Abstract
Puberty is a critical period of development during which the reemergence of gonadotropin releasing hormone secretion from the hypothalamus triggers a cascade of hormone-dependent processes. Maturation of specific brain regions including the prefrontal cortex occurs during this window, but the complex mechanisms underlying these dynamic changes are not well understood. Particularly, the potential involvement of epigenetics in this programming has been under-examined. The epigenome is known to guide earlier stages of development, and it is similarly poised to regulate vital pubertal-driven brain maturation. Further, as epigenetic machinery is highly environmentally responsive, its involvement may also lend this period of growth to greater vulnerability to external insults, resulting in reprogramming and increased disease risk. Importantly, neuropsychiatric diseases commonly present in individuals during or immediately following puberty, and environmental perturbations including stress may precipitate disease onset by disrupting the normal trajectory of pubertal brain development via epigenetic mechanisms. In this review, we discuss epigenetic processes involved in pubertal brain maturation, the potential points of derailment, and the importance of future studies for understanding this dynamic developmental window and gaining a better understanding of neuropsychiatric disease risk.
Introduction
The brain undergoes critical organizational changes during the pubertal window, when reemergence of gondadotropin releasing hormone (GnRH) triggers a cascade of hormone-dependent processes. While previous reports have primarily focused on the classic role of hormones in driving neural and behavioral maturation during puberty, epigenetic mechanisms may also play an important role in guiding pubertal brain development. Further, epigenetic machinery is highly responsive to the environment and therefore may lend to this period of growth a greater vulnerability to external insults. As epidemiological studies demonstrate, individuals who experience early life adversity prior to and during puberty are at increased risk for psychiatric disease, especially affective disorders (Heim et al., 2010; Kendler and Eaves, 1986; Kendler and Gardner, 2011; Kendler et al., 1993; Stein et al., 1996; Wise et al., 2001).
The epigenome has been implicated in development from its earliest phase, as epigenetic stability is globally perturbed when gametes fuse, allowing the newly formed zygote to reacquire totipotency (reviewed in (Cantone and Fisher, 2013)). Disruption of the normal epigenetic environment during early development has serious consequences, and epigenetic dysfunction is a significant factor in precipitating human genetic disorders (as reviewed in (Berdasco and Esteller, 2013)). The epigenome is similarly poised during puberty to both regulate development and to potentially affect disease risk, though these regulatory mechanisms of pubertal development are largely understudied. However, recent evidence linking polycomb group protein-driven transcriptional silencing to the timing of pubertal onset in female rodents offers some insight into the relationship between the epigenome and puberty (Lomniczi et al., 2013). In this review, we focus on the proposed role of epigenetic mechanisms in driving pubertal brain development, both under normal conditions and in the face of external perturbations.
Maturation of the nervous system during puberty
Following a period of relative quiescence during childhood, massive brain reorganization and maturation occurs during puberty. Typical development of adolescent brain structure and activity has been examined in humans, where puberty is associated with a peak and subsequent decline in cortical grey matter and a continual, though sexually dimorphic, increase in cortical white matter volume, in both the frontal and parietal lobes (Giedd et al., 1999; Perrin et al., 2008; Pfefferbaum et al., 1994). Task-dependent brain activity also changes during adolescence. For example, improved performance on executive function tasks measuring working memory and response inhibition is associated with increased activity in the prefrontal and parietal cortices (Adleman et al., 2002; Kwon et al., 2002; Luna et al., 2001; Rubia et al., 2000). The development of important limbic brain areas, including the prefrontal cortex, hippocampus, and amygdala, has been demonstrated in animal models as well (Isgor et al., 2004; Lee et al., 2003; Matsuoka et al., 2010; Scherf et al., 2013).
Differences in pubertal brain development between males and females highlight the role of gonadal hormones during this window. Though the sex-specific programming of neural maturation is widespread, the majority of studies examining sex differences during puberty focus on the neural circuitry controlling the activation of reproductive behaviors. Evidence in rats suggests that new cells are added in a sex-dependent manner to brain regions that control reproductive behavior, with more cells being added to the male sexually dimorphic nucleus of the preoptic area and medial amygdala and more cells being added to the female anteroventral periventricular nucleus of the hypothalamus (Ahmed et al., 2008). These sex differences in the number of newly added cells directly correspond to sex differences in adult volume, suggesting that the effects programmed during puberty are long lasting. Gonadectomy prior to puberty eliminates such sex differences, indicating that gonadal hormones are key in driving the addition of new cells during puberty that sustain these sexual dimorphisms in adulthood. Studies in sheep have similarly described sex-specific changes in the morphology of specific limbic system brain nuclei during puberty (Nuruddin et al., 2013). Following GnRH release, both male and female sheep show reduced amygdala volume, although this loss is more substantial in females. These changes are dependent upon GnRH action at its receptor, as pharmacological blockade of the hypothalamic-pituitary-gonadal axis via a GnRH agonist results in a larger amygdala volume in both males and females. Together, these data suggest that aspects of normal brain development are dependent upon intact gonadal hormone levels, and represent an important organizational effect of the gonadal hormone surge during puberty.
