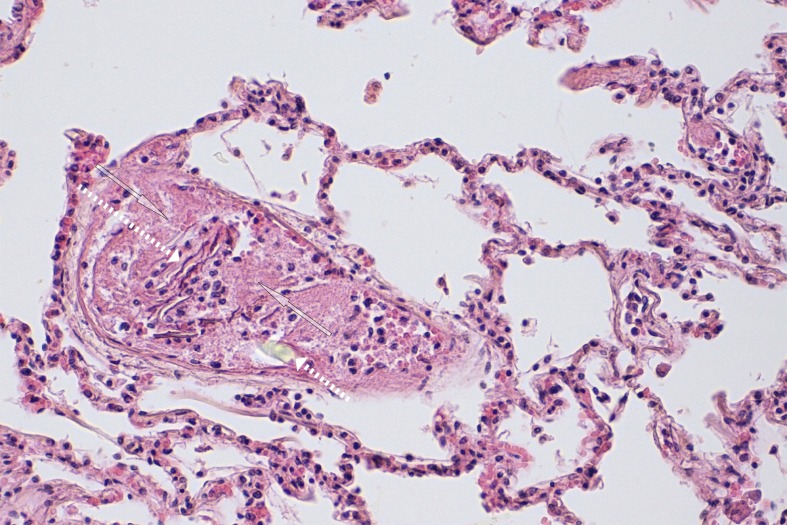
Because macropathological findings are nonspecific, cause of death should not be determined without careful histological examination. Detection of formed amniotic fluid components such as usually lamellar, adjacent epidermal squames, meconium components, or lanugo hairs (Figure 2) in the pulmonary bloodflow constitutes histological evidence of AFE (3, 28, 36). The fluid component of amniotic fluid cannot be detected histologically. It is important that a representative number of samples (at least one sample from each lung segment, 28) be taken.
Figure 2.
A blood vessel enclosed by lamellar epithelial squames (long dotted arrow) embedded in a fibrin thrombus (two transparent arrows). The lower part of the picture shows a transparent, cylindrical structure corresponding to a lanugo hair (short dotted arrow). Hematoxylin eosin staining: 200x. Survival time: 8 hours
Embolic material is found mainly in the pulmonary arterioles and capillaries. Fibrin thrombi, sometimes in connection with amniotic fluid components, are universal and can be detected even after a survival time of two hours or more (Figure 2) (38).
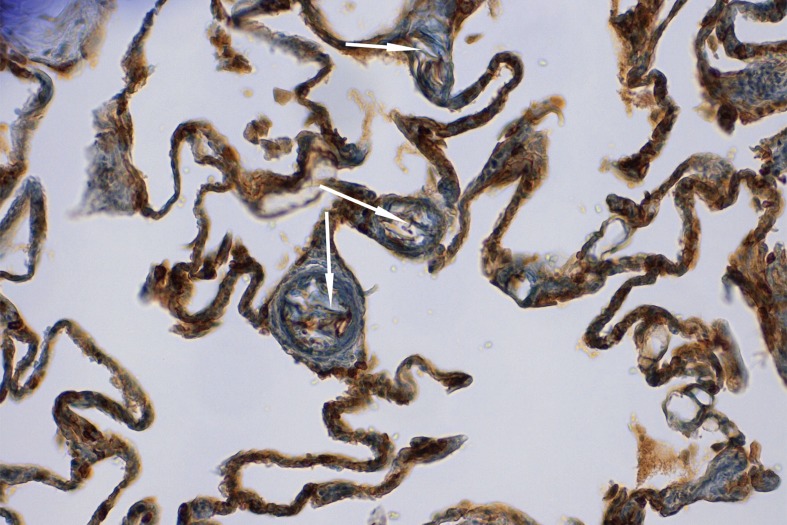
In addition to conventional stains such as hematoxylin eosin as a surveillance stain, Sudan III to show fatty substances, and PAS or alcian blue to visualize mucus, immunohistochemical staining of fetal epithelial cells using cytokeratin is now a standard procedure (28). This allows the severity of AFE to be assessed more precisely. For mild AFE with simultaneous interference by autolysis, epithelial squames in the pulmonary capillaries can only be visualized following immunohistochemical staining with cytokeratin (Figure 3). However, morphologically determined severity of AFE does not correlate with severity of clinical symptoms (38).
Figure 3.
