Abstract
Coronary angiography is the gold standard for defining obstructive coronary disease. However, radiation exposure remains an unwanted hazard. Patients referred for coronary angiography with abdominal circumference <45 inches and glomerular filtration rate >60mL/min were randomized to the Fluorography (n=25) or Cineangiography (n=25) group. Patients in the Fluorography group underwent coronary angiography using retrospectively-stored fluorography with repeat injection under cineangiography only when needed for better resolution per operator’s discretion. Patients in the Cineangiography group underwent coronary angiography using routine cineangiography. The primary endpoint was patient radiation exposure measured by radiochromic film. Secondary endpoints included the radiation output measurement of kerma-area product (KAP) and air kerma at the interventional reference point (Ka,r), and operator radiation exposure measured by dosimeter. Patient radiation exposure (158.2mGy [76.5–210.2] vs 272.5mGy [163.3–314.0], p=0.001), KAP (1323μGy m2 [826–1765] vs 3451μGy m2 [2464–4818], p<0.001), and Ka,r (175 mGy [112–252] vs 558 mGy [313–621], p<0.001)was significantly lower in the Fluorography compared with Cineangiography group (42%, 62%, and 69% relative reduction, respectively). Operator radiation exposure trended in the same direction though statistically non-significant (Fluorography 2.35 μGy [1.24–6.30] vs Cineangiography 5.03μGy [2.48–7.80], p=0.059). In conclusion, the use of fluorography in a select group of patients during coronary angiography with repeat injection under cineangiography only when needed was efficacious at reducing patient radiation exposure.
Keywords: Radiation, Fluoroscopy, Coronary Angiography
Currently, cineangiography is the standard method used to record images during fluoroscopic procedures and is a major contributor to total radiation dose administered during coronary angiography (1). However, current technology now allows for improved visualization under fluoroscopy alone and the option to save these images for review (fluorography), thereby providing a possible lower radiation alternative to cineangiography (2). In this study, we aim to evaluate the efficacy and safety of fluorography imaging compared with standard cineangiography in the reduction of patient and operator radiation exposure during diagnostic coronary angiography.
Methods
Subjects were scheduled for a diagnostic coronary angiogram at a single tertiary care center. Patients with chronic kidney disease defined as an estimated glomerular filtration rate (GFR) <60 mL/minute were excluded as it was unclear if more contrast would be used in the Fluorography compared with Cineangiography group. In addition, an arbitrary abdominal circumference threshold of >45 inches was chosen as an additional exclusion criterion given that image quality decreases with increasing abdominal girth. Finally, patients undergoing emergent coronary angiography were also excluded (Figure 1). New York University School of Medicine’s Institutional Review Board approved this trial, and all patients provided written informed consent. This study is registered at ClinicalTrials.gov (Identifier:NCT01605045).
Figure 1.
Screening, enrollment, and randomization of study population
Participants were stratified by use of trans-femoral versus trans-radial access and randomized in blocks of 4 and 8, using opaque sealed envelopes to 1 of 2 groups: Fluorography group or Cineangiography group. Patients randomized to the Fluorography group had coronary angiography performed using the fluoroscopy-save function (retrospectively-stored fluorography). Operators then had the option to repeat an injection of contrast under cineangiography only if they were unsatisfied with the quality of an image under fluorography alone. Patients randomized to Cineangiography had coronary angiography performed and recorded using standard cineangiography.
Trans-femoral or trans-radial access was obtained under sterile technique, followed by administration of either 500 units unfractionated heparin through a 6 French trans-femoral sheath or a cocktail of 2500 units unfractionated heparin, 2.5mg of verapamil, and 100μg of nitroglycerin through a5 French trans-radial sheath. Contrast was administered via an automated injector (MEDRAD, MEDRAD Inc., Pittsburgh, Pennsylvania), and camera angles used were per operator discretion. Operators were asked to defer further imaging of peripheral arteries and the left ventricle and performance of percutaneous coronary intervention (PCI) until after radiation exposure measures were collected. Pulsed fluoroscopy was used at a frame rate of 15 frames per second. The Artis Zee coronary angiography system equipped with software version VC14 and lead shields (Siemens AG, Germany) and a ceiling mounted lead shield were used for all procedures. The use of a RadPad® (Worldwide Innovations & Technologies Inc., Kansas City, Kansas) protective drape was encouraged when available.