While it is clear that processes initiated or guided by gonadal hormone action are integral to pubertal maturation, sexually dimorphic physiology and behavior may also originate independent of gonadal hormone levels. Investigation of the role of the sex chromosome complement (XX versus XY) independent of the hormonal milieu has been achieved with the use of the “four core” genotype mice, a line of mice where the testes determining factor gene, Sry, has been transposed onto an autosome, producing gonadal females (XX or XY-Sry, with ovaries) and males (XY or XX+Sry, with testes) (De Vries et al., 2002). Studies in these mice have demonstrated a partial dissociation between the role of sex chromosomes and the action of gonadal hormones in brain maturation and associated behaviors. Sex chromosomes contribute directly to the development of sex differences in the arginine vasopressin (AVP) system, social exploration, and reproductive behavior in adults, as indicated by both male and female XY mice being more masculine than XX mice (De Vries et al., 2002). Gonadal hormones primarily mediate other sexual dimorphisms, including cortical thickness and progesterone receptor expression, as mice with testes, irrespective of sex chromosome complement, are more masculine on these measures than mice with ovaries (Markham et al., 2003; Wagner et al., 2004). In contrast, behaviors such as intruder-directed aggression and maternal pup retrieval are determined by the interaction of both sex chromosome complement and gonadal hormone levels, as females but not males with XX differ from females with XY complement (Gatewood, 2006).
The complex processes guiding both sex-dependent and -independent pubertal maturation require precise chromatin regulation, and therefore suggest underlying epigenetic regulation. Modifications to the epigenome, by affecting gene expression without altering DNA sequence, mediate long-lasting changes in gene transcription and may serve as the link between environmental influences and gene transcription (Jessen and Auger, 2011). The most common epigenetic modifications include methylation of cytosines within CpG islands and histone modifications, chiefly the acetylation or methylation of core histone proteins (McCarthy et al., 2009; Meaney and Ferguson-Smith, 2010). Additionally, small noncoding RNAs, including microRNAs (miRs), are increasingly identified as important epigenetic modulators of neurodevelopment, largely due to their vast post-transcriptional regulation of protein-coding genes (Morgan and Bale, 2012).
Studies focused on the epigenetic control of normal pubertal brain maturation are limited; however, one notable example recently linked pubertal onset in females to methylation-driven epigenetic silencing (Lomniczi et al., 2013). The initiation of puberty in females is associated with specific changes in gene transcription, including increased gene expression of kisspeptin (kiss1) in the medial basal hypothalamus. Kiss1 neurons play many important roles in reproductive endocrinology, including the direct innervation and stimulation of GnRH neurons (Oakley et al., 2009). Lomniczi et al. demonstrated that increased promoter methylation and decreased expression of two polycomb group proteins leads to disinhibition of kiss1 expression and onset of estrous cyclicity, signaling pubertal onset. Further, chronic pharmacological inhibition of DNA methylation prior to puberty onset results in a pubertal delay. Upstream of DNA methylation regulation, disruption of histone deacetylation has been similarly shown to induce pubertal failure (Ojeda et al., 2010). Further evidence of the epigenetic control of pubertal onset was offered in the regulation of the GnRH gene itself, where distinct changes in methylation of the GnRH promoter were demonstrated across puberty in the rhesus monkey (Kurian and Terasawa, 2013). Additional insight into the epigenetic regulation of pubertal brain maturation can be inferred by exploring the role of epigenetic machinery in processes integral to pubertal development—gonadal hormone activity and the establishment of sex differences.
