Abstract
Background
Symptomatic intracranial hemorrhage (sICH) occurs uncommonly after ischemic stroke therapy with Tissue plasminogen activator (TPA). Clotting factor administration may be a treatment option.
Objective
To determine if treatment with clotting factors was associated with improved outcomes in sICH.
Methods
We conducted a retrospective cohort study within University of Texas at Houston Stroke registry involving consecutive patients from February 1, 2007 to June 30, 2011 with TPA related sICH; including cases with subsequent intra-arterial therapy.
Intervention
clotting factor administration; fresh frozen plasma or cryoprecipitate.
Outcomes
modified Rankin Score mRS at discharge, death, and hematoma expansion.
Results
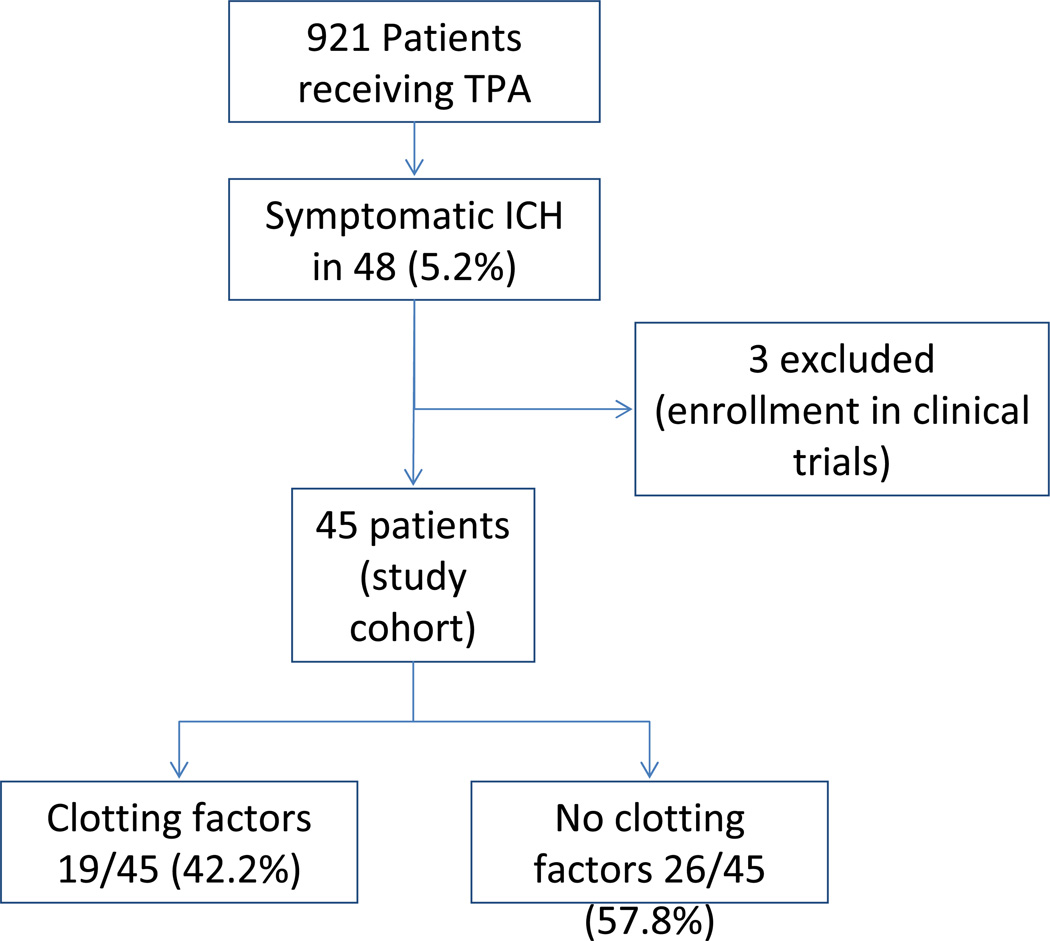
Of 921 patients treated with TPA, 48 (5.2%) had sICH and 45 met criteria for the study. Nineteen patients received clotting factors (42.2%) (18 received FFP and 7 received cryoprecipitate), while 26 (57.8%) patients received conservative management without clotting factors. None of the patients treated with clotting factors and only 2 of those who did not receive clotting factors had a good outcome; mRS ≤2. All the patients treated with clotting factors and most of those not treated were left bedridden or dead (mRS 4–6); 19 (100%) vs. 22 (85%). Mortality was 9 (47.4%) vs. 9 (34.6%) respectively. There was no difference in hematoma expansion between the two groups.
Conclusions
We found no evidence that treatment of sICH with clotting factors has a favorable effect on clinical or radiological outcomes. However, the sample was small due to the low frequency of sICH. New treatments are urgently needed for this uncommon yet serious condition.