Patient demographics and anthropometric data were recorded prospectively for all participants. Medical and surgical histories were obtained via chart review and confirmed by patient interview, while laboratory data were collected from chart review. Procedural characteristics were recorded in real-time. Degree of coronary artery diameter stenosis was visually estimated by operating attending physician.
The primary measure was patient radiation exposure, measured by a 14″ by 17″ Gafchromic® XR RV3 film sheet(International Specialty Products, Wayne, New Jersey) placed underneath the patient during the procedure. Film sheets were quantitatively analyzed with use of the FilmQA-XR™ software to quantitatively determine the amount of peak skin dose radiation exposure. Secondary measures included kerma-area product (KAP) measured in μGy m2 and air kerma at the interventional reference point (Ka,r) measured in mGy by built-in software in the Siemens cardiac angiography system. Operator radiation exposure was measured in μR by a dosimeter placed in the left upper pocket of the lead apron worn by the primary operator. This was converted to a dose in units of μGy using the appropriate f-factor of 115. All measures of radiation were recorded immediately after diagnostic coronary angiography was completed.
The primary safety measure was the rate of 30-day major adverse cardiac events (MACE), defined as a composite of all-cause mortality, occurrence of new myocardial infarction, or occurrence of repeat coronary angiography, determined by telephone interviews 30 days after the procedure. A secondary safety measure was contrast use during coronary angiography, which was measured in real-time.
Each patient had 1 image repeated in the alternate modality with the same angulation for assessment of diagnostic quality. In the Fluorography group, 1 picture not already repeated under cineangiography per operator discretion was re-imaged using cineangiography. In the Cineangiography group,1 picture was repeated using fluorography. The designation of which image to repeat was determined at random prior to the procedure but not shared with the operator until after diagnostic imaging was complete. Once radiation data was collected, the operator was informed of which image to repeat using the alternate modality. The fluorography and cineangiography images were then scrambled (no image appeared consecutively with its pair) and reviewed by 2angiographers blinded to patient data, randomized group, and overall study design. The epicardial coronary artery of interest on left coronary artery injection was defined as the circumflex artery in caudal views, left anterior descending artery in cranial views, and left main artery in left anterior oblique caudal views.
Sample size was calculated based on observational data of mean radiation output (4199.4 ± 1340.4μGy m2)during coronary angiography using standard cineangiography performed over 6 months in our catheterization laboratory. Using a 2-sided 2-sample t-test, the number of patients needed in each group to observe a 25% decrease in radiation exposure in the Fluorography versus Cineangiography group at 80% power and a 0.05 significance level was estimated at 25 (total n=50). Categorical variables are presented as proportions and compared using Fisher’s exact test, while skewed continuous variables (Shapiro-Wilks test) are presented as median [interquartile range] and compared using Mann Whitney test. Association between patient skin dose and KAP was assessed using Spearman’s correlation test. Significance level was set at 0.05, and statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL).
Results
Baseline demographic, clinical, and procedural characteristics, including RadPad use, between patients in the Fluorography and Cineangiography groups were largely similar (Tables 1 and 2), except for a greater proportion of patients with a history of PCI in the Fluorography compared with the Cineangiography group.
Table 1.
Clinical characteristics of patients in the fluorography compared with standard cineangiography group
| Variable | Fluorography (n=25) | Cineangiography (n=25) | p-value |
|---|---|---|---|
| Age (years) | 62 [53–73] | 65 [54–72] | 0.82 |
| Men | 16 (64%) | 22 (88%) | 0.10 |
| White | 9 (36%) | 11 (44%) | |
| Black | 1 (4%) | 5 (20%) | 0.29 |
| Hispanic | 2 (8%) | 1 (4%) | |
| Asian | 13 (52%) | 8 (32%) | |
| Body mass index (kg/m2) | 26 [23–29] | 26 [22–28] | 0.29 |
| Abdominal circumference (inch) | 37 [35–42] | 38 [36–40] | 0.77 |
| Glomerular filtration rate (mL/min) | 84 [72–95] | 82 [76–100] | 0.39 |
| Prior percutaneous coronary intervention | 9 (36%) | 2 (8%) | 0.04 |
| Prior coronary artery bypass graft surgery | 2 (8%) | 2 (8%) | 1.00 |
| Indication for procedure | |||
| Atypical chest pain/dyspnea | 16 (64%) | 14 (56%) | |
| Stable angina | 7 (28%) | 5 (20%) | 0.48 |
| Unstable angina | 0 | 1 (4%) | |
| Preoperative evaluation | 0 | 2 (8%) | |
| Valvular disease | 2 (8%) | 2 (8%) | |
Table 2.