DNA Methylation
Much of the sex-specific brain programming during puberty depends upon the availability of gonadal hormone receptors, and, importantly, several studies suggest that the DNA methylation status of gonadal hormone receptors may influence their expression. Increased methylation has been associated with decreased expression of the estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and the progesterone receptor (Prewitt and Wilson, 2007; Westberry et al., 2011; 2010). The developmental time course of methylation of these genes has been examined in the rat. While there do not appear to be changes in methylation of either ERβ or progesterone receptor from birth through adulthood in the preoptic area or medial basal hypothalamus, areas of high receptor expression critical for the production of male and female sexual behavior, ER methylation increases over time, suggesting that its regulation is important in the processes of early neurodevelopment (Schwarz et al., 2010). The methylation status of ERα also increases during early development in the cortex, with significantly more methylation at postnatal day (PN) 18 and 25 compared to PN 2 and ERα mRNA levels that negatively correlate with methylation (Prewitt and Wilson, 2007; Westberry et al., 2010). Further, data suggest that ERα methylation is initiated by DNA methyltransferase (DNMT) 3a, the known de novo methylation enzyme, and maintained by DNMT1, the maintenance enzyme (Westberry et al., 2010). DNMT3A levels peak at PN 10 and fall back to low expression by PN 25, while DNMT 1 levels increase concomitantly and remain high in adulthood. A similar increased methylation and reduced expression of ERβ has been reported throughout the cortex during the natural progression of reduced circulating hormones in aging (Westberry et al., 2011). This increased ERβ methylation was also associated with increased cortical DNMT 1 and 3α expression, again suggesting a mechanism whereby local methylation levels are interrelated with gonadal status.
Additionally, changes in DNA methylation may drive sex differences in hormone receptor expression and the programming of related behaviors. As early as PN 1, females have increased methylation of ERα compared to males, suggesting that sex differences in the processes governed by this receptor, such as programming reproductive behaviors, are epigenetically driven. Disruption of local DNA methylation stability has been used to examine sex differences in important pubertal behaviors, such as social play (Forbes-Lorman et al., 2012; Kurian et al., 2008). The sexually dimorphic organization of the amygdala influences many social behaviors into adulthood (Forbes-Lorman et al., 2012). Specifically, the expression of AVP in the amygdala is typically higher in males, and it is linked to many social behaviors displayed predominantly in males, especially juvenile social play. Transiently reduced expression of the methyl binding protein MeCP2 at PN 1-3 eliminated the sex differences in AVP expression and in juvenile social play behaviors (Kurian et al., 2008). Similarly, gonadectomized males showed significant alterations in methylation patterns and gene expression in the bed nucleus of the stria terminalis (BNST), another sexually dimorphic brain region programmed during puberty, with castrated rats having decreased methylation and increased expression of ERα and increased methylation and reduced expression of AVP compared to intact males (Auger et al., 2011). These effects were reversed by testosterone replacement, supporting a possible role for gonadal hormones to regulate gene expression through epigenetic mechanisms.
It should be noted that while the classic dogma for the relationship between DNA methylation and gene expression is one of increasing methylation resulting in decreased expression, there is now growing evidence supporting a more complex relationship (reviewed in (McCarthy and Nugent, 2013)). For example, methylation status may be indicative of past, not future, gene expression and therefore not be predictive. Additionally, the association of methylation status with gene repression may vary with the location of methylation along the gene. Further, research has shown that methylation, via MeCP2, can attract transcriptional activators such as CREB1 and therefore increase gene expression (Chahrour et al., 2008). Thus, interpretation of methylation data should be carefully undertaken with the caveat of our yet incomplete understanding of the full effects of methylation.
Histone acetylation
Histone acetylation is an important epigenetic modification critical to all gene transcription, with specific histone marks playing a role in early development and likely contributing to pubertal maturation as well. There is a sex difference in the medial preoptic area in the acetylation of histones associated with promoters of ERα and aromatase genes and in the binding of histone deacetylase (HDAC) 2 and 4 to their promoters at PN 1-3 (Matsuda et al., 2011). HDAC binding is increased in males compared to females, indicating that males have lower acetylation of these promoters. The increased HDAC binding in males is key to programming male sexual behavior, and global HDAC inhibition in neonatal rats results in a disruption of adult behavior. Another brain region, the BNST, which is larger in volume and contains more cells in males than in females, is dependent on testosterone exposure during early neonatal life (Murray et al., 2009). Treatment with the HDAC inhibitor, valproic acid, on PN 1 significantly reduced both the volume and cell number in the BNST of males and testosterone-treated females, thereby eliminating the sex difference in the size of this brain region. In addition, this reversal in morphology was linked with a reversal in reproductive behaviors associated with the BNST. These results demonstrate that histone acetylation during early postnatal life plays a critical role in brain masculinization, and provides a potential mechanism by which the sexually dimorphic brain organization during puberty may be similarly regulated by gonadal hormone-mediated changes in histone marks.
microRNAs
Appropriate regulation of gene expression results from a controlled balance between transcriptional and post-transcriptional mechanisms. miRs are small non-coding RNAs that regulate posttranscriptional gene expression by affecting the stability or translational efficiency of specific mRNA targets (Bartel, 2004). An individual miR can directly target more than a hundred different mRNA targets. In fact, one genome-wide bioinformatics study annotated more than 45,000 conserved miR binding sites in the 3’ UTR of 60% of human genes (Friedman et al., 2008). Together, these characteristics indicate that this mode of regulation can enact far-reaching programmatic effects and should be viewed as a major component of an integrated gene expression regulatory mechanism (Baek et al., 2008; Selbach et al., 2008).