Keywords: Cerebrovascular disease, Stroke, symptomatic intracerebral hemorrhage, thrombolysis, fresh frozen plasma, acute ischemic stroke
Introduction
Symptomatic intracranial hemorrhage (sICH) is a well-recognized complication of thrombolysis for acute ischemic stroke treatment. Tissue plasminogen activator (TPA) is currently the only drug therapy proven to improve patient outcomes after acute ischemic stroke. [1, 2] Intraarterial therapy (IAT), intraarterial thrombolysis and mechanical thrombectomy, also carry a risk of sICH. [3–6] Although it is an infrequent complication, occurring in 2.2–6.4% of patients receiving TPA for ischemic stroke, much of the reluctance to administer TPA to stroke patients is due to fear of sICH. [1, 2, 7–9] Patients that experience sICH are at a high risk of poor outcome. [10]
While considerable attention has been given to patient selection to avoid unfavorable outcomes, research focused on treatment of thrombolysis-related symptomatic intracranial hemorrhage (TPA-sICH) is lacking.[11–13] Currently, hemostatic therapy by administering clotting factors, fresh frozen plasma FFP or cryoprecipitate, has been incorporated in clinical protocols to treat this complication. [14] The efficacy of this practice has not been studied well. It is unclear if administering clotting factors alters the clinical course of this complication.
Aims
We sought to identify whether administering clotting factors for TPA-sICH alters patient outcomes.
Methods
Patients and setting
This is a retrospective study based on the prospectively collected University of Texas at Houston Stroke registry. The registry contains systematically collected data on a total of 921 consecutive acute ischemic stroke patients treated with IV alteplase within 6 hours from symptom onset admitted to Memorial Hermann hospital at the Texas Medical Center from February 1, 2007 and June 30, 2011. In the present study, we included patients admitted with a diagnosis of acute ischemic stroke who then developed sICH defined as any CT or MRI evidence of intracranial hemorrhage associated with any deterioration in neurological signs or symptoms, and additionally those with parenchymal hemorrhage type 2 (PH2) on imaging regardless of symptomatic status. [1, 15, 16] Patients could have either supra- or infra-tentorial sICH within the infarct or remote from it. We excluded patients who only had asymptomatic hemorrhagic infarction or asymptomatic parenchymal hemorrhage type 1. The Institution Review Boards at the University of Texas at Houston and Memorial Hermann hospital granted approval for the study.
Interventions, risk factors, confounding variables
For our analysis we separated patients into a.) those who were treated with clotting factors; FFP or cryopreciptate within hours of sICH onset and b.) those who were not treated with clotting factors. We considered various confounding variables; stroke risk factors, ischemic stroke prognostic factors and intracerebral hemorrhage prognostic factors. [17–19] Additionally, we collected data on blood and platelet transfusions.
Outcomes
The main outcome of interest was good outcome after sICH, defined as mRS ≤2 at hospital discharge. Due to the high disability rate with sICH, only two patients had mRS ≤2, we also did analyses for outcomes of different thresholds; in particular very poor outcome mRS 5–6. Other outcomes of interest were mortality and hematoma expansion. Hematoma expansion was calculated from first scan that showed ICH and last available scan during hospitalization. The timing of the follow up scan to assess hematoma expansion was not standardized. As no established criteria for hematoma expansion in thrombolysis related intracranial hemorrhage exists, we used the definitions of hematoma expansion in spontaneous intracerebral hemorrhage. The most commonly used definitions were used; 6 mL growth, 12.5 mL growth and 33% growth.[10]
Data Collection and data cleaning
NIHSS certified stroke team physicians assessed pretreatment and 24-hour NIHSS. Certified abstractors prospectively obtained modified Rankin scale at discharge. We evaluated presence and location of intracranial hemorrhage by review of neuroradiology report and examining the images. Hematoma volume was measured by author YJA blinded to the treatment assignment using the ABC/2 method. [20]
The following data points were available from the stroke registry: demographics (age, gender, race), stroke risk factors (diabetes mellitus, hypertension, hyperlipidemia, atrial fibrillation and smoking), home medications, NIHSS, mRS at discharge, mortality, laboratory variables (baseline glucose, platelets, INR, PTT), presence and type of intracranial hemorrhage and surgical procedures (ventriculostomy and craniotomy).
Data that was unavailable from the registry was retrospectively abstracted from the medical records: Glasgow coma scale (GCS) at sICH onset, administration of clotting factors, blood transfusion, platelet transfusion, imaging variables (initial & final sICH volume, sICH location, presence of intraventricular hemorrhage, presence of hydrocephalus) fibrinogen levels and d-dimer levels. We abstracted initial fibrinogen levels before clotting factor administration and last fibrinogen level within 3 days of clotting factor administration.