Immunohistochemically marked epithelial squames in pulmonary arterioles (arrows). Cytokeratin, 200x
An absence of histological evidence of amniotic fluid components in the lung in the first three days following clinical manifestation of AFE and maternal death rules out AFE. In case of an anaphylactoid reaction, the transfer of a small, histomorphologically undetectable amount of amniotic fluid into the maternal circulation may be the cause, but in such cases there would be no histological evidence of DIC. If the mother survives for longer, it should be borne in mind that as yet there is no reliable information on how long amniotic fluid components remain in the maternal circulation (38).
Table 3. Differential diagnoses by clinical symptoms.
| Clinical manifestation/symptoms | Amniotic fluid embolism | Pulmonary embolism | Myocardial infarction | Peripartal cardiomyopathy |
|---|---|---|---|---|
| Manifestation | During labor/birth → hours postpartum | 2 to 15 times more common during labor than pregnancy | 21% peripartally 34% postpartally | Third trimester: approx. 9% to 80% up to 4 months postpartum |
| Risk factors | +/nonspecific | +++/specific | +++/specific | +/nonspecific |
| Cardiac arrest | ++ | + → ++ | + | + |
| Chest pain | – | ++ → +++ | +++ | ++ |
| Cardiac arrhythmia | + → ++ | ++ → +++ | +++ | ++ |
| Dyspnea | +++ | + → +++ | + → ++ | ++ |
| Hypotension | +++ | + → ++ | + → ++ | +/– |
| Neurological symptoms | ++ | + secondary | (+) secondary | (+) secondary |
| Coagulopathy | ++ | – | – | – |
| Acute fetal distress | + → ++ | (+) secondary | (+) secondary | No data |
–: None or rare; +: Occasional; ++: Common; +++: Very common; Table from Rath W.: Fruchtwasserembolie, Lungenembolie (Amniotic Fluid Embolism, Pulmonary Embolism). In: Feige A., Rath W., Schmidt S (eds.): Kreißsaal-Kompendium, Stuttgart, New York, Thieme 2013; 142–9 (e17). Reproduced with the kind permission of Thieme Publishers
Key Messages.
Amniotic fluid embolism is one of the leading causes of maternal death resulting directly from childbirth, accounting for 5% to 15% of cases. Case-related mortality is between 11% and 44%.
The main risk factors are maternal age ≥ 35 years, Cesarean section, placenta previa, and multiple pregnancy.
Diagnosis is based on clinical symptoms after other causes have been excluded. The main symptoms are acute dyspnea/cyanosis, severe hypotension/cardiac arrest, fetal distress, and hemorrhage caused by coagulopathy during and after labor. The most important differential diagnoses are versus pulmonary embolism and myocardial infarction.
Immediate interdisciplinary treatment with cardiopulmonary resuscitation, hemodynamic stabilization using crystalloid-based volume replacement and early vasopressor use, correction of hemostasis disorders, and undelayed Cesarean section play a decisive role in prognosis.
In the event of sudden maternal death due to an unknown cause, autopsy must be requested, in which amniotic fluid embolism can be reliably diagnosed using immunohistochemical techniques.
Acknowledgments
Translated from the original German by Caroline Devitt, M.A.
Footnotes
Conflict of interest statement
Prof. Rath has received consultancy fees from Ferring and lecture fees from CSL Behring. He has received reimbursement of travel expenses from CSL Behring.
Prof. Hofer has received reimbursement of travel expenses from CSL Behring and lecture fees from CSL Behring, NovoNordis, and Biotest Roche.
Dr. Sinicina declares that no conflict of interest exists.