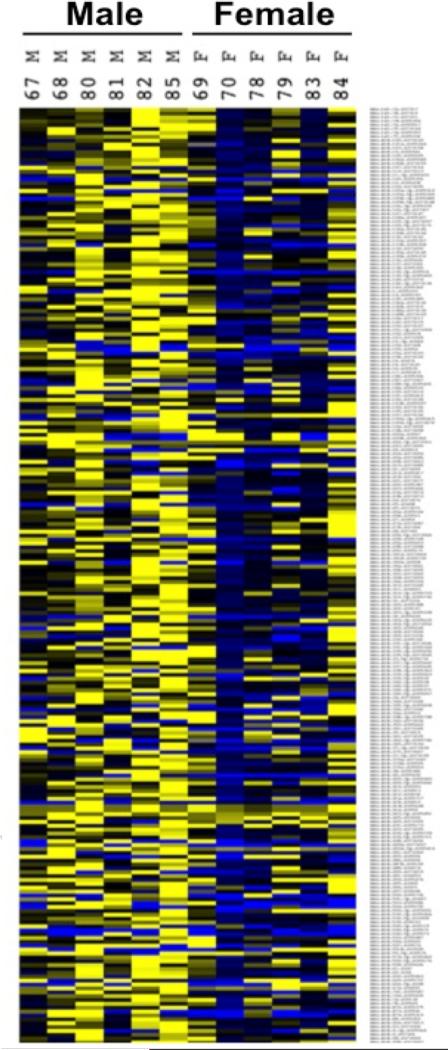
Initial studies characterizing the impact of gonadal steroids, including dihydrotestosterone, progesterone, and estradiol on miR expression patterns have generally involved the analyses of steady-state mature miR levels, often in hormone-responsive tumor samples (Klinge, 2012; Kuokkanen et al., 2010; Waltering et al., 2010). More recently, studies have demonstrated that the miR environment of the brain is responsive to gonadal hormones and/or sex during windows of dynamic hormonal change, including the perinatal window of brain masculinization and menopause (Morgan and Bale, 2011; Rao et al., 2013). In agreement with these studies, we have recently examined sex differences in miR expression patterns in the PN 28 prefrontal cortex, a brain region undergoing sex-biased development during puberty, and found remarkable sex-specific patterns of expression here (Figure 1). Unbiased hierarchical clustering of the expression patterns of 249 of the most abundant miRs completely segregated male and female samples into distinct clusters. These findings support the likely importance of these epigenetic mediators in the sexually dimorphic development of the brain during the peripubertal period.
Figure 1. Robust sex differences in miRNA expression patterns in prepubertal male and female prefrontal cortex at PN 28.
Analyses of the miRNA environment as analyzed by miRNA Taqman qRT-PCR Array of 239 most abundant rodent miRNAs (ABI) on micropunches from PN 28 PFC C57:129 F1 hybrid mice was compared by Pearson Correlational Hierarchical Clustering. Yellow color indicates increased levels compared to average male expression. Blue indicates reduced levels compared to average male expression. All data are normalized to control miRs sno135 and sno202, N=6.
The adolescent brain epigenome: poised to respond to environmental perturbations
In addition to guiding normal pubertal brain maturation, the epigenome may shift the trajectory of nervous system development following environmental challenge during the pubertal period. Alterations in epigenetic machinery following external perturbations have been well demonstrated during earlier stages of development. For example, in typically developing rat litters, neonatal males receive more maternal grooming than females (Moore, 1984; Moore and Morelli, 1979). This behavior has since been associated with increased ERα methylation (Kurian et al., 2010; Moore, 1984; Moore and Morelli, 1979). Further, simulated maternal grooming can masculinize female ER methylation and expression patterns in PN10 rats, and these grooming-induced changes in offspring methylation have been associated with juvenile social play behavior (Edelmann and Auger, 2011; Edelmann et al., 2013; Kurian et al., 2010). Recent studies characterizing developmental susceptibility to external perturbations during puberty itself similarly support involvement of epigenetic mechanisms.