For Quality Control, data re-abstraction was performed on 10% of randomly selected records (n=5). The original data was compared with re-abstracted data using a statistical program and discrepancies were adjudicated by a clinical expert not involved in the authorship of the study. For variables that exceeded 0.5% error rate, all the records were re-abstracted, compared and discrepancies were adjudicated. We corrected confirmed errors and the corrected data set was used for the statistical analysis.
Ischemic stroke treatment
All patients were treated with intravenous rt-PA 0.9 mg/kg; 10% bolus and remainder given over 1 hour. The stroke team provided subsequent care in neurological intensive care and stroke units. Patients meeting National Institute of Neurological Disorders and Stroke tPA Stroke Trial (NINDS) criteria were treated as part of standard of care. After publication of ECASS-3 the time-window for routine treatment was extended with observation of ECASS-3 criteria. Off-label use was considered in patients who did not meet NINDS or ECASS-3 criteria on an individual basis if they did not meet criteria for enrolment in any prospective clinical trials. Patients or their next-of-kin granted consent for off-label treatment. Patient selection for off-label treatment was left to the treating physician using non-contrast CT, ASPECTS score, CT angiography or MRI. Patients with large vessel occlusion on CT angiography were considered for intra-arterial therapy (IAT) on an individual basis. Intra-arterial therapy (IAT) was not standardized and included combinations of different devices and drugs.
Statistical analysis
Frequencies and percentages were reported for categorical variables. Means with standard deviations or median with interquartile range (IQR) were presented for continuous data. Comparison between treatment group and non-treatment group were performed using Chi-square and Fischer's exact tests for categorical variables and independent samples t-tests and Mann-Whitney U tests for continuous variables. 95% confident intervals of differences in means or proportions were presented and Hodges-Lehmann estimate of the median differences and their confidential intervals were calculated. Exact logistic regression analysis, univariate and multivariate, was conducted to determine associations of treatment for sICH with outcomes after adjustments from possible confounders. Variables with P<0.25 in univariate analysis were included in multivariate analysis. Multivariate regression was performed through stepwise selection, and variables which met change-in-estimate (CIE) criteria were selected. The parameter estimates of coefficients from exact logistic analysis were presented. Statistical analysis was performed using SAS 9.3 (Cary, NC).
Results
In this study cohort, symptomatic intracranial hemorrhage occurred in 48 (5.2%) patients. Three patients were excluded because of enrollment into clinical trials testing experimental interventions. Out of the 45 patients, 29 (64.4%) patients met criteria for PH2, and 32 (71.1%) met NINDS criteria for sICH. Prior to having intracranial hemorrhage, 35 (77.8%) patients received intravenous TPA alone and 10 (22.2%) received intravenous TPA followed by intra-arterial therapy. Clotting factors were administered to 19 (42.2%) patients; 12 received FFP without cryoprecipitate, 1 received cryoprecipitate without FFP and 6 received both, while 26 (57.8%) patients with sICH were not treated with clotting factors. None of the patients received factor VIIa or prothrombin complex concentrate. Platelet transfusions were administered to 4 patients; all of whom were also treated with clotting factors. Blood transfusions were administered in 4 patients who were treated with clotting factors and 2 patients who were not treated with clotting factors. (figure 1).
Figure 1.
Study flow diagram
*Symptomatic ICH was defined as patients experiencing symptomatic intracerebral hemorrhage SICH by NINDS definition, and additionally those with Parenchymal hemorrhage type 2 PH2 on imaging regardless of symptomatic status
Ten patients treated with TPA between 3 and 4.5 hours developed sICH. Of these patients, only 1 patient had ECASS-3 exclusions; for age greater than 80 years and being on an oral anticoagulant. None of the patients treated with IV TPA between 3 and 4.5 hours who developed sICH had NIHSS >25, severe hypertension BP >185mmHg systolic or >110mmHg diastolic, or a combination of diabetes mellitus with previous stroke. Only 1 patient who developed sICH was treated with TPA beyond 4.5 hours. Of the patients treated with TPA between 3 and 4.5 hours who developed sICH 6 (60%) were treated with clotting factors; all 6 received FFP and 3 also received cryoprecipitate. The remaining four (40%) patients were not treated with clotting factors.
Stroke risk factors and ischemic stroke prognostic factors
Apart from post-TPA NIHSS which was higher in patients treated with clotting factors and smoking which was more common in patients not treated with clotting factors, there were no significant differences in stroke risk factors or variables that might influence outcome between the treatment and non-treatment groups; including age, history of diabetes, baseline glucose, pre-TPA NIHSS, and proportion of patients undergoing intra-arterial therapy (table 1).