References
- 1.Meyer JR. Embolia pulmonar amnio caseosa. Bras Med. 1926;2:301–303. [Google Scholar]
- 2.Steiner PE, Lushbaugh C. Maternal pulmonary embolism by amniotic fluid as a cause of obstetric shock and unexpected death in obstetrics. JAMA. 1941;117:1245–1254. doi: 10.1001/jama.255.16.2187. [DOI] [PubMed] [Google Scholar]
- 3.Knight M, Tufnell D, Brocklehurst P, Spark P, Kurinczuk J. on behalf of the UK Obstetric Surveillance System: Incidence and risk factors for amniotic-fluid embolism. Obstet Gynecol. 2010;115:910–917. doi: 10.1097/AOG.0b013e3181d9f629. [DOI] [PubMed] [Google Scholar]
- 4.Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolisms: a population-based study on 3 million births in the United States. AJOG. 2008;199(49):e1–e8. doi: 10.1016/j.ajog.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 5.Kramer MS, Rouleau J, Liu S, Bartholomew S, Joseph KS. for the Maternal Health Study Group of the Canadian Perinatal Surveillance System: Amniotic fluid embolism: incidence, risk factors, and impact on perinatal outcome. BJOG. 2012;119:874–879. doi: 10.1111/j.1471-0528.2012.03323.x. [DOI] [PubMed] [Google Scholar]
- 6.Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. AJOG. 2009;201 doi: 10.1016/j.ajog.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark SL, Belfort MA, Dildy GA. Maternal death in the 21st century prevention and relationship to caesarean delivery. AJOG. 2008;199 doi: 10.1016/j.ajog.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 8.The Confidential Enquiry into Maternal and Child Health (CEMACH) London: CEMACH; 2007. Saving Mother’s lives reviewing maternal deaths to make childhood safer 2003-2006. [Google Scholar]
- 9.AG „Mütterliche Todesfälle“ 2011. 2012. AQUA-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen. [Google Scholar]
- 10.Stolk KH, Zwart JJ, Schutte J, van Roosmalen J. Severe maternal morbidity and mortality from amniotic fluid embolism in the Netherlands. Acta Obstet Gynecol Scand. 2012;91:991–995. doi: 10.1111/j.1600-0412.2012.01442.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. AJOG. 1995;172:1158–1167. doi: 10.1016/0002-9378(95)91474-9. [DOI] [PubMed] [Google Scholar]
- 12.Knight M, Berg C, Brocklehursr P, et al. Amniotic fluid embolism incidence, risk factors and outcomes: a review and recommendations. BMC Pregnancy & Childbirth. 2012;12 doi: 10.1186/1471-2393-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tufnell D, Knight M, Plaat F. Amniotic fluid embolism—an update. Anaesthesia. 2011;66:3–6. doi: 10.1111/j.1365-2044.2010.06597.x. [DOI] [PubMed] [Google Scholar]
- 14.Tufnell DJ. United Kingdom Amniotic Fluid Embolism Register. BJOG. 2005;112:1625–1629. doi: 10.1111/j.1471-0528.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Kamitomo M. Amniotic fluid embolism: a comparison between patients who survived and those who died. J Intern Med Res. 2009;37:1515–1521. doi: 10.1177/147323000903700529. [DOI] [PubMed] [Google Scholar]
- 16.Toy H. Amniotic fluid embolism. Eur J Gen Med. 2009;6:108–115. [Google Scholar]
- 17.Clark SL. Amniotic fluid embolism. Clin Obstet Gynecol. 2010;53:322–328. doi: 10.1097/GRF.0b013e3181e0ead2. [DOI] [PubMed] [Google Scholar]
- 18.Benson MD. A hypothesis regarding complement activation and amniotic fluid embolism. Medical Hypotheses. 2007;68:1019–1025. doi: 10.1016/j.mehy.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Benson MD. Current concepts of immunology and diagnosis in amniotic fluid embolism. Clin Dev Immunol. 2012 doi: 10.1155/2012/946576. doi: 10.1155/2012/946576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen KB, Chang SS, Tseng YL, et al. Amniotic fluid induces platelet-neutrophil aggregation and neutrophil activation. AJOG. 2013;208. 318:e1–e7. doi: 10.1016/j.ajog.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Gist RS, Stafford IP, Leibowitz AB, Beilin Y. Amniotic fluid embolism. Anesth Analg. 2009;108:1599–1602. doi: 10.1213/ane.0b013e31819e43a4. [DOI] [PubMed] [Google Scholar]
- 22.Kramer MS, Rouleau J, Baskett TF, Joseph KS. Amniotic fluid embolism and medical induction of labour: A retrospective, population-based cohort study. Lancet. 2006;368:1444–1448. doi: 10.1016/S0140-6736(06)69607-4. [DOI] [PubMed] [Google Scholar]
- 23.Ecker JL, Solt K, Fitzsimons MG, Mac Gillivray TE. Case 40-2012: A 43-years-old woman with cardiorespiratory arrest after a cesarean section. N Engl J Med. 2012;367:2528–2536. doi: 10.1056/NEJMcpc1201413. [DOI] [PubMed] [Google Scholar]