Studies investigating long-term changes in the brain and in behavior from environmental exposures during puberty have focused on adverse stimuli commonly encountered during adolescence, including cannabis, alcohol, and/or chronic stress. Extensive links between substance use during adolescence and adult behavioral dysfunction, including an increased likelihood of alcohol or drug dependence, have been drawn in human and animal studies (DeWit et al., 2000; Hall and Lynskey, 2005). Here too, the induction of long-term outcomes suggests involvement of the epigenome. Adolescent male rats exposed to Δ(9)-tetrahydrocannabinol every third day from PN 28-49 showed enhanced heroin self-administration and associated dysregulation of the proenkephalin system in the nucleus accumbens in adulthood (Tomasiewicz et al., 2012). This brief Δ(9)-tetrahydrocannabinol exposure produced long-term changes in the pattern of normal H3K9 dimethylation associated with gene expression and behavioral reprogramming. Similarly, adolescent alcohol exposure evoked long-term histone modifications and related transcriptional changes, where male rats exhibit upregulated histone acetyl transferase activity in the prefrontal cortex, an increased amount of acetylated histone H3 and H4, and increased H3K4 dimethylation in the promoter regions of genes involved in reward signaling, including cFos, Cdk5, and FosB (Pascual et al., 2012). In addition, it is important to note that stimuli typically associated with positive outcomes, such as physical exercise, also affect the developmental trajectory of the adolescent brain through epigenetic mechanisms. In fact, one week of voluntary wheel running in adolescent male mice produced increased acetylation of histone H3 and decreased DNMTs and HDACs in the hippocampus and cerebellum (Abel and Rissman, 2013).
Chronic stress during adolescence may similarly exploit epigenetic mechanisms to elicit adult neurobehavioral deficits. Tran et al. described heightened visceral pain behaviors resembling irritable bowel syndrome following chronic water avoidance stress in pubertal rodents, associating behavioral changes with increased glucocorticoid receptor methylation and reduced corticotropin-releasing factor methylation in the amygdala (Tran et al., 2013). Niwa et al. characterized a mouse model investigating the interaction of the DISC1 mutation, a genetic risk factor for schizophrenia, with 3 weeks of social isolation stress during puberty (Niwa et al., 2013). Stressed mice with a dominant-negative DISC1 showed adult behavioral deficits across a variety of dimensions, including pre-pulse inhibition, forced swim behavior, and locomotor activity. The gene × environment interaction also produced hypermethylation of the tyrosine hydroxylase gene in mesocortical dopaminergic projection cells. Interestingly, both the behavioral and epigenetic deficits elicited by social isolation stress in DISC1 mice are reversed by chronic blockade of glucocorticoid receptors.
Importantly, long-term neurobehavioral changes often occur in a sex-specific fashion, underscoring a potential interaction of epigenetic-driven maturation with known hormonal changes during puberty. Male and female rats selectivity bred for resilience or susceptibility to the forced swim test and subsequently exposed to a mixed modality chronic stress paradigm display differential effects on behavior in the forced swim and sucrose preference based on both sex and lineage (Harrell et al., 2013). Chronic intermittent restraint stress during puberty in rats similarly elicits a sex-specific effect, with stressed females, but not males, exhibiting a long-term blunting of neurogenesis in the dentate gyrus (Barha et al., 2010). These data characterize puberty as a period of considerable sex-specific vulnerability to stress, with lasting impacts on the maturing neural systems and consequences for adult behavior via epigenetic mechanisms.
Neuropsychiatric disease symptom onset often occurs during or immediately following puberty. Thus, a more thorough understanding of epigenetic mechanisms involved in this maturational window may provide novel insight into disease risk factors. Dysregulation of stress neurocircuitry is one of the most common endophenotypes across neuropsychiatric disease, with both hyper- and hypo-reactivity of the hypothalamic-pituitary-adrenal (HPA) axis being reported across disorders (Arborelius et al., 1999; Corbett et al., 2009; Moghaddam, 2002; Nestler et al., 2002; Walker et al., 2008). Maturation of the adult stress response has been examined in rodents (Foilb et al., 2011; Goldman et al., 1973; Romeo et al., 2006; 2004a; 2004b; Vázquez and Akil, 1993). Differential control at many levels of the HPA axis, including neural activation of the paraventricular nucleus, glucocorticoid-dependent negative feedback, and baseline corticotropin-releasing factor expression, have been associated with the rapid change in stress responsivity, suggesting that puberty stimulates a wholesale change in the regulation of the HPA axis (Goldman et al., 1973; Lui et al., 2012; Romeo et al., 2005; 2007; Viau et al., 2005). Gonadal hormone levels also impact stress reactivity differently in adult and prepubertal animals, highlighting further that processes critical to the organization of stress neurocircuitry likely involve epigenetic mechanisms (Carey et al., 1995; Handa et al., 1994; McCormick et al., 2002; Redei et al., 1994; Romeo et al., 2004b; 2004a; Viau and Meaney, 1996).