Procedural characteristics of patients in the fluorography compared with standard cineangiography group
| Variable | Fluorography (n=25) | Cineangiography (n=25) | p-value |
|---|---|---|---|
| Acess site | |||
| Radial | 12 (48%) | 10 (40%) | 0.54 |
| Femoral | 13 (52%) | 14 (56%) | |
| Radial-femoral crossover | 0 | 1 (4%) | |
| Primary operator | |||
| First year fellow | 9 (36%) | 11 (44%) | |
| Physician’s assistant | 8 (32%) | 1 (4%) | 0.08 |
| Interventional fellow | 6 (24%) | 10 (40%) | |
| Attending physician | 2 (8%) | 3 (12%) | |
| Difficulty navigating periphery | 1 (4%) | 4 (16%) | 0.35 |
| Right dominance | 23 (92%) | 23 (92%) | 1.00 |
| No views to visualize right coronary artery | |||
| 1 | 3 (12%) | 6 (24%) | 0.63 |
| 2 | 15 (60%) | 13 (52%) | |
| 3 | 5 (20%) | 4 (16%) | |
| No views to visualize left coronary artery | |||
| 3 | 2 (8%) | 4 (16%) | 0.58 |
| 4 | 15 (60%) | 16 (64%) | |
| 5 | 5 (20%) | 2 (8%) | |
| Radiation protection drape | 7 (28%) | 7 (28%) | 1.00 |
| Use of collimation | 20 (80%) | 16 (64%) | 0.35 |
| Degree of coronary artery narrowing | |||
| ≤30% diameter stenosis | 12 (48%) | 16 (64%) | |
| 40–69% diameter stenosis | 6 (24%) | 2 (8%) | |
| No coronary arteries ≥70% narrowed | 0.29 | ||
| 1 | 4 (16%) | 6 (24%) | |
| 2 | 1 (4%) | 1 (4%) | |
| 3 | 2 (8%) | 0 | |
| Proceed to percutaneous coronary intervention | 5 (20%) | 4 (16%) | 1.00 |
| Repeat injection under cineangiography | 12 (48%) | -- | -- |
Among the 25 patients in the Fluorography group, 12 (48%) underwent repeat of at least 1 image under cineangiography per operator discretion (8 patients with 1 image, 3 patients with 2 images, and 1 patient with 4 images repeated under cineangiography). Despite this, the measures of radiation exposure were significantly lower in the Fluorography group compared with the Cineangiography group.
The primary measure of patient peak skin dose was 42% lower in the Fluorography group compared with the Cineangiography group (Figure 2A). Similarly, KAP was 62% lower in the Fluorography group when compared with the Cineangiography group(Figure 2B). Patient peak skin dose and KAP significantly correlated with each other (Spearman’s rho=0.81, p<0.001). Ka,r was also significantly lower in the Fluorography group when compared with the Cineangiography group (175 mGy [112–252] vs 558 mGy [313–621], p<0.001). Median operator radiation dose exposure was non-significantly lower in the Fluorography compared with Cineangiography groups (Figure 2C).
Figure 2.
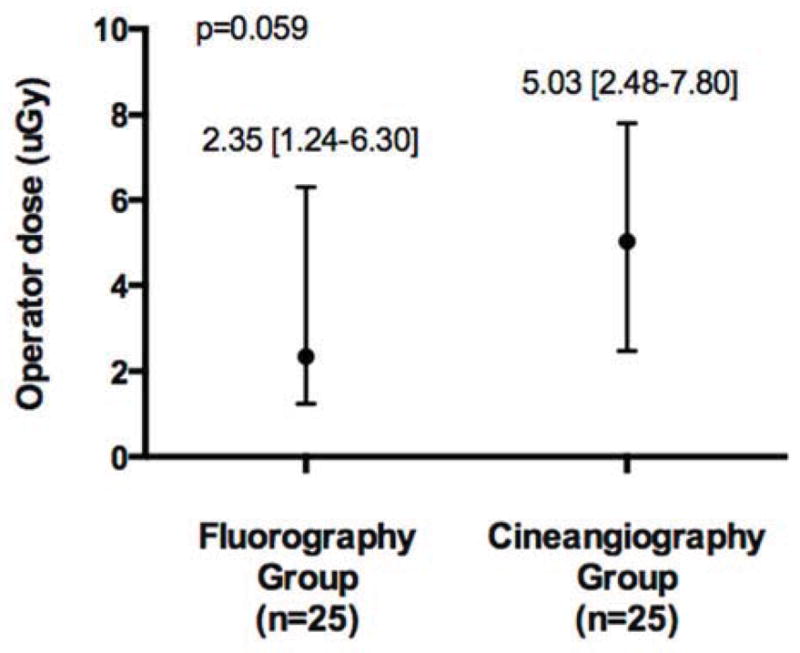
A) Patient peak skin dose, B) kerma air product, and C) operator radiation exposure in the fluorography (n=25) compared with standard cineangiography(n=25) groups (data presented as median [interquartile range] or number (percentage))
Contrast use was similar in the Fluorography compared with Cineangiography groups (57 mL [50–73] vs 51 mL [44–69], p=0.26). At 30-days, there were no clinical events in the Fluorography group and 1 event (non-cardiac death) in the Cineangiography group.