- 24.Stafford I, Sheffield J. Amniotic fluid embolism. Obstet Gynecol Clin North Am. 2007;34:545–553. doi: 10.1016/j.ogc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.O’ Shea, Eappen S. Amniotic fluid embolism. Int Anesthesiol Clin. 2007;45:17–28. doi: 10.1097/AIA.0b013e31802b8853. [DOI] [PubMed] [Google Scholar]
- 26.Dean LS, Rogers RP, Harley FA, Hood DD. Case Scenario: amniotic fluid embolism. Anesthesiology. 2012;116:186–192. doi: 10.1097/ALN.0b013e31823d2d99. [DOI] [PubMed] [Google Scholar]
- 27.Davies S. Amniotic fluid embolism: a review of the literature. Can J Anaesth. 2001;48:88–98. doi: 10.1007/BF03019822. [DOI] [PubMed] [Google Scholar]
- 28.Sisodia SM, Bendale KA, Khan WAZ. Amniotic fluid embolism: a cause of sudden maternal death and police inquest. Am J Forensic Med Pathol. 2012;33:330–334. doi: 10.1097/PAF.0b013e31825fb201. [DOI] [PubMed] [Google Scholar]
- 29.Malhotra P, Agarwei R, Awasthi A, Behera D. Delayed presentation of amniotic fluid embolism: lessons from a case diagnosed at autopsy. Respirology. 2007;12:148–150. doi: 10.1111/j.1440-1843.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Liu S, Ma M. Procoagulant activity and phosphatidylserine of amniotic fluid. Thromb Haemost. 2009;101:845–851. [PubMed] [Google Scholar]
- 31.Uszynski M, Uszynski W. Coagulation and fibrinolysis in amniotic fluid: physiology and observations on amniotic fluid embolism, preterm fetal membrane rupture, and pre-eclampsia. Semin Thromb Hemost. 2011;37:165–174. doi: 10.1055/s-0030-1270345. [DOI] [PubMed] [Google Scholar]
- 32.Estelles A, Gilabert J, Andres C, Espana F, Aznar J. Plasminogen activator inhibitors type 1 and type 2 in amniotic fluid during pregnancy. Thromb Haemost. 1990;64:281–285. [PubMed] [Google Scholar]
- 33.Collins NF, Bloor M, Mc Donnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anest. 2013;22:71–76. doi: 10.1016/j.ijoa.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Leighton BL, Wall MH, Lockhart EM, Phillips LE, Zatta AJ. Use of recombinant factor VIIa in patients with amniotic fluid embolism: a systematic review of case reports. Anesthesiology. 2011;115:1201–1208. doi: 10.1097/ALN.0b013e31821bdcfd. [DOI] [PubMed] [Google Scholar]
- 35.Franchitto N, Minville V, Dédouit F, Telmon N, Rougé D. Medical responsibility in the operating room: the example of an amniotic fluid embolism. J Forensic Sci. 2012;57:1120–1123. doi: 10.1111/j.1556-4029.2012.02098.x. [DOI] [PubMed] [Google Scholar]
- 36.Jecmenica D, Baralic I, Alempijevic D, Pavlevic S, Kiurski M, Terzic M. Amniotic fluid embolism - apropos two consecutive cases. J Forensic Sci. 2011;56:247–251. doi: 10.1111/j.1556-4029.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 37.Turillazzi E, Greco P, Neri M, Pomara C, Riezzo I, Fineschi V. Amniotic fluid embolism: still a diagnostic enigma for obstetrician and pathologist? Acta Obstet Gynecol Scand. 2009;88:839–841. doi: 10.1080/00016340902971474. [DOI] [PubMed] [Google Scholar]
- 38.Sinicina I, Pankratz H, Bise K, Matevossian E. Forensic aspects of post-mortem histological detection of amniotic fluid embolism. Int J Legal Med. 2010;124:55–62. doi: 10.1007/s00414-009-0351-x. [DOI] [PubMed] [Google Scholar]
- e1.Roberts CL, Algert CS, Knight M, Morris JM. Amniotic-fluid embolism in an Australian population-based cohort. BJOG. 2010;117:1417–1421. doi: 10.1111/j.1471-0528.2010.02656.x. [DOI] [PubMed] [Google Scholar]
- e2.Rossi AC, Mullin P. The etiology of maternal mortality in developed countries: a systematic review of the literature. Arch Gynecol Obstet. 2012;285:1499–1503. doi: 10.1007/s00404-012-2301-y. [DOI] [PubMed] [Google Scholar]
- e3.Clark SL. Strategies for reducing maternal mortality. Semin Perinatol. 2012;36:42–47. doi: 10.1053/j.semperi.2011.09.009. [DOI] [PubMed] [Google Scholar]
- e4.Welsch H, Wischnik A, Lehner R. Müttersterblichkeit. In: Schneider H, Husslein P, Schneider KTM, editors. Die Geburtshilfe. 4th edition. Berlin, Heidelberg, New York: Springer; 2011. pp. 1207–1224. [Google Scholar]
- e5.Perozzi KJ, Englert NC. Amniotic fluid embolism: An obstetric emergency. Crit Care Nurse. 2004;24:54–61. [PubMed] [Google Scholar]
- e6.Statistisches Bundesamt. Natürliche Bevölkerungsbewegung. Fachserie 1, Reihe 1.1, 2011. 2012 www.destatis.de/DE/Publikationen (last accessed on 27 Januay 2013) [Google Scholar]
- e7.Faktencheck Gesundheit. Kaiserschnittgeburten - Entwicklung und regionale Verteilung, Bertelsmann-Stiftung. https://faktencheck-gesundheit.de.