Conclusion
Dynamic processes of brain maturation occur during puberty, and epigenetic mechanisms are clearly involved in regulating this sex-specific development (Figure 2). Epigenetic machinery is poised to exert programmatic control over brain development during puberty and is environmentally responsive, likely guiding maturation under both normal and adverse conditions. Therefore, further examination into the specific role of epigenetics in regulating pubertal brain maturation may help elucidate aspects of this unique window in brain development and novel molecular targets vulnerable in neuropsychiatric disease risk.
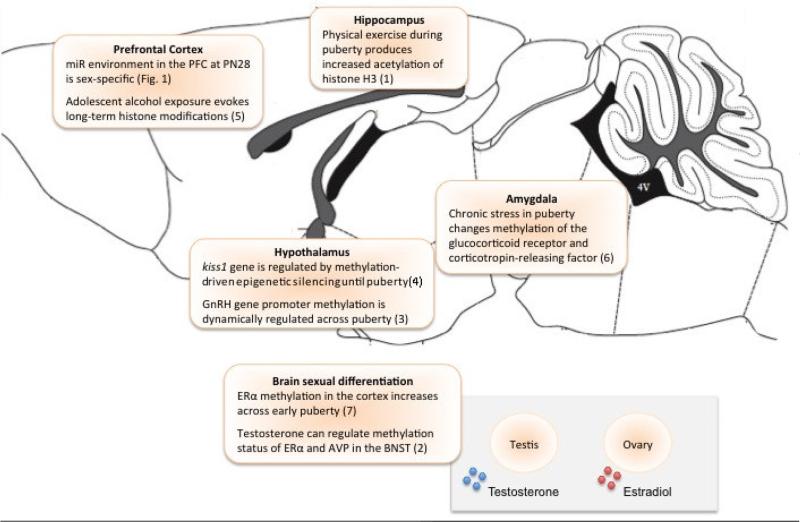
Figure 2. Epigenetic mechanisms are broadly involved in pubertal-driven brain maturation.
Schematic summarizing the role of the epigenome in the development of the brain, both under normal circumstances and in the face of external perturbations. Research has shown that the development a wide variety of brain regions involves epigenetic mechanisms. The diagram was modified from the mouse brain atlas of Paxinos and Watson (Paxinos and Watson, 2007). AVP – arginine vasopressin; BNST – bed nucleus of the stria terminalis; ER – estrogen receptor alpha; GnRH – gonadotropin releasing hormone; PFC – prefrontal cortex. (1Abel & Rissman, 2013, 2Auger et al, 2011, 3Kurian & Terasawa, 2013, 4Lomniczi et al 2013, 5Pascual et al, 2012, 6Tran et al, 2013, 7Westberry et al, 2010)
Highlights.
Puberty is a critical period of brain development
Puberty is a time of greater risk for neuropsychiatric disease
Epigenectic mechanisms are involved in normal maturational processes
Therefore, epigenetic mechanisms are a likely target for environmental perturbation
This review discusses epigenetic processes in pubertal brain maturation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel JL, Rissman EF. Running-induced epigenetic and gene expression changes in the adolescent brain. International Journal of Developmental Neuroscience. 2013;31:382–390. doi: 10.1016/j.ijdevneu.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, Reiss AL. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, DonCarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Coss D, Auger AP, Forbes-Lorman RM. Epigenetic control of vasopressin expression is maintained by steroid hormones in the adult male rat brain. Proc Natl Acad Sci USA. 2011;108:4242–4247. doi: 10.1073/pnas.1100314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LAM. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2010;21:1216–1227. doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Berdasco M, Esteller M. Genetic syndromes caused by mutations in epigenetic genes. Hum Genet. 2013;132:359–383. doi: 10.1007/s00439-013-1271-x. [DOI] [PubMed] [Google Scholar]
- Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20:282–289. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J. Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong STC, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Schupp CW, Levine S, Mendoza S. Comparing cortisol, stress, and sensory sensitivity in children with autism. Autism Res. 2009;2:39–49. doi: 10.1002/aur.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain, Behavior, and Immunity. 2011;25:1299–1304. doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann MN, Demers CH, Auger AP. Maternal Touch Moderates Sex Differences in Juvenile Social Play Behavior. PLoS ONE. 2013 doi: 10.1371/journal.pone.0057396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foilb AR, Lui P, Romeo RD. The transformation of hormonal stress responses throughout puberty and adolescence. J. Endocrinol. 2011;210:391–398. doi: 10.1530/JOE-11-0206. [DOI] [PubMed] [Google Scholar]
- Forbes-Lorman RM, Rautio JJ, Kurian JR, Auger AP, Auger CJ. Neonatal MeCP2 is important for the organization of sex differences in vasopressin expression. Epigenetics. 2012;7:230–238. doi: 10.4161/epi.7.3.19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2008;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26:2335–2342. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Goldman L, Winget C, Hollingshead GW, Levine S. Postweaning development of negative feedback in the pituitary-adrenal system of the rat. Neuroendocrinology. 1973;12:199–211. doi: 10.1159/000122169. [DOI] [PubMed] [Google Scholar]
- Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug and alcohol review. 2005 doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol. Behav. 1994;55:117–124. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Harrell CS, Hardy E, Boss-Williams K, Weiss JM, Neigh GN. Sex and lineage interact to predict behavioral effects of chronic adolescent stress in rats. Behav. Brain Res. 2013;248:57–61. doi: 10.1016/j.bbr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev. Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Jessen HM, Auger AP. Sex differences in epigenetic mechanisms may underlie risk and resilience for mental health disorders. Epigenetics. 2011 doi: 10.4161/epi.6.7.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Eaves J. Symptoms of Anxiety and Depression in a Volunteer Twin Population. Arch Gen Psychiatry. 1986;34:213–221. doi: 10.1001/archpsyc.1986.01800030023002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. A longitudinal etiologic model for symptoms of anxiety and depression in women. Psychological medicine. 2011;41:2035–2045. doi: 10.1017/S0033291711000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, Neale MC, Heath AC, Eaves LJ. The prediction of major depression in women: toward an integrated etiologic model. Am J Psychiatry. 1993;150:1139–1148. doi: 10.1176/ajp.150.8.1139. [DOI] [PubMed] [Google Scholar]
- Klinge CM. miRNAs and estrogen action. Trends Endocrinol Metab. 2012;23:223–233. doi: 10.1016/j.tem.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen S, Chen B, Ojalvo L, Benard L. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82:791–801. doi: 10.1095/biolreprod.109.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Bychowski ME, Forbes-Lorman RM, Auger CJ, Auger AP. Mecp2 organizes juvenile social behavior in a sex-specific manner. J Neurosci. 2008;28:7137–7142. doi: 10.1523/JNEUROSCI.1345-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian JR, Terasawa E. Epigenetic Control of Gonadotropin Releasing Hormone Neurons. Frontiers in Endocrinology. 2013;4 doi: 10.3389/fendo.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Reiss AL, Menon V. Neural basis of protracted developmental changes in visuo-spatial working memory. Proc Natl Acad Sci USA. 2002;99:13336–13341. doi: 10.1073/pnas.162486399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady D, Koenig JI. Corticosterone alters N-methyl-d-aspartate receptor subunit mRNA expression before puberty. Brain Res Mol Brain Res. 2003;115:55–62. doi: 10.1016/s0169-328x(03)00180-3. [DOI] [PubMed] [Google Scholar]
- Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, Knoll JG, Wright H, Pfeifer GP, Ojeda SR. Epigenetic control of female puberty. Nat Neurosci. 2013;16:281–289. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui P, Padow VA, Franco D, Hall BS, Park B, Klein ZA, Romeo RD. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol. Behav. 2012;107:104–111. doi: 10.1016/j.physbeh.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, Keshavan MS, Genovese CR, Eddy WF, Sweeney JA. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Markham JA, Jurgens HA, Auger CJ, De Vries GJ, Arnold AP, Juraska JM. Sex differences in mouse cortical thickness are independent of the complement of sex chromosomes. Neuroscience. 2003;116:71–75. doi: 10.1016/s0306-4522(02)00554-7. [DOI] [PubMed] [Google Scholar]
- Matsuda KI, Mori H, Nugent BM, Pfaff DW, McCarthy MM, Kawata M. Histone deacetylation during brain development is essential for permanent masculinization of sexual behavior. Endocrinology. 2011;152:2760–2767. doi: 10.1210/en.2011-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Sumiyoshi T, Tsunoda M, Takasaki I, Tabuchi Y, Uehara T, Itoh H, Suzuki M, Kurachi M. Change in the expression of myelination/oligodendrocyte-related genes during puberty in the rat brain. J Neural Transm. 2010;117:1265–1268. doi: 10.1007/s00702-010-0461-0. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Nugent BM. Epigenetic contributions to hormonally mediated sexual differentiation of the brain. Journal of Neuroendocrinology. 2013 doi: 10.1111/jne.12072. n/a–n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat Neurosci. 2010;13:1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Moore CL. Maternal contributions to the development of masculine sexual behavior in laboratory rats. Dev. Psychobiol. 1984;17:347–356. doi: 10.1002/dev.420170403. [DOI] [PubMed] [Google Scholar]