Table 1.
Characteristics of 45 Patients with symptomatic intracerebral hemorrhage
| Treated with Clotting factors n=19 |
No Clotting factors n=26 |
Difference in means, medians or proportions (95% CI.) |
|
|---|---|---|---|
| Stroke risk factors | |||
| Age, mean±SD | 69.5±13.9 | 66.8±13.6 | 2.7 (−6.7, 12.1) |
| Coronary artery disease, n (%) | 4 (21.1%) | 4 (15.4%) | 5.7 (−17.3, 28.6) |
| Diabetes mellitus, n (%) | 5 (25%) | 3 (11.5%) | 14.8 (−8.5, 38.1) |
| Hypertension, n (%) | 12 (66.7%) | 20 (83.3%) | −16.7 (−43.1, 9.7) |
| Smoking†, n (%) | 1 (5.3%) | 9 (34.6%) | −29.4 (−50.2, −8.5) |
| Ischemic stroke prognostic factors | |||
| Glucose, mean±SD | 137.9±55.8 | 149.6±58.9 | −11.7 (−46.6, 23.1) |
| Circulation, n (%) | |||
| Anterior | 16 (84.2%) | 22 (84.6%) | 0.4 (−29.4, 28.3) |
| Posterior | 2 (10.5%) | 4 (15.4%) | −4.9 (−33.1, 24.5) |
| Both | 1 (5.3%) | 0 | 5.3 (−24.1, 34.1) |
| NIHSS pre-TPA, median (IQR) | 15.5 (9, 18) | 14 (10, 21) | 0 (−4, 4) |
| NIHSS post-TPA, median† (IQR) | 27 (21.5, 38.5) | 17 (8, 37) | 9 (5, 12) |
| Intraarterial Therapy, n (%) | 4 (21.1%) | 6 (23.1%) | 2.0 (−26.5, 22.4) |
| ICH associated factors | |||
| PH2, n (%) | 12 (63.2%) | 17 (68%) | −4.8 (−33.2, 23.5) |
| SICH, n (%) | 14 (73.7%) | 18 (72%) | 1.7 (−24.8, 28.2) |
| GCS at time of ICH, median (IQR) | 7 (5.5, 9) | 9 (7, 13) | −2 (−3, −1) |
| Initial ICH volume, mean±SD | 48.1±41.4 | 35.3±61.8 | 12.8 (−19.0, 44.6) |
| Intraventricular hemorrhage†, n (%) | 13 (68.4%) | 8 (30.8%) | 37.6 (10.2, 65.1) |
| Hydrocephalus†, n (%) | 14 (73.7%) | 8 (30.8%) | 42.9 (16.3, 69.5) |
| Anticoagulation prior to ICH, n (%)‡ | 0 | 2 (7.7%) | −7.7 (−17.9, 2.6) |
| Time TPA to ICH (hours), mean ± SD | 7.8±6.0 | 10.9±7.9 | −3.1 (−7.5, 1.4) |
| Platelets, mean±SD | 227.6±60.8 | 222.5±70.8 | 5.1 (−34.6, 44.9) |
| PTT, mean±SD | 31.2±8.2 | 28±4.2 | 3.2 (−0.9, 7.5) |
| INR, mean±SD | 1.4±0.4 | 1.2±0.3 | 0.2 (−0.1, 0.4) |
| Fibrinogen, initial level† mean±SD | 224.9±121.7 | 385±163.3 | −160.2 (−282.1, − 38.4) |
| Ventriculostomy, n (%) | 3 (15.8%) | 2 (7.7%) | 8.1 (−11.2, 27.4) |
| Hemicraniectomy, n (%) | 4 (21.1%) | 4 (16%) | 5.1 (−18.2, 28.4) |
| Pre-TPA antiplatelets | 6 of 15 (40%) | 13 of 23 (56.5%) | 16.5 (−48.5, 15.5) |
IQR indicates Interquartile range
P<0.05
Only 2 patients had anticoagulation prior to ICH, both were on warfarin prior to TPA and had subtherapeutic INR. No therapeutic anticoagulation was given after TPA
Intracerebral hematoma prognostic factors
Patients who were treated with clotting factors were more likely to have had intraventricular hemorrhage or hydrocephalus at baseline than patients who were not treated with clotting factors 13 (68%) vs. 8 (31%) and 14 (74%) vs. 8 (31%), respectively. There were no other significant differences in intracerebral hematoma prognostic factors such as proportion of PH2, GCS at time of sICH and initial ICH volume. The proportions of patients undergoing craniotomy were similar between the two groups (table 1).