Both reviewers classified 4 out of 47 (8.5%) fluorography images as indeterminate but their corresponding cineangiography image as determinate for coronary artery lesion assessment. However, the reviewers only had 1 of these 4 fluorography images in common. (Figure 3)
Figure 3.


Examples of A) fluorography and B) corresponding cineangiography image frames used in the quality review in the current study
Discussion
This is the first randomized trial to evaluate a novel method of reducing patient and operator radiation exposure by performing coronary angiography with fluorography rather than cineangiography alone. The results demonstrate that despite the need for repeat imaging of at least 1 picture under cineangiography in 48% of patients randomized to the Fluorography group, utilizing fluorography first is still associated with a significant reduction in radiation dose exposure when compared with the current standard practice of routine cineangiography.
Effective dose is an important indicator of the global risk of radiation exposure to patients. A recent study by Smith-Bindman et al retrospectively analyzed 25.8 million person-years from different regions of the United States and demonstrated an increase in the use of advanced diagnostic imaging with an overall doubling of mean effective patient radiation dose from 1996 to 2010 (1). By the end of the study period, 6.8% and 3.9% of patients undergoing diagnostic imaging received high (>20–50 mSv) or very high (>50 mSv) annual radiation exposure, respectively, and nearly 20% of patients ≥45 years of age received high or very high radiation exposure annually. The use of angiography was associated with 14.6% of average per capita radiation dose, thereby representing a meaningful target for reducing patient radiation exposure. In the current study, using reported average KAP conversion factors (0.185 mSv Gy−1cm−1or 0.22 mSvGy−1cm−1), the median effective dose in the Fluorography group was 2.4 mSv to 2.9 mSv (equivalent of 44 chest x-rays) and Cineangiography group was 6.4 mSv to 7.6 mSv (equivalent of 117 chest x-rays) (3–4). Although relatively small absolute numbers, the additive effects with other imaging modalities or repeat angiography procedures can lead to substantial annual or lifetime exposure.
Tissue reactions, such as skin erythema, ulcers, and dermal atrophy, occur once a threshold dose is achieved, and the risk and severity of injury increases with radiation exposure (5). Although diagnostic coronary angiography alone does not commonly reach the reported thresholds for these tissue reactions, ad-hoc complex PCI is commonly performed and the combination of diagnostic coronary angiography and PCI may reach such thresholds (6–10). It is also interesting to note that with exposure of as little as 500 mGy of radiation to the chest, it is possible for microvascular damage of the myocardium to occur and perhaps accelerate the atherosclerosis process, highlighting the judicious use of radiation in younger patients (11). Malignancy and heritable effects, on the other hand, are considered stochastic effects, where risk increases with exposure, but at an unknown threshold. Any given patient undergoing a cardiovascular workup may commonly have a stress nuclear study or cardiac computed tomography examination, followed by a diagnostic coronary angiogram. In addition, almost 1/3 of patients undergo at least 2 cardiac catheterization procedures(8). Attempts at reducing radiation exposure during any one of the procedures is, therefore, increasingly important (12).
Although the sample size evaluated in this study is small, there was a low rate of fluorography images read as indeterminate by either reviewer. Moreover, physicians generally evaluate a lesion in multiple views, while, in this study, each reviewer had only 1 view to evaluate during the quality review. It is likely that if all views taken of the coronary artery of interest were available to the reviewers, the rate of lesions read as indeterminate would have been even lower. The trial enrolled a selective patient population, a large number of which did not have significant coronary artery disease, and, therefore, it is unclear if the results of this study would be applicable to all patients undergoing coronary angiography. In addition, this study was not powered to evaluate the differential impact of the Fluorography strategy on the imaging of different coronary arteries and bypass grafts or different views(straight versus oblique angles). Finally, although the use of the RadPad was encouraged, it was only used in a little more than a quarter of the patients. However, its use was exactly the same in each group.