- Moore CL, Morelli GA. Mother rats interact differently with male amd female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. Res. 2011;31:11748–11755. doi: 10.1523/JNEUROSCI.1887-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CP, Bale TL. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol Sex Differ. 2012;3:1–9. doi: 10.1186/2042-6410-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EK, Hien A, De Vries GJ, Forger NG. Epigenetic Control of Sexual Differentiation of the Bed Nucleus of the Stria Terminalis. Endocrinology. 2009;150:4241–4247. doi: 10.1210/en.2009-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, Cascella NG, Kano S-I, Ozaki N, Nabeshima T, Sawa A. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. doi: 10.1126/science.1226931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuruddin S, Bruchhage M, Ropstad E, Krogenæs A, Evans NP, Robinson JE, Endestad T, The Sex On Brain European Research Group – SOBER. Westlye LT, Madison C, Haraldsen IRH. Effects of peripubertal gonadotropin-releasing hormone agonist on brain development in sheep-A magnetic resonance imaging study. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine reviews. 2009 doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda SR, Lomniczi A, Sandau U, Matagne V. Endocrine Development, Endocrine Development. KARGER; Basel: 2010. New concepts on the control of the onset of puberty; pp. 44–51. [DOI] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Perrin JS, Hervé PY, Leonard G, Perron M, Pike GB, Pitiot A, Richer L, Veillette S, Pausova Z, Paus T. Growth of white matter in the adolescent brain: role of testosterone and androgen receptor. J Neurosci. 2008;28:9519–9524. doi: 10.1523/JNEUROSCI.1212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Prewitt AK, Wilson ME. Changes in estrogen receptor-alpha mRNA in the mouse cortex during development. Brain Res. 2007;1134:62–69. doi: 10.1016/j.brainres.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao YS, Mott NN, Wang Y, Chung WCJ, Pak TR. MicroRNAs in the aging female brain: A putative mechanism for age-specific estrogen effects. Endocrinology. 2013 doi: 10.1210/en.2013-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei E, Li L, Halasz I, McGivern RF, Aird F. Fast glucocorticoid feedback inhibition of ACTH secretion in the ovariectomized rat: effect of chronic estrogen and progesterone. Neuroendocrinology. 1994;60:113–123. doi: 10.1159/000126741. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology. 2005;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, Jasnow AM, McEwen BS. Age- and stress-induced changes in corticotropin-releasing hormone mRNA expression in the paraventricular nucleus of the hypothalamus. Neuroendocrinology. 2007;85:199–206. doi: 10.1159/000102950. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Karatsoreos IN, McEwen BS. Pubertal maturation and time of day differentially affect behavioral and neuroendocrine responses following an acute stressor. Horm Behav. 2006;50:463–468. doi: 10.1016/j.yhbeh.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004a;79:125–132. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Lee SJ, McEwen BS. Differential stress reactivity in intact and ovariectomized prepubertal and adult female rats. Neuroendocrinology. 2004b;80:387–393. doi: 10.1159/000084203. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Smyth JM, Delgado MR. The amygdala: an agent of change in adolescent neural networks. Horm Behav. 2013 doi: 10.1016/j.yhbeh.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Anderson G, Hazen AL, Ross CA, Eldridge G, Forde DR. Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am J Psychiatry. 1996;153:275–277. doi: 10.1176/ajp.153.2.275. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Jacobs MM, Wilkinson MB, Wilson SP, Nestler EJ, Hurd YL. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biological Psychiatry. 2012;72:803–810. doi: 10.1016/j.biopsych.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology. 2013;38:898–906. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Vázquez DM, Akil H. Pituitary-adrenal response to ether vapor in the weanling animal: characterization of the inhibitory effect of glucocorticoids on adrenocorticotropin secretion. Pediatr Res. 1993;34:646–653. doi: 10.1203/00006450-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner CK, Xu J, Pfau JL, Quadros PS, De Vries GJ, Arnold AP. Neonatal mice possessing an Sry transgene show a masculinized pattern of progesterone receptor expression in the brain independent of sex chromosome status. Endocrinology. 2004;145:1046–1049. doi: 10.1210/en.2003-1219. [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- Waltering KK, Porkka KP, Jalava SE, Urbanucci A, Kohonen PJ, Latonen LM, Kallioniemi OP, Jenster G, Visakorpi T. Androgen regulation of micro - RNAs in prostate cancer. Prostate. 2010;71:604–614. doi: 10.1002/pros.21276. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor beta expression in the rat cortex during aging. Neuroreport. 2011;22:428–432. doi: 10.1097/WNR.0b013e328346e1cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise L, Zierler S, Krieger N, Harlow B. Adult onset of major depressive disorder in relation to early life violent victimisation: a case-control study. The Lancet. 2001;358:881–887. doi: 10.1016/S0140-6736(01)06072-X. [DOI] [PubMed] [Google Scholar]