We reviewed the available MRIs for evidence of cerebral amyloid angiopathy by Boston criteria.[21] MRI was available in 9 of 19 patients treated with clotting factors and 18 of 26 patients not treated with clotting factors. Among those treated with clotting factors there was one patient with probable cerebral amyloid angiopathy. Among those who were not treated with clotting factors there was only 1 patient with possible cerebral amyloid angiopathy. Also, among the patients receiving intra-arterial therapy there was no evidence of vascular malformations or aneurysms on angiography.
Outcomes
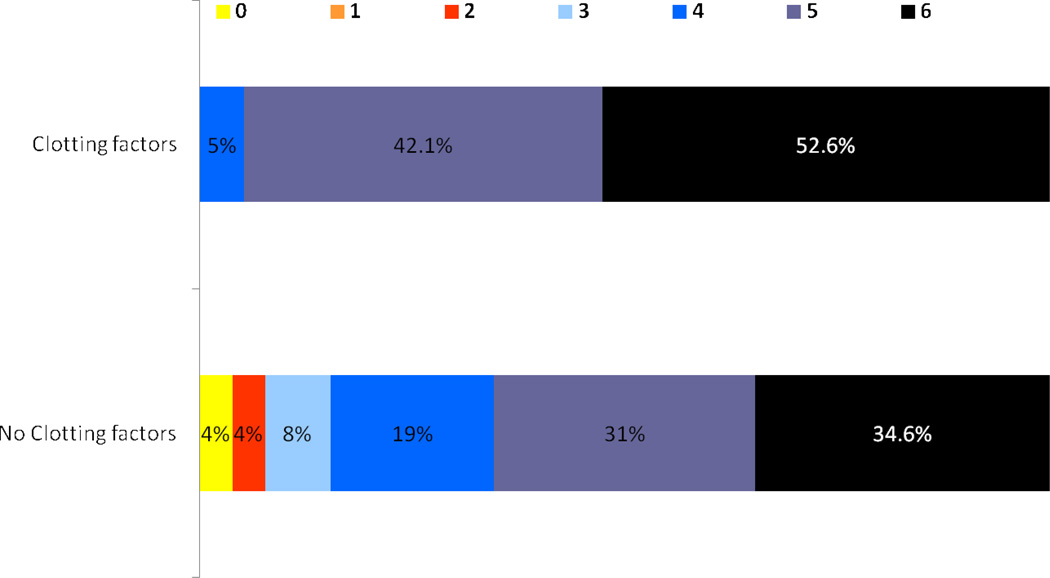
There were only 2 patients who had a good outcome at discharge, mRS ≤2; both had PH2 and neither had received clotting factors. There were no significant differences in other outcomes at discharge. Mortality was high and not significantly different between the two groups; 9 (47%) in those treated with clotting factors vs. 9 (35%) in those not treated with clotting factors, OR 1.7, 95% CI 0.507–5.7. (figure 2, table 2).
Figure 2.
Modified Rankin score at discharge in patients treated with clotting factors and those without such treatment.
Table 2.
Outcome of patients with symptomatic intracerebral hemorrhage
| Outcome measure | Treated with Clotting factors n=19 |
No Clotting factors n=26 |
Difference in (means, medians or proportions), Odds ratios OR, (95%CI) |
|---|---|---|---|
| mRS, median (IQR) | 6 (5–6) | 4 (4–6) | 0 (0, 1) |
| mRS 4–6, proportion | 19 (100%) | 22 (85%) | 15 (−14.5, 43.1) |
| mRS 5–6 (Unadjusted)* | OR 9.53 (1.09–83.44) | ||
| mRS 5–6 (Adjusted) † | OR 5.98 (0.61–58.41) | ||
| Death, proportion | 9 (47.4%) | 9 (34.6%) | 12.8 (−16.2, 41.7) |
| Death (Unadjusted) | OR 1.7 (0.507–5.7) | ||
| Death (Adjusted) ‡ | OR 0.246 (0.005–2.121) | ||
| Fibrinogen, final level | 369.7.2±133.2 | 500±107.5 | −130.3 (−283.5, 23.0) |
mRS, indicates modified Rankin scale
IQR indicates interquartile range
near-compete separation limited adjustment for multiple risk factors
Adjusted for post-TPA NIHSS
Adjusted for presence of GCS(≥9 versus ≤ 8), initial ICH volume(≥30 versus <30), hydrocephalus, presence of intraventricular hemorrhage and PTT.