- e8.Heyes E, Aida G, Crocker A. The morbidity adherent placenta: diagnosis and management. Curr Opin Obstet Gynecol. 2011;23:448–453. doi: 10.1097/GCO.0b013e32834cef7a. [DOI] [PubMed] [Google Scholar]
- e9.Aqua-Institut: Geburtshilfe. Aqua-Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen. Göttingen: 2013. Qualitätsindikatoren - Bundesauswertung zum Erfassungsjahr 2012; 16/1 pp. [Google Scholar]
- e10.Awad JT, Shorten GD. Amniotic fluid embolism and isolated coagulopathy: atypical presentation of amniotic fluid embolism. Eur J Anaesthesiol. 2001;18:410–413. doi: 10.1046/j.0265-0215.2001.00859.x. [DOI] [PubMed] [Google Scholar]
- e11.Yang JS, Kim HS, Chang KH, Ryu HS, Joo HJ. Amniotic fluid embolism with isolated coagulopathy. J Reprod Med. 2006;51:64–66. [PubMed] [Google Scholar]
- e12.Clark SL. New concepts of amniotic fluid embolism: a review. Obstet Gynecol Surv. 1990;45:360–368. doi: 10.1097/00006254-199006000-00003. [DOI] [PubMed] [Google Scholar]
- e13.Harnett MJ, Hepner DL, Datta S, Kodali BJ. Effect of amniotic fluid on coagulation and platelet function in pregnancy. Anaesthesia. 2005;60:1068–1072. doi: 10.1111/j.1365-2044.2005.04373.x. [DOI] [PubMed] [Google Scholar]
- e14.Biron-Andreani C, Morau E, Schved JF, Hedon B, Dechaud H. Amniotic fluid embolism with haemostasis complications: primary fibrinogenolysis or disseminated intravascular coagulation? Pathophysiol Haemost Thromb. 2003;33:170–171. doi: 10.1159/000077826. [DOI] [PubMed] [Google Scholar]
- e15.Annecke T, Geisenberger T, Kürzl R, Penning R, Heindl B. Algorithm-based coagulation management of catastrophic amniotic fluid embolism. Blood Coagulation and Fibrinolysis. 2010;21:95–100. doi: 10.1097/MBC.0b013e328332cfe2. [DOI] [PubMed] [Google Scholar]
- e16.Legrand M, Rossignol M, Dreux S, et al. Diagnostic accuracy of insulin-like growth factor binding protein-1 for amniotic fluid embolism. Crit Care Med. 2012;40:2059–2063. doi: 10.1097/CCM.0b013e31824e6737. [DOI] [PubMed] [Google Scholar]
- e17.Rath W. Fruchtwasserembolie, Lungenembolie. In: Feige A, Rath W, Schmidt S, editors. Kreißsaal-Kompendium. Stuttgart: New York, Thieme; 2013. pp. 142–149. [Google Scholar]
- e18.Regilz-Zagrosek V, Seeland U, Geibel-Zehender A, Gohlke-Bärwolf C, Kruck I, Schaefer C. Herz-Kreislauferkrankungen in der Schwangerschaft. Deutsches Ärzteblatt. 2011;108:267–273. doi: 10.3238/arztebl.2011.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Crofts JF, Winter C, Sowter MC. Practical simulation training for maternity care - where we are and where next. BJOG. 2011;118:11–16. doi: 10.1111/j.1471-0528.2011.03175.x. [DOI] [PubMed] [Google Scholar]
- e20.van der Ven J, Houterman S, Steinweg RA, et al. TOSTI-Trial Group: Reducing errors in health care: cost-effectiveness of multidisciplinary team training in obstetric emergencies (TOSTI-study); a randomised controlled trial. BMC Pregnancy Childbirth. 2010;10 doi: 10.1186/1471-2393-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]