Acknowledgments
Funding Sources
Dr. Shah was partially funded by a NIH/NHLBI grant (T32HL098129 in 2012; UL1 TR000038 in 2013).
We would like to acknowledge the contributions of Gregory Katz, MD in conducting the software set-up required to measure patient radiation exposure using radiochromic film.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith-Bindman R, Miglioretti DL, Johnson E, Lee C, Feigelson HS, Flynn M, Greenlee RT, Kruger RL, Hornbrook MC, Roblin D, Solberg LI, Vanneman N, Weinmann S, Williams AE. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousins C, Miller DL, Bernardi G, Rehani MM, Schofield P, Vano E, Einstein AJ, Geiger B, Heintz P, Padovani R, Sim KH ICRP. Radiological protection in cardiology. ICRP Publication 120. Ann ICRP. 2013;42(1) doi: 10.1016/j.icrp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Katritsis D, Efstathopoulos E, Betsou S, Korovesis S, Faulkner K, Panayiotakis G, Webb-Peploe MM. Radiation exposure of patients and coronary arteries in the stent era: A prospective study. Catheter Cardiovasc Interv. 2000;51:259–264. doi: 10.1002/1522-726x(200011)51:3<259::aid-ccd2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Leung KC, Martin CJ. Effective doses for coronary angiography. Br J Radiol. 1996;69:426–431. doi: 10.1259/0007-1285-69-821-426. [DOI] [PubMed] [Google Scholar]
- 5.Koenig TR, Wolff D, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: Part 1, characteristics of radiation injury. Am J Roentgenology. 2001;177:3–11. doi: 10.2214/ajr.177.1.1770003. [DOI] [PubMed] [Google Scholar]
- 6.Shah B, Bangalore S, Feit F, Fernandez G, Coppola J, Attubato MJ, Slater J. Radiation exposure during coronary angiography via transradial or transfemoral approaches when performed by experienced operators. Am Heart J. 2013;165:286–292. doi: 10.1016/j.ahj.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laskey WK, Williams DO, Vlachos HA, Cohen H, Holmes DR, King SB, 3rd, Kelsey SF, Slater J, Faxon D, Al-Bassam M, Block E, Detre KM. Dynamic Registry Investigators. Changes in the practice of percutaneous coronary intervention: a comparison of enrollment waves in the National Heart, Lung, and Blood Institute (NHLBI) Dynamic Registry. Am J Cardiol. 2001;87:964–969. doi: 10.1016/s0002-9149(01)01430-8. [DOI] [PubMed] [Google Scholar]
- 8.Padovani R, Bernardi G, Quai E, Signor M, Toh HS, Morocutti G, Spedicato L. Retrospective evaluation of occurrence of skin injuries in interventional cardiac procedures. Radiat Prot Dosimetry. 2005;117:247–250. doi: 10.1093/rpd/nci757. [DOI] [PubMed] [Google Scholar]
- 9.Vano E, Goicolea J, Galvan C, Gonzalez L, Meiggs L, Ten JI, Macaya C. Skin radiation injuries in patients following repeated coronary angioplasty procedures. Br J Radiol. 2001;74:1023–1031. doi: 10.1259/bjr.74.887.741023. [DOI] [PubMed] [Google Scholar]
- 10.Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326–341. doi: 10.1148/radiol.2542082312. [DOI] [PubMed] [Google Scholar]
- 11.International Commission on Radiological Protection. Statement on Tissue Reactions. ICRP; Seoul, Korea: 2011. [last accessed 23 August 2013]. Available at: < http://www.icrp.org/docs/ICRP%20Statement%20on%20Tissue%20Reactions.pdf>. [Google Scholar]
- 12.Imanishi Y, Fukui A, Niimi H, Itoh D, Nozaki K, Nakaji S, Ishizuka K, Tabata H, Furuya Y, Uzura M, Takahama H, Hashizume S, Arima S, Nakajima Y. Radiation-induced temporary hair loss as a radiation damage only occurring in patients who had the combination of MDCT and DSA. Eur Radiol. 2005;15:41–46. doi: 10.1007/s00330-004-2459-1. [DOI] [PubMed] [Google Scholar]