Most of the patients in both groups had poor outcomes; mRS >3 in the majority. Therefore, we considered other thresholds for defining poor outcome. There was no significant difference in outcome even when other thresholds were considered. Being bedridden or dead, mRS 4–6, occurred in 19 (100%) patients treated with clotting factors and 22 patients (85%) not treated with clotting factors (table 2, figure 2). When using the threshold of mRS 5–6, treatment with clotting factors was associated with a small statistically significant different odds of having a very poor outcome, OR 9.53 95% CI 1.09–83.44. However, this analysis does not include a correction for multiple testing and the confidence intervals are very broad. After adjusting for difference in post-TPA NIHSS, there was no significant effect of clotting factors on outcome (table 2). Adjusting for other confounders was not possible due to near-complete separation (18 patients among total 19 patients in treatment group had mRS 5–6). The results from multivariate regression analysis showed, after adjusting for GCS at sICH onset (≤8 versus >8), initial ICH volume (≥30 versus <30 cc), intraventricular hemorrhage, hydrocephalus and PTT, there was no treatment effect on mortality OR =0.246, 95% CI: 0.005–2.121 (table 2). The results from multivariable regression also showed that patients with GCS >8 at sICH onset less likely to have mortality OR=0.065, 95%CI: 0.001–0.901, p-value=0.0381. Due to complete separation (all 19 patients in treatment group had mRS 4–6), multivariate regression analysis was not possible for other outcome thresholds (mRS 4–6) or by using other outcome variables (table 2).
Surrogate markers
Fibrinogen data was available for 16/19 (80%) patients treated with clotting factors and 11/26 (42.3%) patients not treated. Where serial data were available, initial and final fibrinogen levels were significantly lower in patients treated with clotting factors. Patients treated with clotting factors had lower initial fibrinogen levels; 224.9±121.1 vs. 385.1±163.3, difference in mean of −160.2 95% CI −282.1–38.4. (table 1). Fibrinogen levels after treatment were high in both groups but higher in patients not treated with clotting factors; 369.7±133.2 vs. 500.0±107.5, difference in mean of −130.3, 95% CI. −283.5–23.0). Hematoma expansion data were available for 32/45 (71.1%); 12/19 (63.2%) of those treated with clotting factors and 20/25 (80%) of those not treated with clotting factors. There was a mean of 2.9 days between CT scans; standard deviation of 2.95. The proportions of patients experiencing hematoma expansion was variable according to the definition used (table 3) but overall we found no significant differences in hematoma expansion between those treated and not treated with clotting factors.
Table 3.
Hematoma expansion in patients with symptomatic intracerebral hemorrhage
| Treated with Clotting factors n=12 |
No Clotting factors n=20 |
Difference in proportions (95%CI) |
|
|---|---|---|---|
| Hematoma expansion, n (%) | |||
| by 6ml criteria | 5 (41.7) | 4 (20%) | 21.7 (−15.1, 54.5) |
| by 12ml criteria | 2 (16.7) | 2 (10%) | 6.7 (−29.3, 41.6) |
| by 33% criteria | 4 (33.3) | 7 (35%) | −1.7 (−37.1, 33.9) |
Discussion
The majority of the patients with t-PA related sICH had a poor outcome confirming findings of other studies. [10] In the present study, we were unable to demonstrate a robust effect of clotting factors on disability. This highlights the need for interventions that reduce disability from this complication. The disability reflects primary injury caused by the initial ischemic stroke and secondary injury caused by sICH. SICH occurs more often in patients with severe ischemic stroke. Interestingly, reports suggest that the overall prognosis may be driven by the severity of the initial ischemic stroke rather than the presence of sICH. [10, 22] Therefore, while measuring proportions of patients with disability is clinically important it may be an insensitive measure of efficacy of clotting factor treatment. The sample size of our study was too small to assess for effect of clotting factors in patients with sICH and low initial NIHSS. This is an obstacle for future research in sICH given that it is an uncommon event; both in clinical trials and clinical practice.[1, 2, 8, 9, 23] Separating the risk of disability attributable to either the primary injury of the initial stroke or secondary injury caused by incident sICH remains challenging. Another limitation regarding measurement of outcomes in our study is that disability data was only available at hospital discharge. It is conceivable that longer term follow up might provide different results and allow detection of a difference between treatment and non-treatment groups. Length of follow up is relevant as efficacy of TPA in ischemic stroke is demonstrable at 3 months but not at discharge. Given that our study showed that most patients had mRS>3, the disability threshold used for this study was likely too optimistic; mRS ≤2. Taken together with recent literature, these results suggest using other outcomes may be more sensitive to detect a difference; for example using mRS≤4 as an outcome measure in studies of therapies that may avert catastrophic sICH outcomes. This is supported by evidence that current surrogate markers for spontaneous ICH such as hematoma expansion are more predictive for poorer outcomes than for good outcomes. [24] However, in the present cohort, we were unable to demonstrate a robust effect on disability even when using other thresholds such as mRS 5–6 or mortality (table 2). Even though the analysis suggested a possible effect using mRS 5–6 threshold, caution should be exercised as this analysis is unadjusted for error from conducting multiple analyses and the confidence intervals are very broad. Prognostic risk factors are useful in guiding clinical decisions and in patient stratification for interventional trials. The results from the present cohort suggested patients with coma at sICH onset (GCS≤8) had higher chance of mortality. Additionally, the results indicated that coma at sICH onset (GCS≤8), initial sICH volume ≥30ml, presence of intraventricular hemorrhage and presence of hydrocephalus were potential confounders for the effects of clotting factors on mortality.
We sought to detect a biological effect of clotting factors using hematoma expansion and fibrinogen levels as surrogate measures of efficacy. In our cohort, we detected a mixed effect on surrogates. The difference in initial fibrinogen levels between patients treated with clotting factors and patients who were not likely reflects bias of the treating physicians. What is demonstrated is that levels improved in both groups. This may reflect effect of clotting factors on fibrinogen levels; as well as the natural recovery of fibrinogen after effects of fibrinolysis subside. The final fibrinogen level, while greater than 200 in all patients in the treatment group, was still lower than in patients not treated with clotting factors. Conclusions about effects on fibrinogen are limited by incomplete data in patients not treated with clotting factors. With regards to hematoma expansion, we were unable to detect a treatment effect in spite of changes in fibrinogen levels. It is important to note that the time course, frequency and outcome of hematoma expansion in sICH are not well established. Knowledge of these parameters would assist in defining appropriate outcome measures for clinical studies. Additionally, appropriate surrogate markers such as, hematoma expansion/retraction on imaging or laboratory tests that give global measures of thrombotic/thrombolytic/anticoagulant status, need to be validated for potential use in early phase studies. In spontaneous ICH, hematoma expansion has been shown to be a specific yet insensitive measure of poor outcome; the predictive value in TPA-sICH is undetermined. [24]
In our study, there were several sources of bias that were unavoidable due to the retrospective design in spite of using a prospective registry. The treatment allocation was based on clinical judgment of treating physicians and therefore nonrandom. At our center, we incorporated guidance that clotting factors be administered to patients with low fibrinogen levels; especially those less than 100. In spite of similar baseline NIHSS, patients who were treated with clotting factors had a higher Post-TPA NIHSS, a higher incidence of intraventricular hemorrhage and hydrocephalus. High NIHSS at baseline is a marker of poor outcome in ischemic stroke.[25, 26] Intraventricular hemorrhage and hydrocephalus are both poor prognostic risk factors in spontaneous intracerebral hemorrhage. [27] It is plausible that this may have counteracted potential benefit conferred by clotting factors in TPA-sICH. With regards to hematoma expansion, the timing of repeat CT scan to assess for expansion was not standardized. This may have reduced our ability to detect a symptomatic expansion and late expansion; creating bias against patients that had a good clinical course. However, there were only 2 patients with a good clinical course, neither of whom received clotting factors. Additionally, as this was a retrospective review we were unable to obtain accurate data on the exact duration from sICH detection to clotting factor administration. We were also unable to standardize the dose of clotting factors.
In conclusion, TPA-sICH carries a poor prognosis with no effective therapies. There is no evidence in this study that clotting factors alter the prognosis or have a measurable biological effect on imaging surrogate markers. An explanation may be that TPA-sICH is not related to a clotting factor deficiency but rather to other mechanisms; such as shifting of hemostatic mechanisms towards fibrinolysis or loss of neurovascular unit integrity. Potential therapies worth further study include: shifting the hemostatic-balance towards thrombosis using anti-fibrinolytics or other agents. More rapid or selective administration of factors using prothrombin complex concentrate or activated factor VII may prove to be viable interventions as well. Measures that influence neurovascular unit integrity, by targeting matrix-metalloproteinases for example, merit additional investigation in preventative and therapeutic paradigms. Interventions for hydrocephalus and intraventricular hemorrhage such as CSF diversion are also of investigational consideration in TPA-sICH. Moreover, prospective studies are necessary to delineate the clinical and imaging course of TPA-sICH to define appropriate outcomes and provide sample-size estimates for interventional trials.
Acknowledgments
Funding source:
The University of Texas at Houston Stroke registry is supported by NIH SPOTRIAS P50 NS 044227 and NIH Training Grant, 5 T32 NS007412
Abbreviations
- FFP
fresh frozen plasma
- GCS
Glasgow coma scale
- IQR
Interquartile range
- mRS
modified Rankin scale
- NIHSS
NIH Stroke Scale
- NINDS
National Institute of Neurological Disorders and Stroke
- OR
odds ratio
- PH
parenchymal hemorrhage
- sICH
symptomatic intracranial hemorrhage
- TPA
tissue plasminogen activator
- TPA-sICH
tissue plasminogen activator related symptomatic intracranial hemorrhage
Footnotes
Conflict of interest:
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Ethical Approval:
Institution Review Board IRB granted exemption approval for the study protocol and for the request for waiver of informed consent for our research.
Author Contributions:
Study concept and design: SIS, YJA, NVB, VM. Acquisition of data: YJA, NVB, DDN. Analysis and interpretation of data YJA, HP, NS, FSV, NVB, SIS. Statistical analysis: HP, FSV. Drafting of the manuscript: YJA. Critical revision of the manuscript for important intellectual content: YJA, NS, HP, SIS. Study supervision: SIS.
Disclosures:
Dr. Alderazi reports no disclosures
Dr. Barot reports no disclosures
Dr. Peng reports no disclosures
Dr. Vahidy reports no disclosures
Dr. Navalkele reports no disclosures
Dr. Sangha reports no disclosures
Dr. Misra reports no disclosures
Dr. Savitz reports no disclosures
Contributor Information
Yazan J Alderazi, Email: yazanalderazi@yahoo.com.
Nirav Kumar V. Barot, Email: onenirav@gmail.com.
Hui Peng, Email: Hui.Peng@uth.tmc.edu.
Farhaan S. Vahidy, Email: Farhaan.Vahidy@uth.tmc.edu.
Digvijaya D Navalkele, Email: navdeep1sangha@yahoo.com.
Navdeep Sangha, Email: Digvijaya.D.Navalkele@uth.tmc.edu.
Vivek Misra, Email: misrav@uthscsa.edu.
Sean I. Savitz, Email: Sean.I.Savitz@uth.tmc.edu.
Reference
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. Jama. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 4.The enumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–2768. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 5.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 6.Flint AC, Duckwiler GR, Budzik RF, et al. Mechanical thrombectomy of intracranial internal carotid occlusion: pooled results of the MERCI and Multi MERCI Part I trials. Stroke. 2007;38:1274–1280. doi: 10.1161/01.STR.0000260187.33864.a7. [DOI] [PubMed] [Google Scholar]
- 7.Brown DL, Barsan WG, Lisabeth LD, et al. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46:56–60. doi: 10.1016/j.annemergmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 10.Strbian D, Sairanen T, Meretoja A, et al. Patient outcomes from symptomatic intracerebral hemorrhage after stroke thrombolysis. Neurology. 2011;77:341–348. doi: 10.1212/WNL.0b013e3182267b8c. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 12.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 13.Souza LC, Payabvash S, Wang Y, et al. Admission CT perfusion is an independent predictor of hemorrhagic transformation in acute stroke with similar accuracy to DWI. Cerebrovasc Dis. 2012;33:8–15. doi: 10.1159/000331914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein JN, Marrero M, Masrur S, et al. Management of thrombolysis-associated symptomatic intracerebral hemorrhage. Arch Neurol. 2010;67:965–969. doi: 10.1001/archneurol.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke. 1999;30:2280–2284. doi: 10.1161/01.str.30.11.2280. [DOI] [PubMed] [Google Scholar]
- 16.Seet RC, Rabinstein AA. Symptomatic Intracranial Hemorrhage following Intravenous Thrombolysis for Acute Ischemic Stroke: A Critical Review of Case Definitions. Cerebrovasc Dis. 2012;34:106–114. doi: 10.1159/000339675. [DOI] [PubMed] [Google Scholar]
- 17.Hemphill JC, 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 18.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 19.Christoforidis GA, Slivka A, Mohammad Y, et al. Size matters: hemorrhage volume as an objective measure to define significant intracranial hemorrhage associated with thrombolysis. Stroke. 2007;38:1799–1804. doi: 10.1161/STROKEAHA.106.472282. [DOI] [PubMed] [Google Scholar]
- 20.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.str.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 21.Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 22.Saver JL. Hemorrhage after thrombolytic therapy for stroke: the clinically relevant number needed to harm. Stroke. 2007;38:2279–2283. doi: 10.1161/STROKEAHA.107.487009. [DOI] [PubMed] [Google Scholar]
- 23.Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowlatshahi D, Demchuk AM, Flaherty ML, et al. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adams HP, Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Saver JL, Smith EE, et al. Relationship of national institutes of health stroke scale to 30-day mortality in medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1:42–50. doi: 10.1161/JAHA.111.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diringer MN, Edwards DF, Zazulia AR. Hydrocephalus: a previously unrecognized predictor of poor outcome from supratentorial intracerebral hemorrhage. Stroke. 1998;29:1352–1357. doi: 10.1161/01.str.29.7.1352. [DOI] [PubMed] [Google Scholar]